Abstract
Akt/PKB is a critical regulator of cardiac function and morphology, and its activity is governed by dual phosphorylation at active loop (Thr308) by phosphoinositide-dependent protein kinase-1 (PDK1) and at carboxyl-terminal hydrophobic motif (Ser473) by a putative PDK2. P21-activated kinase-1 (Pak1) is a serine/threonine protein kinase implicated in the regulation of cardiac hypertrophy and contractility, and was shown previously to activate Akt through an undefined mechanism. Here we report Pak1 as a potential PDK2 that is essential for Akt activity in cardiomyocytes. Both Pak1 and Akt can be activated by multiple hypertrophic stimuli or growth factors in a phosphatidylinositol-3-kinase (PI3K)-dependent manner. Pak1 overexpression induces Akt phosphorylation at both Ser473 and Thr308 in cardiomyocytes. Conversely, silencing or inactivating Pak1 gene diminishes Akt phosphorylation in vitro and in vivo. Purified Pak1 can directly phosphorylate Akt only at Ser473, suggesting that Pak1 may be a relevant PDK2 responsible for AKT Ser473 phosphorylation in cardiomyocytes. In addition, Pak1 protects cardiomyocytes from cell death, which is blocked by Akt inhibition. Our results connect two important regulators of cellular physiological functions and provide a potential mechanism for Pak1 signaling in cardiomyocytes.
Keywords: Akt/PKB, P21-activated kinase, Cardiomyocytes
INTRODUCTION
Akt/protein kinase B (PKB) is a cardinal intracellular kinase that regulates diverse cellular functions in the heart including myocyte growth and survival, contractile function, and coronary angiogenesis(1). Akt is activated by dual phosphorylation at active loop (T308) by phosphoinositide-dependent protein kinase-1 (PDK1)(2) and at carboxyl-terminal hydrophobic motif (S473) by a putative PDK2(3). Among more than 10 kinases proposed to function as the potential PDK2, the mammalian target of rapamycin complex 2 (mTORC2) has gained popularity as a physiological kinase that is required for Akt S473 phosphorylation(3, 4). However, theses studies were performed in tumor or transformed cell lines and embryonic tissues. It remains possible that kinases other than mTORC2 could be responsible for S473 phosphorylation in cardiomyocytes as phosphorylation of Akt S473 could be cell type or signaling pathway specific(3).
P21-activated kinases (Paks) are a family of serine/threonine protein kinases that are activated by external stimuli through various cell surface receptors including G protein-coupled receptors and receptor tyrosine kinases in a small GTPase dependent or independent manner(5). At least three Pak isoforms exist in the heart. Pak activity is increased by hyperosmotic shock(6) and hypoxia-reoxygenation(7) in cultured cardiomyocytes. Both Pak1 and Pak3 are able to enhance the calcium sensitivity of cardiac muscle cells albeit through apparently distinct mechanisms(8). Moreover, Pak1 has been implicated in Rac1-induced cardiac hypertrophy in transgenic mice(9). Nevertheless, the specific role of Pak in cardiac physiology and pathology has not been directly studied, and the down stream effectors of Pak remain uncertain. Interestingly, Pak1 shares a few similar functions with Akt in other cell types(5, 10). Also, Pak1 has been shown to activate Akt in pulmonary smooth muscle cells, although a mechanism of the activation was not defined(11). In this study, we tested the hypothesis that Pak1 is a potential S473 specific kinase that is essential for Akt activity in cardiomyocytes.
MATERIALS AND METHODS
Cell culture
Neonatal rat ventricular cardiomyocytes (NRVC) were prepared as described(12). NRVC were cultured in serum-free media for 24 hours before treated with each of the following agents for 5 to 15 minutes: α-1-adrenergic agonist phenylephrine (PE, 25 μmol/L), endothelin-1 (ET-1, 100 nmol/L), β-adrenergic agonist isopreterenol (ISO, 10 μmol/L), phorbol 12-myristate 13-acetate (PMA, 100 nmol/L), insulin-like growth factor 1 (IGF-1, 50 ng/ml), insulin (INS, 100 nmol/L), and platelet-derived growth factor (PDGF, 20 ng/ml). PI3K inhibitor wortmanin or LY294002 was added at 100 nmol/L or 20 μmol/L 30 minutes before addition of IGF-1 or INS. The specific Akt inhibitor X (Calbiochem, Cat. No. 124020, San Diego, CA) was used at 2 μmol/L.
Construction of replication-deficient adenoviruses
Adenoviruses expressing active Myc-Pak1-T423E and kinase dead Myc-Pak1-K299R were constructed as described(13). Adenovirus expressing a short hairpin RNA (shRNA) was designed for silencing both human and rodent Pak1 genes. The following oligonucleotides 5'-CGGCTCGAGAAAAAAAATCACAGACCATGTGTACACAGATTTcAAGCTTcAAATCTGTATACACACGGTCTGTGATTATCTCTATCACTGATAGGG AA-3' and 5'-ACCTCTAGAAATTCGAACGCTGACGTCATCAA-3' were used to amplify a Pak1 specific shRNA following the PCR-Shagging procedures(14). The ∼500 bp PCR product was cloned into the adenoviral shuttle vector pAdTrack, and converted to adenovirus by standard methods(13). Adenoviral infection of NRVC was performed for overexpression or gene silencing as described(12).
Gene silencing with Small Interfering RNA (siRNA)
The pre-designed siRNAs targeting rat Pak1(ID#198264), mTOR (ID#199097) as well as a scrambled siRNA were obtained from Ambion (Austin, TX). SiRNA transfection was performed using Lipofectamine RNAiMAX (Invitrogen).
Western Blot Analysis
was performed as described(12). All primary antibodies were purchased from Cell Signaling Technology except for GAPDH (Research Diagnostics).
Measurement of myocyte death
cardiomyocyte death was measured by staining with propidium iodide (PI, 1 μg/ml, Roche). PI was added directly to the culture medium, and myocytes were photographed under both phase contrast and fluorescent conditions. Apoptosis was assessed by the cleavage of Poly (ADP-ribose) polymerase (PARP) and caspase3.
GST fusion proteins and in vitro phosphorylation assays
GST-Akt and GST-Pak1 fusion proteins were made as described(15). Pak1 was cleaved off with thrombin and 40 ng of purified Pak1 was used to phosphorylate GST-Akt fusion protein (10 μg) in vitro. The latter was subjected to Western blot analysis with phospho-Akt-S473 (pAkt473), phospho-Akt-T308 (pAkt308) and GST antibodies, respectively.
Pak1 kinase Assays
Pak1 activity was measured by immuno-complex kinase assay using myelin basic protein (MBP) as the substrate with 10 μCi [γ-32P] ATP(15).
Pak1−/− mice
A Pak1−/− mouse was generated by targeted deletion of the p21-binding domain from the Pak1 gene. Pak1−/− mice are viable, and will be described in detail elsewhere. Pak1−/− mice and C57BL/6 wildtype mice were injected i.p. with IGF-1 at 4 mg/kg, and sacrificed 1 hour later for determining cardiac Akt activation.
Transverse Aortic Constriction (TAC)
TAC was performed in 8-week-old mice of FVB/N or C57BL/6 strain as described(15).
Statistical Analysis
Data are analyzed by one-way analysis of variance and expressed as mean ± SE with P<0.05 as the significant level.
RESULTS
Pak1 and Akt are activated by multiple hypertrophic stimuli and growth factors
To explore the role of Pak1 in cardiomyocytes, we cultured NRVC under serum-free conditions, and tested the ability of multiple hypertrophic agents and growth factors to activate Pak1 as determined by immuno-complex kinase assay and phosphorylation of Pak1 at Threonin-423 (T423), a site essential for Pak1 activity. Both Pak1 kinase activity (Figure 1A) and T423 phosphorylation (pPak1, Figure 1B) were markedly induced by all the drugs tested. These include PE, ET-1, ISO, PMA, IGF-1, INS, and PDGF. Akt phosphorylation at S473 and T308 was also increased by these treatments, which was associated with increased phosphorylation of AKT down-stream targets, including glycogen synthase kinase-3β (GSK-3β) and p70 S6 kinase (S6K). The growth factor-induced activation of both Pak1 and Akt was dramatically attenuated by PI3K inhibitor wortmanin or LY294002 (Figure 1C&D), indicating that the activation is PI3K-dependent. We measured Pak1 kinase activity in mouse hearts before and after transverse aortic constriction (TAC) that produces pressure load on the heart. Cardiac Pak1 activity was markedly increased by TAC in both C57BL/6 and FVBN mice, which was accompanied by increased Akt phosphorylation (Figure 1F). Together, these results suggest that Pak1 and Akt are activated concurrently in cardiomyocytes by the same stimuli.
Figure-1.
A. Pak1 is activated by multiple hypertrophic stimuli and growth factors in NRVC. NRVC were cultured under serum-free condition and stimulated with indicated agonists for 5 to 15 minutes. Pak1 activity was determined by immuno-complex kinase assay using MBP as the substrate in the presence of 10 μCi [γ-32P] ATP. 32P-labeled MBP was detected by autoradiography. The numbers above each immuno-blot are fold changes relative to control vehicle-treated cells as determined by densitometry. Results are means (n=4, SE not shown). * indicates p<0.05 compared with control (Con). B. Phosphorylation of Pak1-T423, Akt-S473 or T308, GSK-3β-S9 and S6K-T389 was determined by Western blot analysis. C. IGF-1 and insulin (INS)-induced Pak1 kinase activity was attenuated by PI3K inhibitor wortmanin (WT) or LY294002 (LY). Shown are autoradiographs of p32-labeled MBP. D. IGF-1 and INS-induced phosphorylation of Pak1 and Akt was blocked by LY as shown with western blot analysis. E. Pak1 activity was increased in pressure overloaded mouse hearts. FVB/N or C57B/6 mice underwent transverse aortic constriction (TAC), and Pak1 activity was determined by immuno-complex kinase assay 1 or 3 days after TAC. Results are means (n=3, SE not shown). * indicates p<0.05 compared with sham-operated mice. F. Cardiac Akt activity was increased by TAC as shown by Western blot analysis for pAkt473. G. Endogenous Pak1 is physically associated with Akt in cardiomyocytes. Protein lysates from either NRVC (left half of the image) or FVB/N mouse heart (right half) were incubated with Pak1 or Akt antibodies (Ab), and precipitated with protein A/G agarose beads (IP), which was followed by Western blot analysis (WB) with Pak1 or Akt Ab. The input is one tenth of the amount of protein lysates used for IP. Note: Akt Ab is efficient to pull down Akt and Pak1 proteins from NRVC but not from heart lysates. H. Purified Pak1 can phosphorylate a wild type Akt but not a mutant Akt with serine converted to alanine at the amino acid residue 473. The in vitro phosphorylation of GST-Akt-S fusion protein by purified Pak1 was performed in a cell-free system. Akt phosphorylation was determined by Western blot analysis with phospho-specific antibodies. I. Purified Pak1 can directly phosphorylate Akt-S473 but not T308 in GST-Akt-F fusion protein that contain the full length Akt.
Pak1 interacts with Akt in vivo and directly phosphorylates Akt at S473 in vitro
We tested if endogenous Pak1 is physically associated with Akt in cardiomyocytes by reciprocal immuno-precipitation (co-IP) followed by Western blot analysis. As shown in Figure 1G, both Pak1 and Akt antibodies are able to pull down Pak1 or Akt protein from NRVC cell lysates although only Pak1 is sufficient to do this in mouse heart lysates. To test if Pak1 functions as an Akt kinase in vitro, three GST-Akt fusion proteins were used as substrates in in vitro phosphorylation assays with purified Pak1. GST-Akt-F contains full length Akt; GST-Akt-S carries the C-terminal 80 amino acids of Akt that contains S473 but not T308; and the S473A mutant of GST-AKT-S has a S-A mutation at S473. Purified Pak1 can directly phosphorylate GST-AKT-S at S473. However, S-A mutation at S473 eliminates phosphorylation at this residue either with or without Pak1 (Figure 1H). With GST-Akt-F as the substrate, Pak1 induces robust phosphorylation of Akt at S473 but lacks the ablity to phosphorylate T308 (Figure 1I). These results suggest Pak1 as a potential Akt S473-specific kinase.
Pak1 is sufficient to induce Akt phosphorylation in NRVC
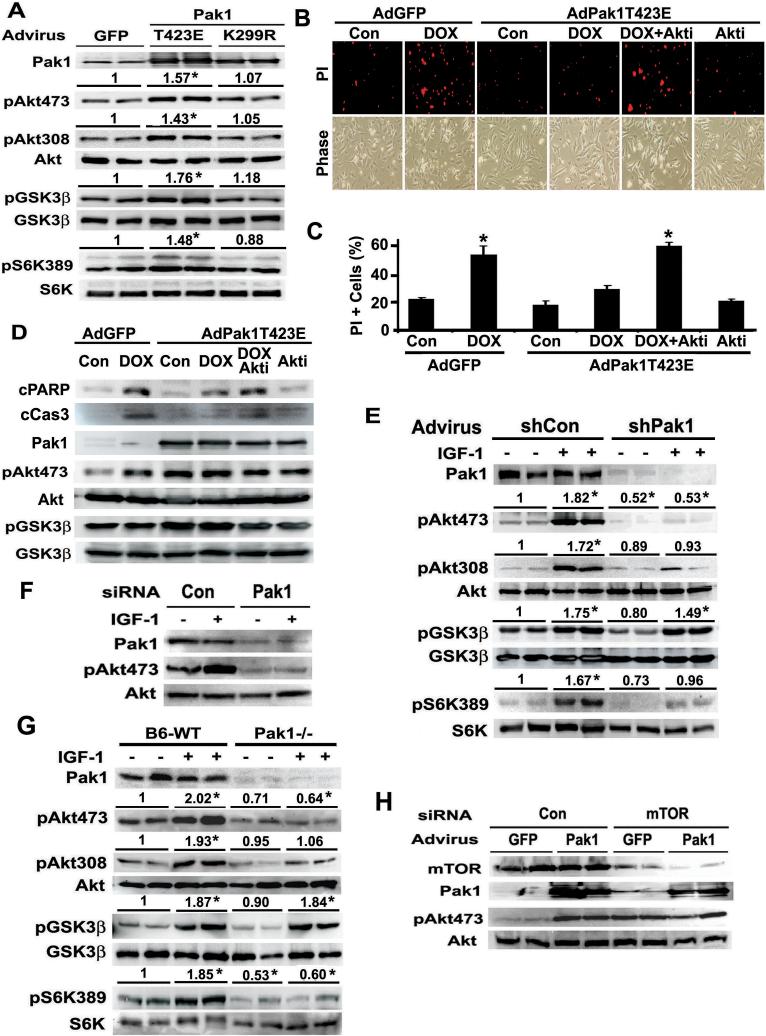
To test the ability of Pak1 to phosphorylate Akt in vivo, NRVC were transduced respectively with adenovirus expressing GFP, Pak1-T423E, or Pak1-K299R. Expression of active Pak1-T423E markedly increased Akt phosphorylation at both S473 and T308, and the phosphorylation of GSK-3β and S6K (Figure 2A). Coupled with the in vitro phosphorylation data, Pak1 is likely an Akt S473-specific kinase that also indirectly increases T308 phosphorylation. The kinase activity seemed required for Pak1 to activate Akt as kinase-dead Pak1-K299R did not affect Akt phosphorylation.
Figure-2.

A. Adenovirus-mediated gene transfer of active Pak1-T423E, but not kinase-dead Pak1-K299R, was sufficient to activate Akt signaling in cultured cardiomyocytes. NRVC were infected with indicated adenoviruses, and the phosphorylation of Akt, GSK-3β and S6K was determined by Western blot analysis 24 hours after infection. The numbers above each immuno-blot are fold changes relative to AdGFP-infected cells as determined by densitometry. Results are mean (n=4, SE not shown). * indicates p<0.05 compared with GFP. B. Overexpression of Pak1 in NRVCs protects from anticancer drug doxorubicin (DOX)-induced myocyte death. NRVCs were infected with AdGFP or AdPak1-T423E, and exposed to DOX (1 μmol/L) for 16 hours with or without the Akt inhibitor X (Akti, 2 μmol/L). Myocyte death was determined by propidium iodide (PI, red) staining with fluorescent microscopy. C. PI positive NRVCs were expressed as the percentage of total myocytes counted under phase contrast. Shown are means ± SE from 5 independent experiments. *p<0.01 vs control. D. Pak1-T423E inhibits DOX-induced apoptosis as shown by reduced cleavage of PARP and caspase3. NRVC were infected with indicated adenovirus and treated with indicated drugs as in B and C. Cleaved PARP (cPARP) and caspase3 (cCas3) were determined by Western blot analysis. E. IGF-1-induced Akt phosphorylation was dramatically diminished by shRNA-mediated Pak1 silencing in cultured cardiomyocytes. NRVC were infected with adenovirus expressing shPak1 or shCon, and Akt activation by IGF-1 was determined 72 hours after viral infection. The numbers above each immuno-blot are fold changes relative to shCon-expressing cells in the absence of IGF-1 as determined by densitometry. Results are means (n=4, SE not shown). * indicates p<0.05 compared with shCon. F. Synthetic siRNA-mediated Pak1 Gene silencing diminished IGF-1-induced Akt phosphorylation. NRVC were transfected with 50 nmol/L of siRNA targeting Pak1, and the ability of IGF-1 to induce Akt phosphorylation was determined 60-hour later by Western blot analysis. G. Targeted deletion of Pak1 gene markedly diminished Akt activity in mouse hearts. Wild type control mice (B6-WT) and Pak1−/− mice were injected i.p. with IGF-1 (4 mg/kg), and Akt activity was determined by Western blot analysis 1 hour after injection. The numbers above each immuno-blot are fold changes relative to B6-WT in the absence of IGF-1 as determined by densitometry. Results are means (n=4, SE not shown). * indicates p<0.05 compared with B6-WT. H. SiRNA-mediated knockdown of mTOR protein did not prevent Pak1-induced Akt phsophorylation at S473. NRVC were transfected with a synthetic siRNA targeting rat mTOR, and the ability of Pak1-T423E to induce Akt phosphorylation was determined by Western blot analysis.
Pak1 promotes cardiomyocyte survival
We tested if Pak1 overexpression in NRVC is able to reduce cardiotoxicity of doxorubicin (DOX), an anticancer drug that can kill NRVC. Adenovirus-mediated gene transfer of Pak1-T423E dramatically attenuated DOX-induced myocyte death as shown by reduced PI positive cells and cleavage of PARP and caspase3 (Figure 2BCD), suggesting that Pak1 conveys a robust survival signal in NRVC. The Pak1-confered protection was blocked by the Akt inhibitor X (Akti), suggesting that the protective effect of Pak1 is mediated, at least in part, by Akt signaling. These results demonstrate an important role of Pak1-Akt signaling in cardiomyocyte survival.
Akt signaling is impaired by Pak1 gene silencing or targeted deletion
To test if Pak1 is required for Akt activation, NRVCs were infected with an adenovirus expressing a short hairpin RNA for Pak1(shPak1), and the phosphorylation of Akt and its downstream targets was examined upon IGF-1 stimulation. ShPak1 reduced Pak1 protein levels by roughly 90% (Figure 2E). Correspondingly, IGF-1-induced Akt phosphorylation at S473 and T308 was dramatically diminished by Pak1 gene silencing, so was the phosphorylation of S6K at T389. This result was reproduced by synthetic siRNA-mediated Pak1 gene silencing (Figure 2F), further validating the requirement of Pak1 for Akt activation. Similarly, IGF-1-induced Akt activity was markedly reduced in the hearts of gene-targeted Pak1−/− mice (Figure 2G), suggesting that Pak1 is required for the efficient phosphorylation of Akt at both S473 and T308. Nevertheless, Pak1 seems not required for GSK3β phosphorylation, suggesting that Pak1-induced Akt activation may be specific for the pathway involving S6K, but not that involving GSK3β.
Pak1 can activate Akt in NRVC with diminished mTOR protein levels
As shown in Figure 2H, siRNA-mediated knockdown of mTOR protein did not prevent Pak1-induced Akt S473 phsophorylation, suggesting that Pak1 activation of Akt may not be mediated by mTORC2, a widely accepted Akt S473 kinase.
DISCUSSION
Akt is a well established regulator of myocardial growth and survival, contractile function, and coronary angiogenesis(1). The role of Pak1 in cardiomyocytes has just begun to be explored. Interestingly, Pak1 and Akt share a few similar functions in other cell types(5, 10). For example, both Pak1 and Akt can promote cell growth and survival(5, 10); both can phosphorylate the same pro-apoptotic protein BAD(5, 10). Notably, Pak1 is able to activate Akt(11); while Akt can phosphorylate Pak1(5). These results indicate an intimate and complex relationship between Pak1 and Akt. In the present study, we show that both Pak1 and Akt can be activated by multiple hypertrophic stimuli and growth factors in a PI3K-depenedent manner, suggesting that Pak1 and Akt may lie in the same signaling pathway in cardiomyocytes. Indeed, using both gain- and loss-of-function approaches in vitro and in vivo, we demonstrate that Pak1 is sufficient to activate Akt, and is essential for growth factor-induced Akt activity in cardiomyocytes. Moreover, purified Pak1 can directly phosphorylates Akt at S473 but not T308 in vitro, suggesting Pak1 as a relevant PDK2 specific for Akt S473 phosphorylation. These results also suggest that Pak1-induced Akt phosphorylation at T308 in cardiomyocytes is an indirect effect that is likely mediated by PDK1. The involvement of PDK1 in Pak1-induced Akt activation was suggested previously(11). Due to the failure of kinase-dead Pak1 to efficiently induce Akt phosphorylation, the kinase activity seems required for Pak1 to activate Akt although a scaffolding role may be equally important in this process. The functional significance of Pak1-Akt signaling is underscored by the observation that the pro-survival effect of Pak1 is diminished by Akt inhibition. In summary, our results connect two important regulators of cellular physiological functions and raise the possibility that Akt may act as a down stream effector that at least partly mediates Pak1 signaling in cardiomyocytes.
ACKNOWLEDGMENTS
SK is supported by an American Heart Association (AHA) postdoctoral fellowship. JC was supported by grants from the NIH (R01 CA117884) and the Department of Defense (W81XWH-06-1-0213). QL was supported by Grant P20 RR-017662 from the National Center for Research Resources, a component of the NIH, AHA Scientist Development Grant 0435308N, and Juvenile Diabetes Research Foundation Grant 1-2007-741. We thank Dr. Timothy O'Connell and Andy Cypher for preparing cultured cardiomyocytes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 3.Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005;289:E187–196. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 4.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 6.Clerk A, Sugden PH. Activation of p21-activated protein kinase alpha (alpha PAK) by hyperosmotic shock in neonatal ventricular myocytes. FEBS Lett. 1997;403:23–25. doi: 10.1016/s0014-5793(97)00020-3. [DOI] [PubMed] [Google Scholar]
- 7.Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y. Hypoxia and hypoxia/reoxygenation activate p65PAK, p38 mitogen-activated protein kinase (MAPK), and stress-activated protein kinase (SAPK) in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1997;239:840–844. doi: 10.1006/bbrc.1997.7570. [DOI] [PubMed] [Google Scholar]
- 8.Sheehan KA, Ke Y, Solaro RJ. P21 Activated Kinase-1 and its Role in Integrated Regulation of Cardiac Contractility. Am J Physiol Regul Integr Comp Physiol. 2007 doi: 10.1152/ajpregu.00253.2007. [DOI] [PubMed] [Google Scholar]
- 9.Sussman MA, Welch S, Walker A, Klevitsky R, Hewett TE, Price RL, Schaefer E, Yager K. Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1. J Clin Invest. 2000;105:875–886. doi: 10.1172/JCI8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24:7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- 11.Gorlach A, BelAiba RS, Hess J, Kietzmann T. Thrombin activates the p21-activated kinase in pulmonary artery smooth muscle cells. Role in tissue factor expression. Thromb Haemost. 2005;93:1168–1175. doi: 10.1160/TH05-01-0006. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, Lackey T, Huang Y, Bisping E, Pu WT, Boxer LM, Liang Q. Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. Faseb J. 2006;20:800–802. doi: 10.1096/fj.05-5426fje. [DOI] [PubMed] [Google Scholar]
- 13.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi: 10.1101/gad.981002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Q, Bueno OF, Wilkins BJ, Kuan CY, Xia Y, Molkentin JD. c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. Embo J. 2003;22:5079–5089. doi: 10.1093/emboj/cdg474. [DOI] [PMC free article] [PubMed] [Google Scholar]