Abstract
Hypothalamic POMC neurons regulate energy balance via interactions with brain melanocortin receptors (MC-Rs). POMC neurons express the MC3-R which can function as an inhibitory autoreceptor in vitro. We now demonstrate that central activation of MC3-R with icv infusion of the specific MC3-R agonist, [D-Trp8]-γ-MSH, transiently suppresses hypothalamic Pomc expression and stimulates food intake in rats. Conversely, we also show that icv infusion of a low dose of a selective MC3-R antagonist causes a transient decrease in feeding and weight gain. These data support a functional inhibitory role for the MC3-R on POMC neurons that leads to changes in food intake.
Keywords: POMC, MSH, melanocortin-3-receptor, feeding
1. Introduction
1.1 The melanocortin system
The hypothalamic melanocortin system involving proopiomelanocortin (POMC), the central melanocortin receptors MC3- and MC4-R and the endogenous melanocortin receptor antagonist AGRP, plays a major role in regulating feeding behavior and energy balance. POMC, a precursor protein synthesized in multiple tissues including the arcuate nucleus, is post-translationally cleaved in a tissue-specific manner to yield the melanocortin neuropeptides α-MSH, γ3-MSH, and β-MSH (7,13,32,33). α-MSH is an MC3/4-R agonist that inhibits feeding and stimulates energy expenditure while γ3-MSH is a potent and somewhat selective MC3-R agonist whose effects have yet to be fully characterized. In humans, POMC mutations that result in deficiency of POMC and its cleavage products, cause a syndrome of early-onset obesity, adrenal insufficiency, and red hair pigmentation (23). Mouse models with POMC deficiency display a similar phenotype of obesity, absence of normal adrenal gland development and function, and yellowish coat pigmentation (35). These mice experience substantial weight loss when treated with α-MSH (35). Inactivation of the MC4-R in both mice and humans also results in an obese phenotype, and additional rodent studies have demonstrated that the majority of the anorectic effects of α-MSH and its analogues are primarily mediated by MC4-R (1,8,9,30). However, the manner in which the MC3-R modulates energy homeostasis is not entirely clear.
1.2 The role of the melanocortin-3 receptor (MC3-R)
While mice deficient in MC4-R demonstrate an obesity syndrome characterized by hyperphagia, hyperinsulinemia, as well as increased lean body mass and longitudinal growth, MC3-R knockout mice are relatively hypophagic with increased feed efficiency. MC3-R null mice exhibit reduced lean body mass and are only mildly hyperinsulinemic (8,16). MC3-R deficient mice also have a higher respiratory quotient compared to wild type animals when placed on a high fat diet suggesting that the MC3-R may affect fat oxidation (5). Moreover, mice lacking both MC3 and MC4 receptors are significantly heavier than mice lacking only MC4-R (8). In addition, MC4-R knockout mice are relatively protected from disease induced cachexia while MC3-R deficient animals demonstrate enhanced susceptibility with exacerbated weight loss (26,27,29). These data imply that MC3 and MC4-R possess distinct, non-redundant functions in mediating energy balance.
Several rodent studies have demonstrated that the role of the MC3-R in regulating feeding is complex and not straight forward. Feeding studies with MC3-R knockout mice have yielded quite varied results that have included no difference in food consumption, modest hypophagia, and even slight hyperphagia compared to wildtype animals (5,6,9). MC3-R signaling has also been implicated in mediating some of the effects of leptin on feeding (36). Zhang et al showed that the absence of MC3-R impairs the ability of leptin to reduce food intake and postulated that MC3-R which is found on both AGRP/NPY and POMC neurons may have a role in setting leptin response thresholds among these neuronal populations (36).
The finding that MC3-Rs are expressed in both AGRP and POMC neurons within the arcuate has raised the possibility that one role of MC3-R is to mediate crosstalk between POMC and AGRP neurons (2,18). Subsequently, in vitro data demonstrating increased inhibition of POMC neuronal activity with MC3-R activation by the selective MC3-R agonist [D-trp8]-γ-MSH suggested an autoinhibitory function for MC3-R (11). It would thus be predicted that MC3-R activation would inhibit POMC activity and presumably stimulate feeding. An increase in food intake is precisely what Marks et al observed after acute injection of the specific MC3-R agonist [D-Trp8]-γ-MSH administered peripherally to mice (28). However, it should be noted that the MC3-R is expressed in multiple peripheral tissues (10). Thus, as the authors themselves acknowledge, the observed increase in food intake may potentially have been mediated by activation of peripheral MC3-Rs. Whether selective central MC3-R stimulation would decrease POMC levels and stimulate feeding was unknown. Here, using a rat model we have now been able to show that central administration of [D-Trp8]-γ-MSH increases food intake. We also demonstrate for the first time that selective central MC3-R activation with [D-Trp8]-γ-MSH in the rat suppresses hypothalamic Pomc expression providing in vivo evidence to support the hypothesis of MC3-R as an autoinhibitory receptor. In addition, we investigated whether central infusion of a relatively selective MC3-R antagonist would, conversely, disinhibit POMC with subsequent anorectic effects.
2. Materials and methods
2.1 Animals
All animal experiments were approved by the Columbia University Institutional Animal Care and Use Committee. Male Sprague-Dawley rats weighing 250–275 g were purchased from Charles River (Wilmington, MA, USA). Animals had ad libitum access to water and Purina Rodent Chow 5001 (12% fat, 28% protein, 60% carbohydrate; Purina Mills, St Louis, MO, USA). They were acclimatized to a natural light/dark cycle for at least 1 week prior to any surgery. In all experiments, rats were anesthetized with pentobarbital (50mg/kg) by intraperitoneal (i.p.) injection for icv cannula placement. A 28-guage stainless steel cannula connected by a vinyl catheter tubing to a 7-day osmotic pump (ALZET model 2001, Cupertino, CA, USA) delivering 1 μL/hr of normal saline was inserted stereotaxically into the right lateral ventricle (coordinates from bregma: lateral 1.3 mm; caudal 0.8 mm; depth from dura 3.5 mm). After the initial surgery, animals were individually housed and allowed to recover for 7 days. The animals were then divided into treatment groups of equivalent average weight and daily food intake and anesthetized by 0.25 ml of ketamine(1 g/16.5 mL)/xylazine (130 mg/16.5 mL; Exp. 1) or by inhaled isoflurane (Exp. 2 and MC3-R antagonist experiments) when the saline pumps were replaced by new pumps delivering either continuous infusion of saline or peptide. Each animal received either a new 3-day or 7-day osmotic pump (ALZET model 1003D, model 2001, Cupertino, CA, USA), depending on the experiment, filled with saline or peptide. Food intake and body weight were recorded daily. Animals exhibiting signs of illness and whose food consumption fell to less than 17 g on any given day were excluded from final analysis.
2.2 [D-Trp8]-γ-MSH and MC3-R antagonist peptides
[D-Trp8]-γ-MSH (15) (Phoenix Pharmaceuticals, Belmont, CA, USA) from lyophilized stock was dissolved in normal saline immediately prior to icv infusion. Compound PG-932, Ac-Nle-c[Asp-Pro-DNal(2′)-Arg-Trp-Lys]-Pro-Val-NH2, a selective MC3R antagonist was synthesized as previously described (14). We have carefully reevaluated its binding affinity and second messenger (adenylate cyclase (AC)) properties at the hMC3R, hMC4R, and hMC5R receptors in HEK cells. At the hMC3R its binding affinity IC50 is 3.91 nM and its EC50 in the AC function is >10,000 (it is a pure neutral antagonist); at the hMC4R its IC50 is 20 nM in binding affinity and its EC50 is 500 nM (partial agonist in the AC assay); at the hMC5R its IC50 is 6.7 nM in binding affinity and its EC50 in the AC function assay is 8.1 nM (full agonist). Therefore it would be expected to antagonize only the hMC3R activities in vivo. The lyophilized PG-932 stock was also dissolved in normal saline immediately prior to infusion.
2.3 [D-Trp8]-γ-MSH Experiments
2.3.1 Experiment 1
After 7 days of ICV saline infusion, rats were subdivided into two groups of equivalent body weight prior to mini-pump exchange. One group (n = 8; mean weight 316 ± 3.8 g) then received 3-day mini -pumps delivering [D-Trp8]-γ-MSH at 0.5 μg/μL/hr (7.6 nmol/day), while the control group (n = 8; mean weight 318 ± 5.9 g) received saline infusion at 1.0 μL/hr. For placement of pumps delivering either peptide or normal saline, animals were anesthetized with ketamine/xylazine. After a 66 hour infusion period, rats were sacrificed by decapitation after a 30-s exposure to CO2.
2.3.2 Experiment 2
Mean body weight prior to pump exchange was equivalent between [D-Trp8]-γ-MSH (323 ± 3.4 g) and saline (323 ± 3.2g) treated animals. One group (n = 10) received 3-day mini-pumps delivering D-Trp8-γ-MSH at 0.5 μg/μL/hr (7.6 nmol/day), while the control group (n = 9) received saline infusion at 1.0 μL/hr. For pump placement, animals were anesthetized by inhaled isoflurane. Before placing the new osmotic pumps, 10 μL of either saline or [D-Trp8]-γ-MSH were injected into the vinyl catheter tubing attaching the pump to the icv cannula. Thus, total infusion time prior to sacrifice was extended in this experiment to 84 hours.
2.4 MC3-R antagonist (PG-932) experiments
2.4.1 High dose MC3-R antagonist infusion
Baseline weight was equivalent between both the treatment (320 ± 6.0 g) and saline control (319 ± 6.7 g) groups (n = 10/group). Before placing new 7-day osmotic pumps, 10 μL of either saline or MC3-R antagonist were injected into the vinyl catheter tubing that attaches the pump to the icv cannula. PG-932 was infused at 0.5 μg/μL/hr (9.8 nmol/day). Rats were sacrificed after a 7-day infusion period.
2.4.2 Low dose MC3-R antagonist infusion
Rats were divided into three groups receiving either saline (n = 6) or MC3-R antagonist infused at a rate of 0.5 ng/hr (9.8 pmoles/day; n = 6) or 2.0 ng/hr (39 pmoles/day; n = 7). Body weight was equivalent in all three groups (mean weight, 318 g). Before placing new 3-day osmotic pumps, 10 μL of either saline or MC3-R antagonist was injected into the vinyl catheter tubing that attaches the pump to the icv cannula. After the 3-day infusion period, rats were sacrificed.
2.5 Plasma insulin and leptin
Trunk blood was collected into heparinized tubes immediately after decapitation and centrifuged at 4° C to separate plasma that was then stored at −20° C. Plasma insulin concentrations were measured via a radioimmunoassay (RIA) kit using rat insulin as a standard (Sensitive Insulin RIA Kit, Linco, St Charles, MO, USA). Plasma leptin levels were measured with the rat Linco RIA Kit.
2.6 Measurement of hypothalamic Pomc and Agrp RNA levels
The MBH was dissected and total RNA was isolated as previously described (22). RNA used to generate standard curves and 32P-labeled RNA probes were synthesized using commercial transcription kits (Promega, Madison, WI, USA). Sense and antisense Agrp mRNAs were synthesized from plasmid vectors containing T3 and T7 promoters and a 175-bp rat Agrp cDNA fragment (21). Sense and antisense Pomc mRNAs were synthesized from a plasmid vector containing the Sp6 promoter and a 538-bp Pomc cDNA fragment spanning part of exon 3 (3). Sense and antisense Cyclophilin (Cyc) mRNAs were synthesized using plasmid vectors containing T3 and T7 promoters and a 117-bp rat Cyc cDNA fragment (12). Sense RNAs were quantified spectrophotometrically and used to generate standard curves in the hybridization assays. Hybridizations with Pomc, Agrp and Cyc riboprobes were performed together in the same tube followed by the addition of S1 nuclease. Samples were then phenol-chloroform extracted, precipitated and electrophoresed on a 4% nondenaturing acrylamide gel. The protected Pomc and Agrp bands were quantified by phosphoimager analysis and compared with the standard curve. Because the protected hybrids were smaller than the cellular transcripts, results were normalized to the full-length RNA species: 0.7 kb for full-length mouse Agrp cytoplasmic RNA; 1.0 kb for full-length mouse Pomc cytoplasmic RNA. Cyc mRNA was measured as an internal control and Pomc and Agrp results are expressed as pg/100 pg Cyc.
2.7 Statistical Analysis
Statistical analysis was performed with Student’s t test. ANOVA for repeated measures was used when values over different times were analyzed. P < 0.05 was considered statistically significant. Results are reported as mean values ± SEM.
3. Results
3.1 Effects of [D-trp8]-γ-MSH on Feeding, Body Weight, and Hypothalamic Pomc and Agrp
3.1.1 Experiment 1
In Experiment 1, saline or [D-Trp8]-γ-MSH was infused at a rate of 0.5 μg/hr (7.6 nmol/day) for a total infusion time of 66 hours prior to sacrifice. There was an equivalent fall in food intake in both groups on day 1 that is characteristic of the animals’ response to the ketamine/xylazine anesthesia used on the day of pump exchange in this experiment (Figure 1). On day 2 of this study, there was a trend towards greater food intake by [D-Trp8]-γ-MSH rats versus saline controls (25.9 ± 1.5 g vs. 23.6 ± 1.2 g; p = 0.24, Figure 2). Mean daily body weight throughout the infusion period as well as cumulative weight gain were equivalent between the [D-Trp8]-γ-MSH and saline groups. The mean cumulative weight gain was 12.2 ± 2.5 g ([D-Trp8]-γ-MSH) vs. 10.9 ± 1.8 g (saline). After 66 hours of peptide infusion, there was a significant decline in Pomc mRNA concentration in [D-Trp8]-γ-MSH rats (1.42 ± 0.15 pg/100 pg Cyc) compared to controls (2.13 ± 0.2 pg/100 pg Cyc; p = 0.012, Figure 1). Agrp mRNA levels were not significantly different compared to saline rats (Figure 1). There was no difference between [D-Trp8]-γ-MSH and saline treated animals in plasma leptin (2.3 ± 0.3 vs. 2.0 ± 0.2 ng/mL) or insulin (2.8 ± 0.5 vs. 2.6 ± 0.2 ng/mL) levels.
Figure 1. Effects of ICV [D-Trp8]-γ-MSH Infusion in Experiment 1.
(A). Food Intake: On day 1, there was a decline in food intake characteristic of the animals’ response to ketamine/xylazine anesthesia in both the [D-Trp8]-γ-MSH and the saline control groups. On day 2, a trend toward greater food intake among [D-Trp8]-γ-MSH rats vs. saline controls was noted but this was not significant (n = 8/group; p = 0.24). (B & C). Hypothalamic Pomc and Agrp Expression (expressed as pg/100 pg Cyc): Pomc mRNA levels were significantly decreased in [D-Trp8]-γ-MSH animals compared to controls (*p = 0.012); Agrp mRNA levels tended to be lower in the [D-Trp8]-γ-MSH group but this was not significant (p = 0.1).
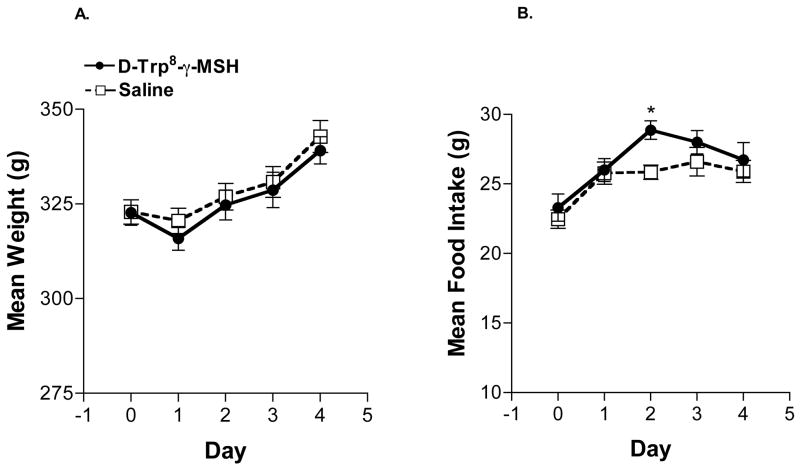
Figure 2. Effects of ICV [D-Trp8]-γ-MSH Infusion in Experiment 2.
(A). Mean Body Weight: [D-Trp8]-γ-MSH infusion did not have any significant effect on body weight or weight gain which was similar to that of saline controls. (B). Mean Food Intake: A significant increase in food intake among [D-Trp8]-γ-MSH rats (28.7 ± 0.6 g; *p = 0.003; n = 10) compared to saline controls (25.7 ± 0.6 g; n = 9) was observed on day 2 of infusion. In contrast to Exp. 1, there was also no decline in food intake by either [D-Trp8]-γ-MSH or saline rats on the day after pump exchange in this experiment using inhaled isoflurane anesthesia.
3.1.2 Experiment 2
In Experiment 2, while the dose of [D-Trp8]-γ-MSH remained the same at 0.5 μg/hr (7.6 nmol/day), the infusion time was extended to 84 hours. Another point of difference from Experiment 1, was the use of inhaled isoflurane as the anesthetic during the pump exchange. Notably, there was no decline in food intake by either [D-Trp8]-γ-MSH or saline rats on the day after pump exchange performed with inhaled isoflurane anesthesia. A statistically significant increase in food intake among rats infused with [D-Trp8]-γ-MSH (28.7 ± 0.6 g) compared to saline controls (25.7 ± 0.6; p = 0.003) was observed on day 2 of infusion (Figure 2). There was no difference in body weight or cumulative weight gain between treatment groups. Mean cumulative weight gain among [D-Trp8]-γ-MSH rats was 17.3 ± 1.5 g vs. 19.1 ± 2.1 g among saline controls. There was no difference between [D-Trp8]-γ-MSH and saline treated animals in plasma leptin (1.2 ± 0.2 vs. 0.8 ± 0.1 ng/mL) or insulin (2.1 ± 0.1 vs. 2.2 ± 0.1 ng/mL) levels. With peptide infusion extended to 84 hours, no significant differences between [D-Trp8]-γ-MSH and controls in Pomc (1.9 ± 0.2 vs. 2.1 ± 0.3 pg/100 pg Cyc, respectively) or Agrp (1.6 ± 0.2 vs. 1.8 ± 0.1 pg/100 pg Cyc) mRNA concentrations were detected.
3.2 Effects of a Relatively Selective MC3-R Antagonist (PG-932) on Feeding, Body Weight, and Hypothalamic Pomc and Agrp
Infusion of PG-932 at a high dose of 0.5 μg/hr (9.8 nmol/day) resulted in a significant increase in food intake (PG-932: 327.2 ± 9.2 vs. saline: 198.1 ± 8.2; p < 0.0001) and cumulative weight gain (PG-932: 102.1 ± 3.1 g vs. saline: 39.5 ± 3.5; p < 0.0001, Figure 3). Leptin and insulin levels were also significantly higher among rats treated with PG-932 compared to saline (Table 1). Hypothalamic Agrp levels were significantly lower in the PG-932 group compared to saline (p < 0.0001) while Pomc tended to be higher in the PG-932 group (p = 0.07; Table 1).
Figure 3. Effects of ICV High Dose PG-932 Infusion.
Infusion of 0.5 μg/hr of peptide PG-932 vs. saline. (A) Weight Gain: PG-932 caused significant increases in cumulative weight gain (p < 0.0001) and (B) Food Intake: PG-932 resulted in a significant increase in cumulative food intake (p < 0.0001)(n = 10/group).
Table 1.
Effect of Central Infusion of MC3-R Antagonist (9.8 nmol/day)
| PG-932 | Saline | |
|---|---|---|
| Insulin (ng/mL) | 11.2 ± 1.4* | 2.2 ± 0.3 |
| Leptin (ng/mL) | 20.2 ± 1.1* | 2.7 ± 0.2 |
| POMC (pg/100 pg Cyc) | 2.5 ± 0.2 | 1.9 ± 0.2 |
| AGRP (pg/100 pg Cyc) | 0.8 ± 0.1* | 1.6 ± 0.1 |
p < 0.0001 compared to saline controls.
These results were consistent with antagonist effects at MC4-R resulting in increased feeding and weight gain. When a dose response study was conducted, this demonstrated an effect of increasing food intake at escalating doses over a range of 20 to 500 ng/hr (data not shown). The effect of infusing the antagonist at 0.5 vs. 2.0 ng/hr compared to saline was then further investigated.
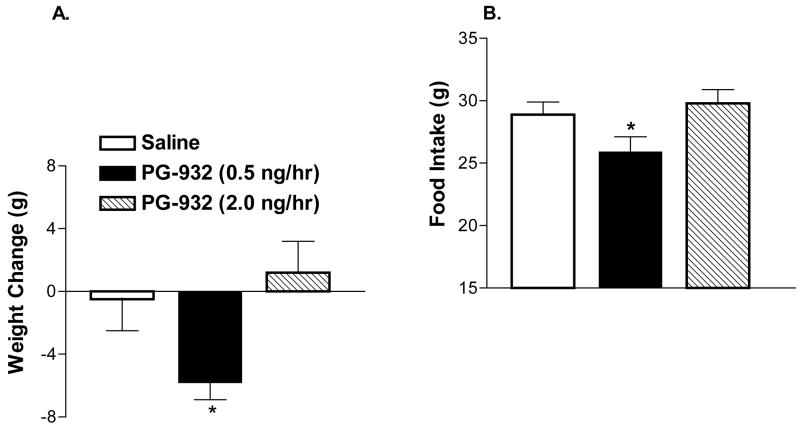
Within 24 hours, rats infused with the 0.5 ng/hr (9.8 pmoles/day) dose demonstrated a significant weight loss of −5.8 ± 1.1 g compared to both saline (−0.5 ± 2.0 g) and the 2.0 ng/hr (39 pmoles/day) group (1.2 ± 2.0 g) (Figure 4; p < 0.05). 24 hours after infusion a significant reduction in food intake in the 0.5 compared to the 2.0 ng/hr was also noted with rats consuming 25.9 ± 1.2 g and 29.8 ± 1.1 g, respectively (p < 0.05). A tendency towards decreased food intake among 0.5 ng/hr rats compared to saline controls who consumed 28.9 ± 1.0 over the first 24 hours was also noted (p = 0.07; Figure 4). After the first 24 hour period of infusion, no significant difference in daily food intake or weight gain was again noted. However, overall, there was a tendency for food intake to decrease over time (72h) in rats treated with the 0.5 ng/hr dose compared to saline (p = 0.055 by ANOVA, repeated measures).
Figure 4. Effects of ICV Low Dose PG-932 Infusion.
(A) Body Weight: Rats treated with PG-932 at a dose of 0.5 ng/hr demonstrated significant weight loss (−5.8 ± 1.1 g; *p < 0.05, n = 6) compared to saline (−0.5 ± 2.0 g; n = 6) as well as to rats infused with PG-932 at 2.0 ng/hr (1.2 ± 2.0 g; n = 7) (p < 0.05). (B) Food Intake: A significant decline in food intake in the rats treated with the 0.5 ng/hr dose compared to the 2.0 ng/hr dose was noted 24 hours after infusion (*p < 0.05) as well as a tendency towards decreased food intake compared to saline controls (p = 0.07).
4. Discussion
4.1 ICV administration of [D-trp8]-γ-MSH
These studies examined the effects of central administration of a specific MC3-R agonist [D-Trp8]-γ-MSH on food intake and hypothalamic expression of Pomc and Agrp in the rat. Early studies had suggested that the MC3-R did not participate in regulating ingestive behavior (1,9,30). However, Marks et al recently demonstrated that peripheral administration of [D-Trp8]-γ-MSH stimulated food intake in wild type mice (28). This increase in feeding was of short duration that did not persist beyond 4–6 hours. They found that peripheral [D-Trp8]-γ-MSH had no effect on feeding when administered to MC3-R knockout mice, whereas in MC4-R deficient mice the effect of stimulating food intake was maintained (28). These data indicated that the effects of [D-Trp8]-γ-MSH on feeding were indeed being mediated by MC3-R. However, as MC3-R is also present in the periphery, it was not entirely certain if these results were the product of peripheral or central MC3-R action.
We have now demonstrated in rats that food intake is stimulated when [D-Trp8]-γ-MSH is given ICV, consistent with a central mechanism of action. This increase in food intake occurs within the first 24–48 hours of continuous infusion of the peptide, and similar to the results of Marks et al, this effect on feeding is transient (28). Our results are also consistent with the finding by Marks et al that with repeated daily injections of [D-Trp8]-γ-MSH, the stimulatory effect on food intake was no longer seen.
In Experiment 1, food intake tended to be higher in [D-Trp8]-γ-MSH rats compared to saline treated animals, whereas in Experiment 2, the increase was statistically significant. It is possible that the fall in food intake experienced by both [D-Trp8]-γ-MSH and saline treated animals in response to the ketamine/xylazine anesthesia used during the pump exchange in Experiment 1 may have attenuated the magnitude of the modest feeding difference induced by [D-Trp8]-γ-MSH, thus obscuring a statistically significant effect. Because the animals recover much more rapidly from inhaled isoflurane anesthesia compared to ketamine/xylazine, there was no decline in feeding immediately after pump exchange.
In vitro, the selective MC3-R agonist [D-Trp8]-γ-MSH inhibits POMC neuronal activity, a finding that prompted the hypothesis that MC3-R functions as an autoinhibitory receptor on POMC neurons (11). We have now generated in vivo evidence in a rat model to support this theory. ICV administration of [D-Trp8]-γ-MSH suppresses hypothalamic Pomc gene expression compared to rats receiving only ICV saline. Although Agrp neurons in the arcuate also express MC3-R, no significant effect of [D-Trp8]-γ-MSH was observed on hypothalamic Agrp gene expression. Whether, the reduction in Pomc is a direct effect of [D-Trp8]-γ-MSH activation of MC3-R on POMC neurons or a product of increased GABA mediated inhibition on POMC by [D-Trp8]-γ-MSH activation of MC3-R on AGRP/NPY neurons, as suggested by the electrophysiological data from Cowley et al, or a combination of both, still remains to be determined (11). Finally, the reduction in Pomc cannot be attributed to changes in levels of the peripheral hormones leptin or insulin as they did not differ between treatment or control groups.
Suppression of Pomc expression by [D-Trp8]-γ-MSH is a transient effect. Although rats subjected to a longer infusion time of 84 hours tended to have lower Pomc levels, the degree of suppression was statistically significant only in the experiment with a shorter infusion time. The transient nature of Pomc suppression parallels the short duration of the feeding effect. It is conceivable that receptor down regulation may be responsible for the short-lived suppression of Pomc, but other explanations are also possible. For example, a reduction in POMC levels may very well be accompanied by a concurrent decrease in the release of the POMC derived peptide β-EP. β-EP also exerts autoinhibitory effects on POMC neurons which are known to express mu-opioid receptors. Moreover, β-EP, as well as other opioids, has been shown to hyperpolarize POMC neurons in a manner similar to what has been reported for D-trp8-γ-MSH (4,11,19). Thus, a concomitant decline in β-EP might actually tend to restore Pomc expression levels. Conversely, mu-opioid antagonism has been shown to stimulate Pomc gene expression and peptide release (17,25). Thus, both γ-MSH and β-EP may exert inhibitory effects on POMC via different inhibitory autoreceptors. It is therefore possible that [D-Trp8]-γ-MSH induced suppression of Pomc results in decreased β-EP release, leading to restoration of Pomc expression and termination of the temporary increase in feeding behavior. Ultimately, further study is needed to clarify the mechanisms that contribute to the short-term decline in Pomc observed with [D-Trp8]-γ-MSH administration.
4.2 ICV administration of PG-932, an MC3-R antagonist
To examine whether antagonism of MC3-R would inhibit feeding, rats were infused with PG-932, an MC3-R antagonist. Opposite to what was expected, an infusion dose of 0.5 μg/hr (9.8 nmol/day) resulted in significant increases in food intake and body weight with elevations in leptin and insulin. A significant suppression of hypothalamic Agrp mRNA levels and a tendency for a rise in Pomc levels were also noted, as might be expected as a consequence of the increase in body weight and leptin levels that were observed. These effects are remarkably similar in magnitude to what we have seen previously with chronic ICV AGRP infusion in the rat using the same minipump infusion protocol (22) and are consistent with antagonism at MC4-R with high dose PG-932 infusion. Although PG-932 functionally has selectivity for MC3-R, as assessed by assays with human MC3-R and MC4-R transfected into HEK cells, this compound might have some activity at MC4-R (IC50 of 20 nM) at relatively high doses, though it is only a weak partial agonist. Moreover, it is known that there are differences in binding affinity between human and rodent MC-Rs. Schiöth et al demonstrated that while rat and human MC3-R did not have any major differences in binding properties, rat MC4-R in general had higher affinity than human MC4-R for several MSH peptides tested (31). Cell type can also affect binding affinity. For example, when assays were performed with human MC3- and MC4-R transfected into CHO cells, PG-932 demonstrated 7-fold greater selectivity for hMC4-R than hMC3-R (14). It is thus likely, in contrast to studies with the human MC4-R in vitro, that PG-932 can act in the rat as an MC4-R antagonist when administered at high doses in vivo.
Thus, in order to attempt to isolate effects to the MC3-R, a very low dose of peptide was infused. Rats were treated with either 0.5 ng/hr (a dose 1000 fold less than the dose demonstrating effects consistent with MC4-R antagonism) or 2.0 ng/hr and compared to saline controls. Rats treated with 0.5 ng/hr of PG-932 exhibited significant weight loss compared to saline controls as well as compared to rats infused with PG-932 at 2.0 ng/hr within the first 24 hours. Overall, there was also a tendency for a decline in food intake among rats treated with 0.5 ng/hr of PG-932, as well. Consistent with MC3-R as an inhibitory autoreceptor on POMC neurons, these results suggest that selective MC3-R antagonism may transiently suppress food intake with a modest reduction in weight gain.
4.3 Effects of MC3-R Modulation on Feeding Behavior
Although our findings are consistent with the hypothesis that MC3-R functions as an inhibitory autoreceptor on POMC neurons, the MC3-R is also expressed by other neurons in regions of the hypothalamus outside of the arcuate and our findings may be mediated by these receptors as well. The role of the MC3-R in regulating feeding behavior and energy balance is complex and not fully understood. Chen et al have documented hypophagia among MC3-R knockout mice fed a regular chow diet (8). In contrast, Butler et al did not discern any difference in food intake between MC3-R deficient mice and wildtypes on rodent chow with relatively low dietary fat content (12.5% kJ/fat), but did note the development of slight hyperphagia that occurred primarily during the lights-on phase among MC3-R deficient mice provided a purified high fat diet (5,6). MC3-R knockout mice have also been shown to demonstrate a transient hyperphagia as the initial response to leptin administration with a subsequent delayed anorectic response to leptin (36). In addition, there is some evidence that AGRP can stimulate feeding in MC4-R null mice, an orexigenic effect presumably mediated by antagonism at the MC3-R (20, 30). However, results from studies with genetic knockout models must be interpreted with caution as they can be subject to influences from developmental compensation and may not be solely isolable to selective MC3-R blockade. The role of MC3-R in modulating feeding is clearly complex and can differ depending upon diet composition, diurnal feeding patterns, as well as genetic background. Further studies with more specific and potent MC3-R antagonists and with conditional or site specific MC3-R genetic approaches are warranted to more fully understand the role of the MC3-R in regulating energy balance.
4.3 Conclusions
We have demonstrated that the selective MC3-R agonist [D-Trp8]-γ-MSH centrally delivered suppresses Pomc mRNA levels and can modestly stimulate feeding in rats. The feeding effect is transient as is the reduction in Pomc mRNA mediated by central [D-Trp8]-γ-MSH administration. Our findings corroborate those of Marks et al who showed an increase in food intake in mice treated peripherally with [D-Trp8]-γ-MSH (28). Our data also provide physiologic in vivo evidence to support the hypothesis that one function of MC3-R is as an inhibitory autoreceptor on POMC neurons. Correspondingly, we also found that administration of an MC3-R antagonist produces a modest and transient anorectic effect. At the same time, however, MC3-R knockout mice are known to be obese. Thus, the role of MC3-R in modulating melanocortin tone and energy homeostasis is complex. MC3-R appears to have dual functions, both restraining melanocortinergic tone by inhibiting POMC neurons as well as playing a critical role in curtailing the development of obesity. Further investigation with selective MC3-R antagonists should help refine our understanding of how MC3-R modulation contributes to the pathogenesis of two debilitating disorders of energy balance, both cachexia and obesity.
Acknowledgments
This work was supported by NIH Grants DK57561, MH55708 (S.L.W.) and DK17420 (V.J.H.)
Abbreviations
- POMC
proopiomelanocortin
- AGRP
agouti related protein
- [D-Trp8]-γ-MSH
gamma-melanocyte stimulating hormone
- PG-932
a melanocortin 3 receptor antagonist
- MC3-R
melanocortin 3 receptor
- MC4-R
melanocortin 4 receptor
- β-EP
β-endorphin
- NPY
Neuropeptide Y
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott CR, Rossi M, Kim MS, AlAhmed SH, Taylor GM, Ghatei MA, et al. Investigation of the melanocyte stimulating hormones on food intake: lack of evidence to support a role for the melanocortin-3-receptor. Brain Research. 2000;869:203–210. doi: 10.1016/s0006-8993(00)02386-6. [DOI] [PubMed] [Google Scholar]
- 2.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, et al. Anatomy of an endogenous antagonist: relationship between agouti-related protein and proopiomelanocortin in brain. Journal of Neuroscience. 1999;19:1–7. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum M, Roberts JL, Wardlaw S. Androgen regulation of proopiomelanocortin gene expression and peptide content in the basal hypothalamus. Endocrinology. 1989;124:2283–2288. doi: 10.1210/endo-124-5-2283. [DOI] [PubMed] [Google Scholar]
- 4.Bouret S, Prevot V, Croix D, Jegou S, Vaudry H, Stefano GB, et al. Mu-opioid receptor mRNA expression in proopiomelanocortin neurons of the rat arcuate nucleus. Brain Res Mol Brain Res. 1999;70:155–158. doi: 10.1016/s0169-328x(99)00132-1. [DOI] [PubMed] [Google Scholar]
- 5.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 6.Butler AA. The melanocortin system and energy balance. Peptides. 2006;27:281–90. doi: 10.1016/j.peptides.2005.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Critical Reviews in Neurobiology. 1997;11:35–57. doi: 10.1615/critrevneurobiol.v11.i1.30. [DOI] [PubMed] [Google Scholar]
- 8.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan X, Yu H, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nature Genetics. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 9.Chen AS, Metzger JM, Trumbauer ME, Guan XM, Frazier EG, Marsh DJ, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Research. 2006;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- 10.Chhajlani V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochemistry and Molecular Biology International. 1996;38:73–80. [PubMed] [Google Scholar]
- 11.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 12.Danielson PE, Forss-Petter S, Brow MA, Calavetta L, Douglass J, Milner RJ, et al. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988;7:261–7. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- 13.Emeson RB, Eipper BA. Characterization of pro-ACTH/endorphin-derived peptides in rat hypothalamus. Journal of Neuroscience. 1986;6:837–849. doi: 10.1523/JNEUROSCI.06-03-00837.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grieco P, Balse-Srinivasan P, Han G, Weinberg D, MacNeil T, Van der Ploeg LH, et al. Extensive structure-activity studies of lactam derivatives of MT-II and SHU-9119: their activity and selectivity at human melanocortin receptors 3,4, and 5. J Pept Res. 2003;62:199–206. doi: 10.1034/j.1399-3011.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 15.Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human mc3 receptor selectivity. J Med Chem. 2000;43:4998–02. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 16.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, et al. Targeted disruption of the melanocortin- receptor results in obesity in mice. Cell. 1997;88:131–41. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe SB, Sobieszcztk S, Wardlaw SL. Effect of opioid antagonism on beta-endorphin processing and proopiomelanocortin-peptide release in the hypothalamus. Brain Res. 1994;648:24–31. doi: 10.1016/0006-8993(94)91900-3. [DOI] [PubMed] [Google Scholar]
- 18.Jegou S, Boutelet I, Vaudry H. Melanocortin-3 receptor mRNA expression in pro-opiomelanocortin neurones of the rat arcuate nucleus. J Neuroendocrinol. 2000;12:501–505. doi: 10.1046/j.1365-2826.2000.00477.x. [DOI] [PubMed] [Google Scholar]
- 19.Kelly MJ, Loose MD, Ronnekleiv OK. Opioids hyperpolarize beta-endorphin neurons via mu-receptor activation of a potassium conductance. Neuroendocrinology. 1990;52:268–275. doi: 10.1159/000125597. [DOI] [PubMed] [Google Scholar]
- 20.Kim MS, Rossi M, Abbot Cr, AlAhmed SH, Smith DM, Bloom SR. Sustained orexigenic effect of Agouti related protein may be not mediated by the melanocortin 4 receptor. Peptides. 2002;23:1069–76. doi: 10.1016/s0196-9781(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 21.Korner J, Wardlaw SL, Liu SM, Conwell IM, Leibel RL, Chua SC., Jr Effects of leptin receptor mutation on Agrp gene expression in fed and fasted lean and obese (LA/N-faf) rats. Endocrinology. 2000;141:2465–2471. doi: 10.1210/endo.141.7.7580. [DOI] [PubMed] [Google Scholar]
- 22.Korner J, Wissig S, Kim A, Conwell IM, Wardlaw SL. Effects of agouti-related protein on metabolism and hypothalamic neuropeptide gene expression. Journal of Neuroendocrinology. 2003;15:1116–1121. doi: 10.1111/j.1365-2826.2003.01113.x. [DOI] [PubMed] [Google Scholar]
- 23.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in human. Nature Genetics. 1998;19:155–57. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence CB, Rothwell NJ. Anorexic but not pyrogenic actions of interleukin-1 are modulated by central melanocortin-3/4 receptors in the rat. Journal of Neuroendocrinology. 2001;13:490–95. doi: 10.1046/j.1365-2826.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- 25.Markowitz CE, Berkowitz KM, Jaffe SB, Wardlaw SL. Effect of opioid receptor antagonism on proopiomelanocortin_peptide levels and gene expression in the hypothalamus. Mol Cell_Neurosci. 1992;3:184–90. doi: 10.1016/1044-7431(92)90037-3. [DOI] [PubMed] [Google Scholar]
- 26.Marks DL, Butler AA, Turner R, Brookhart G, Cone RD. Differential role of melanocortin receptor subtypes in cachexia. Endocrinology. 2003;144:1513–23. doi: 10.1210/en.2002-221099. [DOI] [PubMed] [Google Scholar]
- 27.Marks DL, Cone RD. The role of the melanocortin-3 receptor in cachexia. Annals of the New York Academy of Sciences. 2003;994:258–266. doi: 10.1111/j.1749-6632.2003.tb03188.x. [DOI] [PubMed] [Google Scholar]
- 28.Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–264. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks DL, Ling N, Cone RD. Role of the central melanocortin system in cachexia. Cancer Research. 2001;61:1432–8. [PubMed] [Google Scholar]
- 30.Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, et al. Response of melanocortin -4 receptor-deficient mice to anorectic and orexigenic peptides. Nature Genetics. 1999;21:119–121. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- 31.Schiöth HB, Bouifrouri AA, Rudzish R, Muceniece R, Watanobe H, Wikberg JE, et al. Pharmacological comparison of rat and human melanocortin 3 and 4 receptors in vitro. Regul Pept. 2002;106:7–12. doi: 10.1016/s0167-0115(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 32.Smith AI, Funder JW. Proopiomelanocortin processing in the pituitary, central nervous system, and peripheral tissues. Endocrine Reviews. 1988;9:159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 33.Wardlaw S. Obesity as a Neuroendocrine Disease: Lessons to be learned from proopiomelanocortin and melanocortin receptor mutations in mice and men. Journal of Clinical Endocrinology and Metabolism. 2000;86:1442–1446. doi: 10.1210/jcem.86.4.7388. [DOI] [PubMed] [Google Scholar]
- 34.Wisse BE, Frayo RS, Schwartz M, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–01. doi: 10.1210/endo.142.8.8324. [DOI] [PubMed] [Google Scholar]
- 35.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nature Medicine. 1999;5:1066–70. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Kilroy GE, Henagan TM, Prpic-Uhing V, Richards WG, Bannon AW, et al. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB. 2005;19:1482–91. doi: 10.1096/fj.05-3851com. [DOI] [PubMed] [Google Scholar]