Abstract
Rad52 is essential for all homologous recombination and DNA double strand break repair events in S. cerevisiae. This protein is multifunctional and contains several domains that allow it to interact with DNA as well as with different repair proteins. However, it has been unclear how Rad52 enters the nucleus. In the present study, we have used a combination of mutagenesis and sequence analysis to show that Rad52 from S. cerevisiae contains a single functional pat7 type NLS essential for its nuclear localization. The region containing the NLS seems only to be involved in nuclear transport as it plays no role in repair of MMS induced DNA damage. The NLS in Rad52 is weak, as monomeric protein species that harbor this NLS are mainly located in the cytosol. In contrast, multimeric protein complexes wherein each subunit contains a single NLSRad52 sort efficiently to the nucleus. Based on the results we propose a model where the additive effect of multiple NLSRad52 sequences in a Rad52 ring-structure ensures efficient nuclear localization of Rad52.
1. INTRODUCTION
The integrity of the genome is constantly challenged by DNA damage induced by reactive metabolic intermediates and environmental agents. Among the different types of DNA lesions that can occur, DNA double strand breaks are particularly dangerous, as they may cause cell death or provoke genomic rearrangements. In Saccharomyces cerevisiae, DNA double strand breaks are mainly repaired by pathways that involve homologous recombination (HR). HR depends on the genes of the RAD52 epistasis group, RAD50, RAD51, RAD52, RAD54, RAD55, RAD57, RAD59, RDH54, RFA1, MRE11 and XRS2 [1]. Among these, mutations in RAD52 show the most severe phenotype, reflecting the involvement of this gene in multiple HR pathways. The importance of Rad52 is stressed by the fact that it is conserved from yeast to human.
Several biochemical properties of Rad52 are germane for its HR role, including DNA binding and an ability to interact with the Rad51 recombinase, Rad59 protein, and the single-strand DNA binding protein RPA. These attributes enable Rad52 to promote the annealing of RPA-coated ssDNA and to function with Rad51 in the displacement of RPA from ssDNA [2], [3], [4], [5], [6], [7], [8], [9]. The highly conserved N-terminus of Rad52 contains domains that allow it to self-associate and form ring-structures, to bind Rad59, to bind DNA and to facilitate DNA annealing (Figure 1) [7], [2], [8], [9], [10], [11]. The middle- and C-terminal regions of yeast and human Rad52 proteins have been shown to contain the RPA and Rad51 interaction domains, respectively, but are otherwise not well conserved in primary sequence [12], [13], [2], [14, 15], (Krejci et al., submitted). Notably, it has remained unclear how Rad52 is transported into the nucleus.
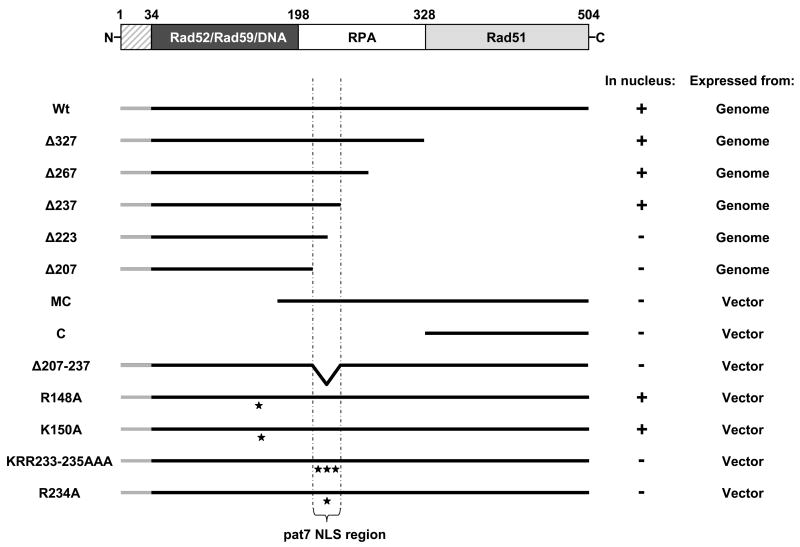
Figure 1.
Functional map of S. cerevisiae Rad52 and an overview of the cellular localization of all Rad52 mutants. A schematic representation of Rad52 from S. cerevisiae is presented in the top. The hatched region covering aa residues 1-33 is not expressed. The dark region spanning aa residues 34 – 198 corresponds to the region of Rad52 that is highly evolutionary conserved. The regions in Rad52 that are involved in protein-protein interactions and in binding to DNA are indicated. A diagram showing all individual Rad52 deletion and mutated species relative to wild-type Rad52 is presented below. All Rad52 species are C-terminally extended by YFP (not shown in the figure). Rad52-YFP (Wt), Rad52-Δ327-YFP (Δ327), Rad52-Δ267-YFP (Δ267), Rad52-Δ237-YFP (Δ237), Rad52-Δ223-YFP (Δ223), Rad52-Δ207-YFP (Δ207), Rad52MC-YFP (MC), Rad52C-YFP (C), Rad52-Δ207-237-YFP (Δ207-237), Rad52-R148A-YFP (R148A), Rad52-K150A-YFP (K150A), Rad52-KRR233-235AAA-YFP (KRR233-235AAA) and Rad52-R234A-YFP (R234A). The position of single aa residue substitutions are indicated with stars and the pat7 NLS region is indicated below the figure. To the right, the cellular localization of individual Rad52 species and whether it is expressed from the genomic RAD52 locus or from a vector is shown. Nuclear and cytosolic localization of a given Rad52 species is indicated by + and -, respectively.
Most nuclear proteins larger than 40–60 kDa require active transport to enter the nucleus [16]. This transport is facilitated by nuclear transport receptors, importins, which recognize nuclear localization signals, NLSs, which are typically composed by clusters of basic amino acid (aa) residues[16]. The complex of a cargo protein and a nuclear transport receptor is then shuttled from the cytosol into the nucleus through the nuclear pore complex by forming transient interactions with nucleoporins that line the channel of the pore. In a previous search for NLS motifs in DNA repair proteins, no putative NLS in S. cerevisiae Rad52 was identified and it was proposed that Rad52 is escorted into the nucleus via an interaction with another protein factor that harbors such a transport signal [17]. We have located the region in Rad52 required for its nuclear localization. Combining these domain mapping results with a complementary sequence analysis, we have identified a single “pat7” type NLS in Rad52 and shown that it is essential and sufficient for efficient Rad52 transport into the nucleus. Interestingly, the functionality of this NLS seems to be dependent on Rad52 oligomerization being mediated by the N-terminus of the protein.
2. MATERIALS AND METHODS
2.1 Genetic methods and strains
All media were prepared as described by Sherman [18] with minor modifications as the synthetic medium contained twice the amount of leucine (60 mg/L). All strains are isogenic to W303 [19] except they are RAD5 [20], [21], and ADE2 (see Table 1). Integrated RAD52 mutants were constructed and fused to YFP using the cloning-free PCR-based allele replacement method previously described by Erdeniz and colleagues [22], [23]. Correct integration of the mutations were verified by PCR and sequencing (MWG-Biotech AG).
Table 1.
Yeast strains used in this study.
| a Strain | Genotype |
|---|---|
| UM74-3B | MATa bar1::LEU2 his3-11,15 leu2-3,112 trp1-1 ura3-1 RAD52-YFP |
| UM94-9C | MATa his3-11,15 leu2-3,112 trp1-1 ura3-1 rad52-Δ327-YFP |
| UM93-12D | MATa his3-11,15 leu2-3,112 trp 1-1 ura3-1 rad52-Δ267-YFP |
| UM227-9D | MATalpha his3-11,15 leu2-3,112 lys2Δ ura3-1 rad52-Δ237-YFP |
| UM262-3B | MATa his3-11,15 leu2-3,112 trp1 ura3-1 rad52-Δ223-YFP |
| UM261-9C | MATalpha his3-11,15 leu2-3,112 lys2Δ ura3-1 Rad52-Δ207-YFP |
| UM69-1A | MATa bar1::LEU2 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 rad52-Δ207-CFP |
| UM94-5D | MATa his3-11,15 leu2-3,112 trp1-1 ura3-1 RAD52 |
| UM263-7C | MATalpha his3-11,15 leu2-3,112 lys2Δ ura3-1 RAD52-YFP |
| UMR128 | MATalpha bar1::LEU2 his3-11,15 trp1-1 ura3-1 rad52-R234A-YFP |
| w3849-10C | MATa bar1::LEU2 his3-11,15 ura3-1 RAD52-CFP |
| UM101-15B | MATa his3-11,15 leu2-3,112 trp1-1 ura3-1 rad52::HIS5 |
All strains are derivatives of W303-1A and W303-1B. In addition to the genotype listed above all strains are RAD5 and ADE2.
2.2 Plasmid construction
Plasmids expressing MmRad52-YFP and KlRad52-YFP
Plasmids were constructed from the CEN6 based plasmid pWJ1213 [24] by replacing S. cerevisiae RAD52 with RAD52 from Kluveromyces lactis and Mus musculus preserving the S. cerevisiae RAD52 promoter. Both genes are lacking stop codons to ensure fusion to YFP. The new vectors, pIPL2 and pIPL3, harboring RAD52-YFP from either K. lactis or M. musculus were transformed into a rad52Δstrain (UM101-15B) and nuclear localization visualized by fluorescent microscopy.
Plasmids expressing Rad52-YFP
A series of plasmids expressing Rad52-YFP with mutations in areas predicted to be involved in nuclear transport of Rad52 were constructed. All plasmids were constructed by inserting an AgeI-SphI digested RAD52 fragment into an AgeI-SphI vector fragment of pWJ1213. Plasmids harbouring rad52-R148A-YFP and rad52-K150A-YFP were constructed from two plasmids (p52mut-R148A and p52mut-K150A) previously constructed by Mortensen [25]. To create fragments encoding the mutations rad52-KRR233-235AAA, rad52-R234A and rad52-Δ207-37, sequences flanking the mutation were amplified by PCR. The primers used to construct the RAD52 plasmids are listed in supplementary material. The PCR fragments were inserted into pWJ1213 with AgeI and SphI to generate the plasmids pWJ1213-rad52-KRR233-235AAA-YFP, pWJ1213-rad52-R234A-YFP and pWJ1213-rad52-Δ207-37-YFP.
Plasmids expressing NLS-tagged Rad52-YFP mutants
To tag rad52-Δ207-237-YFP with the NLSSV40 sequence [26], [27], a PCR fragment encoding rad52-Δ207-237-YFP-NLS was constructed by PCR using pWJ1213-rad52-Δ207-237 as template. The PCR fragment was inserted into vector pWJ1213 to generate pWJ1213-rad52-Δ207-37-YFP-NLS using AgeI and XhoI. A plasmid harbouring rad52-R234A-YFP-NLS was constructed by digestion of pWJ1213-rad52-Δ207-37-YFP-NLS with SphI and XhoI. The resulting fragment was inserted into a SphI-XhoI vector fragment of pWJ1213-rad52-R234A-YFP to generate the plasmid pWJ1213-rad52-R234A-YFP-NLS.
The CEN6 based RAD52-YFP expression vector, pWJ1213, and the 2-micron-based RAD52-YFP vector pWJ1214 were used to clone rad52MC-YFP and rad52C-YFP (primers are listed in Supplementary material).
Plasmids expressing mono- and tetrameric DsRed tagged with the NLS of Rad52
Plasmids expressing either monomeric DsRed (pCupmono-DsRed-NLSRad52) or tetrameric DsRed (Pcup-tetra-DsRed-NLSRad52) tagged at the C-terminus with NLSRad52 (PNKRRQL) were constructed from pCM1513 (A kind gift from C. Müller) leading to a protein under the control of the inducible Cup1 promoter. A PCR fragment harbouring monomeric DsRed-NLSRad52 was constructed by PCR using pCM1513 as a template (primers are listed in supplementary material), and a fragment harboring tetrameric DsRed-NLSRad52 using pPgpdA-DsRed as a template [28]. Both the monomeric and the tetrameric DsRed containing PCR fragments were digested with HpaI-HindIII and ligated into a HpaI-HindIII vector fragment of pCM1513. Plasmids were transformed into the UM94-5D and nuclear localization was visualized by fluorescence microscopy.
2.3 MMS assay
To assess sensitivity of NLS-mutants to methyl methanesulfonate (MMS) (M4016 from Sigma), plasmids harbouring wild type RAD52, rad52-Δ207-37-YFP, rad52-Δ207-37-YFP-NLSSV40, rad52-R234A-YFP, rad52-R234A-YFP-NLSSV40 and an empty plasmid were transformed into a rad52Δ strain (UM101-15B). Cells were grown overnight at 30 °C in selective media (SC-His), washed with sterile water and resuspended in an appropriate volume. Subsequently, six 10-fold dilutions were made of cell suspensions containing 108 cells per ml and 5μl of each dilution was spotted on SC-His plates containing no or 0.0025% MMS. Plates were incubated at 30 °C for 2 days before examination [29].
2.4 Fluorescence microscopy and imaging
Microscopy was essentially performed as previously described [30]. Cells were grown in SC medium prior to microscopy, except when RAD52 molecules were expressed from a plasmid. In this case, cells were grown in SC-His or SC-Leu medium to select for the plasmid. When fluorescent molecules were expressed under the control of the Cup1 promoter, CuSO4 was added to the inoculation media to a final concentration of 0.2 mM Cu2+ to induce expression. In all experiments cells were grown at 23°C to allow efficient formation of the chromophore. DNA in living cells was stained for visualization by adding 10 μg/ml DAPI to the culture 30 min prior to imaging. Selected strains were made rho0 (mitochondrial DNA negative) before staining to eliminate any signal from mitochondrial DNA.
2.5 Co-expression of RAD52 strains
rad52-Δ207-CFP (UM69-1A) was crossed with UM261-9C, UM227-9D, and UM263-7C to generate diploids rad52-Δ207-CFP/rad52-Δ207-YFP, rad52-Δ207-CFP/rad52-Δ237-YFP and rad52-Δ207-CFP/RAD52-YFP. Diploid cells were grown overnight in SC media and the subcellular localization of the proteins examined by using fluorescence microscopy.
2.6 Purification of Rad52MC
(His)6-RAD52MC was constructed in pRSET-c and purified from E. coli strain Rosetta (Novagen) as described (Krejci et. al, submitted).
2.7 Gel filtration analysis of Rad52MC
(His)6-tagged Rad52MC (100 μg) was fractionated in a 36-ml Sephacryl S300 column in K buffer containing 150mM KCl, collecting 0.4 ml fractions at 0.1 ml/min. The column fractions were analyzed by 10% SDS-PAGE and staining with Coomassie Blue. The column was calibrated with thyroglobulin (663 kDa), catalase (223 kDa) and ovalbumin (43 kDa).
3. RESULTS
3.1 Nuclear localization of Rad52 is mediated by a region in the middle section of the protein
Rad52 of S. cerevisiae is predominantly localized in the nucleus in all phases of the cell cycle, even in the absence of genotoxins [23] and Figure 2. To delimit the region in Rad52 required for its nuclear localization, a series of five mutant strains expressing YFP tagged C-terminally truncated Rad52 species expressed from the endogenous RAD52 locus were constructed (Figure 1). These truncation alleles terminate at aa residue positions ranging from 207 to 327, compared to wild-type Rad52, which terminates at position 504. In addition, two N-terminally truncated species, one starting from aa residue 169 (Rad52MC-YFP) and one from aa residue 327 (Rad52C-YFP) were constructed. All mutant strains were subjected to fluorescence microscopy to determine the cellular localization of the fusion proteins. Of these truncations, Rad52-Δ327-YFP was expected to sort into the nucleus because the MMS sensitivity of a rad52-Δ327 strain has previously been shown to be fully suppressed by over-expression of Rad51 [13], [31]. In agreement with this, we find that Rad52-Δ327-YFP localizes in the nucleus. Interestingly, we find that the truncation species Rad52-Δ237-YFP and Rad52-Δ267-YFP also sort correctly into the nucleus whereas the two shortest truncations, Rad52-Δ207-YFP and Rad52-Δ223-YFP mainly localize in the cytosol (Figure 1, 2 and supplementary material). In addition, the N-terminal truncations Rad52MC-YFP and Rad52C-YFP also fail to sort efficiently to the nucleus. To rule out the possibility that mis-sorting of the Rad52 truncation species could be explained by their nuclear transport being compromised by the YFP moiety of the fusion protein, we constructed a Rad52-YFP species containing an internal deletion, Rad52-Δ207-237-YFP, and expressed it from a single copy plasmid in a rad52Δ strain. This species also fails to sort correctly to the nucleus (Figure 1). Together these results indicate that a region involved in nuclear localization is located between or close to aa residues 207-237, a region of the protein that has not previously been assigned any Rad52 function. However, the fact that Rad52MC-YFP, which contains aa residues 207-237, accumulates in the cytosol (Figure 1 and 2) suggests that nuclear localization of Rad52 may also depend on other Rad52 features, see below.
Figure 2.
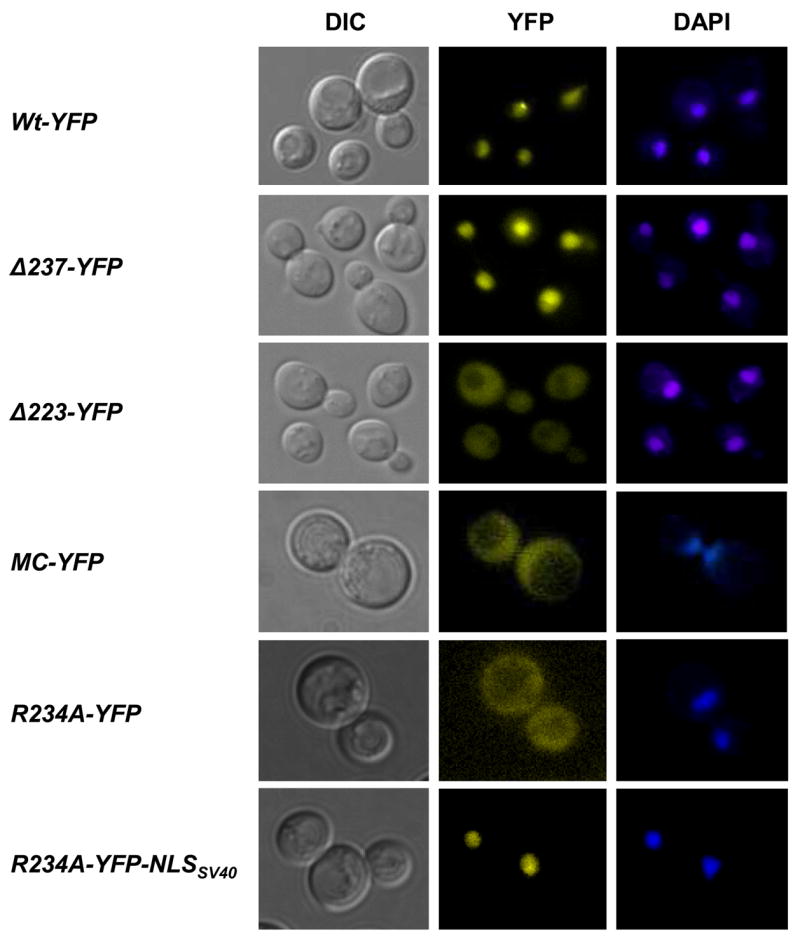
Cellular localization of Rad52 mutant proteins. Microscopy of cells expressing Rad52-YFP or corresponding mutant derivatives as indicated. Pictures shown are pseudocolored monochrome images.
3.2 A nuclear localization signal is present in the middle of Rad52
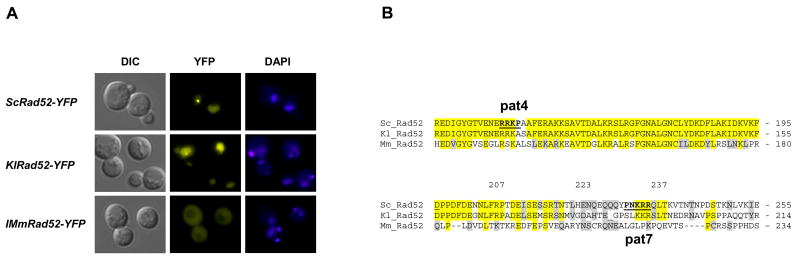
Next, we investigated the possibility that the domain responsible for nuclear localization of Rad52 is sufficiently conserved to retain interspecies functionality. Hence, K. lactis and M. musculus Rad52 species (KlRad52 and MmRad52) tagged with YFP were individually expressed under the control of the Saccharomyces cerevisiae RAD52 promoter from single copy plasmids in S. cerevisiae rad52Δ strains. When these strains were examined by fluorescence microscopy, we observed that KlRad52-YFP locates in the nucleus whereas MmRad52-YFP mainly remains in the cytosol (Figure 3A) suggesting that a common mechanism ensures nuclear localization of Rad52 and KlRad52, but not MmRad52, in S. cerevisiae. In agreement with this view, sequence comparisons of the aa residues 207-237 in Rad52 to the corresponding Rad52 sequences from K. lactis and M. musculus show that only Rad52 and KlRad52 share identical stretches of aa residues in this region (Figure 3B). Importantly, we find a proline residue at position 231 in Rad52 that is situated close to a stretch of three positively charged aa residues (PNKRR). At the corresponding position, a similar motif is present in KlRad52 (PSLKKR), but not in MmRad52. Both motifs qualify as pat7 NLS sequences [32] and therefore constitute sequences that may be involved in nuclear localization of KlRad52 and Rad52 in S. cerevisiae. The fact that the putative NLS sequence in Rad52 (and in KlRad52) only contains three basic aa residues explains why it was not recognized in the more stringent NLS search performed previously by Boulikas [33], [17] who defined a monopartite NLS motif as a cluster of at least four basic residues within a hexapeptide. The existence of a pat7 type NLS in Rad52 prompted us to perform a new sequence search for other putative NLS sequences in the entire Rad52 primary sequence using two different algorithms PSORT II and Predict NLS [32], [34]. Using PSORTII, one additional candidate sequence, a pat4 sequence (RRKP) [32] at position 148-151 was identified. However, this motif is not conserved in KlRad52 and MmRad52 (Figure 3B).
Figure 3.
Localization of Rad52, KlRad52 and MmRad52 in S. cerevisiae cells. (A) rad52Δ cells expressing S. cerevisiae, K. lactis or M. musculus Rad52-YFP fusion proteins from plasmids as indicated.. Pictures shown are pseudocolored monochrome images. (B) A sequence comparison of the region of Rad52, which is important for nuclear targeting, to the corresponding regions of KlRad52 and MmRad52. Identical or similar aa residues are highlighted by yellow and grey, respectively. The positions of the two predicted NLS sequences, pat4 and pat7, in S. cerevisiae Rad52 are indicated as bold underscored aa residues.
3.3 A single NLS ensures nuclear localization of yeast Rad52
Three basic aa residues (K233-R234-R235) constitute the core of the predicted pat7 NLS identified in Rad52. To verify that these aa residues in fact are part of an NLS, the triple mutant Rad52-KRR233-235AAA-YFP and the single mutant Rad52-R234A-YFP were individually expressed from single copy plasmids in rad52Δ strains. In agreement with this motif being an NLS, both Rad52-KRR233-235AAA-YFP and Rad52-R234A-YFP were observed to remain mainly in the cytosol (Figure 1 and 2). To rule out the possibility that mis-sorting was due to the Rad52 mutants being expressed from a plasmid, the R234A mutation was introduced into the genomic version of RAD52-YFP. In the resulting strain, Rad52-R234A-YFP was also observed to localize in the cytosol (unpublished data). Next, we investigated the role of the pat4 NLS motif spanning aa residues 148 -151 in nuclear sorting. Two mutant Rad52 species, Rad52-R148A-YFP and Rad52-K150A-YFP, were expressed from plasmids in rad52Δ strains and their cellular location determined. Both mutants were found to be present in the nucleus showing that this pat4 NLS motif plays little or no role in the nuclear localization of Rad52 (Figure 1). In fact, this result was expected as two Rad52 mutant strains, rad52-R148A and rad52-R149A, were previously shown to repair γ-ray induced damage and to perform mitotic homologous recombination at wild-type levels [25] showing that sufficient Rad52 protein must reach the nucleus to maintain these functions. We therefore conclude that a single NLS of the pat7 type spanning aa residues 231-235 is responsible for targeting Rad52 to the nucleus.
3.4 The NLSRad52 can direct a heterologous cytosolic protein to the nucleus
To demonstrate that the PNKRR motif in Rad52 acts as a functional NLS, we extended the C-terminus of monomeric DsRed, which is normally located in the cytosol with an NLSRad52, and asked whether the presence of this motif would direct it to the nucleus. Surprisingly, this species fails to sort efficiently to the nucleus (Figure 4). In contrast, if monomeric DsRed is C-terminally fused to the well characterized NLS sequence from SV40 virus, NLSSV40 [26, 27], then monomeric DsRed localizes in the nucleus (data not shown). This result suggests that a single NLSRad52 is insufficient to mediate the transport of monomeric DsRed to the nucleus. Since some proteins contain several NLS sequences to ensure efficient nuclear sorting, we fused NLSRad52 to the C-terminus of tetrameric DsRed to determine whether this protein complex that contains a total of four NLSRad52 sequences would localize to the nucleus. Unlike monomeric DsRed-NLSRad52 tetrameric DsRed-NLSRad52 is highly concentrated in the nucleus (Figure 4) showing that the NLSRad52 can act as a functional NLS if it is present in one copy in each of the subunits of a homo-multimeric protein complex.
Figure 4.
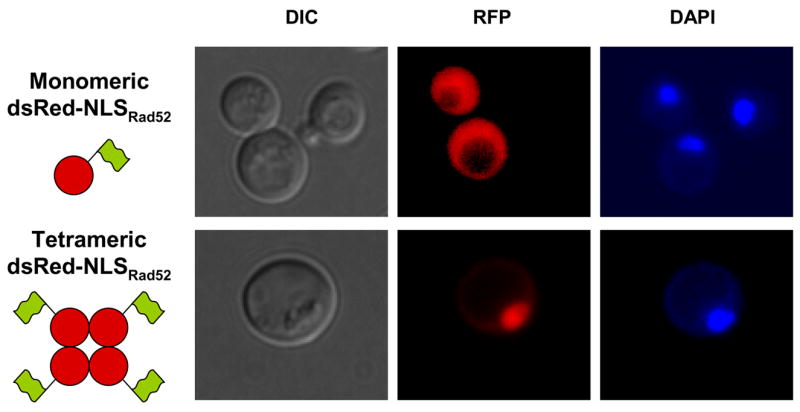
The pat7 NLS sequence in Rad52 is a functional NLS. The cellular localization of monomeric and tetrameric DsRed tagged with NLSRad52. DsRed is depicted as a filled red circle and the NLSRad52 sequences as green flags.
3.5 The role of Rad52 multimerization in nuclear localization of Rad52
Rad52 exists as heptameric ring-structure and the observation that the NLSRad52 promotes efficient nuclear localization of tetrameric, but not monomeric, DsRed suggests that Rad52 multimerization may also be important for the nuclear localization of Rad52. It would therefore be interesting to test whether a Rad52 mutant that fails to form ring-structures, yet contains the NLSRad52, sorts to the nucleus. In fact, the fragment Rad52MC–YFP described above may represent such a mutation as it lacks the N-terminal self-association domain responsible for ring-structure formation, but contains the NLSRad52 sequence. Importantly, this Rad52 fragment fails to concentrate in the nucleus (Figure 1 and 2). Since the C-terminus of human Rad52 contains a self-association domain that allows Rad52 ring structures to further multimerize [12], we tested whether Rad52MC exists as a monomer or a multimer as judged by a gel-filtration experiment. As shown in Figure 5A, Rad52MC elutes at a volume corresponding to that expected if it forms a tetra- or pentamer. To investigate whether this C-terminal self-association domain contributes to the nuclear localization of Rad52, we co-expressed Rad52MC-YFP and Rad52-CFP in the same strain. Of these two protein species, only Rad52-CFP concentrates in the nucleus (Figure 5B) indicating that the interaction between Rad52MC-YFP and Rad52-CFP is not sufficiently strong to ensure Rad52-CFP mediated nuclear localization of Rad52MC-YFP. Similarly, MC-NLSSV40 fails to mediate nuclear localization of Rad52-R234A-YFP (data not shown). Finally, we asked whether the ability of a sorting defective Rad52 mutant to participate in a multimeric ring-structure could promote its nuclear localization if it is co-expressed with a sorting proficient Rad52 species. This possibility was tested by co-expressing Rad52-Δ207-CFP with Rad52-Δ237-YFP and by co-expressing Rad52-Δ207-CFP together with wild-type Rad52-YFP. In both cases, the NLS defective Rad52 mutants were mainly located in the nucleus (Figure 5C) showing that Rad52 multimerization may facilitate nuclear targeting.
Figure 5.
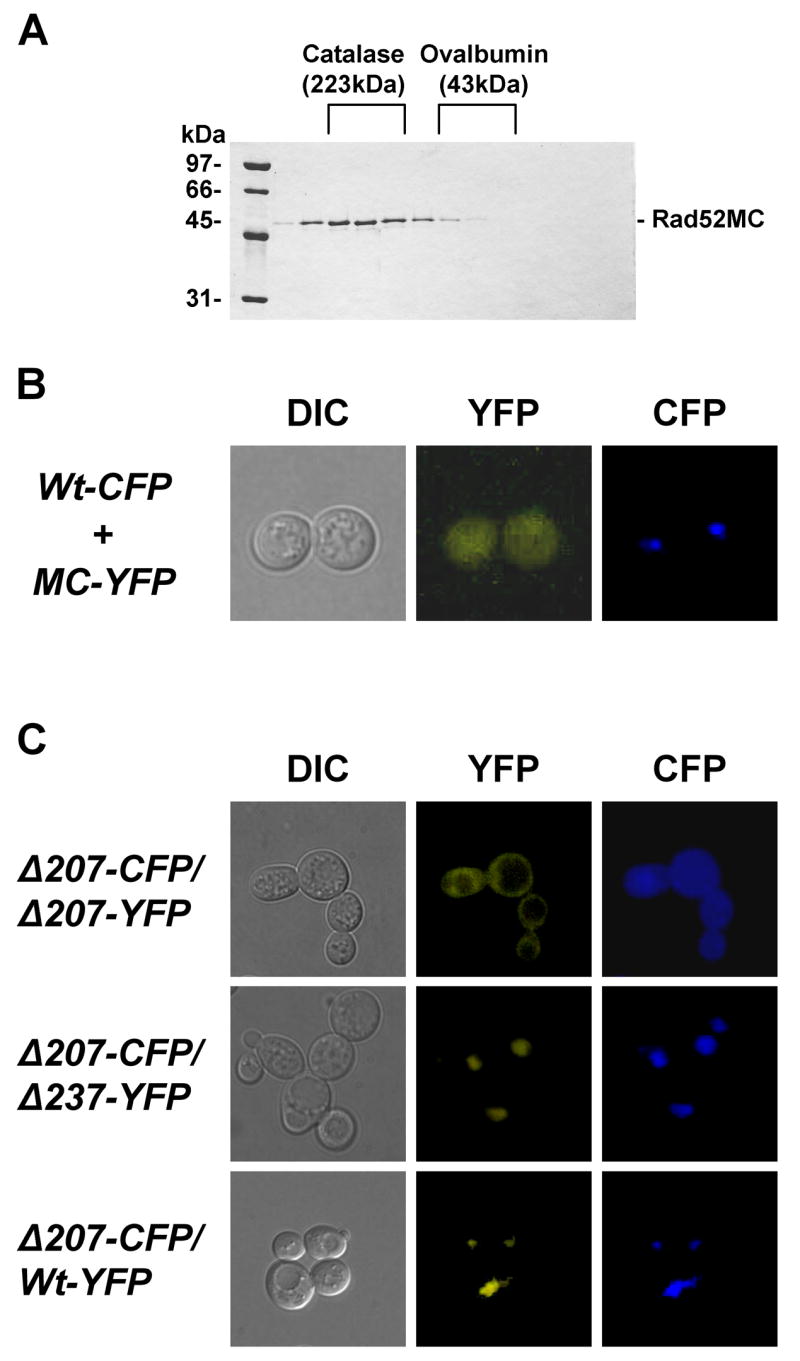
Rad52 self-association mediates nuclear transport. (A) Elution profile of Rad52MC after gelfiltration. Fractions eluting at the same volume as the marker proteins catalase and ovalbumin are indicated. (B) Expression of Rad52MC-YFP from a plasmid in a RAD52-CFP strain. Co-localization of Rad52 species tagged with either CFP or YFP. (C) Microscopy of heterozygous diploid strains co-expressing Rad52-Δ207-CFP and Rad52-Δ207-YFP, Rad52-Δ207-CFP and Rad52-Δ237-YFP and Rad52-Δ207-CFP and Rad52-YFP.
3.6 The NLS region of Rad52 is not involved in the DNA DSB repair functions of Rad52
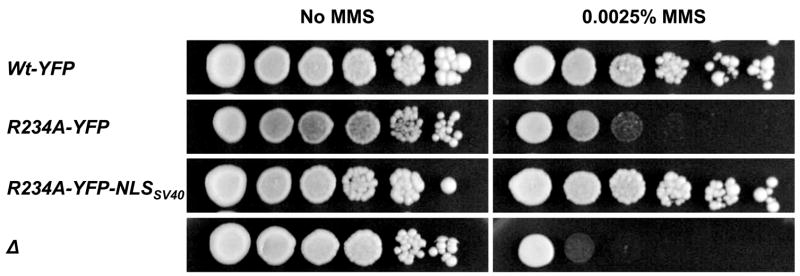
Finally, we investigated whether the region of Rad52 containing the NLS motif participates directly in DNA repair in addition to its role in nuclear transport. To address this possibility, we tagged Rad52-R234A-YFP C-terminally with the NLSSV40 to mediate its nuclear transport by a non-Rad52 sequence. As expected, this fusion protein is indeed concentrated in the nucleus (Figure 2). The ability of the resulting strain, rad52-R234A-YFP-NLSSV40, to perform Rad52 functions during repair of MMS induced damage was determined and compared to that of rad52-R234A-YFP. As expected rad52-R234A-YFP strains, like rad52Δ strains, are sensitive to MMS reflecting the absence of Rad52 in the nucleus. In contrast, rad52-R234A-YFP-NLSSV40 strains, like wild-type strains, were not affected by this treatment (Figure 6). In fact, it is possible to delete the entire region, aa residues 207-237, and still maintain efficient DNA repair, as rad52Δ strains transformed with a plasmid expressing Rad52-Δ207-237-YFP-NLSSV40 are resistant to MMS whereas rad52Δ strains expressing Rad52-Δ207-237-YFP are not (Supplementary material). These results show that the NLS region in Rad52 does not contribute directly to repair of MMS induced DNA damage.
Figure 6.
A mutation in the pat7 NLS sequence in Rad52 does not affect its ability to repair MMS induced DNA damage. A spot assay of rad52Δ strains transformed with plasmids expressing RAD52-YFP rad52-R234A-YFP or rad52-R234A-YFP-NLSSV40 as indicated. A strain transformed with an empty plasmid is also included in the analysis. Serial 10-fold dilutions of each transformed strain were spotted on selective media containing either no or 0.0025% MMS. Pictures were captured after 2 days.
4. DISCUSSION
In the present study, we have explored the mechanism of Rad52 nuclear localization by using a combination of mutagenesis and sequence analysis. We have identified a single pat7 type NLS, aa residues 231-235 (PNKRR), which is required and sufficient for efficient nuclear localization of Rad52. This NLSRad52 is a functional NLS sequence as tetrameric DsRed tagged with NLSRad52 is sorted efficiently to the nucleus. In some cases, an NLS sequence may provide a dual function in a protein. For example, in many proteins the NLS sequences overlap nucleic acid binding domains [35]. We observed, that Rad52 mutants, where the NLSRad52 has been eliminated, efficiently repair MMS induced DNA damage when they are tagged with NLSSV40. This suggests that the NLSRad52 only contributes to nuclear sorting of Rad52.
The observation that the NLSRad52 only contains three basic aa residues suggests that it may be a weak signal for nuclear transport. In agreement with this, we find that a single NLSRad52, in contrast to NLSSV40, does not mediate efficient nuclear localization of monomeric DsRed. In addition, the presence of four weak NLSRad52 sequences in tetrameric DsRed-NLSRad52 efficiently mediates nuclear transport of this protein complex. This result predicts that individual Rad52 subunits also do not accumulate in the nucleus. In agreement with this view, we observed that the Rad52 N-terminal truncation, Rad52MC-YFP, fails to sort to the nucleus despite the fact that it contains the NLSRad52 (Figure 2). This Rad52 mutant protein does not contain the N-terminal protein oligomerization domains responsible for ring-structure formation [7], [8], [9], [15], [36] and the self-association of the MC part of Rad52 observed in vitro ([12] and this study) does not seem to be sufficient for nuclear localization in vivo. Taken together, we conclude that Rad52 multimerization is an essential aspect of the nuclear localization mechanism of Rad52 because the NLSRad52 is a weak NLS.
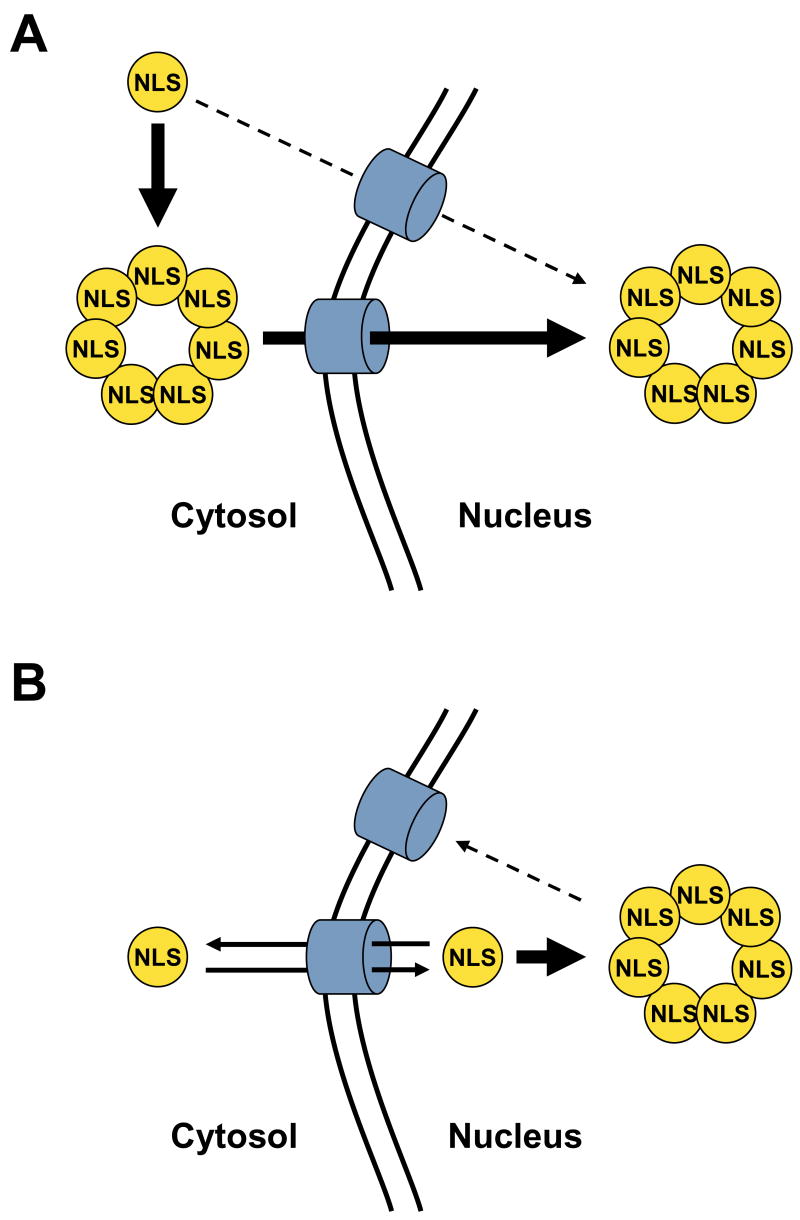
Two models can explain how Rad52 multimerization drives efficient nuclear localization (see Figure 7). In the first model, the additive NLS signal model (Figure 7A), the heptameric ring is formed in the cytosol, and the additive effect of seven weak NLS sequences within a single Rad52 ring-structure drives efficient nuclear targeting by e.g. increasing the frequency of interactions to the nuclear transport receptors. Rad52 monomers remain in the cytoplasm because each monomer only contains a single weak NLS of Rad52, which is insufficient to mediate any significant transport into the nucleus. In the second model, the nuclear retention model (Figure 7B), the heptameric ring is formed in the nucleus. In this model, the weak Rad52-NLS allows Rad52 to enter the nucleus at a slow rate as a monomer. Moreover, in this model, Rad52 monomers exit the nucleus at a similar rate preventing monomers from accumulating in the nucleus. In contrast, Rad52 subunits, which are incorporated into heptameric ring-structures, are trapped in the nucleus e.g. due to the larger size of this complex compared to monomers. A similar model has been proposed to explain nuclear localization of the pathogenic fusion protein CBFβ-SMMHC (core binding factor beta fused to smooth muscle myosin heavy chain) [37].
Figure 7.
The illustrations show two models explaining how Rad52 multimerization may facilitate nuclear localization of Rad52. Rad52 monomers are depicted as filled yellow circles. The fact that each Rad52 molecule contains a single NLS sequence is indicated by the presence of a single “NLS” in each Rad52 monomer. The nuclear pore complex is shown as blue cylinders. (A) The additive NLS model. (B) The nuclear retention model. The models are explained in detail in the text.
The effects predicted by the two models may both contribute to the nuclear localization of Rad52. However, we note that the nuclear retention model does not easily explain the observation that Rad52-Δ207-CFP and Rad52-Δ237-YFP both localize in the nucleus when they are co-expressed. For example, in the nuclear retention model, Rad52-Δ207-CFP enters the nucleus independently of Rad52-Δ237-YFP. Hence, Rad52-Δ207-CFP must be able to enter the nucleus independently of the NLSRad52 sequence. This is unlikely as the size of this fusion protein is approximately 47 kDa. Moreover, since Rad52-Δ207-CFP does not accumulate in the nucleus when it is expressed on its own, the retention model predicts that Rad52-Δ207-CFP fails to multimerize in the nucleus since it is not retained. This seems unlikely as the nuclear retention model at the same time predicts that Rad52-Δ207-CFP must be efficiently incorporated into multimers together with Rad52-Δ237-YFP to ensure its nuclear retention in the co-expression experiment. In contrast, in the additive NLS model, efficient co-localization of Rad52-Δ207-CFP and Rad52-Δ237-YFP in the nucleus is easily explained. In this model Rad52-Δ207-CFP and Rad52-Δ237-YFP both form ring-structures in the cytosol, but the Rad52-Δ207-CFP ring-structures do not enter the nucleus, as they do not contain any NLS sequences. However, when the two species are co-expressed they form chimeric multimers in the cytosol that enter the nucleus via NLS sequences present in the Rad52-Δ237-YFP molecules. In this context it is important to note that the DsRed-NLSRad52 tetramer efficiently sorts to the nucleus showing that four NLSRad52 sequences are adequate for efficient nuclear transport. Since wild-type Rad52 rings contain seven [36], and the Rad52 truncations may even contain a higher number of subunits, [12], [38], [39] then most chimeric complexes should have enough NLSRad52 sequences to relocate efficiently to the nucleus. Finally, the nuclear retention model would likley depend on a mechanism preventing Rad52 from multimerizing in the cytoplasm to ensure efficient sorting. A similar level of complication is not required if nuclear transport occurs according to the additive NLS model. For these reasons, we favour a scenario where the mechanism predicted by the additive NLS signal model provides the driving force of Rad52 nuclear targeting.
The ability of Rad52 to form ring-like structures is evolutionary conserved from yeast to humans [40], [9], [12], which raises the question whether the nuclear sorting mechanism is also conserved. A pat7 NLS sequence similar to the one in S. cerevisiae, is present at the same position in Rad52 from K. lactis, A. gossypii and C. glabrata suggesting that the mechanism of nuclear transport of Rad52 is conserved in these yeasts. In fact, we find that KlRad52 is sorted to the nucleus of S. cerevisiae. This result was expected as it has previously been demonstrated that expression of KlRad52 in S. cerevisiae partially suppresses the severe phenotype of rad52Δ strains [13]. On the other hand, Rad52 homologs from higher eukaryotes, e.g. chicken, zebra fish, mouse and human do not contain an NLS at this position, but rather they have a single putative NLS sequence in their C-terminal end suggesting that the Rad52 nuclear sorting mechanism may have diverged during evolution. Whether efficient nuclear targeting of Rad52 in higher eukaryotes is dependent on Rad52 multimerization therefore remains elusive. Lastly, S. cerevisiae contains a Rad52 homolog, Rad59 [41]. Inspection of the Rad59 sequence by PSORT II and Predict NLS did not reveal any NLS (data not shown). Interestingly, it has previously been observed that Rad59 fails to localize to the nucleus in the absence of Rad52 [42], and since Rad52 and Rad59 interacts physically [10] it is likely that Rad52 and Rad59 are transported to the nucleus as a complex..
Supplementary Material
Suppl. Table 1. Primers used in this study. Adaptamer sequences are shown in small letters.
Suppl. 1. Localization of Rad52 mutant proteins in cells. Microscopy of cells expressing Rad52-Δ327-YFP, Rad52-Δ267-YFP and Rad52-Δ207-YFP, respectively. Pictures shown are pseudocolored monochrome images.
Suppl. 2. A deletion in the middle of the Rad52 sequence does not affect its ability to repair MMS induced DNA damage. A spot assay of rad52Δ strains transformed with plasmids expressing RAD52-YFP, rad52-Δ207-37-YFP and rad52-Δ207-37-YFP-NLSSV40 as indicated. A strain transformed with an empty plasmid is also shown. Serial 10-fold dilutions of each transformed strain were spotted on selective media containing either no or 0.0025% MMS. Pictures were captured after 2 days.
Acknowledgments
This work was supported by the Danish Research Council for Technology and Production Sciences (U.H.M), The Alfred Benzon Foundation (U.H.M), The Hartmann Foundation (U.H.M), The Technical University of Denmark (S.C.L.H), The Danish Natural Science Research Council (M.L), The Villum Kann Rasmussen Foundation (M.L), and NIH grants ES07061 (P.S), GM50237 and GM67055 (R.R). We thank Elvira Chapka for excellent technical help and members of the Mortensen laboratory for comments on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Symington LS. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol Mol Biol Rev. 2002;66:630–70. doi: 10.1128/MMBR.66.4.630-670.2002. table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortensen UH, Bendixen C, Sunjevaric I, Rothstein R. DNA strand annealing is promoted by the yeast Rad52 protein. Proc Natl Acad Sci U S A. 1996;93:10729–10734. doi: 10.1073/pnas.93.20.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugiyama T, New JH, Kowalczykowski SC. DNA annealing by RAD52 protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc Natl Acad Sci U S A. 1998;95:6049–6054. doi: 10.1073/pnas.95.11.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kagawa W, Kurumizaka H, Ikawa S, Yokoyama S, Shibata T. Homologous pairing promoted by the human Rad52 protein. J Biol Chem. 2001;276:35201–35208. doi: 10.1074/jbc.M104938200. [DOI] [PubMed] [Google Scholar]
- 5.Sung P. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J Biol Chem. 1997;272:28194–28197. doi: 10.1074/jbc.272.45.28194. [DOI] [PubMed] [Google Scholar]
- 6.New JH, Sugiyama T, Zaitseva E, Kowalczykowski SC. Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A. Nature. 1998;391:407–410. doi: 10.1038/34950. [DOI] [PubMed] [Google Scholar]
- 7.Shen Z, Peterson SR, Comeaux JC, Zastrow D, Moyzis RK, Bradbury EM, Chen DJ. Self-association of human RAD52 protein. Mutat Res. 1996;364:81–89. doi: 10.1016/0921-8777(96)00025-0. [DOI] [PubMed] [Google Scholar]
- 8.Shinohara A, Shinohara M, Ohta T, Matsuda S, Ogawa T. Rad52 forms ring structures and co-operates with RPA in single-strand DNA annealing. Genes Cells. 1998;3:145–156. doi: 10.1046/j.1365-2443.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- 9.Van DE, Hajibagheri NM, Stasiak A, West SC. Visualisation of human rad52 protein and its complexes with hRad51 and DNA. J Mol Biol. 1998;284:1027–1038. doi: 10.1006/jmbi.1998.2203. [DOI] [PubMed] [Google Scholar]
- 10.Davis AP, Symington LS. The yeast recombinational repair protein Rad59 interacts with Rad52 and stimulates single-strand annealing. Genetics. 2001;159:515–525. doi: 10.1093/genetics/159.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes-Ledesma F, Malagon F, Aguilera A. A novel yeast mutation, rad52-L89F, causes a specific defect in Rad51-independent recombination that correlates with a reduced ability of Rad52-L89F to interact with Rad59. Genetics. 2004;168:553–557. doi: 10.1534/genetics.104.030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ranatunga W, Jackson D, Lloyd JA, Forget AL, Knight KL, Borgstahl GE. Human RAD52 exhibits two modes of self-association. J Biol Chem. 2001;276:15876–15880. doi: 10.1074/jbc.M011747200. [DOI] [PubMed] [Google Scholar]
- 13.Milne GT, Weaver DT. Dominant negative alleles of RAD52 reveal a DNA repair/recombination complex including Rad51 and Rad52. Genes Dev. 1993;7:1755–1765. doi: 10.1101/gad.7.9.1755. [DOI] [PubMed] [Google Scholar]
- 14.Krejci L, Song B, Bussen W, Rothstein R, Mortensen UH, Sung P. Interaction with Rad51 is indispensable for recombination mediator function of Rad52. J Biol Chem. 2002;277:40132–40141. doi: 10.1074/jbc.M206511200. [DOI] [PubMed] [Google Scholar]
- 15.Park MS, Ludwig DL, Stigger E, Lee SH. Physical interaction between human RAD52 and RPA is required for homologous recombination in mammalian cells. J Biol Chem. 1996;271:18996–19000. doi: 10.1074/jbc.271.31.18996. [DOI] [PubMed] [Google Scholar]
- 16.Gorlich D, Mattaj IW. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 17.Boulikas T. Nuclear import of DNA repair proteins. Anticancer Res. 1997;17:843–863. [PubMed] [Google Scholar]
- 18.Sherman FGHJ. Methods in Yeast Genetics. Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 19.Thomas BJ, Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989;56:619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- 20.Fan HY, Cheng KK, Klein HL. Mutations in the RNA polymerase II transcription machinery suppress the hyperrecombination mutant hpr1 delta of Saccharomyces cerevisiae. Genetics. 1996;142:749–759. doi: 10.1093/genetics/142.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou H, Rothstein R. Holliday junctions accumulate in replication mutants via a RecA homolog-independent mechanism. Cell. 1997;90:87–96. doi: 10.1016/s0092-8674(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 22.Erdeniz N, Mortensen UH, Rothstein R. Cloning-free PCR-based allele replacement methods. Genome Res. 1997;7:1174–1183. doi: 10.1101/gr.7.12.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lisby M, Rothstein R, Mortensen UH. Rad52 forms DNA repair and recombination centers during S phase. Proc Natl Acad Sci U S A. 2001;98:8276–8282. doi: 10.1073/pnas.121006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Q, During L, de Mayolo AA, Lettier G, Lisby M, Erdeniz N, Mortensen UH, Rothstein R. Rad52 and Rad59 exhibit both overlapping and distinct functions. DNA Repair (Amst) 2007;6:27–37. doi: 10.1016/j.dnarep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen UH, Erdeniz N, Feng Q, Rothstein R. A molecular genetic dissection of the evolutionarily conserved N terminus of yeast Rad52. Genetics. 2002;161:549–562. doi: 10.1093/genetics/161.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalderon D, Richardson WD, Markham AF, Smith AE. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984;311:33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- 27.Kalderon D, Smith AE. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984;139:109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- 28.Mikkelsen L, Sarrocco S, Lubeck M, Jensen DF. Expression of the red fluorescent protein DsRed-Express in filamentous ascomycete fungi. FEMS Microbiol Lett. 2003;223:135–139. doi: 10.1016/S0378-1097(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 29.Prakash L, Prakash S. Isolation and characterization of MMS-sensitive mutants of Saccharomyces cerevisiae. Genetics. 1977;86:33–55. doi: 10.1093/genetics/86.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lisby M, ntunez de MA, Mortensen UH, Rothstein R. Cell cycle-regulated centers of DNA double-strand break repair. Cell Cycle. 2003;2:479–483. [PubMed] [Google Scholar]
- 31.Asleson EN, Okagaki RJ, Livingston DM. A core activity associated with the N terminus of the yeast RAD52 protein is revealed by RAD51 overexpression suppression of C-terminal rad52 truncation alleles. Genetics. 1999;153:681–692. doi: 10.1093/genetics/153.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks GR, Raikhel NV. Protein import into the nucleus: an integrated view. Annu Rev Cell Dev Biol. 1995;11:155–188. doi: 10.1146/annurev.cb.11.110195.001103. [DOI] [PubMed] [Google Scholar]
- 33.Boulikas T. Nuclear localization signals (NLS) Crit Rev Eukaryot Gene Expr. 1993;3:193–227. [PubMed] [Google Scholar]
- 34.Cokol M, Nair R, Rost B. Finding nuclear localization signals. EMBO Rep. 2000;1:411–415. doi: 10.1093/embo-reports/kvd092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacasse EC, Lefebvre YA. Nuclear localization signals overlap DNA- or RNA-binding domains in nucleic acid-binding proteins. Nucleic Acids Res. 1995;23:1647–1656. doi: 10.1093/nar/23.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stasiak AZ, Larquet E, Stasiak A, Muller S, Engel A, Van Dyck E, West SC, Egelman EH. The human Rad52 protein exists as a heptameric ring. Curr Biol. 2000;10:337–340. doi: 10.1016/s0960-9822(00)00385-7. [DOI] [PubMed] [Google Scholar]
- 37.Kummalue T, Lou J, Friedman AD. Multimerization via its myosin domain facilitates nuclear localization and inhibition of core binding factor (CBF) activities by the CBFbeta-smooth muscle myosin heavy chain myeloid leukemia oncoprotein. Mol Cell Biol. 2002;22:8278–8291. doi: 10.1128/MCB.22.23.8278-8291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kagawa W, Kurumizaka H, Ishitani R, Fukai S, Nureki O, Shibata T, Yokoyama S. Crystal structure of the homologous-pairing domain from the human Rad52 recombinase in the undecameric form. Mol Cell. 2002;10:359–371. doi: 10.1016/s1097-2765(02)00587-7. [DOI] [PubMed] [Google Scholar]
- 39.Singleton MR, Wentzell LM, Liu Y, West SC, Wigley DB. Structure of the single-strand annealing domain of human RAD52 protein. Proc Natl Acad Sci U S A. 2002;99:13492–13497. doi: 10.1073/pnas.212449899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinohara A, Ogawa T. Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature. 1998;391:404–407. doi: 10.1038/34943. [DOI] [PubMed] [Google Scholar]
- 41.Bai Y, Symington LS. A Rad52 homolog is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev. 1996;10:2025–2037. doi: 10.1101/gad.10.16.2025. [DOI] [PubMed] [Google Scholar]
- 42.Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Table 1. Primers used in this study. Adaptamer sequences are shown in small letters.
Suppl. 1. Localization of Rad52 mutant proteins in cells. Microscopy of cells expressing Rad52-Δ327-YFP, Rad52-Δ267-YFP and Rad52-Δ207-YFP, respectively. Pictures shown are pseudocolored monochrome images.
Suppl. 2. A deletion in the middle of the Rad52 sequence does not affect its ability to repair MMS induced DNA damage. A spot assay of rad52Δ strains transformed with plasmids expressing RAD52-YFP, rad52-Δ207-37-YFP and rad52-Δ207-37-YFP-NLSSV40 as indicated. A strain transformed with an empty plasmid is also shown. Serial 10-fold dilutions of each transformed strain were spotted on selective media containing either no or 0.0025% MMS. Pictures were captured after 2 days.