Abstract
Age-related changes in the hippocampus increase vulnerability to impaired learning and memory. Our goal is to understand how a genetic vulnerability to cognitive impairment can be modified by aging and sex. Mice with a mutation in the cAMP response element binding (CREB) protein gene (CREBαδ- deficient mice) have a mild cognitive impairment and show test condition-dependent learning and memory deficits. We tested 3 ages of CREBαδ- deficient and wild-type (WT) mice in 2 Morris water maze (MWM) protocols: MWM4 and MWM2. All CREBαδ- deficient mice performed well in the easier MWM4, except for the aged females that performed poorly. In the harder MWM2, young male and female and middle-aged male CREBαδ- deficient mice performed well, but aged male and all middle-aged and aged female CREBαδ- deficient mice were impaired. These results show that mice with a genetic vulnerability to impaired learning and memory exhibit increased vulnerability with age that is most apparent among females. Thus, a genetic predisposition to cognitive impairment may render females more vulnerable than males to such deficits with age.
Keywords: Aging, Sex Differences, Spatial, Learning, Memory, Morris water maze
The rising number of older people in the population has generated an increased awareness of age-related alterations in cognitive ability, especially learning and memory deficits. These deficits have a profound impact on the quality of life for elderly adults, their families and caregivers, and a huge economic impact on the health care system. Not all elderly people show cognitive impairment, but a subset of the elderly exhibit a wide range of cognitive alterations from the recently accepted syndrome mild cognitive impairment to progressive, debilitating, neurodegenerative dementias such as Alzheimer’s disease (Grundman et al, 2004; Rivas-Vazquez et al, 2004). In normal aging and age-related neurodegenerative processes such as AD, the earliest reported symptom is most commonly the inability to learn and recall new information. Exploring the mechanisms involved in age-related learning and memory alterations is critical, including determination of neuropsychological, biochemical, and molecular criteria for distinguishing normal cognitive aging from mild cognitive impairment and predicting degree of vulnerability to various cognitive impairments such as dementia. In particular, animal models can be used to better define the interplay between genetic vulnerability and environmental factors, including the impact of experience and physiological factors such as gonadal hormones.
Normal brain aging has been associated with a decline in some cognitive abilities but stability or even improvement in others. For example, processing speed decreases, while language abilities remain stable (Ganguli et al, 1991). Cognitive abilities of men and women that change with age have been generally found to show parallel patterns and similar rates of decline with normal aging (Aartsen et al, 2004; Finkel et al, 2006; Gerstorf et al, 2006), and gender differences in cognitive abilities such as the female advantage in verbal memory are maintained into old age. Spatial tasks, in which men typically perform better than women, were not included in many of these studies, but a group of researchers has found that the robust sex difference favoring males remained despite the impact of aging on spatial learning and memory (Driscoll et al, 2003, 2005).
Despite broadly comparable patterns of cognitive decline with normal aging for males and females, some groups of females may be more vulnerable than males to cognitive dysfunction as they age. Most remarkable is the observation that Alzheimer’s Disease is 2-3 fold higher in women than in men after the age of 65 years (Anderson et al, 1999; Ott et al, 1995; Wade et al, 1987). This gender difference persists even after correcting for differences in life expectancy. There is also limited evidence that women have an earlier onset and faster progression of Alzheimer’s Disease (Garcia-Segura et al, 2001).
This gender difference in cognitive decline may be impacted, at least in vulnerable populations, by the marked fluctuations in estrogen and progesterone levels in women as they transition through menopause and the reduction of these hormones post-menopause. Indeed, several studies have demonstrated estrogen’s benefits in improving memory of female humans and mice (Harburger et al, 2006; Resnick et al, 1997; Sherwin and Tulandi, 1996; Xu and Zhang, 2006), and these effects may result from multiple convergent mechanisms. In addition to the typical action of steroid nuclear receptors, estrogen can act via non-nuclear receptors coupled to second messenger systems and via rapid non-genomic mechanisms to phosphorylate cAMP response element binding protein (CREB) and, thereby, regulate gene expression underlying memory formation (Kelly and Levin, 2001; Szego et al, 2006; Zhao et al, 2005; Zhou et al, 2005). A close parallel has been found between the temporal conditions by which estrogen improves memory and the conditions for estrogen to induce new excitatory synaptic connections in the hippocampus (HPC), a key structure involved in learning and memory (McEwen et al, 2001). Thus, a decline in estrogen levels with aging likely places vulnerable females at greater risk for cognitive dysfunction.
One of the first brain structures to show age-related functional decline in normal humans is the HPC. Similarly, in aged rats and mice, numerous reports show impairment of HPC-dependent tasks such as the Morris water maze (Brightwell et al, 2004; Fordyce and Wehner, 1993; Frick et al, 2000; Hebda-Bauer et al, 1999; Koistinaho et al, 2001; Magnusson, 1998; Sauer et al, 1999; Wolff et al, 2004), contextual fear conditioning (Kudo et al, 2005; Monti et al, 2005), the Barnes circular maze (Barnes et al, 1990; McLay et al, 1999), and the radial arm maze (Ammassari-Teule et al, 1994; Culley et al, 2004; Geinisman et al, 1986). Spatial learning and memory impairments are observed as early as middle age (Frick et al, 2003; Verbitsky et al, 2004). In contrast, no impairment with aging is found in non-HPC dependent tasks such as simple cued-discrimination tasks (Frick et al, 2000). Some old animals are behaviorally impaired while others perform as well as young control animals (Brightwell et al, 2004: Fisher et al, 1992; Hebda-Bauer et al, 1999; Issa et al, 1990; Nicholson et al, 2004). The degree of spatial learning and memory impairment in some old animals has been correlated with the extent of age-related physiological and morphological changes in the HPC (Barnes and McNaughton, 1985; Deupree et al, 1993; Dunbar et al, 1993; Fisher et al, 1992; Fordyce and Wehner, 1993; Nicholson et al, 2004; Wilson et al, 2003). The reported correlations demonstrate that larger quantities of age-related changes (e.g., rigidity in HPC place cell spatial representation, decreased maintenance of long-term-potentiation, decreased numbers of choline acetyltransferase-labeled neurons, lower acetyltransferase activity, decreased size of perforated postsynaptic densities in HPC axospinous synapses, or decreased HPC-bound protein kinase C activity) are associated with larger spatial learning and memory deficits.
Aging is related to multiple cell signaling alterations. Such alterations may, at least in part, be due to altered constitutive expression of proteins important for memory consolidation or to the inability of these proteins to respond appropriately to a learning experience.
Dysregulation of some memory-related proteins and transcription factors in the HPC of aged rats has recently been reported (Lund et al, 2004; Monti et al, 2005). Substantial evidence shows that CREB-dependent modulation via phosphorylation of Ser133, is required for the cellular events underlying long-term memory (Bourtchuladze et al, 1994; Dash et al, 1990; Guzowski and McGaugh, 1997; Mizuno et al, 2002; Yin et al, 1994), and dysregulation of the phosphorylation state of CREB in the HPC of aged rats is correlated with their impaired memory Monti et al, 2005). Thus, studying the impact of altered CREB function in aging males and females is directly relevant to understanding differential vulnerability to cognitive decline across genders.
Mice with a targeted disruption of the α and δ isoforms of the CREB gene (CREBαδ- deficient mice) are impaired in the Morris water maze (MWM), a test of spatial learning and memory (Bourtchuladze et al, 1994) and have an altered ability to code space, as demonstrated by decreased spatial selectivity and stability of hippocampal place cells (Cho et al, 1998). This impairment, however, is not evident under all testing conditions or genetic backgrounds. CREBαδ- deficient mice are impaired when given two trials per day with a one-minute inter-trial interval (ITI), but perform normally with spaced training (Kogan et al, 1997) and more trials (Hebda-Bauer et al, 2005). The memory deficit in CREBαδ- deficient mice is, therefore, not absolute. Under highly demanding conditions, the mice are unable to acquire or retain adequate information for learning and memory and are impaired. Under less demanding conditions, the mice are unimpaired. This may be due to related transcription factors (e.g., cAMP response element modulator or the CREB β isoform which are up-regulated and may make up for the deficit (Blendy et al, 1996; Mantamadiotis et al, 2002). In addition, we have recently reported that past experience in the MWM—depending on its nature (i.e., whether mice were successful or failed to learn)—significantly facilitates or hinders future MWM performance (Hebda-Bauer et al, 2005). Spatial learning and memory performance of CREBαδ- deficient mice in different genetic backgrounds have been reported to show either a genetically dose-dependent effect (Gass et al, 1998) or no impairment at all (Graves et al, 2002). Further, various CREB mutants (in the 129SvEv x C57BL/6 background) with either a marked reduction or complete loss of HPC CREB exhibit only modestly impaired water maze learning (Balschun et al, 2003).
Thus, mice with a CREBαδ- mutation on a C57BL/6 x 129SvJ background can be viewed as having a genetic vulnerability to impaired learning and memory that is highly influenced by environmental conditions and previous experience. How this genetic vulnerability to cognitive impairment, beginning early in life, affects cognitive aging and whether the effects are different in males and females is not known. Therefore, we examined the effects of aging and sex in CREBαδ- deficient mice by assessing learning and memory performance of males and females and their wild-type littermates at three different ages--young, middle-aged, and aged--in the Morris water maze.
EXPERIMENTAL PROCEDURES
Subjects
CREBαδ- deficient mice were originally generated in the laboratory of Dr. Gunther Schutz (Hummler et al, 1994). They were initially obtained for our laboratory from Dr. Alcino Silva as F2 progeny derived from a cross between CREBαδ- deficient heterozygotes in the C57BL/6 background (>87%) and wild-type 129SvJ mice. Thus, the genetic background of the wild type and mutant mice subsequently bred and used for the current study consists of approximately a 50% contribution of genes from each of the C57BL/6 and 129SvJ strains. Approximately 15% of the newborn pups are homozygous for the CREBαδ- mutation.
The wild type (WT; +/+) and CREBαδ- homozygous (-/-) deficient mice used in the current study were of three different ages: 4-6 months old (young; N = 24 +/+, 24 -/-), 12-14 months old (middle-aged; N = 28 +/+, 28 -/-) and 20-22 months old (aged; N = 28 +/+, 27 -/-). Mice were group-housed in a temperature- and humidity-controlled room with free access to food and water. They were maintained on a 14:10 light/dark cycle (lights on at 0600, lights off at 2000 hours). The health of the mice was monitored regularly. All mice maintained normal home cage activity and, therefore, none were removed from the study. Breeding was set-up so that mice of all three ages were tested at the same time. The WT mice and CREBαδ- deficient mice of all three ages were randomly assigned to one of two behavioral testing protocols (i.e., MWM2 or MWM4). All behavioral testing was conducted between 0800 and 1600 hours. The experiments were conducted in accordance with the guidelines of the University Committee on the Use and Care of Animals at the University of Michigan.
CREBαδ- polymerase chain reaction (PCR) genotyping
Mice were genotyped by PCR analysis. Tail biopsies were obtained at weaning and digested in 600 μl of TNES (10 mM Tris, pH 7.5, 400 mM NaCl, 100 mM EDTA, and 0.6% SDS) and 35 μl of Proteinase K (10 mg/ml) overnight at 57°C. The next day, 166.7 μl of saturated NaCl was added and mixed. After centrifugation (14,000 rpms for 5 minutes) and recovery of the supernatant were performed twice, equal volume of 100% EtOH was added and the DNA was spooled, dipped briefly in 70% EtOH, allowed to dry, and then resuspended in TE (10 mM tris, 1 mM EDTA). One μl of the DNA was used directly in a PCR reaction. The following PCR primers were used for genotyping CREBαδ- deficient mice: CREB1 = 5’-CCATATTATTGTAGGTAACTAAATGA-3’, CREB2 = 5’-ATGTATTTTTATACCTGGGC-3’, and NEO = 5’-ATGATGGATACTTTCTCGGCAAGG-3’. The following PCR conditions were used in a Peltier Thermal Cycler (PTC-2000, MJ Research): 4°C for 180 seconds; 94°C for 90 seconds; 40 cycles of 93°C for 45 seconds, 47°C for 45 seconds, and 72°C for 90 seconds; then 72°C for 600 seconds.
Behavioral testing
The Morris water maze (MWM) was used to test for spatial learning and memory. The inside of the 91 cm diameter tub was painted white and filled with 26° ± 2° Celsius water made white with powdered milk to control for intra-maze cues. Animals must use a stationary array of cues outside the tub to find a submerged platform that they cannot see, hear, or smell. These cues, and the location of the platform, remained constant during testing.
All mice were given a preliminary trial the day before regular testing began to acclimate them to water and let them know that a platform can be found. For this trial, a mouse was placed in the water for 10 seconds, allowed to swim around, and then placed on the platform for only one to two seconds. The hidden platform was put in a different location from where it was located for the regular trials. If a mouse found the platform before the 10-second mark, it was removed immediately.
A regular trial began by placing a mouse in the water at one of two (MWM2) or four (MWM4) randomly assigned starting positions. After a mouse located the platform, it was allowed to remain on the platform for 30 seconds. If a mouse had not located the platform within 60 seconds, it was removed from the water and placed on the platform for 30 seconds. Mice received two (MWM2) or four (MWM4) trials per day with a one (MWM2) or three to five (MWM4) minute inter-trial interval (ITI) for four days. A videotracking system (Ethovision, Noldus Technology) was used to measure swim time, distance traveled, swim speed, number of crossings over the old vs. the new goal location, search error (i.e., cumulative distance: sum of distances to goal taken every second minus the value obtained for an ideal direct swim; Gallagher et al, 1993), and percent time in goal quadrant. Data are expressed as the mean of trials per day unless otherwise specified.
Twenty-four hours after completion of regular testing, all mice received four cued-platform trials to assess sensorimotor abilities and motivation to escape the water independent of spatial learning and memory ability. The platform was located in the same place as that for the regular trials and marked with a black cube. After a mouse located the cued-platform, it was allowed to remain on the cued-platform for 30 seconds. If a mouse had not located the cued-platform within 60 seconds, it was removed from the water and placed on the cued-platform for 30 seconds. The ITI was three to five minutes like that for the MWM4 protocol.
Twenty-four hours after the cued-platform trials, the platform was removed for the probe trial and all mice were allowed to swim for 60 seconds to assess their memory for the platform location. Time spent and distance traveled in the four quadrants were measured. The number of platform crossings in the goal quadrant and over comparable platform locations in the other three quadrants were measured to assess the accuracy toward the trained submerged platform location.
Data analysis
Data were analyzed using SAS statistical software. Three- and four-way analysis of variance (ANOVA) with repeated measures using the General Linear Model and Mixed Model procedures with planned contrasts were used to analyze regular trial performance across days. Planned contrasts were used to determine genotype, age, sex, and protocol differences on each day. ANOVAs were also used to analyze probe trial performance. Data are expressed as the mean of the two (MWM2) or four (MWM4) trials for each day.
RESULTS
Genotype differences in swim speed during regular trials
Significant differences in swim speed were found in the MWM4 and MWM2 protocols between CREBαδ- deficient and WT mice. In the MWM4, a two-way ANOVA with repeated measures reveals a significant effect for genotype, but not age, and a time by genotype interaction (Genotype: F(1/50)=17.59, p<0.001; Age: F(2/50)=2.41, p>0.05; Time x Genotype Interaction: F(3/150)=3.19, p<0.05). Posthoc analyses reveal that CREBαδ- deficient mice swam faster than WT counterparts on all four test days in the MWM4 (data not shown). In the MWM2, a two way ANOVA with repeated measures reveals a significant effect for age (Age: F(2/62)=3.68, p<0.05). Posthoc analyses show that aged mice swam slower than young mice on the fourth MWM2 day (data not shown). The higher swim speed of CREBαδ- deficient mice than their WT counterparts in the MWM2 was also significant (F(1/62)=3.83, p=0.05). Swim time was not used in the analysis because of these swim speed differences. Thus, other measures used are distance traveled and degree of search error (i.e., cumulative distance) for regular trials and percent distance and number of platform crossings in each quadrant during the probe trial. Since distance traveled and degree of search error show similar results, only degree of search error is detailed here. The number of platform crossings in each quadrant during the probe trial are shown in figures because it is a more sensitive measure of memory than distance traveled in each quadrant.
MWM4 versus MWM2: easy versus hard protocol
Our laboratory has previously reported that the MWM2 protocol is harder than the MWM4 protocol for CREBαδ- deficient mice (Hebda-Bauer et al, 2005). CREB-mediated transcription has an impact on the number of trials and the ITI required for the formation of spatial memories in mice. Maximal CREB activation in the HPC and cortex may take three to eight minutes after synaptic activation (Moore et al, 1996). Thus, the one minute ITI of the MWM2 task, compared to the three to five minute ITI of the MWM4 task, is more challenging for CREBαδ- deficient mice because one minute is not enough time to maximize the residual CREB activity (approximately 15% of normal; Blendy et al, 1996) left in their brains. The MWM4 task may facilitate learning because of the longer ITI and the increased number of trials (thereby, giving more opportunities for CREB activation).
The data from the current study confirm that CREBαδ- deficient and WT mice of all three age groups perform worse in the MWM2 protocol compared to their respective counterparts in the MWM4 protocol. A three-way ANOVA with repeated measures for degree of search error reveals a significant effect for protocol and trial, but not age or sex for WT mice (Protocol: F(1/68)= 5.39, p<0.05; Trial: F(3/204)= 62.46, p<0.001; Age: F(2/68)= 2.17, p>0.05; Sex(1/68)=0.14, p>0.05). Figure 1 illustrates how the learning curves of the WT mice from the two protocols are different. WT mice in the MWM2 are slower to learn the task than their counterparts in the MWM4 by exhibiting a significantly greater degree of search error on day two. Early learning is clearly adaptive, and the majority of learning normally occurs from the first to the second test day in the MWM task. Interestingly, WT mice do not show age-related changes in learning and memory in either MWM protocol. CREBαδ- deficient mice, like their WT counterparts, also display significant protocol differences (F(1/67)= 17.95, p<0.001). The performance difference between the two MWM protocols shows that exposure to only two learning trials per day with a one minute ITI is challenging for both WT and CREBαδ- deficient mice of different ages.
Fig. 1.
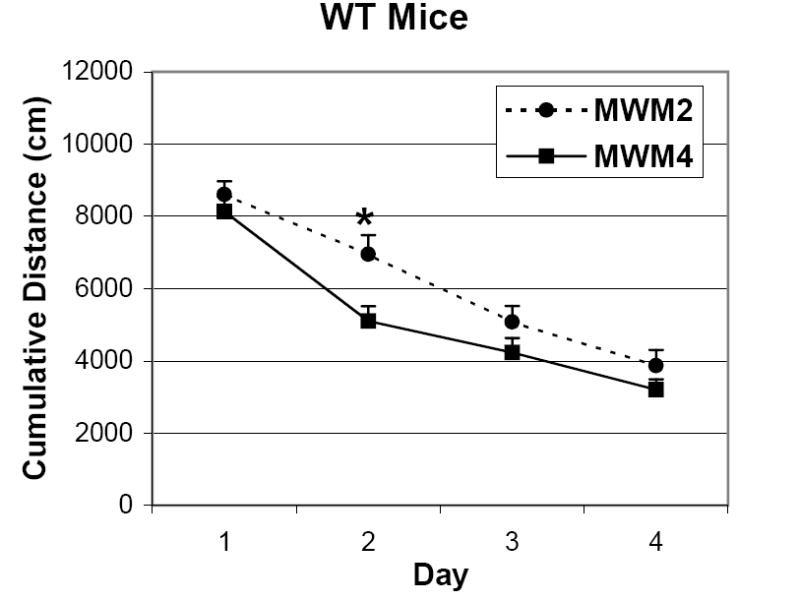
Comparison of learning curves of WT mice from the MWM4 versus the MWM2 protocol. Data show the mean degree of search error ± SEM. The MWM2 protocol is more challenging than the MWM4 protocol for WT and CREBαδ- deficient mice regardless of age and sex. An example of this protocol difference is shown by WT mice in the MWM2 that exhibit a significantly different learning curve with a greater degree of search error on day two than their counterparts in the MWM4. * p<0.05.
The effects of sex and age on learning and memory of CREBαδ- deficient mice
CREBαδ- deficient mice are known to perform well in the easier MWM4 protocol (Hebda-Bauer et al, 2005) and the data from this study replicate the good MWM4 performance of young male and female CREBαδ- deficient mice (Figs. 2A and D, respectively). In the MWM4, a three-way ANOVA with repeated measures for degree of search error reveals significant effects for age and a sex by age interaction, with a genotype effect approaching significance (Age: F(2/66)= 3.96, p<0.05; Age x Sex: F(2/66)= 3.14, p<0.05; Genotype: F(1/66)= 3.54, p=0.06). Figure 2 shows the learning curves in degree of search error for male (A-C) and female (D-F) WT and CREBαδ- deficient mice split by age. Although all young mice perform well in the MWM4 (except for WT females compared to CREBαδ- deficient females on day 3), middle-aged male and female CREBαδ- deficient mice display much less learning than WT mice from day 1 to day 2 as depicted by posthoc analyses revealing a significantly higher degree of search error for CREBαδ- deficient mice on day 2 compared to their WT counterparts (Fig. 2B and E). Early learning clearly has adaptive benefits; thus, although their extent of learning reached that of the WT mice on later days, male and female middle-aged CREBαδ- deficient mice are impaired early in MWM4 testing. Among aged mice, male CREBαδ- deficient mice perform as well as their WT counterparts in the MWM4 (Fig. 2C). In contrast, aged female CREBαδ- deficient mice are impaired as shown by a significantly greater degree of search error than other mice on test days 3 and 4 (Fig. 2F). Thus, the current data clearly show more impaired learning and memory with aging in female than in male CREBαδ- deficient mice in an easier, less demanding task.
Fig. 2.
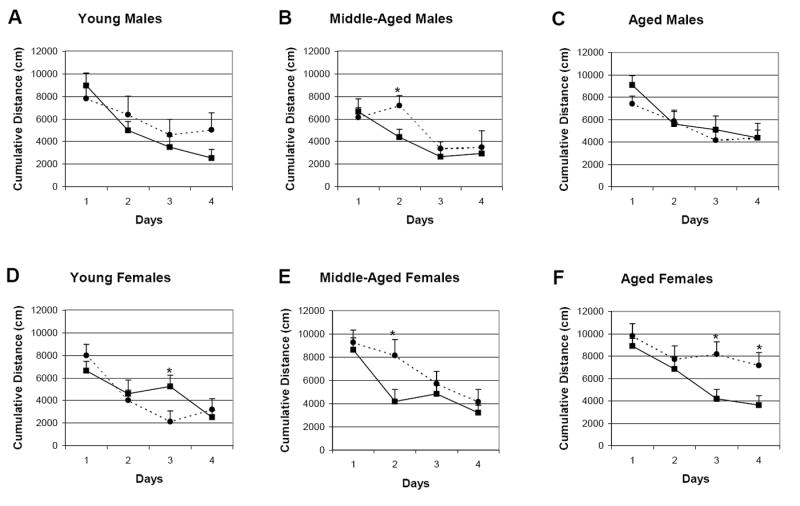
Learning curves of male and female WT and CREBαδ- deficient mice in the MWM4 protocol split by age. Data show the mean degree of search error ± SEM. Solid lines with squares depict the WT mice. Dotted lines with circles depict the CREBαδ- deficient mice. Males and Females are designated as M and F, respectively. (A-C) Male CREBαδ- deficient mice of all three ages performed as well as their WT counterparts in the MWM4, with one exception. Middle-aged male CREBαδ- deficient mice showed an early learning impairment as exhibited by a significantly greater degree of search error on day two. They showed no improvement in the degree of search error from day one to day two, but with more testing they eventually learned to the same extent as their WT counterparts. (D) Young female CREBαδ- deficient mice performed as well, or better (on day three) as their WT counterparts in the MWM4. (E) Middle-aged female CREBαδ- deficient mice showed an early learning impairment as exhibited by a significantly greater degree of search error on day two, but with more testing they eventually learned to the same extent as their WT counterparts. (F) Aged female CREBαδ- deficient mice were impaired as shown by a significantly greater degree of search error than their WT counterparts on test days three and four. * p<0.05.
The impairment of young CREBαδ- deficient mice in the MWM2 is somewhat milder in this study than we and others had previously reported (Hebda-Bauer et al, 2005; Kogan et al, 1997). Interestingly, this had the advantage of allowing us to detect age- and sex-related performance differences. In the MWM2, a three-way ANOVA with repeated measures for degree of search error reveals significant effects for genotype, age, and sex and a genotype by sex interaction (Genotype: F(1/69)= 17.64, p<0.001; Age: F(2/69)= 6.71, p<0.01; Sex: F(1/69)= 5.01, p<0.05; Genotype x Sex Interaction: F(1/69)= 4.17, p<0.05). Figure 3 shows the learning curves in degree of search error for male (A-C) and female (D-F) WT and CREBαδ- deficient mice split by age. Among the male CREBαδ- deficient mice, only the aged display impaired performance in the MWM2. Posthoc analyses reveal that aged male CREBαδ- deficient mice exhibited a significantly greater degree of search error on three of the four test days compared to their WT counterparts. In contrast, middle-aged and aged female CREBαδ- deficient mice show impaired performance in the MWM2. Although posthoc analyses reveal only a trend for worse performance of young female CREBαδ- deficient than WT mice on days two and three (p=0.09), the degree of search error is significantly greater for middle-aged and aged female CREBαδ- deficient mice on two and three of the four test days, respectively. Thus, all aged CREBαδ- deficient mice show impairment in the MWM2, and females clearly show an earlier age-related learning and memory deficit than their male counterparts.
Fig. 3.
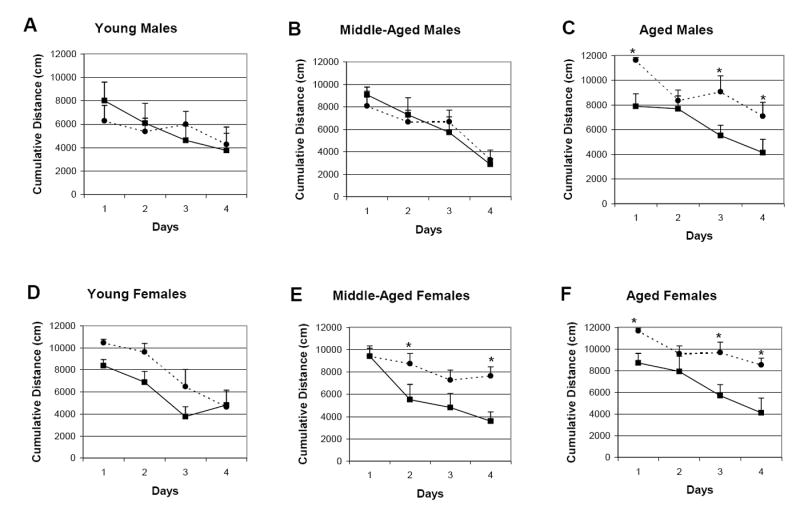
Learning curves of male and female WT and CREBαδ- deficient mice in the MWM2 protocol split by age. Data show the mean degree of search error ± SEM. Solid lines with squares depict the WT mice. Dotted lines with circles depict the CREBαδ- deficient mice. Males and Females are designated as M and F, respectively. (A-C) Young and middle-aged male CREBαδ- deficient mice learned as well as their WT counterparts, but the aged are impaired. Aged male CREBαδ- deficient mice exhibited a significantly greater degree of search error than their WT counterparts on three of the four test days. (D) In the MWM2, young female CREBαδ- deficient mice showed a greater degree of search error than their WT counterparts that is approaching significance (p=0.09) on days two and three. (E) and (F) Middle-aged and aged female CREBαδ- deficient mice were impaired in the MWM2 as shown by a significantly greater degree of search error on two and three of the test days, respectively. * p<0.05.
Figure 4 highlights the sex and age differences in the extent of learning among CREBαδ- deficient mice in the MWM4 and MWM2 protocols on the fourth day of testing. Male CREBαδ- deficient mice achieve a similar level of learning in the MWM4, as measured by degree of search error, to that of the WT mice by the fourth test day (Fig. 4A). The extent of learning in the MWM4 for male CREBαδ- deficient mice is not dependent on age. The harder MWM2 uncovers a learning and memory impairment in only the oldest male CREBαδ- deficient mice (p<0.05; Fig. 4B).
Fig. 4.
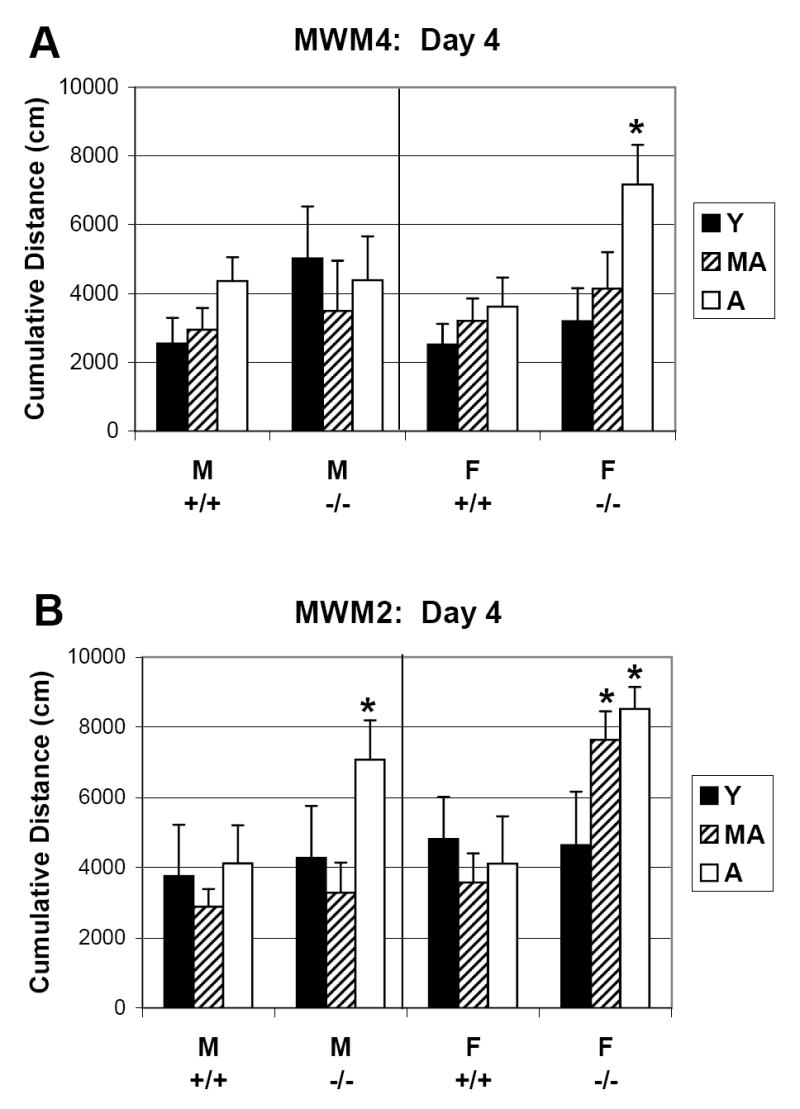
Sex differences in the extent of learning among CREBαδ- deficient mice in the MWM4 and MWM2 protocols. Data show the mean degree of search error on test day four ± SEM. WT and CREBαδ- deficient mice are designated as +/+ and -/-, respectively. Males and Females are designated as M and F, respectively. Young, middle-aged, and aged mice are designated as Y, MA, and A, respectively. (A) WT mice had achieved a similar amount of learning by the fourth MWM4 test day, as exhibited by a lack of significant sex or age differences in the degree of search error. Among CREBαδ- deficient mice, females but not males showed a clear impairment in the oldest age group, as shown by a significantly greater degree of search error on the fourth MWM4 test day compared to the other mice. (B) In the MWM2, all WT mice showed a similar degree of search error. Among CREBαδ- deficient mice, females showed a learning deficit at a younger age than the males: middle-aged and aged females, while only aged males exhibited a significantly greater degree of search error. * p<0.05.
In contrast, female CREBαδ- deficient mice show an age-related impairment in both MWM protocols. Aged female CREBαδ- deficient mice exhibit a significantly greater degree of search error on day four in the MWM4 (p<0.05; Fig. 4A), while both middle-aged and aged female CREBαδ- deficient mice are impaired in the MWM2 (p<0.05; Fig. 4B). Notably, the level of deficit that the old females exhibit in the easy task is comparable to the deficit shown by the aged males in the more difficult task. Thus, female CREBαδ- deficient mice clearly show a higher vulnerability than their male counterparts to impaired learning and memory, with the easier MWM4 task revealing deficits in the oldest group and the MWM2 exposing deficits at an earlier age than the males.
Probe trial performance
Animals’ memory for the location of the goal was assessed in the probe trial during which the goal was removed. All WT mice from the MWM4 and MWM2 showed a significant preference for the goal quadrant, as measured by percent distance in each of the quadrants, except for aged males from the MWM2 (data not shown). Another probe trial measure, the number of platform crossings in the goal quadrant compared to hypothetical platform crossings in the other quadrants, is a more sensitive memory measure since it determines the degree of preciseness in the search for the goal. The data reveal that young and middle-aged, but not aged, male WT mice from either MWM protocol performed well (Fig. 5A and C). Female WT mice from the MWM4 protocol showed a significant preference for the goal quadrant while their WT counterparts from the MWM2 did not (Fig. 5A and C). We conclude that the MWM4 protocol generally facilitates long-term memory better than the MWM2 protocol among female WT mice.
Fig. 5.
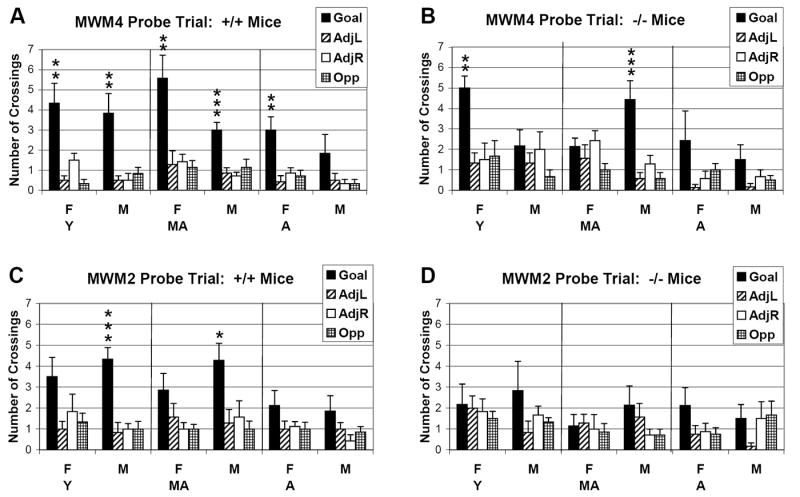
Number of platform crossings during the probe trial for WT and CREBαδ- deficient mice in the MWM4 and MWM2 protocols. WT and CREBαδ- deficient mice are designated as +/+ and -/-, respectively. Young, middle-aged, and aged mice are designated as Y, M, and A, respectively. Males and Females are designated as M and F, respectively. (A) All WT mice from the MWM4 protocol showed a preference for the goal quadrant during the probe trial, as shown by a significantly higher number of crossings over the platform location in the goal quadrant compared to hypothetical platform crossings in the other quadrants, with one exception. The preference for the goal quadrant in aged WT males did not reach significance. (B) In contrast, only young female and middle-aged male CREBαδ- deficient mice in the MWM4 showed a preference for the goal quadrant during the probe trial. (C) Among WT mice from the harder MWM2, only young and middle-aged male WT mice showed a significant preference for the goal quadrant. (D) None of the CREBαδ- deficient mice from the MWM2 showed a significant preference for the goal quadrant in the probe trial. * p<0.05, ** p<0.01, *** p<0.001.
CREBαδ- deficient mice from the MWM2 and the MWM4 had significant difficulty during the probe trial. Although all male CREBαδ- deficient mice performed well during the MWM4 acquisition phase and only the aged were impaired in the MWM2 acquisition phase, the probe trial revealed cognitive deficits. Only the middle-aged males showed a significant preference for the goal quadrant, as measured by percent distance traveled (MWM4 and MWM2; data not shown) and number of platform crossings (MWM4 only; Fig. 5B).
Among female CREBαδ- deficient mice from the MWM4, the young (Y) and middle-aged (MA) showed a significant preference for the goal quadrant as measured by percent distance traveled (Y and MA; data not shown) and number of platform crossings (Y only; Fig. 5B). None of the female CREBαδ- deficient mice from the MWM2 showed a preference for the goal quadrant (Fig. 5D), except for percent distance traveled by aged females (data not shown). Thus, the majority of the CREBαδ- deficient mice that performed well during the regular MWM trials did not perform well during the probe trial.
Cued-platform performance
Some aged rodents may not perform well in the typical spatial learning and memory version of the Morris water maze due to sensorimotor deficits; thus, all mice in the current study received four cued-platform trials after completion of the regular spatial learning trials. A significant effect for age, but not genotype, sex, or protocol was found for swim speed during the cued-platform trials (Age: F(2/405)= 4.15, p<0.05; Genotype: F(1/405)= 0.00, p=NS; Sex: F(1/405)= 0.68, p=NS; Protocol: F(1/405)= 0.20, p=NS). Thus, distance traveled and cumulative distance, but not swim time, were used in examining each mouse’s performance. There was no increase in the number of mice that showed poor cued-platform performance as a function of age. Correlation analysis of mouse performance on the last regular test day and during the probe trial with that of performance during cued-platform trials reveals very low and non-significant correlations. Thus, mice exhibiting poor cued-platform performance did not necessarily show impaired spatial learning and memory, and vice versa. Thus, the findings of cognitive deficits could not be explained based on performance during the cued-platform trials.
DISCUSSION
The data from the current study are the first to show how vulnerability to cognitive impairment in mice with a CREB deficiency at birth increases with age in a sex-dependent manner. Aging does not affect acquisition of male CREBαδ- deficient mice in the easier of two spatial learning and memory tasks, but the oldest males perform poorly in the task with higher cognitive demand. In contrast, female CREBαδ- deficient mice show age-related deficits in learning both tasks, with a deficit in the more challenging task observed at an earlier age than that found for the males. This earlier decline in spatial learning performance was found exclusively in female CREBαδ- deficient mice, since WT mice did not show sex or age-related acquisition differences. During the probe trial, young and middle-aged, but not aged, male WT mice performed well regardless of MWM protocol. However, female WT mice exhibited better long-term memory following the MWM4 versus the MWM2 protocol. By contrast, the majority of CREBαδ- deficient mice exhibited poor long-term memory, and this included mice that performed well during either MWM protocol.
The current data extend our previous report of the MWM4 protocol being easier than the MWM2 protocol in young CREBαδ- deficient mice (Hebda-Bauer et al, 2005) to that of older CREBαδ- deficient mice and WT mice. The present study allowed a direct comparison of aging effects on spatial learning and memory performance of mice with and without a CREB deficiency in test conditions that differ in the amount of cognitive demand required to successfully complete the task (i.e., high cognitive demand: MWM2; lower cognitive demand: MWM4). Acquisition of the MWM2 protocol was found to be more challenging than the MWM4 protocol in all ages of WT and CREBαδ- deficient mice. Memory performance in the probe trial clearly showed how the MWM2 protocol is more challenging for WT females and all CREBαδ- deficient mice. Interestingly, the majority of CREBαδ- deficient mice from the easier MWM4, except for young females and middle-aged males, demonstrated impaired memory in the probe trial. Impairment of young male, but not female, CREBαδ- deficient mice in the probe trial after the MWM4 suggests that CREB signaling seems more important for males than females at this age and is consistent with recent reports of a male-specific role of calcium/calmodulin kinase kinase β (necessary for activation of CREB) in spatial memory of mice (Mizuno et al, 2007; Peters et al, 2003). It is not known why only the middle-aged males of the older CREBαδ- deficient mice performed well during the probe trial following the MWM4.
It is conceivable that the difficulty encountered by the CREBαδ- deficient mice during the probe trials may reflect, in part, their cognitive rigidity. Thus, the cued-platform, which was in the same place as that for the regular trials, may have interfered with the memory of the CREBαδ- deficient mice during the probe trial since the cue trials took place between the regular trials and the probe trial. Rodents with an intact HPC demonstrate flexibility in the use of spatial and non-spatial strategies to perform a spatial task, with spatial strategies predominant (O’Keefe and Nadel, 1978). Mice with a CREB deficiency have an altered ability to code space, as demonstrated by decreased spatial selectivity and stability of hippocampal place cells (Cho et al, 1998). This altered ability to code space may compromise their flexibility in learning strategies and, thus, increase their susceptibility to impaired memory performance. Nevertheless, this decreased flexibility is unlikely to be the primary factor, as performance differences between the two MWM protocols were demonstrated in acquisition rate.
This is the first study to report the effects of aging on learning and memory in mice with approximately a 50/50 background of the C57Bl/6 and 129Sv/J strains (i.e., the WT controls of the current study). Interestingly, middle-aged and aged WT mice were not impaired in acquisition in either MWM protocol, in contrast to the widely reported age-related impairment of spatial learning and memory in rodents, including the C57Bl/6 and 129Sv strains of mice (Ammassari-Teule et al, 1994; Fordyce and Wehner, 1993; Frick et al, 2000; Magnusson, 1998; Sauer et al, 1999; Wolff et al, 2004). With age, C57Bl/6 mice demonstrate increasing impairment in the Morris water maze, more heterogeneity of performance within an age group, and a slower rate of learning (as seen in aged rats). In contrast, performance of WT mice in the current study was quite homogeneous across age groups. The only evidence of an age-related change was found in the aged males from both MWM protocols that showed impaired memory during the probe trial. The mild age-related effects on spatial memory performance in 12 to 14-and 22 to 24-month-old WT mice on a combined C57Bl/6 and 129Sv/J background of the current study are likely due to hybrid vigor (Wolfer et al, 2002).
Interestingly, WT females of all three ages showed some impairment in the probe trial after the MWM2 protocol. The few studies that have examined sex differences in spatial memory of mice, have reported sex differences in older mice, but not among young mice (Benice et al, 2005; Frick et al, 2000; Jonasson, 2005). However, the MWM2 protocol of the current study is clearly more challenging than the MWM4 protocol and likely the other studies’ protocols since they also consisted of more than two training trials and, thus, may not have been as sensitive to long-term memory deficits in the probe trial. The challenging MWM2 protocol uncovered a male advantage in spatial memory that is consistent with human studies (Driscoll et al, 2003, 2005).
The current study allowed a direct comparison of normal age-related changes in this mixed background with changes occurring from minimal CREB activity alone (young CREBαδ- deficient mice) and with concurrent age-related changes (old CREBαδ- deficient mice). Although we and others have shown the impairment of young CREBαδ- deficient mice in the MWM2 protocol (Hebda-Bauer et al, 2005; Kogan et al, 1997) the deficit was milder in this study. This worked to our advantage by enabling us to observe age-related worsening in performance in mice with a vulnerability to cognitive deficits. The acquisition of a spatial learning and memory task is intact in young, middle-aged, and aged male CREBαδ- deficient mice as long as the task does not require a high cognitive demand (i.e., MWM4), with one exception. Middle-aged CREBαδ- deficient mice, unlike the WT mice, did not learn much from the first to the second test day; however, they caught up to the WT mice by the next day. The higher cognitive demand in the MWM2 protocol elicited a strong spatial learning and memory impairment of only the oldest (i.e., 22 to 24-month-old) male CREBαδ- deficient mice. Thus, the level of cognitive demand in spatial learning tasks can determine how well aged males with a CREB deficiency perform, from normal to very impaired.
The striking finding from this study is the sex difference in acquisition of the Morris water maze of CREBαδ- deficient mice that appears with aging. In a previous study (Hebda-Bauer et al, 2005) we reported sex differences in acquisition of MWM performance of young CREBαδ- deficient mice, but only after an unsuccessful learning experience (i.e., after the MWM2 protocol) and not with an initial learning and memory task. In the current study, sex differences were revealed by the differential impact of aging on the females. In contrast to the good performance of all three ages of male CREBαδ- deficient mice in the easier MWM4, aged female CREBαδ- deficient mice displayed little learning. Middle-aged females showed an early learning impairment in the MWM4, similar to that of their male counterparts. The majority of young animals normally show the most learning from the first to the second or third test days, and early learning is clearly beneficial to an organism’s survival. Minimal to no learning has been reported to be an indicator of vulnerability to further learning and memory deficits (Hebda-Bauer et al, 1999), and this may be the case for female but not male CREBαδ- deficient mice since only the female CREBαδ- deficient mice show great impairment in acquisition of the MWM4 with aging. With a higher cognitive demand in the MWM2, female CREBαδ- deficient mice exhibited an increasing impairment with age. Young females showed a tendency for slower learning, but reached the level of the WT mice by the last day. Both middle-aged and aged female CREBαδ- deficient mice exhibited little learning across test days, showing great impairment. Thus, female CREBαδ- deficient mice demonstrate higher vulnerability to cognitive impairment than their male counterparts by showing impairment in a low demanding task when old and an earlier impairment in a highly demanding task when middle-aged. Female CREBαδ- deficient mice are clearly most vulnerable to age-related spatial learning and memory deficits.
Sex differences in spatial learning and memory greatly vary among species and behavioral tasks (Jonasson, 2005), but some evidence exists for a greater or earlier decline in spatial learning and memory of female mice than male mice in the Morris water maze (Benice et al, 2006; Frick et al, 2000). In one study, 17- and 25-month old female C57Bl/6 mice were impaired in the distance-traveled measure of the MWM, while only the oldest males (i.e., 25 months old) were impaired (Frick et al, 2000). In another study, 10 to 12-month-old male and female C57Bl/6 mice showed intact MWM performance, but 18 to 20-month-old females showed a decreased rate of learning (Benice et al, 2006). A substantial age difference in long-term memory was reported in females during the probe trial, in contrast to the small age difference found in the males. In the current study, the only age-related sex difference found among WT mice was during the probe trial in which the oldest males were impaired. Only a combination of aging plus a long-term CREB deficiency resulted in sex-dependent age differences in spatial learning and memory during acquisition, rendering the females more susceptible to impairment.
Among humans, males are reported to exhibit superior spatial abilities to females, but both sexes show parallel patterns and similar rates of cognitive decline with normal aging (Driscoll et al, 2003, 2005). Given the observation that Alzheimer’s disease is 2-3 fold higher in women after age 65 (Anderson et al, 1999; Ott et al, 1995; Wade et al, 1987), some women may be more vulnerable than males to cognitive decline as they age despite these comparable patterns of cognitive decline. In contrast to human data, female and male WT mice in the current study demonstrated comparable spatial learning and memory, with one exception. Training in the harder MWM2 task elicited long-term memory deficits in the probe trial unique to females, uncovering some evidence for a male advantage in spatial memory like that found in humans. Although the hybrid background of the mice in the current study likely attenuated age-related changes, a CREB deficiency combined with aging provided an example of how some females can be more vulnerable to cognitive deficits and exhibit them at an earlier age than males.
Age-related effects on the levels of constitutively active (phosphorylated, pCREB) and non-phosphorylated CREB have been reported in rats but not mice. Although the levels are reported to be higher, lower, or show no change in the HPC of aged rats, the learning-induced upregulation of pCREB is more consistently shown to be attenuated with aging (Foster et al, 2001; Hattiangady et al, 2005; Kudo et al, 2005; Monti et al, 2005). Decreased pCREB levels have even been reported as early as middle age (i.e., 12 months of age), with a further decline from middle to old age (i.e., 24 months of age). Especially relevant to the current study, CREB1 (the main transcriptional activator form of CREB) protein levels are decreased in aged rats that exhibit MWM impairment (Brightwell et al, 2004). This decrease in CREB protein levels is exclusive to aged-impaired rats, because aged rats that perform as well as young adult rats show similar CREB protein levels to that of the young rats. Increasing CREB levels via somatic gene transfer of CREB into the HPC at eight weeks of age has recently been shown to prevent memory loss during normal aging (Mouravlev et al, 2006). The majority of these studies are largely correlative in nature, and the long-term effects of decreased CREB gene expression and function on learning and memory have not been reported. Although it is not known whether CREB mRNA or protein (phosphorylated or unphosphorylated) levels differ among various ages of WT mice on the mixed C57Bl/6 and 129Sv/J background, it would not be surprising to find little to no change with age given the relatively intact spatial learning and memory of the aged WT mice in the current study.
The earlier spatial learning and memory decline of female versus male CREBαδ- deficient mice may be related to the cessation of estrous cycling in combination with a CREB deficiency. The middle-aged female mice in the current study were 12 to 14 months of age; well within the normal range (i.e., 11 to 16 months of age) of estrous cycle cessation in C57Bl/6 mice (Felicio et al, 1984). The time prior to and shortly after cessation constitutes a time of hormonal flux in mice, just like in humans. The fluctuating levels of estrogen and progesterone during the early stages of estrous cycle cessation likely alter HPC connectivity, thereby triggering age-related neurobiological changes that may interfere with learning and memory. A close parallel has been found between the temporal conditions by which estrogen improves memory of female rats in a delayed matching-to-place task and the conditions for estrogen to induce new excitatory synaptic connections in the HPC (McEwen et al, 2001). Consistent with age-related hormonal changes, intermittent, but not continuous, estradiol treatments are detrimental to spatial memory of aged ovariectomized female mice (Gresack and Frick, 2006). Memory changes and concentration difficulties are commonly reported during perimenopause in women (Sullivan Mitchell and Fugate Woods, 2001), and those who begin hormone replacement therapy (i.e., are given estrogen) at this time have improved memory (Resnick et al, 1997). Thus, gonadal hormone changes occurring during middle age may accelerate age-related neurobiological changes that interfere with learning and memory.
In addition to the typical action of steroid nuclear receptors, estrogen can act via non-nuclear receptors coupled to second messenger systems and via rapid non-genomic mechanisms to phosphorylate CREB and, thereby, regulate gene expression underlying memory formation (Kelly and Levin, 2001; Szego et al, 2006; Zhao et al, 2005; Zhou et al, 2005). Estrogen has been found to change components of the CREB signaling pathway (i.e., Ca2+/calmodulin kinase IV and pCREB) in the CA1 and CA3 regions of the HPC (Zhou et al, 2005) and increase CREB phosphorylation in basal forebrain cholinergic neurons (Szego et al, 2006). Thus, the interaction between changing estrogen and estrogen receptor levels during middle age (Bergman et al, 1991) coupled with a long-term CREB deficiency likely renders female CREBαδ- deficient mice very susceptible to impaired cognitive function. A CREB deficiency at birth results in an animal that has many molecular and cellular compensatory changes, which likely initially diminishes the severity of cognitive impairment. CREB is only one of several transcription factors in the CREB/ATF family and the CREBαδ- mutation results in up-regulated cAMP response element modulator and CREB β (Blendy et al, 1996; Mantamadiotis et al, 2002). Age-related brain alterations, especially those affected by the changing hormonal milieu, however, serve to enhance the vulnerability of female mice with this mutation to exhibit cognitive impairment. A direct way to test this hypothesis is to ascertain whether treatment with gonadal hormones in middle-aged females can abrogate the learning difficulties in the CREBαδ- deficient mice.
In conclusion, the results from this study reveal that mice with a genetic vulnerability to impaired learning and memory, which is highly influenced by environmental conditions and previous experience, show more extensive learning and memory impairment with aging. The impairment displayed by these aged CREBαδ- deficient mice continues to be influenced by the degree of cognitive demand required to solve a learning task, with tasks requiring higher demand resulting in the most impairment. This impairment is also most apparent among females such that it appears at an earlier age for females than males with old females showing deficits in both low and high cognitive demand tasks. Thus, a genetic predisposition to cognitive impairment may render females more vulnerable than males to such deficits with age. Since the nature of a previous learning experience influences learning and memory performance of young CREBαδ- deficient mice (Hebda-Bauer et al, 2005) and prior learning experience facilitates performance when the same task is subsequently tested (Dellu et al, 1997; Markowska and Savonenko, 2002; Vicens et al, 1999, 2002), exploring the effects of an early age positive learning experience on learning and memory performance in middle and old age would help determine if the age and sex differences in spatial learning and memory would be attenuated in mice with a long-term CREB deficiency. Moreover, it would be of interest to investigate the ability of hormone replacement therapy to prevent age-related cognitive abilities in the vulnerable female animals.
Acknowledgments
This research was supported by a MTRC Grant, Nathan Shock Center of Excellence in Aging, University of Michigan; NIMH PO1MH42251; NIDA RO1DA3386; and NIDA T32-DA07268.
List of Abbreviations
- CREB
cAMP response element binding protein
- CREBαδ- deficient mice
Mice with a mutation in the alpha and delta isoforms of the cAMP response element binding protein gene
- WT
wild-type
- MWM
Morris water Maze
- HPC
hippocampus
- ITI
inter-trial interval
- +/+
wild type
- -/-
homozygous deficient
- PCR
polymerase chain reaction
- EDTA
ethylenediamine tetraacetic acid
- SDS
Sodium dodecyl sulfate
- TNES
Buffer containing Tris, NaCl, EDTA, and SDS
- DNA
Deoxyribonucleic acid
- MWM2
Morris water maze protocol with 2 trials per day and a 1 minute inter-trial interval
- MWM4
Morris water maze protocol with 4 trials per day and a 3-5 minute inter-trial interval
- ANOVA
analysis of variance
- pCREB
phosphorylated cAMP response element binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript.The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elaine K. Hebda-Bauer, Email: hebda@umich.edu.
Stanley J. Watson, Email: watsons@umich.edu.
Huda Akil, Email: akil@umich.edu.
References
- Aartsen MJ, Martin M, Zimprich D. Gender differences in level and change in cognitive functioning. Results from the Longitudinal Aging Study Amsterdam. Gerontology. 2004;50:35–38. doi: 10.1159/000074387. [DOI] [PubMed] [Google Scholar]
- Ammassari-Teule M, Fagioli S, Rossi-Arnaud C. Radial maze performance and open-field behaviours in aged C57BL/6 mice: further evidence for preserved cognitive abilities during senescence. Physiol Behav. 1994;55:341–345. doi: 10.1016/0031-9384(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JR, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. EURODEM Incidence Research Group. Neurology. 1999;53:1992–1997. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, Frey JU, Lipp HP. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci. 2003;23:6304–6314. doi: 10.1523/JNEUROSCI.23-15-06304.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Markowska AL, Ingram DK, Kametani H, Spangler EL, Lemken VJ, Olton DS. Acetyl-1-carnitine. 2: Effects on learning and memory performance of aged rats in simple and complex mazes. Neurobiol Aging. 1990;11:499–506. doi: 10.1016/0197-4580(90)90110-l. [DOI] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Bergman MD, Karelus K, Felicio LS, Nelson JF. Age-related alterations in estrogen receptor dynamics are independent of cycling status in middle-aged C57BL/6J mice. J Steroid Biochem Mol Biol. 1991;38:127–133. doi: 10.1016/0960-0760(91)90117-n. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Kaestner KH, Schmid W, Gass P, Schutz G. Targeting of the CREB gene leads to up-regulation of a novel CREB mRNA isoform. Embo J. 1996;15:1098–1106. [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brightwell JJ, Gallagher M, Colombo PJ. Hippocampal CREB1 but not CREB2 is decreased in aged rats with spatial memory impairments. Neurobiol Learn Mem. 2004;81:19–26. doi: 10.1016/j.nlm.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Cho YH, Giese KP, Tanila H, Silva AJ, Eichenbaum H. Abnormal hippocampal spatial representations in alphaCaMKIIT286A and CREBalphaDelta- mice. Science. 1998;279:867–869. doi: 10.1126/science.279.5352.867. [DOI] [PubMed] [Google Scholar]
- Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–314. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Vallee M, Le Moal M, Simon H. Facilitation of cognitive performance in aged rats by past experience depends on the type of information processing involved: a combined cross-sectional and longitudinal study. Neurobiol Learn Mem. 1997;67:121–128. doi: 10.1006/nlme.1996.3750. [DOI] [PubMed] [Google Scholar]
- Deupree DL, Bradley J, Turner DA. Age-related alterations in potentiation in the CA1 region in F344 rats. Neurobiol Aging. 1993;14:249–258. doi: 10.1016/0197-4580(93)90009-z. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Dunbar GL, Rylett RJ, Schmidt BM, Sinclair RC, Williams LR. Hippocampal choline acetyltransferase activity correlates with spatial learning in aged rats. Brain Res. 1993;604:266–272. doi: 10.1016/0006-8993(93)90378-z. [DOI] [PubMed] [Google Scholar]
- Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31:446–453. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, Berg S, Pedersen NL. Surprising lack of sex differences in normal cognitive aging in twins. Int J Aging Hum Dev. 2006;62:335–357. doi: 10.2190/C39X-9QHY-49DM-X9GJ. [DOI] [PubMed] [Google Scholar]
- Fischer W, Chen KS, Gage FH, Bjorklund A. Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Effects of aging on spatial learning and hippocampal protein kinase C in mice. Neurobiol Aging. 1993;14:309–317. doi: 10.1016/0197-4580(93)90116-s. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci. 2001;21:4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–198. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Ratcliff G, Huff FJ, Belle S, Kancel MJ, Fischer L, Seaberg EC, Kuller LH. Effects of age, gender, and education on cognitive tests in a rural elderly community sample: norms from the Monongahela Valley Independent Elders Survey. Neuroepidemiology. 1991;10:42–52. doi: 10.1159/000110246. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Gass P, Wolfer DP, Balschun D, Rudolph D, Frey U, Lipp HP, Schutz G. Deficits in memory tasks of mice with CREB mutations depend on gene dosage. Learn Mem. 1998;5:274–288. [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Aged rats need a preserved complement of perforated axospinous synapses per hippocampal neuron to maintain good spatial memory. Brain Res. 1986;398:266–275. doi: 10.1016/0006-8993(86)91486-1. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Herlitz A, Smith J. Stability of sex differences in cognition in advanced old age: the role of education and attrition. J Gerontol B Psychol Sci Soc Sci. 2006;61:P245–249. doi: 10.1093/geronb/61.4.p245. [DOI] [PubMed] [Google Scholar]
- Graves L, Dalvi A, Lucki I, Blendy JA, Abel T. Behavioral analysis of CREB alphadelta mutation on a B6/129 F1 hybrid background. Hippocampus. 2002;12:18–26. doi: 10.1002/hipo.10003. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res. 2006;1115:135–147. doi: 10.1016/j.brainres.2006.07.067. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci U S A. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195:353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Hebda-Bauer EK, Morano MI, Therrien B. Aging and corticosterone injections affect spatial learning in Fischer-344 X Brown norway rats. Brain Res. 1999;827:93–103. doi: 10.1016/s0006-8993(99)01310-4. [DOI] [PubMed] [Google Scholar]
- Hebda-Bauer EK, Watson SJ, Akil H. Cognitive performance is highly sensitive to prior experience in mice with a learning and memory deficit: failure leads to more failure. Learn Mem. 2005;12:461–471. doi: 10.1101/lm.94105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummler E, Cole TJ, Blendy JA, Ganss R, Aguzzi A, Schmid W, Beermann F, Schutz G. Targeted mutation of the CREB gene: compensation within the CREB/ATF family of transcription factors. Proc Natl Acad Sci U S A. 1994;91:5647–5651. doi: 10.1073/pnas.91.12.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schutz G, Silva AJ. Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol. 1997;7:1–11. doi: 10.1016/s0960-9822(06)00022-4. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Ort M, Cimadevilla JM, Vondrous R, Cordell B, Koistinaho J, Bures J, Higgins LS. Specific spatial learning deficits become severe with age in beta -amyloid precursor protein transgenic mice that harbor diffuse beta -amyloid deposits but do not form plaques. Proc Natl Acad Sci U S A. 2001;98:14675–14680. doi: 10.1073/pnas.261562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo K, Wati H, Qiao C, Arita J, Kanba S. Age-related disturbance of memory and CREB phosphorylation in CA1 area of hippocampus of rats. Brain Res. 2005;1054:30–37. doi: 10.1016/j.brainres.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Lund PK, Hoyt EC, Bizon J, Smith DR, Haberman R, Helm K, Gallagher M. Transcriptional mechanisms of hippocampal aging. Exp Gerontol. 2004;39:1613–1622. doi: 10.1016/j.exger.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Aging of glutamate receptors: correlations between binding and spatial memory performance in mice. Mech Ageing Dev. 1998;104:227–248. doi: 10.1016/s0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schutz G. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Protective effect of practice on cognition during aging: implications for predictive characteristics of performance and efficacy of practice. Neurobiol Learn Mem. 2002;78:294–320. doi: 10.1006/nlme.2002.4064. [DOI] [PubMed] [Google Scholar]
- McEwen B, Akama K, Alves S, Brake WG, Bulloch K, Lee S, Li C, Yuen G, Milner TA. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc Natl Acad Sci U S A. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Harlan RE, Kastin AJ, Zadina JE. Tests used to assess the cognitive abilities of aged rats: their relation to each other and to hippocampal morphology and neurotrophin expression. Gerontology. 1999;45:143–155. doi: 10.1159/000022077. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Maekawa N, Saito K, Seishima M, Nabeshima T. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav Brain Res. 2002;133:135–141. doi: 10.1016/s0166-4328(01)00470-3. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Antunes-Martins A, Ris L, Peters M, Godaux E, Giese KP. Calcium/calmodulin kinase kinase beta has a male-specific role in memory formation. Neuroscience. 2007;145:393–402. doi: 10.1016/j.neuroscience.2006.11.056. [DOI] [PubMed] [Google Scholar]
- Monti B, Berteotti C, Contestabile A. Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus. 2005;15:1041–1049. doi: 10.1002/hipo.20099. [DOI] [PubMed] [Google Scholar]
- Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem. 1996;271:14214–14220. doi: 10.1074/jbc.271.24.14214. [DOI] [PubMed] [Google Scholar]
- Mouravlev A, Dunning J, Young D, During MJ. Somatic gene transfer of cAMP response element-binding protein attenuates memory impairment in aging rats. Proc Natl Acad Sci U S A. 2006;103:4705–4710. doi: 10.1073/pnas.0506137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe JaN L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- Ott A, Breteler MM, van Harskamp F, Claus JJ, van der Cammen TJ, Grobbee DE, Hofman A. Prevalence of Alzheimer’s disease and vascular dementia: association with education. The Rotterdam study. Bmj. 1995;310:970–973. doi: 10.1136/bmj.310.6985.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M, Mizuno K, Ris L, Angelo M, Godaux E, Giese KP. Loss of Ca2+/calmodulin kinase kinase beta affects the formation of some, but not all, types of hippocampus-dependent long-term memory. J Neurosci. 2003;23:9752–9760. doi: 10.1523/JNEUROSCI.23-30-09752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- Rivas-Vazquez RA, Mendez C, Rey GJ, Carrazana EJ. Mild cognitive impairment: new neuropsychological and pharmacological target. Arch Clin Neuropsychol. 2004;19:11–27. [PubMed] [Google Scholar]
- Sauer H, Francis JM, Jiang H, Hamilton GS, Steiner JP. Systemic treatment with GPI 1046 improves spatial memory and reverses cholinergic neuron atrophy in the medial septal nucleus of aged mice. Brain Res. 1999;842:109–118. doi: 10.1016/s0006-8993(99)01851-x. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- Sullivan Mitchell E, Fugate Woods N. Midlife women’s attributions about perceived memory changes: observations from the Seattle Midlife Women’s Health Study. J Womens Health Gend Based Med. 2001;10:351–362. doi: 10.1089/152460901750269670. [DOI] [PubMed] [Google Scholar]
- Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Abraham IM. Estrogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbitsky M, Yonan AL, Malleret G, Kandel ER, Gilliam TC, Pavlidis P. Altered hippocampal transcript profile accompanies an age-related spatial memory deficit in mice. Learn Mem. 2004;11:253–260. doi: 10.1101/lm.68204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicens P, Bernal MC, Carrasco MC, Redolat R. Previous training in the water maze: differential effects in NMRI and C57BL mice. Physiol Behav. 1999;67:197–203. doi: 10.1016/s0031-9384(99)00059-1. [DOI] [PubMed] [Google Scholar]
- Vicens P, Redolat R, Carrasco MC. Effects of early spatial training on water maze performance: a longitudinal study in mice. Exp Gerontol. 2002;37:575–581. doi: 10.1016/s0531-5565(01)00217-0. [DOI] [PubMed] [Google Scholar]
- Wade JP, Mirsen TR, Hachinski VC, Fisman M, Lau C, Merskey H. The clinical diagnosis of Alzheimer’s disease. Arch Neurol. 1987;44:24–29. doi: 10.1001/archneur.1987.00520130016010. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336–340. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- Wolff M, Costet P, Gross C, Hen R, Segu L, Buhot MC. Age-dependent effects of serotonin-1A receptor gene deletion in spatial learning abilities in mice. Brain Res Mol Brain Res. 2004;130:39–48. doi: 10.1016/j.molbrainres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang Z. Effects of estradiol benzoate on learning-memory behavior and synaptic structure in ovariectomized mice. Life Sci. 2006;79:1553–1560. doi: 10.1016/j.lfs.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang H, Cohen RS, Pandey SC. Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures. Neuroendocrinology. 2005;81:294–310. doi: 10.1159/000088448. [DOI] [PMC free article] [PubMed] [Google Scholar]


