Abstract
β-Tryptase, a product of the TPSAB1 and TPSB2 genes, is a trypsin-like serine protease that is a major and selective component of the secretory granules of all human mast cells, accounting for as much as 25% of cell protein. Once mast cells are activated, β-tryptase is released along with histamine and heparin proteoglycan. β-Tryptase is a unique enzyme with a homotetrameric structure in which active sites face into the central cavity of the four monomers, stabilized by heparin-proteoglycan. This structure makes β-tryptase resistant to most biological inhibitors of serine proteases. Without stabilization, at neutral pH β-tryptase converts to inactive monomers. Tryptase levels are elevated in bronchoalveolar lavage (BAL) fluid obtained from atopic asthmatics and in serum during systemic anaphylactic shock. Several synthetic small molecular weight β-tryptase inhibitors reduced Ag-induced airway hypersensitivity in animals, suggesting β-tryptase is involved in the pathogenesis of airway inflammation. Although the major biologic substrate(s) of β-tryptase remain ambiguous, the protease can digest several proteins of potential biologic importance, including fibrinogen, fibronectin, pro-urokinase, pro-matrix metalloprotease-3 (proMMP-3), protease activated receptor-2 (PAR2) and complement component C3. Recently, monomers of β-tryptase with enzymatic activity have been detected in vitro. Here we discuss how β-tryptase monomers with enzymatic activity were identified as well as their potential role in vivo.
Keywords: human, β-tryptase, mast cells
1. Introduction
Mature β-tryptase (10-35 pg per human mast cell) consists of four monomers, each monomer stabilized in its active conformation and in its tetrameric form by heparin-proteoglycan [1,2]. This protease is stored in the secretory granules of all human mast cells and is released from these cells when they are activated to degranulate [3,4]. Purified β-tryptase tetramers in the absence of heparin spontaneously convert to inactive monomers at neutral pH in a physiological salt solution. However, the tetrameric structure is stable in high salt solution (>0.5 M NaCl). The crystal structure of β-tryptase reveals details of interaction sites between the monomers [5,6]. The active site of each subunit of the tetramer faces into a central pore, which measures about 50 × 30 Å, and thereby restricts access of biological inhibitors to the active sites. The interactions between two monomer:monomer interfaces include a mixture of hydrophobic and ionic interactions that are reasonably strong, while the other two monomer:monomer interactions are exclusively hydrophobic with domains rich in Tyr and Pro. These latter monomer pairs appear to be stabilized by the binding of heparin to a groove of positively charged amino acids on the periphery of the tetramer that span the two monomers.
Human mast cells have been divided to two types based on the enzymes contained in the granules. Mast cells with β-tryptase (35 pg/cell), chymase (4.5 pg/cell), carboxypeptidase A3 (5-16 pg/cell) and cathepsin G are called MCTC cells. Mast cells only with β-tryptase (10 pg/cell) are called MCT cells. MCTC cells are the predominant type in skin and small bowel submucosa, whereas MCT cells are the predominant type overall in the lung. However, MCTC cells account for most of the mast cell hyperplasia reported in bronchial smooth muscle of asthmatics [7]. MCTC cells also express CD88 (C5aR) and, consequently, are activated by C5a [8]. Chymase, a chymotrypsin-like protease, is stored in mast cell secretory granules along with carboxypeptidase A3 in a complex with proteoglycan [9]. Heparin facilitates the processing of prochymase [10,11] and β-protryptase [12] by dipeptidylpeptidase I (DPPI, Cathepsin C), a cysteine protease. Chymase converts angiotensin I to angiotensin II [13,14], processes type I procollagen to collagen fibrils [15] and degrades various cytokines [16]. Cathepsin G is serine protease expressed by MCTC cells [17] as well as by neutrophils and monocytes. Mast cell carboxypeptidase A3 is zinc-dependent exopeptidase [18-22] with a similar catalytic profile as pancreatic carboxypeptidase A. β-Hexosaminidase, like β-tryptase, is stored in the secretory granules of both MCTC type and MCT type, and can be used as a maker of degranulation for mast cells of at least 20% purity [3].
Human mast cell tryptase is derived from two genes clustered on chromosome 16p13.3, TPSB2 that is monomorphic for β-tryptase and TPSAB1 that is dimorphic for α-tryptase and β-tryptase; each consisting of six exons and five introns [3,23-25]. Human α-tryptases have been divided into α1 and α2 subtypes, β-tryptases into βI, βII and βIII subtypes. Among the α-tryptases and among the β-tryptases, there is 98% homology between amino acid sequences, while comparing α to β-tryptase sequences one finds approximately 90% homology. Each α/β-tryptase gene encodes a 275 amino acid peptide with a 30 amino acid leader sequence; 18 amino acids of pretryptase, 10 amino acids of protryptase and 2 amino acids of pro' tryptase [12]. The mature catalytic portion of tryptase consists of 245 amino acids. Critical differences between α-tryptases and β-tryptases are that α-tryptases do not appear to undergo autocatalytic processing from pro to pro' because the Gln-3 is not hydrolyzed by a trypsin-like enzyme. In contrast, autocatalytic cleavage at the Arg-3 of β-protryptase occurs at acidic pH (6-6.5) in the presence of heparin. Then the remaining ValGln dipeptide of β-pro'tryptase can be removed by DPPI at acidic pH. Mature β-tryptase monomers spontaneously form active tetramers in the presence of heparin at pH 6-6.5. Human α-tryptase also differs from β-tryptase in that it possesses Asp215 rather than Gly215 in one of the loops that form its substrate binding cleft [26]. Asp215 dominantly restricts the substrate specificity of engineered mature α-tryptase. Although mature β-tryptase is stored in secretory granules of mast cells, unprocessed α-protryptases are inactive and spontaneously secreted from mast cells [27]. Of potential interest to the human system is that disrupting the mouse N-deacetylase/N-sulfotransferase-2 gene, which is necessary to express fully sulfated heparin [28], showed reductions in the amounts of tryptase, chymase(s) and carboxypeptidase A3 in mast cells.
Another variant of tryptase is a membrane-bound relative, called transmembrane tryptase (TMT/γ-tryptase), a product of the TPSG1 gene, with 48% homology to α/β-tryptases [29,30]. Human γ-tryptase consist of signal peptide of 38 residues, 19 in the pre portion and 19 in the pro portion, and a mature domain of 282 amino acids, including 23 residues of a membrane-spanning portion near the C-terminal. Since it lacks Tyr and Pro enriched domains, γ-tryptase does not form a tetrameric conformation. The protein is highly expressed in intestine and various tumor cell lines. Human δ-tryptase, a product of the TPSD1 gene, is another variant which originally was named mMCP-7 like tryptase, but it has 40 amino acids deleted from the C-terminal relative to α/β-tryptases [31]. Whether the protein is highly expressed as an active protease is uncertain [32,31]. The most distantly related variant is called ε-tryptase, a product of the PRSS22 gene, with only 40% similarity to α/β-tryptases [33]. Human ε-tryptase is also located in chromosome 16p13.3 and consists of a 49 amino acid leader sequence, 32 residue of signal pre peptide and 17 residue of pro peptide, and a mature enzyme of 268 amino acids. Human ε-tryptase lacks the Tyr and Pro rich domains of α/β-tryptases, indicating no tetramer formation, and also lacks a C-terminal hydrophobic domain, indicating it does not reside in the membrane. These variants of tryptases are biochemically and immunologically distinct from α/β-tryptases. Herein we reserve the term tryptase for only α/β-tryptases.
2. Inhibitors and target proteins
The tetrameric conformation of β-tryptase explains its resistance to biological inhibitors, including α1-antitrypsin, antithrombin III (ATIII), α2-macroglobulin (α2M), bovine aprotinin and plant-derived inhibitors such as soybean trypsin inhibitor (SBTI) and lima bean trypsin inhibitor (LBTI) [34]. β-Tryptase activity is inhibited by small synthetic inhibitors such as diisopropylfluorophosphate, phenylmethylsulfonyl fluoride, tosyl-L-lysine chloromethyl ketone and leupeptin [1]. Several synthetic β-tryptase inhibitors were shown in vivo to reduce Ag-induced airway hypersensitivity in sheep [35,36], pig [37] and mouse [38], which indicates tryptase may be involved in the pathogenesis of airway inflammation.
Despite the lack of inhibition by biological inhibitors, β-tryptase can cleave several proteins, at least in vitro. Fibrinogen is rapidly cleaved and inactivated by β-tryptase [39-41]. β-Tryptase activates pro-matrix metalloprotease-3 (proMMP-3) [42], and cleaves low molecular weight [43] and high molecular weight kininogen [44-46]. β-Tryptase degrades fibronectin [47], activates pro-urokinase [48] and generates C3a from complement C3 [49]. Vasoactive intestinal peptide, VIP [50] and calcitonin gene-related peptide [51] are also degraded by β-tryptase. Presumably, structural features of these proteins allow access to the active sites of the β-tryptase tetramer.
β-Tryptase is also a potent stimulant of the proliferation of smooth muscle cells, fibroblasts and epithelial cells [52-54] and stimulates synthesis of type I collagen by human fibroblasts [55,56]. Proteinase-activated receptors (PARs) are G-protein coupled receptors, and four types of PAR have been cloned (PAR1, PAR2, PAR3 and PAR4). When cleaved near their N-termini, a conformational change occurs that allows signal transduction to occur. Activation of PAR2 on the cells by β-tryptase has been well-described [57-61]. However, the efficiency of β-tryptase -mediated activation is much lower than trypsin, which is major enzyme known as PAR2 activator. Indeed, one study found that recombinant βI-tryptase could not activate PAR2-transfected cells [62], and another report showed that sialylation and N-linked glycosylation of PAR2 and the presence of heparin markedly attenuate the ability of β-tryptase to activate this receptor [63,64]. Thus, activation of PAR2 by β-tryptase may not be a physiologic event. However, other mast cell enzymes affect PARs. Cathepsin G activates PAR4 [65]; and chymase inactivates PAR1 [66].
3. Tetramer ↔ monomer
β-Tryptase rapidly loses its enzymatic activity when incubated at neutral pH and physiologic ionic strength in the absence of a stabilizing polyanion such as heparin. Inactivation occurs concomitantly with the dissociation of the β-tryptase tetramer into inactive monomers [2]. Changes in tertiary structure also occur with this type of inactivation, because mAbs against β-tryptase were made that recognize conformational epitopes on active tetramers that are not available on inactive monomers [67]. A kinetic analysis of β-tryptase decay suggested active tetramers first became inactive tetramers (reversible by adding heparin), and then inactive monomers (irreversible) [68,69]. Evidence for active monomers was first reported by Addington et al [70], this conclusion was based upon analyzing monomers that had been loaded onto a gel-filtration column equilibrated with 10 mM Mes buffer, pH 6.1, containing 10 % glycerol, 1M NaCl and 0.01% NaN3. Eluates in the monomer fractions, when assayed in the presence of 100 μM heparin, were enzymatically active. However, the conclusion that active monomers were responsible for this enzymatic activity had to be reconsidered when it was shown that inactive monomers could spontaneously form active tetramers at acidic pH (6-6.5), though not at neutral pH, in the presence of heparin or another polyanion such as Dextran sulfate [71].
When polyanion-mediated reactivation of inactive monomer to active tetramer occurs at acidic pH, all of the enzymatic activity is recovered [71,72]. The optimal pH for reactivation was tested in 50 mM Mes or Hepes buffers containing 0.12 M NaCl, 0.5 mg/ml BSA and 20 μg/ml of heparin at 22°C for l h. The optimal pH was 6, but some reactivation occurred up to pH 7; no activity was recovered at pH 7.4. A similar acidic pH-dependency for tetramer formation was reported later with mouse mast cell protease-6 (mMCP-6, a homologue of human tryptase) [73]. Thus, pH is a key factor to consider for the regulation of β-tryptase activity. Optimal pH for the autocatalytic activation of β-protryptase also was shown to be acidic [12], as was the generation of bradykinin from low molecular weight kininogen [45], and the degradation of fibrinogen [40].
Additional evidence supporting the possibility of active β-tryptase monomers emerged using mMCP-6 [73]. Incubation of mMCP-6 at pH 6.0 with small heparin glycosaminoglycans (∼10 disaccharides per molecule) led to detection of β-tryptase enzymatic activity in the monomer gel filtration fraction that had about 1/5th the activity measured for tetramers. These putative active monomers degraded fibronectin and were inhibited by bovine pancreatic trypsin inhibitor (BPTI), whereas the tetramer did not degrade fibronectin and was not inhibited by BPTI. Such monomers, like the tetramers, were not inhibited by α1-antitrypsin and SBTI. However, whether short heparin glycosaminoglycans are available to β-tryptase in vivo is uncertain. Formation of active monomers using recombinant human β-tryptase was also noted [74]. β-rTryptase (2 μg in 20 μl of 10 mM Mes, pH 6.1 containing 2 M NaCl) was diluted 10-fold in PBS, pH 7.4, and incubated at 37°C for 30 min. Samples were analyzed on Superdex 200 column eluted with PBS, pH 6.0 containing 10 μM high molecular weight heparin. About 80 % of the β-tryptase activity was detected in tetramer fractions, while 20% was detected in monomer fractions. Only monomer fraction activity was inhibited by BPTI. However, inclusion of heparin in the elution buffer at physiological ionic strength raised the possibility of conversion of inactive monomers to active tetramers.
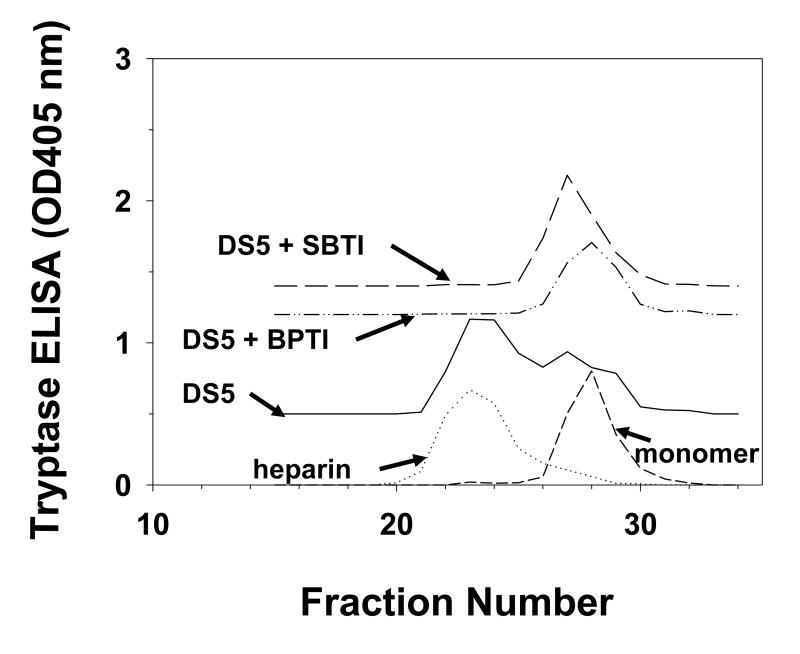
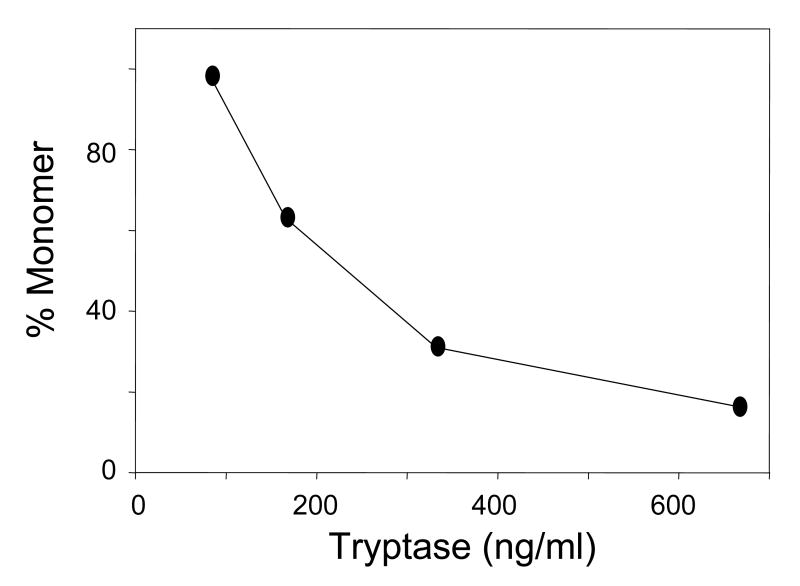
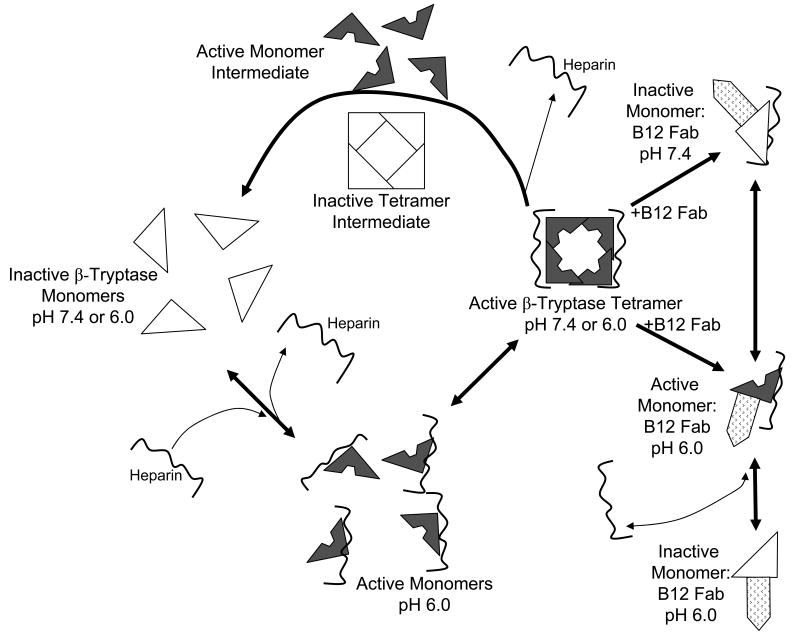
We examined the presence of active monomer by using protease inhibitors and the anti-tryptase mAb named B12, which enhances proteolytic activity at acidic pH [75,72]. Inactive monomers were made by incubating 2-3 μg/ml of β-tryptase tetramer in 10 mM Hepes buffer, pH 7.4, containing 0.12 M NaCl and 0.5 mg/ml BSA at 37°C for 90 min. Monomer formation was confirmed by loss of enzymatic activity and gel-filtration. Reconstitution of active tetramers from inactive monomers was accomplished by adding of one-fifth volume of 0.5 M Mes buffer, pH 6.0 in the presence of heparin or Dextran sulfate. If inactive β-tryptase monomers (2.5 μg/ml) were reactivated in the presence of SBTI, BPTI, ATIII or α2M (10-100 μg/ml) and of heparin at pH 6 for 1 h, reactivation was inhibited by each inhibitor in dose-dependent manner. Tetrameric β-tryptase purified from lung and obtained from reactivated monomers are resistant to these inhibitors. Inhibition of tetramer formation from monomers by BPTI and SBTI was confirmed by gel-filtration analysis (Fig. 1). Gel-filtration on a Superose 12 column was accomplished using 10 mM Mes buffer, pH 6.5, containing 1 M NaCl. High ionic strength was used to prevent conversion of tetramer to monomer and of monomer to tetramer during size separation and to facilitate removal of heparin from the β-tryptase complex. Active monomer formation also was examined with cleavable inhibitors such as ATIII and α2M. As shown in Fig. 2A, β-tryptase monomers are able to cleave ATIII only at pH 6.0. Gel-filtration analysis of this mixture detects the putative ATIII: β-tryptase complex at about 100 kDa (Fig. 2B). When α2M is incubated with inactive monomers in the presence of heparin at pH 6.0, a 98 kDa product is produced, indicating the bait region of α2M is cleaved (Fig. 3). Tetrameric β-tryptase did not produce any cleavage products when incubated with these inhibitors. These results indicate that inactive monomers become active monomers before reformation of active tetramers in the presence of heparin at pH 6.0. One useful biochemical difference between active monomers and tetramers is the susceptibility of monomers but not tetramers to inhibition by SBTI. Thus, the balance of active monomers and tetramer can be examined in a mixture of the two moieties (Fig. 4). After inactive monomers had been incubated with heparin at pH 6.0, 50% of the reconstituted activity is susceptible to inhibition by SBTI at a concentration of β-tryptase of ∼200 ng/ml. When the starting concentration of monomers is less than 100ng/ml, most of the reconstituted activity is susceptible to SBTI inhibition, consistent with formation of active monomers. Without heparin at pH 6.0, as at pH 7.4, tetramers become monomers. The interconversions between monomers and tetramer are summarized in Fig. 5. Active tetramer is converted to inactive monomers at neutral and acidic pH in the absence of heparin. Low concentrations of inactive monomers become active monomers at pH 6.0 in the presence of heparin. When the concentration of active monomers is higher, they convert to active monomers and then to active tetramers. Whether active tetramers convert to inactive monomers through an inactive tetramer intermediate or through an active monomer intermediate is somewhat uncertain. The absence of cleavage products of ATIII and α2M during conversion of β-tryptase tetramers to monomers at neutral pH (Fukuoka and Schwartz, unpublished data) favors the inactive tetramer intermediate, but does not rule out a transient active monomer intermediate. Selwood et al favor inactive tetrameric intermediates by using small synthetic inhibitors to stabilize the putative inactive tetrameric structure in the absence of heparin [76].
Fig. 1.
Analysis of quaternary structure of reactivated β-tryptase. β-Tryptase monomers were assessed directly or after incubation at pH 6.0 in the presence of heparin or Dextran sulfate 5000 or Dextran sulfate 5000 together with SBTI or BPTI. Fractions were analyzed for tryptase by ELISA. Reproduced with permission from Biochemistry, 2004, 43, 10757-10764. © 2004 American Chemical Society.
Fig. 2.
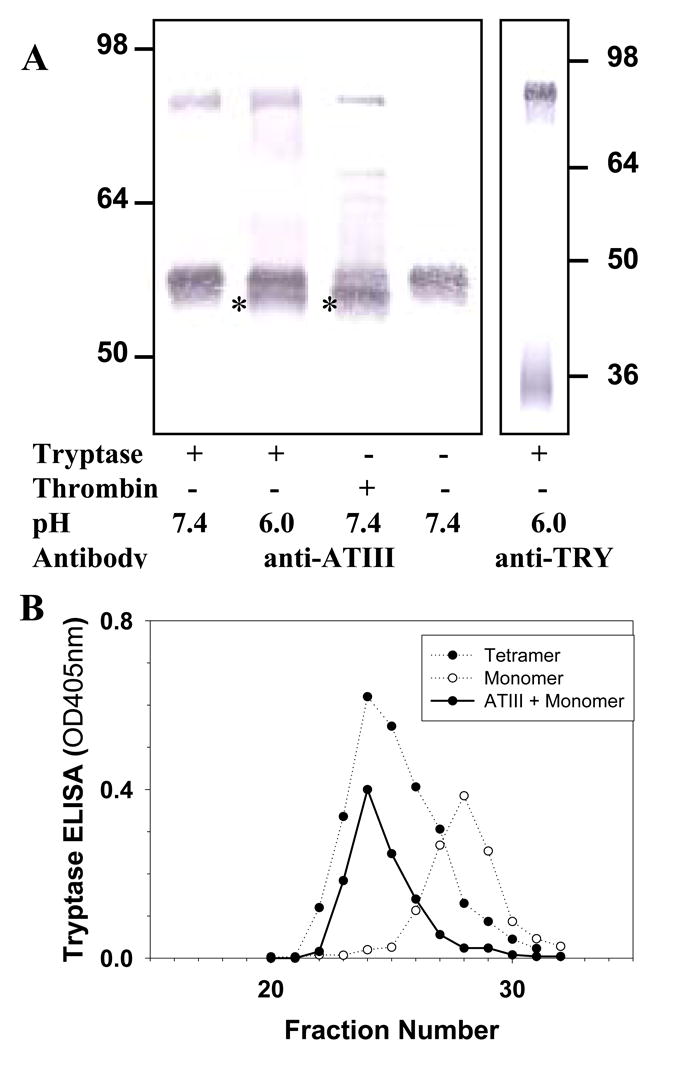
Effect of β-tryptase reactivation on ATIII. A. Western blotting analysis of ATIII and β-tryptase complex using anti-ATIII and anti-tryptase G3. Asterisks mark the degraded ATIII fragment in lanes 2 and 3. B. Gel filtration analysis of ATIII-β-tryptase monomer complex. Fractions were analyzed for tryptase by ELISA. Reproduced with permission from Biochemistry, 2004, 43, 10757-10764. © 2004 American Chemical Society.
Fig. 3.
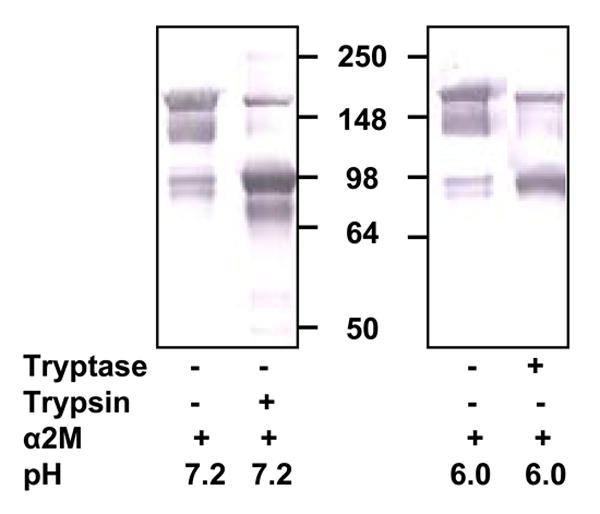
Effect of β-tryptase reactivation on α2M. Inactive monomers of β-tryptase were incubated with α2M in the presence of heparin at pH 6.0 Western blotting analysis was done using rabbit anti-α2M Ab. Lane 1, α2M alone at pH 7.2. Lane 2, trypsin and α2M at pH 7.2. Lane 3, α2M alone at pH 6.0. Lane 4, β-tryptase monomers and α2M at pH 6.0. Reproduced with permission from Biochemistry, 2004, 43, 10757-10764. © 2004 American Chemical Society.
Fig. 4.
Effect of β-tryptase concentration on the equilibrium between β-tryptase monomers and tetramers at acidic pH. Different concentrations of β-tryptase monomers were incubated at pH 6.0 in the presence of heparin. After 15 min at pH 6.0, SBTI or only buffer was added and the activity was measured. Residual activity was presumed to reflect active tetramers, while inhibited activity to reflect SBTI-inhibited active monomers.
Fig. 5.
Inactivation and reactivation pathways of β-tryptase. Active β-tryptase tetramer converts to inactive monomers in the absence of heparin at pH 7.4, probably through an inactive tetrameric intermediate, though an active monomeric intermediate has not been excluded. Inactive β-tryptase monomers become active as monomers at low concentrations when at pH 6.0 in the presence of heparin. Active β-tryptase monomers convert to active tetramers at higher concentrations. B12 Fab mAb modulates β-tryptase activity by converting tetramers to monomers that are enzymatically active at pH 6.0, but inactive at pH 7.4.
B12 Ab is an anti-tryptase mAb with a unique property. B12 mAb inhibits β-tryptase enzymatic activity at pH 7.4. Interestingly, at pH 5.5-6.5 B12 Ab shows minimal inhibition against small synthetic substrates, but enhances fibrinogen cleavage [40]. When the effects of SBTI, ATIII and α2M on the enzymatic activity of the B12 Fab-β-tryptase complex in the presence of heparin at acidic pH are measured, both SBTI and ATIII, but not α2M, inhibit this activity. Perhaps the B12 Fab-β-tryptase complex is too big to be entrapped by α2M. Control IgG and another anti-tryptase mAb called B2 did not increase susceptibility of β-tryptase to inhibition by SBTI and ATIII. Inhibition of β-tryptase by SBTI and activity at acidic but not neutral pH parallel the behavior of β-tryptase monomers, suggesting that B12 might convert tetramers to monomers. To test this hypothesis, the size of B12 Fab-β-tryptase complex was examined by gel-filtration at pH 6.5 at high ionic strength (1 M NaCl). The B12 Fab-tetramer complex eluted at a position compatible with a 1:1 B12 Fab: β-tryptase monomer, regardless whether the starting β-tryptase was monomeric or tetrameric, regardless whether heparin was present, and regardless whether the pH of the incubation was at pH 6.0 or pH 7.4. This supports the hypothesis that B12 binding to tetramer converts the tetramer to monomers independent on pH and heparin stabilization. Different molar ratios of β-tryptase to B12 Fab further illustrates this B12-dependent conversion of tetramers to monomers. At a ratio of 1:1 (tetramer:B12 Fab), a mixture of tetramers and monomer-B12 complexes are evident, while a ratio of 1:4 yield almost all monomer:B12 Fab complexes plus free B12 Fab. Notably, binding of B12 Fab or intact B12 to β-tryptase did not appear to alter the binding to heparin. Presumably B12 binding to β-tryptase interferes with subunit:subunit interactions. At acidic pH, active β-tryptase monomers exist in the presence of heparin when β-tryptase concentrations are less than 100 ng/ml. However, much higher concentrations of active heparin-stabilized β-tryptase monomers can form as a complex with B12 Fab. Whether a natural modulator or autoantibodies exist with a similar B12-like activity is under investigation. B12-stabilized β-tryptase monomers will be useful for further characterization of this form of β-tryptase.
4. Concluding comments
As discussed above, the substrate repertoire of tetrameric β-tryptase is limited because of restricted access to the active sites. However, active monomers and B12-stabilized monomers have a broader repertoire and enhanced activity against protein substrates at acidic pH. Formation of active monomers at acidic pH could be of great importance in vivo, especially at sites of acidic pH such as the airway in asthma patients [77,78], and where there is poor vascularity, such as healing wounds and the margins of solid tumors [79].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981;256:11939–43. [PubMed] [Google Scholar]
- 2.Schwartz LB, Bradford TR. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem. 1986;261:7372–9. [PubMed] [Google Scholar]
- 3.Schwartz LB, Lewis RA, Seldin D, Austen KF. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J Immunol. 1981;126:1290–4. [PubMed] [Google Scholar]
- 4.Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;26:451–63. doi: 10.1016/j.iac.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Pereira PJ, Bergner A, Macedo-Ribeiro S, Huber R, Matschiner G, Fritz H, et al. Human β-tryptase is a ring-like tetramer with active sites facing a central pore. Nature. 1998;392:306–11. doi: 10.1038/32703. [DOI] [PubMed] [Google Scholar]
- 6.Sommerhoff CP, Bode W, Pereira PJ, Stubbs MT, Sturzebecher J, Piechottka GP, et al. The structure of the human betaII-tryptase tetramer: fo(u)r better or worse. Proc Natl Acad Sci USA. 1999;96:10984–91. doi: 10.1073/pnas.96.20.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 8.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, et al. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–8. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein SM, Leong J, Schwartz LB, Cooke D. Protease composition of exocytosed human skin mast cell protease-proteoglycan complexes: Tryptase resides in a complex distinct from chymase and carboxypeptidase. J Immunol. 1992;148:2475–82. [PubMed] [Google Scholar]
- 10.Urata H, Karnik SS, Graham RM, Husain A. Dipeptide processing activates recombinant human prochymase. J Biol Chem. 1993;268:24318–22. [PubMed] [Google Scholar]
- 11.Murakami M, Karnik SS, Husain A. Human prochymase activation. A novel role for heparin in zymogen processing. J Biol Chem. 1995;270:2218–23. [PubMed] [Google Scholar]
- 12.Sakai K, Ren S, Schwartz LB. A novel heparin-dependent processing pathway for human tryptase: autocatalysis followed by activation with dipeptidyl peptidase I. J Clin Invest. 1996;97:988–95. doi: 10.1172/JCI118523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wintroub BU, Schechter NB, Lazarus GS, Kaempfer CE, Schwartz LB. Angiotensin I conversion by human and rat chymotryptic proteinases. J Invest Dermatol. 1984;83:336–9. doi: 10.1111/1523-1747.ep12264144. [DOI] [PubMed] [Google Scholar]
- 14.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–57. [PubMed] [Google Scholar]
- 15.Kofford MW, Schwartz LB, Schechter NM, Yager DR, Diegelmann RF, Graham MF. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem. 1997;272:7127–31. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol. 2005;175:2635–42. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 17.Schechter NM, Irani AMA, Sprows JL, Abernethy J, Wintroub B, Schwartz LB. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. 1990;145:2652–61. [PubMed] [Google Scholar]
- 18.Goldstein SM, Kaempfer CE, Kealey JT, Wintroub BU. Human mast cell carboxypeptidase: purification and characterization. J Clin Invest. 1989;83:1630–6. doi: 10.1172/JCI114061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irani AMA, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB. Human mast cell carboxypeptidase: Selective localization to MCTC cells. J Immunol. 1991;147:247–53. [PubMed] [Google Scholar]
- 20.Natsuaki M, Stewart CB, Vanderslice P, Schwartz LB, Wintroub BU, Rutter WJ, et al. Human skin mast cell carboxypeptidase: Functional characterization, cDNA cloning, and genealogy. J Invest Dermatol. 1992;99:138–45. doi: 10.1111/1523-1747.ep12616776. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds DS, Gurley DS, Austen KF. Cloning and characterization of the novel gene for mast cell carboxypeptidase A. J Clin Invest. 1992;89:273–82. doi: 10.1172/JCI115571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henningsson F, Ledin J, Lunderius C, Wilen M, Hellman L, Pejler G. Altered storage of proteases in mast cells from mice lacking heparin: a possible role for heparin in carboxypeptidase A processing. Biol Chem. 2002;383:793–801. doi: 10.1515/BC.2002.083. [DOI] [PubMed] [Google Scholar]
- 23.Miller JS, Westin EH, Schwartz LB. Cloning and characterization of complementary DNA for human tryptase. J Clin Invest. 1989;84:1188–95. doi: 10.1172/JCI114284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JS, Moxley G, Schwartz LB. Cloning and characterization of a second complementary DNA for human tryptase. J Clin Invest. 1990;86:864–70. doi: 10.1172/JCI114786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pallaoro M, Fejzo MS, Shayesteh L, Blount JL, Caughey GH. Characterization of genes encoding known and novel human mast cell tryptases on chromosome 16p13.3. J Biol Chem. 1999;274:3355–62. doi: 10.1074/jbc.274.6.3355. [DOI] [PubMed] [Google Scholar]
- 26.Huang C, Li L, Krilis SA, Chanasyk K, Tang Y, Li Z, et al. Human tryptases alpha and beta/II are functionally distinct due, in part, to a single amino acid difference in one of the surface loops that forms the substrate-binding cleft. J Biol Chem. 1999;274:19670–6. doi: 10.1074/jbc.274.28.19670. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz LB, Min HK, Ren S, Xia HZ, Hu J, Zhao W, et al. Tryptase Precursors Are Preferentially and Spontaneously Released, Whereas Mature Tryptase Is Retained by HMC-1 Cells, Mono-Mac-6 Cells, and Human Skin-Derived Mast Cells. J Immunol. 2003;170:5667–73. doi: 10.4049/jimmunol.170.11.5667. [DOI] [PubMed] [Google Scholar]
- 28.Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–6. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 29.Wong GW, Tang YZ, Stevens RL. Cloning of the human homolog of mouse transmembrane tryptase. Int Arch Allergy Immunol. 1999;118:419–21. doi: 10.1159/000024152. [DOI] [PubMed] [Google Scholar]
- 30.Caughey GH, Raymond WW, Blount JL, Hau LWT, Pallaoro M, Wolters PJ, et al. Characterization of human gamma-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. J Immunol. 2000;164:6566–75. doi: 10.4049/jimmunol.164.12.6566. [DOI] [PubMed] [Google Scholar]
- 31.Wang HW, McNeil HP, Husain A, Liu K, Tedla N, Thomas PS, et al. delta Tryptase Is Expressed in Multiple Human Tissues, and a Recombinant Form Has Proteolytic Activity. J Immunol. 2002;169:5145–52. doi: 10.4049/jimmunol.169.9.5145. [DOI] [PubMed] [Google Scholar]
- 32.Min HK, Kambe N, Schwartz LB. Human mouse mast cell protease 7-like tryptase genes are pseudogenes. J Allergy Clin Immunol. 2001;107:315–21. doi: 10.1067/mai.2001.112130. [DOI] [PubMed] [Google Scholar]
- 33.Wong GW, Yasuda S, Madhusudhan MS, Li L, Yang Y, Krilis SA, et al. Human tryptase epsilon (PRSS22), a new member of the chromosome 16p13.3 family of human serine proteases expressed in airway epithelial cells. J Biol Chem. 2001;276:49169–82. doi: 10.1074/jbc.M108677200. [DOI] [PubMed] [Google Scholar]
- 34.Alter SC, Kramps JA, Janoff A, Schwartz LB. Interactions of human mast cell tryptase with biological protease inhibitors. Arch Biochem Biophys. 1990;276:26–31. doi: 10.1016/0003-9861(90)90005-j. [DOI] [PubMed] [Google Scholar]
- 35.Clark JM, Abraham WM, Fishman CE, Forteza R, Ahmed A, Cortes A, et al. Tryptase inhibitors block allergen-induced airway and inflammatory responses in allergic sheep. Am J Respir Crit Care Med. 1996 doi: 10.1164/ajrccm.152.6.8520778. [DOI] [PubMed] [Google Scholar]
- 36.Wright CD, Havill AM, Middleton SC, Kashem MA, Dripps DJ, Abraham WM, et al. Inhibition of allergen-induced pulmonary responses by the selective tryptase inhibitor 1,5-bis-{4-[(3-carbamimidoyl-benzenesulfonylamino)-methyl]phenoxy}-pentane (AMG-126737) Biochem Pharmacol. 1999;58:1989–96. doi: 10.1016/s0006-2952(99)00304-4. [DOI] [PubMed] [Google Scholar]
- 37.Sylvin H, Dahlback M, Van DP I, Alving K. The tryptase inhibitor APC-366 reduces the acute airway response to allergen in pigs sensitized to Ascaris suum. Clin Exp Allergy. 2002;32:967–71. doi: 10.1046/j.1365-2222.2002.01239.x. [DOI] [PubMed] [Google Scholar]
- 38.Oh SW, Pae CI, Lee DK, Jones F, Chiang GK, Kim HO, et al. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002;168:1992–2000. doi: 10.4049/jimmunol.168.4.1992. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz LB, Bradford TR, Littman BH, Wintroub BU. The fibrinogenolytic activity of purified tryptase from human lung mast cells. J Immunol. 1985;135:2762–7. [PubMed] [Google Scholar]
- 40.Ren S, Lawson AE, Carr M, Baumgarten CM, Schwartz LB. Human tryptase fibrinogenolysis is optimal at acidic pH and generates anticoagulant fragments in the presence of the anti-tryptase monoclonal antibody B12. J Immunol. 1997;159:3540–8. [PubMed] [Google Scholar]
- 41.Thomas VA, Wheeless CJ, Stack MS, Johnson DA. Human mast cell tryptase fibrinogenolysis: Kinetics, anticoagulation mechanism, and cell adhesion disruption. Biochemistry. 1998;37:2291–8. doi: 10.1021/bi972119z. [DOI] [PubMed] [Google Scholar]
- 42.Gruber BL, Marchese MJ, Suzuki K, Schwartz LB, Okada Y, Nagase H, et al. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84:1657–62. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz LB, Maier M, Spragg J. Interaction of human low molecular weight kininogen with human mast cell tryptase. Adv Exp Med Biol. 1986;198:105–11. doi: 10.1007/978-1-4684-5143-6_15. [DOI] [PubMed] [Google Scholar]
- 44.Maier M, Spragg J, Schwartz LB. Inactivation of human high molecular weight kininogen by human mast cell tryptase. J Immunol. 1983;130:2352–6. [PubMed] [Google Scholar]
- 45.Proud D, Siekierski ES, Bailey GS. Identification of human lung mast cell kininogenase as tryptase and relevance of tryptase kininogenase activity. Biochem Pharmacol. 1988;37:1473–80. doi: 10.1016/0006-2952(88)90008-1. [DOI] [PubMed] [Google Scholar]
- 46.Walls AF, Bennett AR, Sueiras-Diaz J, Olsson H. The kininogenase activity of human mast cell tryptase. Biochem Soc Trans. 1992;20:260S. doi: 10.1042/bst020260s. [DOI] [PubMed] [Google Scholar]
- 47.Lohi J, Harvima I, Keski-Oja J. Pericellular substrates of human mast cell tryptase: 72, 000 Dalton gelatinase and fibronectin. J Cell Biochem. 1992;50:337–49. doi: 10.1002/jcb.240500402. [DOI] [PubMed] [Google Scholar]
- 48.Stack MS, Johnson DA. Human mast cell tryptase activates single-chain urinary-type plasminogen activator (pro-urokinase) J Biol Chem. 1994;269:9416–9. [PubMed] [Google Scholar]
- 49.Schwartz LB, Kawahara MS, Hugli TE, Vik D, Fearon DT, Austen KF. Generation of C3a anaphylatoxin from human C3 by human mast cell tryptase. J Immunol. 1983;130:1891–5. [PubMed] [Google Scholar]
- 50.Caughey GH, Leidig F, Viro NF, Nadel JA. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther. 1988;244:133–7. [PubMed] [Google Scholar]
- 51.Walls AF, Brain SD, Desai A, Jose PJ, Hawkings E, Church MK, et al. Human mast cell tryptase attenuates the vasodilator activity of calcitonin gene-related peptide. Biochem Pharmacol. 1992;43:1243–8. doi: 10.1016/0006-2952(92)90498-8. [DOI] [PubMed] [Google Scholar]
- 52.Hartmann T, Ruoss SJ, Raymond WW, Seuwen K, Caughey GH. Human tryptase as a potent, cell-specific mitogen: Role of signaling pathways in synergistic responses. Am J Physiol Lung Cell Mol Physiol. 1992;262:L528–L534. doi: 10.1152/ajplung.1992.262.5.L528. [DOI] [PubMed] [Google Scholar]
- 53.Cairns JA, Walls AF. Mast cell tryptase is a mitogen for epithelial cells - Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J Immunol. 1996;156:275–83. [PubMed] [Google Scholar]
- 54.Brown JK, Tyler CL, Jones CA, Ruoss SJ, Hartmann T, Caughey GH. Tryptase, the dominant secretory granular protein in human mast cells, is a potent mitogen for cultured dog tracheal smooth muscle cells. Am J Respir Cell Mol Biol. 1995;13:227–36. doi: 10.1165/ajrcmb.13.2.7626290. [DOI] [PubMed] [Google Scholar]
- 55.Cairns JA, Walls AF. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J Clin Invest. 1997;99:1313–21. doi: 10.1172/JCI119290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gruber BL, Kew RR, Jelaska A, Marchese MJ, Garlick J, Ren SL, et al. Human mast cells activate fibroblasts - Tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J Immunol. 1997;158:2310–7. [PubMed] [Google Scholar]
- 57.Mirza H, Schmidt VA, Derian CK, Jesty J, Bahou WF. Mitogenic responses mediated through the proteinase-activated receptor-2 are induced by expressed forms of mast cell α- or β-tryptases. Blood. 1997;90:3914–22. [PubMed] [Google Scholar]
- 58.Mirza H, Yatsula V, Bahou WF. The proteinase activated receptor-2 (PAR-2) mediates mitogenic responses in human vascular endothelial cells. J Clin Invest. 1996;97:1705–14. doi: 10.1172/JCI118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, et al. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2000;278:L193–L201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- 60.Berger P, Perng DW, Thabrew H, Compton SJ, Cairns JA, McEuen AR, et al. Tryptase and agonists of PAR-2 induce the proliferation of human airway smooth muscle cells. J Appl Physiol. 2001;91:1372–9. doi: 10.1152/jappl.2001.91.3.1372. [DOI] [PubMed] [Google Scholar]
- 61.Schmidlin F, Amadesi S, Vidil R, Trevisani M, Martinet N, Caughey G, et al. Expression and function of proteinase-activated receptor 2 in human bronchial smooth muscle. Am J Respir Crit Care Med. 2001;164:1276–81. doi: 10.1164/ajrccm.164.7.2101157. [DOI] [PubMed] [Google Scholar]
- 62.Huang C, De Sanctis GT, O'Brien PJ, Mizgerd JP, Friend DS, Drazen JM, et al. Evaluation of the substrate specificity of human mast cell tryptase beta I and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–84. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 63.Compton SJ, Renaux B, Wijesuriya SJ, Hollenberg MD. Glycosylation and the activation of proteinase-activated receptor 2 (PAR(2)) by human mast cell tryptase. Br J Pharmacol. 2001;134:705–18. doi: 10.1038/sj.bjp.0704303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Compton SJ, Sandhu S, Wijesuriya SJ, Hollenberg MD. Glycosylation of human proteinase-activated receptor-2 (PAR-2): Role in cell surface expression and signalling. Biochem J. 2002 doi: 10.1042/BJ20020706. Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR. Cathepsin G activates protease-activated receptor-4 in human platelets. J Biol Chem. 2000;275:6819–23. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- 66.Schechter NM, Brass LF, Lavker RM, Jensen PJ. Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. J Cell Physiol. 1998;176:365–73. doi: 10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz LB, Bradford TR, Lee DC, Chlebowski JF. Immunologic and physicochemical evidence for conformational changes occurring on conversion of human mast cell tryptase from active tetramer to inactive monomer: Production of monoclonal antibodies recognizing active tryptase. J Immunol. 1990;144:2304–11. [PubMed] [Google Scholar]
- 68.Schechter NM, Eng GY, McCaslin DR. Human skin tryptase: Kinetic characterization of its spontaneous inactivation. Biochemistry. 1993;32:2617–25. doi: 10.1021/bi00061a020. [DOI] [PubMed] [Google Scholar]
- 69.Schechter NM, Eng GY, Selwood T, McCaslin DR. Structural changes associated with the spontaneous inactivation of the serine proteinase human tryptase. Biochemistry. 1995;34:10628–38. doi: 10.1021/bi00033a038. [DOI] [PubMed] [Google Scholar]
- 70.Addington AK, Johnson DA. Inactivation of human lung tryptase: Evidence for a re- activatable tetrameric intermediate and active monomers. Biochemistry. 1996;35:13511–8. doi: 10.1021/bi960042t. [DOI] [PubMed] [Google Scholar]
- 71.Ren SL, Sakai K, Schwartz LB. Regulation of human mast cell β-tryptase: Conversion of inactive monomer to active tetramer at acid pH. J Immunol. 1998;160:4561–9. [PubMed] [Google Scholar]
- 72.Fukuoka Y, Schwartz LB. Human beta-Tryptase: Detection and Characterization of the Active Monomer and Prevention of Tetramer Reconstitution by Protease Inhibitors. Biochemistry. 2004;43:10757–64. doi: 10.1021/bi049486c. [DOI] [PubMed] [Google Scholar]
- 73.Hallgren J, Spillmann D, Pejler G. Structural requirements and mechanism for heparin-induced activation of a recombinant mouse mast cell tryptase, mouse mast cell protease-6: formation of active tryptase monomers in the presence of low molecular weight heparin. J Biol Chem. 2001;276:42774–81. doi: 10.1074/jbc.M105531200. [DOI] [PubMed] [Google Scholar]
- 74.Fajardo I, Pejler G. Formation of active monomers from tetrameric human beta-tryptase. Biochem J. 2003;369:603–10. doi: 10.1042/BJ20021418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukuoka Y, Schwartz LB. The B12 anti-tryptase monoclonal antibody disrupts the tetrameric structure of heparin-stabilized beta-tryptase to form monomers that are inactive at neutral pH and active at acidic pH. J Immunol. 2006;176:3165–72. doi: 10.4049/jimmunol.176.5.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Selwood T, Smolensky H, McCaslin DR, Schechter NM. The interaction of human tryptase-beta with small molecule inhibitors provides new insights into the unusual functional instability and quaternary structure of the protease. Biochemistry. 2005;44:3580–90. doi: 10.1021/bi047765u. [DOI] [PubMed] [Google Scholar]
- 77.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, et al. Endogenous airway acidification. Implications for asthma pathophysiology [see comments] Am J Respir Crit Care Med. 2000;161:694–9. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 78.Gaston B, Hunt JF. How acidopneic is my patient? A new question in the pulmonary laboratory. Am J Respir Crit Care Med. 2002;165:1349–50. doi: 10.1164/rccm.2203052. [DOI] [PubMed] [Google Scholar]
- 79.Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. MRI of the tumor microenvironment. J Magn Reson Imaging. 2002;16:430–50. doi: 10.1002/jmri.10181. [DOI] [PubMed] [Google Scholar]