Abstract
Two-way communication between the conceptus and the mother during early pregnancy is essential if the pregnancy is to survive. In this review, our primary focus is on biochemical communication between the conceptus and mother in the ruminant ungulate species. We emphasize, in particular, the role played by interferon-tau (IFNT) in triggering maternal responses in cattle and sheep and how maternal factors intervene to up-regulate IFNT gene (IFNT) expression in trophoblast. However, we also consider the possibility that different signaling cytokines or the physical presence of trophoblast may induce a partial IFN response in endometrium of those species where there is no evidence for large scale trophoblast IFN production. Conceivably, disparate signaling mechanisms trigger common downstream events necessary to secure a successful pregnancy.
Keywords: Trophoblast, Pregnancy, Endometrium, Interferon Response, Implantation
Introduction
For the early mammalian conceptus to survive, it must intervene in maternal physiology to block ovarian cyclicity and ensure that the production of progesterone from the corpus luteum (CL) is maintained. The conceptus needs also to exploit space offered through the uterine endometrium, intensify its ability to access maternal metabolic resources, and survive scrutiny and potential destruction from a potentially hostile environment. The mother in her turn must avoid excessive exploitation by gaining some measure of control over the demands of the conceptus she nurtures. She must also be capable of assessing the fitness of her future progeny as early as possible during her pregnancy so that decisions can be made about whether the pregnancy should survive. It is not surprising therefore that two-way communication between the conceptus and the mother is established relatively early in embryo development, and that pregnancy loss is at its zenith before implantation is completed. Less than robust signaling from the conceptus and a failure of the mother to either respond to or reciprocate conceptus signals, which would lead to a lack of synchrony between the partners, likely contribute to this wastage [1].
In this review we report how interferons have assumed a role as the primary early signal from the conceptus to the mother in some species of artiodactyl, but also speculate that an “interferon” response by the mother may play a much wider role than previously suspected in establishment of pregnancy in mammals.
Initiation of conceptus signaling
The general aspects of conceptus development from fertilization up to the time that the blastocyst hatches from the zona pellucida are relatively similar across all eutherian mammals [2, 3]. Although there are differences in the timing of these events and where they occur in the reproductive tract of the mother, blastocyst formation is generally initiated when the conceptus enters the uterus. Hatching, which rapidly follows blastocyst formation, allows the conceptus to escape the enclosing zona and exposes the outer surface of trophectoderm directly to the uterine environment. Although there is little doubt that zona-encased conceptuses of many species release compounds with potential biological activity directed towards the lining of the reproductive tract as they progress through these early stages of development reviewed by Duc-Goiran et al [3] such compounds would not appear to be crucial because successful pregnancies can result from blastocysts transfer to appropriately synchronized non-pregnant females in most species where it has been attempted [4]. In cattle [5] and sheep [6], successful transfers of pre-elongation or very early elongation stage conceptuses that are almost two-weeks old and contain many thousands of cells have been made as late as the end of the second week of the recipient’s estrous cycle and only a few days before a new cycle would normally be initiated. Such data suggest that in these ruminants an “in phase” uterine environment, i.e. one in which the embryo is in synchrony with the mother’s ovarian hormonal cycle, is relatively permissive for conceptus development until quite late in the cycle, but that a robust intervention by the conceptus is absolutely necessary by the late luteal phase. In species where a more intimate association with maternal endometrium occurs soon after blastocyst hatching or where the CL is short-lived, productive embryonic signaling is, of necessity, initiated earlier.
After the blastocyst stage there is great variability in the manner that that placentation proceeds. In some species, including humans and mouse, the hatched blastocyst quickly attaches to the uterine epithelium and trophoblast invasion begins, whereas in others, such as the rabbit, implantation is delayed until the blastocyst is much larger [2]. In many other species, the trophoblast is either completely non-invasive, as in the epitheliochorial placenta of the pig [7] and even strepsirhine primates, e.g. lorises and lemurs [8] and shows very limited invasiveness, as in the synepitheliochorial placenta of ruminant species, e.g. cattle and sheep [9], the primary subject of the present paper. The success of superficial placentation, which is regarded as a relatively recent evolutionary adaptation [10, 11], requires that a maternal capillary bed be quickly drawn to the zone of trophoblast attachment to provide gas and nutrient exchange. Moreover, since there is no direct contact of the trophoblast with the maternal blood, macromolecular nutrients and micronutrients that require a macromolecular carrier, such as iron, cannot be acquired directly. To circumvent this difficulty, the mother produces copious amounts of glandular secretions (“histotrophe”), which provide a nutrient bath for the trophoblast [12]. A non-invasive placenta has also led to adaptations in the manner that the conceptus communicates with the mother, since signaling molecules produced by trophoblast do not have direct access to maternal blood and their downstream effects must be received and initially interpreted by the uterine epithelium [13].
IFNT production by the ruminant conceptus
More than two weeks pass before the conceptuses of ruminants begin to attach to the uterine wall and initiate the process of placentation, although the pre-implantation trophoblast grows robustly in size and particularly in length during this time while remaining free within the uterine lumen, much like a parasitic worm in the intestine of a host. Possibly as an outcome of this delay in forming an intimate contact relationship with the maternal endometrium, ruminants have evolved a unique signaling pathway to permit communication with the mother, inform her of her pregnant status, and prevent luteal regression. By the late 1980s it had become clear that the main signal for maternal recognition of pregnancy in these species was a Type I interferon (IFN), later known as IFNT, with structural homology to the earlier identified IFN-α and -β [14, 15], compounds that had been recognized over the previous decades as first responders to viral infections. The peak production of IFNT occurs before uterine attachment but at a time when luteal regression must be blocked to maintain the pregnancy. Infusion of the purified IFN, and later, a recombinant version of the protein into the uterine lumen of non-pregnant ewes and cows verified its capacity to protect the CL from regression and maintain progesterone output. Ovine conceptus secretions from which IFNT had been removed had no such facility [16]. It should be emphasized, however, that IFNT-induced estrous cycle extension, although it may last for several weeks in ewes, is invariably less than the length of a full term pregnancy, emphasizing that luteotrophic anti-luteolytic factors other than IFNT come into play once the pregnancy has been established and may even work cooperatively with IFNT to maintain the CL [17]. Discussion of these less studied additional factors, including placental lactogens and other trophoblast products, such as the trophoblast Kunitz domain proteins (TKDP) [18, 19] and pregnancy-associated glycoproteins (PAG) [20] first secreted about the same time as IFNT is beyond the scope of this review.
It is now clear that the IFNT are unique to the ruminant ungulates, having diverged from ones encoding its closest relative, the IFN-omega (IFNW), about 36 million years ago at a time when the ruminant species themselves began to emerge as a separate lineage within the artiodactyl order [21]. It is tempting to assume that IFN production and its ability to trigger particular downstream signaling pathways in endometrium enabled the superficial placentation of the pecoran ruminants to evolve successfully. As discussed below, it is also clear that the maternal system plays a coordinating role in IFNT production by controlling the timing and magnitude of IFNT output by the conceptus.
Transcriptional control of IFN expression in trophoblast and the role of the mother
In comparison with other Type I IFN, IFNT expression is unique in at least four respects: it is confined to the ruminant ungulates; there is lack of viral inducibility; expression is restricted to embryonic trophectoderm; and high level synthesis is sustained over several days and then terminates. The IFNT are silent in the embryo, the placenta after it forms, and, as far as can be judged, in all tissue of the adult animal. By contrast, expression of related Type I IFN genes occurs in response to virus and other pathogens in many tissues, and the expression of these proteins is generally short-lived.
Control of tissue and temporal expression lies in the 5/-flanking region of the intronless IFNT genes [22]. These control regions are highly conserved across the ruminant species [23]. The trophectoderm-specific expression of IFNT appears to be governed by two specific promoter regions. One region is a distal A/T rich element situated approximately -358 to -322 bp from the transcription start site, which has not been studied in detail. The second is in the proximal region (-91 to -69). Both these regions of the IFNT form strong associations with nuclear proteins in electrophoretic mobility shift assays (EMSA) performed with extracts prepared from elongating ovine and bovine trophoblast [22]. These protein-DNA associations disappear as the IFNT become down-regulated a few days later. Deletion of the -91 to -61 nucleotide sequence silences basal promoter activity [24]. When part of this region was used as a target for yeast one-hybrid screening of a Day 13 conceptus library, the nuclear protein, Ets2, was identified as binding to this site and to activate transcription [24]. This element is adjacent to a putative AP1 site and two helical turns upstream of a binding site for the homeobox protein Dlx3. A combination of Ets2 and Dlx3 promote expression synergistically, allowing a more than 250-fold increase in expression in JAr choriocarcinoma cells, a permissive trophoblast cell line used for transfection experiments (Ezashi, Das et al., submitted 2007). Ets2-transcriptional regulation of the IFNT promoter is also silenced by Oct4, the hallmark transcription factor of pluripotent cells [25]. We have suggested a model in which the down regulation of Oct4 that accompanies the emergence of trophectoderm releases constraints operating over the expression of IFNT, thereby placing the promoter in a permissive state for subsequent up-regulation by positively-acting transcription factors such as Ets2 [25]. Ets2, although essential for efficient transcription of IFNT, is widely distributed among a variety of tissues and present in ovine trophoblast after IFNT production ends [18], suggesting that its presence is necessary but not sufficient for IFNT expression. What transcription factors in addition to Ets2 and Dlx3 participate in the initial up-regulation and, a few days later, to the silencing of the IFNT silencing remains unclear.
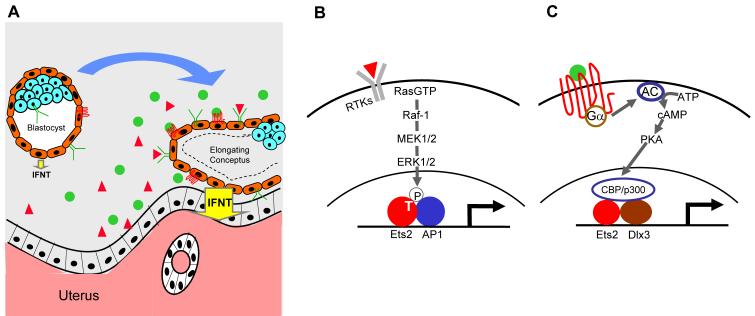
The production of IFNT by bovine and ovine conceptuses begins at the blastocyst stage, where expression on a per cell basis is very low, and then rises as the conceptus begins to elongate [26, 27]. The onset of conceptus elongation and maximal IFNT production is quite variable from animal to animal but is correlated with the rise in maternal concentrations of serum progesterone [28]. Moreover, ewes and cows with an advanced estrous cycle tend to carry larger conceptuses producing copious amounts of IFNT compared to animals where the rise in luteal progesterone is slower [29-31]. There is evidence that factors in maternal uterine secretions that are released in response to rising progesterone up-regulate IFNT expression via activation of Ets2 [32-34] (Figure 1A). It is now established, for example, that activation of RAS and downstream MAP kinases, ERK1/2, can up-regulate IFNT promoter-reporter constructs containing the Ets2-binding element by stimulating the phosphorylation of a highly conserved threonine residue (T72) in the so-called pointed domain [24, 35] (Figure 1B). There is also evidence linking a closely juxtaposed AP1 element to the Ras/MAPK activation of the IFNT [32], and suggesting that exogenous CSF-1, acting through its cognate receptor, CSF1R (c-fms), can drive these processes [32]. Since CSF1 is present in uterine secretions and CSF1R is expressed on bovine trophoblast, it can be expected to activate Ras/MAPK signal transduction pathway and elevate IFNT expression. Activation of protein kinase-A (PKA) can also drive expression through IFNT-promoter constructs through activation of Ets2 (Das et al., In Press, 2007) (Figure 1C). Thus, growth factors that raise intracellular cyclic AMP concentrations or operate through the RAS/MAPK kinase pathway provide a means of coordinating the responsiveness of IFNT gene expression to the state of the uterine environment and indirectly to the maternal endocrine state.
Figure 1.
A model explaining the contribution of maternal uterine secretory products to the up-regulation of IFNT expression in trophoblast of sheep and cattle during the period immediately preceding definitive attachment to the uterine luminal epithelium. A, the conceptus on the left is an expanded ovine blastocyst at about d-10 of pregnancy as maternal progesterone concentrations are rising but before the main phase of uterine secretory activity. The trophectoderm (brown) releases small amounts of IFNT (yellow arrow), and receptors for growth factors are either few or not fully occupied by their cognate ligands. The conceptus on the right is at a later stage, e.g. d-13, beginning to elongate, and producing copious amounts of IFNT (yellow). Growth factors in maternal secretions (red triangles and green circles) are able to occupy receptors on trophectoderm, which include receptor tyrosine kinases (RTK; green) and G-protein coupled receptors (red). B, Ligands bound to RTKs activate the RAS/MAPK signal transduction pathway resulting in activation of transcription factors that associate with the main Ets2 enhancer on the IFNT promoter. C, Ligands bound to certain G-protein coupled receptors cause activation of adenylate cyclase and protein kinase A (PKA) and ultimately nuclear factors involved in transcriptional control of IFNT expression. Data from our laboratory indicate that this signal transduction pathway targets Ets2 and Dlx3 and involves a co-activator (probably either p300 or its close relative CBP).
The action of IFNT on maternal endometrium
There is little evidence that IFNT released by the conceptus has a generalized effect on tissues outside the reproductive tract, although there are mounting indications of an IFN response occurring in the ovary as well as the endometrium of pregnant ewes and of ewes in which IFNT has been infused into the uterine lumen [36]. It is unclear whether this expression of IFN-stimulated genes (ISGs) either plays a role in luteal protection or whether it arises as the result IFNT reaching the ovary from the uterus by some local route.
The main target for IFNT is considered to be the uterine endometrium and not the CL, however, and it is at this location that the release of luteolytic “pulses” of the uterine luteolysin, prostaglandin F2α (PGF), must be blocked if the pregnancy is to survive [37]. The most widely accepted theory to explain how PGF release is modulated predicts that IFNT prevents the up-regulation of estrogen receptor-alpha (ERα), a process that normally occurs in uterine epithelium towards the end of a non-fertile estrous cycle. The resulting lower concentration of ERα then precludes a gain in oxytocin receptor number and blocks a subsequent, full-bodied release of the luteolysin from the endometrium [17]. Although much of the available data are consistent with this hypothesis in the sheep, evidence for a similar mechanism operating in cattle is less clear cut [38]. There is also evidence that IFNT reduces PGF synthesis while simultaneously increasing the production of prostaglandin E (PGE) [39, 40], a prostanoid considered to support rather than antagonize CL function [41]. However, there is wide disagreement on this topic [36]. Our laboratory, for example, has noted that IFNT down regulated expression of the gene (PTGS2) encoding cyclooxygenase-2, which catalyzes the rate limiting step of prostaglandin synthesis, as well as PGF synthase (AKR1C1), in ovine luminal epithelial cells cultured in presence of IFNT, while it up-regulated PGE2 synthase (PGES) [42]. We obtained essentially identical results when comparing endometrium from d-14 pregnant and non-pregnant ewes and after ewes at d-14 of their estrous cycles had been treated with IFNT [36]. These data contrast with those reported by Gray et al. [43], who reported that PTGS2 expression was up-regulated in response to IFNT treatment. We have no explanation for these differences in outcome, although they may relate to differing experimental designs. Overall, our laboratory takes the perspective that IFNT treatment favors PGE production over PGF, and, in agreement with others, blocks an increase in the transcription of the oxytocin receptor (OTR) gene in the endometrium. Together, these mechanisms ensure that the output of PGF during the critical window when the CL is highly susceptible to luteolytic signals is blocked.
IFNT influences many other events in the endometrium than prostaglandin metabolism. In our recent study performed on cultured ovine luminal epithelial cells and employing microarrays based on bovine EST sequences, we collected data on over 15,000 genes of which 1274 (or 8% of the total) were IFNT responsive at a threshold of significance of P< 0.01. We chose to concentrate on the “top” 585 genes (of which 567 were at least partially annotated) where the changes were highly significant (adjusted P < 0.001). Of these genes, 356 were up-regulated, while the reminder showed a reduction in expression. IFNT, like other Type I IFN, raised the transcript concentrations of many genes with a role in antiviral activity, but there were also effects on genes involved in controlling apoptosis, cellular growth, extra-cellular matrix accretion, angiogenesis, blood coagulation, the NFκB pathway and inflammation, and many other processes. In particular, it increased mRNA concentrations of genes related to the VEGFR2 pathway of angiogenesis and down-regulated ones associated with hypoxia, including hypoxia-inducible factors. The data indicate that IFNT likely targets a complex range of physiological processes in endometrium, including ones likely to lead to increased vascularization at sites neighboring the conceptus. Which of these several pathways are critical and which are secondary to establishment of the pregnancy and the development of a functional placenta remains to be tested. In view of what genes we [36, 42] and others [36, 43-46] have observed to be regulated in endometrium of cattle and sheep in response to either IFNT or to the presence of a conceptus secreting IFNT, we predict that the role of IFNT is to transform a full-blown inflammatory response, which that might otherwise overwhelm the conceptus, to a more controlled local reaction that the conceptus can exploit to its own advantage.
IFN-gamma (IFNG) and IFN-delta (IFND) in the Pig
The identification of IFNT in the sheep and the cow led to a search for IFN production by the conceptuses of other species. Our laboratory initially found evidence for antiviral factors associated with elongating d-12 pig [47] and pre-implantation mouse conceptuses [48], but was unable to identify a specific IFN. The nature of the antiviral factor released by mouse blastocysts still remains an enigma, but others successfully demonstrated that elongating pig trophoblast released two IFN beginning on d-12 and ending around d-20 of development [49-51]. The one produced most abundantly was interferon-gamma (IFNG), so called Type II or inflammatory IFN, which is structurally unrelated to IFNT and all other Type I IFN. It has relatively weak antiviral activity and is encoded by a single gene. The second was a previously unknown Type I IFN, now designated IFN-delta (IFND), which is currently believed to have appeared early in eutherian mammalian evolution. IFND is little studied, and its gene has been lost from many branches of the mammalian tree, including primates, suggesting that its function had become redundant [52]. It does, however, have antiviral activity, binds to the Type I IFN receptor, and would be expected to regulate many of the same gene networks that are responsive to IFNT [53]. Trophoblast-secreted IFNG is truncated at the C-terminal, and has a unique glycosylation pattern and lower antiviral activity than IFNG produced by leukocytes [54], suggesting that it has been selected for a special function in pregnancy, although such a role has yet to be defined.
Despite the quite similar temporal patterns of secretion of IFN by porcine, ovine, and bovine conceptuses, the porcine IFN do not make a direct contribution towards prevention of luteolyis. When IFNG and IFND are infused together into the uterine lumen of non-pregnant pigs, they have no ability to extend estrous cycle length [55]. In any case, it is now widely accepted that the main signal for maternal recognition of pregnancy and rescue of the CL in swine is trophoblast estrogen [56]. PGF, rather than being released in an endocrine direction towards the maternal vasculature is shunted in an exocrine manner into the uterine lumen. Nevertheless it seems reasonable to predict that the presence of a powerful inflammatory cytokine such as IFNG and a Type I IFN, such as IFND, does not go unnoticed by the mother and that her responses play some role in contributing towards a successful pregnancy in the pig. Similar processes likely occur in related species with non-invasive forms of placentation.
IFN in maternal conceptus dialog in the mouse and human
IFN production by pre-implantation conceptuses is not well documented in species other than the ruminant and pig, although, as described above, the mouse blastocyst may produce an antiviral response in cells in its immediate environment [48] and antiviral activity associated has been detected in late stage mouse placentas [57]. Importantly, however mice lacking a functional Type I IFN receptor are fertile and can carry a litter of pups to term [58, 59]. It therefore seems unlikely that classical Type I IFN signaling from the conceptus to mother has an essential role in triggering maternal responses in the mouse.
Attempts to identify the release of a soluble IFN by cultured human blastocysts [60, 61] have failed, although production may be below the sensitivity limits of the bioassays employed. IFNA and IFNG have been identified by immunohistochemistry in human syncytiotrophoblast and extravillous trophoblast, especially in tissue from early pregnancies [62, 63], and IFNA and IFNB are readily induced in cytotrophoblast, especially invasive extravillous trophoblast, by Sendai virus [64]. A Type I IFN transcript, identified by in situ hybridization, was reported to be present in first trimester extravillous cytotrophoblast human trophoblast [65], although the claim that the transcript identified was derived from an IFNT was undoubtedly incorrect in view of the fact that the human genome is devoid of such genes [66]. Conceivably, the hybridizing mRNA represented a closely related gene, for example the single functional human IFNW gene (IFNW1), thereby raising the possibility that active production of IFN occurs constitutively in the invasive component of the human placenta. Another possibility is that an as yet poorly characterized Type I IFN, such IFN-Epsilon (IFNE), is involved in trophoblast signaling to maternal endometrial cells [52]. Although the rather skimpy data available do not rule out an involvement of Type I IFN contributing to the trophoblast-maternal dialog in the human, the evidence for a high profile role is in doubt.
Although much of the IFNG expressed during pregnancy in mice is from uterine natural killer cells (uNK), IFNG is expressed by giant trophoblast cells, the early invasive component of trophoblast [67]. Its role in communication with the mother is unknown though. Mice null for either IFNG or lacking the alpha subunit of the IFNG receptor exhibit structural defects at implantation sites, but are fertile [68], suggesting that IFNG, if not essential to pregnancy in mice, contributes to gestational health. More recently, IFNG has been found to be a factor in the modification of spiral arteries, and hence fetal control of blood delivery to the placenta as pregnancy proceeds [69]. Overall, however, there is little to suggest that IFNG is implicated in early communication between conceptuses and the mother.
There is a similar lack of evidence for the participation of trophoblast IFNG in supporting a successful human pregnancy although populations of cells expressing this cytokine, usually of maternal origin, have been described [70]. In general, high IFNG expression in uterine decidual tissue is associated with placental pathology, specifically inflammation, and risk of pregnancy termination, while trophoblast cells seem adapted to resist IFNG action, which might otherwise target them for apoptosis [71].
Evidence for a modified IFN response in endometrium of pregnant rodents and women
There is accumulating evidence that some genes responsive to IFN become up-regulated at implantation sites in the mouse [72-74], rat [75] and in the decidual tissues of pregnant women and baboons [76]. A similar increased expression of IFN-regulated genes has been observed after microarray analysis of human endometrial stromal cells that were either exposed to medium conditioned by extravillous cytotrophoblast [77] or co-cultured with first trimester trophoblast explants [78]. One gene, in particular (ISG15), was common to all these species, and is among the most widely studied genes responsive to IFNT in the sheep [79] and cow [80]. Its gene product, sometimes known as ubiquitin cross-reactive protein, like ubiquitin itself, forms conjugates with proteins. It has both an intracellular as well as extracellular presence in the pregnant uterus of cattle and sheep [80], but its specific function in endometrium during pregnancy is yet to be determined.
Despite the fact that some genes frequently regulated by IFN may be a widespread, possibly universal, feature of pregnant endometrium, it is clear that many other familiar genes anticipated to be expressed following exposure to IFN are not actively transcribed. Conceivably, trophoblast factors other than an IFN, acting locally on endometrial stromal and epithelial cells, are capable of keying into one or more arms of IFN-signal transduction pathways without causing a full blown IFN response. One intriguing possibility is that Toll-like receptors located in endometrium and thought to control local innate immunity [81, 82], are stimulated by the local presence of trophoblast, and initiate the production of pro-inflammatory and anti-viral cytokines that facilitate the process of implantation.
Summary
In summary, successful pregnancy in eutherian mammals requires both changes in ovarian physiology to prevent luteolysis and extensive alterations in the uterine environment to support embryo development. In ruminants, a recently evolved Type 1 IFN, the IFNT, serves as the focal point for maternal-conceptus dialogue in early pregnancy. Its production by the ruminant conceptus has one well established function unique to this sub-order, namely to modulate release of the uterine luteolysin, PGF and maintain the functional integrity of the CL. The control of IFNT production during this critical period of maternal recognition of pregnancy depends upon the expression of an appropriate combination of transcription factors, including the master regulator Ets2, in trophectoderm and the stimulatory action of growth factors produced under the influence of progesterone by the maternal endometrium. A second, less studied aspect of IFNT action appears to be its ability to create a locally receptive endometrium that will provide a cellular environment where the conceptus can grow and develop and avoid immune rejection by the mother. We suggest that the inflammatory-like reaction that occurs at implantation sites during early pregnancy in most, if not all, species of mammal where extended uterine support of the conceptus occurs incorporates aspects of an IFN response and that the scale and type of response will vary according to the degree of intimacy between the trophoblast and the endometrium.
Acknowledgements
The work from this laboratory was supported by NIH Grant HD21896
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Roberts RM, Schalue-Francis T, Francis H, Keisler D. Maternal recognition of pregnancy and embryonic loss. Theriogenology. 1990;33:175–183. [Google Scholar]
- [2].Wimsatt WA. Some comparative aspects of implantation. Biol Reprod. 1975;12:1–40. doi: 10.1095/biolreprod12.1.1. [DOI] [PubMed] [Google Scholar]
- [3].Duc-Goiran P, Mignot TM, Bourgeois C, Ferre F. Embryo-maternal interactions at the implantation site: a delicate equilibrium. Eur J Obstet Gynecol Reprod Biol. 1999;83:85–100. doi: 10.1016/s0301-2115(98)00310-8. [DOI] [PubMed] [Google Scholar]
- [4].Thompson JG, Mitchell M, Kind KL. Embryo culture and long-term consequences. Reprod Fertil Dev. 2007;19:43–52. doi: 10.1071/rd06129. [DOI] [PubMed] [Google Scholar]
- [5].Betteridge K, Eaglesome N, Randall G, Mitchell D, Lugden E. Maternal progesterone levels as evidence of luteotrophic or antiluteolytic effects on embryos transferred to heifers 12-17 days after estrus. Theriogenology. 1978;9:86–93. [Google Scholar]
- [6].Moor RM, Rowson LE. The corpus luteum of the sheep: effect of the removal of embryos on luteal function. J Endocrinol. 1966;34:497–502. doi: 10.1677/joe.0.0340497. [DOI] [PubMed] [Google Scholar]
- [7].Bazer FW, First NL. Pregnancy and parturition. J Anim Sci. 1983;57(Suppl 2):425–60. [PubMed] [Google Scholar]
- [8].King BF. Development and structure of the placenta and fetal membranes of nonhuman primates. J Exp Zool. 1993;266:528–40. doi: 10.1002/jez.1402660605. [DOI] [PubMed] [Google Scholar]
- [9].Wooding FB. Current topic: the synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta. 1992;13:101–13. doi: 10.1016/0143-4004(92)90025-o. [DOI] [PubMed] [Google Scholar]
- [10].Wildman DE, Chen C, Erez O, Grossman LI, Goodman M, Romero R. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc Natl Acad Sci U S A. 2006;103:3203–8. doi: 10.1073/pnas.0511344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Carter AM, Mess A. Evolution of the placenta in eutherian mammals. Placenta. 2007;28:259–62. doi: 10.1016/j.placenta.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [12].Helmer SD, Hansen PJ, Anthony RV, Thatcher WW, Bazer FW, Roberts RM. Identification of bovine trophoblast protein-1, a secretory protein immunologically related to ovine trophoblast protein-1. J Reprod Fertil. 1987;79:83–91. doi: 10.1530/jrf.0.0790083. [DOI] [PubMed] [Google Scholar]
- [13].Roberts RM, Xie S, Mathialagan N. Maternal recognition of pregnancy. Biol Reprod. 1996;54:294–302. doi: 10.1095/biolreprod54.2.294. [DOI] [PubMed] [Google Scholar]
- [14].Roberts RM, Cross JC, Leaman DW. Interferons as hormones of pregnancy. Endocr Rev. 1992;13:432–52. doi: 10.1210/edrv-13-3-432. [DOI] [PubMed] [Google Scholar]
- [15].Bazer FW. Mediators of maternal recognition of pregnancy in mammals. Proc Soc Exp Biol Med. 1992;199:373–84. doi: 10.3181/00379727-199-43371a. [DOI] [PubMed] [Google Scholar]
- [16].Vallet JL, Bazer FW, Fliss MF, Thatcher WW. Effect of ovine conceptus secretory proteins and purified ovine trophoblast protein-1 on interoestrous interval and plasma concentrations of prostaglandins F-2 alpha and E and of 13,14-dihydro- 15-keto prostaglandin F-2 alpha in cyclic ewes. J Reprod Fertil. 1988;84:493–504. doi: 10.1530/jrf.0.0840493. [DOI] [PubMed] [Google Scholar]
- [17].Spencer TE, Johnson GA, Bazer FW, Burghardt RC. Fetal-maternal interactions during the establishment of pregnancy in ruminants. Soc Reprod Fertil Suppl. 2007;64:379–96. doi: 10.5661/rdr-vi-379. [DOI] [PubMed] [Google Scholar]
- [18].Chakrabarty A, Roberts MR. Ets-2 and C/EBP-beta are important mediators of ovine trophoblast Kunitz domain protein-1 gene expression in trophoblast. BMC Mol Biol. 2007;8:14. doi: 10.1186/1471-2199-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].MacLean JA, II, Chakrabarty A, Xie S, Bixby JA, Roberts RM, Green JA. Family of Kunitz proteins from trophoblast: expression of the trophoblast Kunitz domain proteins (TKDP) in cattle and sheep. Mol Reprod Dev. 2003;65:30–40. doi: 10.1002/mrd.10262. [DOI] [PubMed] [Google Scholar]
- [20].Green JA, Xie S, RM R. Pepsin-related molecules secreted by trophoblast. Review of Reproduction. 1998;3:62–69. doi: 10.1530/ror.0.0030062. [DOI] [PubMed] [Google Scholar]
- [21].Roberts RM, Liu L, Alexenko A. New and atypical families of type I interferons in mammals: comparative functions, structures, and evolutionary relationships. Prog Nucleic Acid Res Mol Biol. 1997;56:287–325. doi: 10.1016/s0079-6603(08)61008-9. [DOI] [PubMed] [Google Scholar]
- [22].Leaman DW, Cross JC, Roberts RM. Multiple regulatory elements are required to direct trophoblast interferon gene expression in choriocarcinoma cells and trophectoderm. Mol Endocrinol. 1994;8:456–68. doi: 10.1210/mend.8.4.8052267. [DOI] [PubMed] [Google Scholar]
- [23].Leaman DW, Roberts RM. Genes for the trophoblast interferons in sheep, goat, and musk ox and distribution of related genes among mammals. J Interferon Res. 1992;12:1–11. doi: 10.1089/jir.1992.12.1. [DOI] [PubMed] [Google Scholar]
- [24].Ezashi T, Ealy AD, Ostrowski MC, Roberts RM. Control of interferon-tau gene expression by Ets-2. Proc Natl Acad Sci U S A. 1998;95:7882–7. doi: 10.1073/pnas.95.14.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ezashi T, Ghosh D, Roberts RM. Repression of Ets-2-induced transactivation of the tau interferon promoter by Oct-4. Mol Cell Biol. 2001;21:7883–91. doi: 10.1128/MCB.21.23.7883-7891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Farin CE, Imakawa K, Hansen TR, McDonnell JJ, Murphy CN, Farin PW, et al. Expression of trophoblastic interferon genes in sheep and cattle. Biol Reprod. 1990;43:210–8. doi: 10.1095/biolreprod43.2.210. [DOI] [PubMed] [Google Scholar]
- [27].Ealy AD, Larson SF, Liu L, Alexenko AP, Winkelman GL, Kubisch HM, et al. Polymorphic forms of expressed bovine interferon-tau genes: relative transcript abundance during early placental development, promoter sequences of genes and biological activity of protein products. Endocrinology. 2001;142:2906–15. doi: 10.1210/endo.142.7.8249. [DOI] [PubMed] [Google Scholar]
- [28].Ashworth CJ, Bazer FW. Changes in ovine conceptus and endometrial function following asynchronous embryo transfer or administration of progesterone. Biol Reprod. 1989;40:425–33. doi: 10.1095/biolreprod40.2.425. [DOI] [PubMed] [Google Scholar]
- [29].Nephew KP, McClure KE, Ott TL, Dubois DH, Bazer FW, Pope WF. Relationship between variation in conceptus development and differences in estrous cycle duration in ewes. Biol Reprod. 1991;44:536–9. doi: 10.1095/biolreprod44.3.536. [DOI] [PubMed] [Google Scholar]
- [30].Satterfield MC, Bazer FW, Spencer TE. Progesterone regulation of preimplantation conceptus growth and galectin 15 (LGALS15) in the ovine uterus. Biol Reprod. 2006;75:289–96. doi: 10.1095/biolreprod.106.052944. [DOI] [PubMed] [Google Scholar]
- [31].Mann GE, Lamming GE. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction. 2001;121:175–80. doi: 10.1530/rep.0.1210175. [DOI] [PubMed] [Google Scholar]
- [32].Ezashi T, Roberts RM. Regulation of interferon-tau (IFN-tau) gene promoters by growth factors that target the Ets-2 composite enhancer: a possible model for maternal control of IFN-tau production by the conceptus during early pregnancy. Endocrinology. 2004;145:4452–60. doi: 10.1210/en.2004-0606. [DOI] [PubMed] [Google Scholar]
- [33].Michael DD, Alvarez IM, Ocon OM, Powell AM, Talbot NC, Johnson SE, et al. Fibroblast growth factor-2 is expressed by the bovine uterus and stimulates interferon-tau production in bovine trophectoderm. Endocrinology. 2006;147:3571–9. doi: 10.1210/en.2006-0234. [DOI] [PubMed] [Google Scholar]
- [34].Roberts RM, Ezashi T, Rosenfeld CS, Ealy AD, Kubisch HM. Evolution of the interferon tau genes and their promoters, and maternal-trophoblast interactions in control of their expression. Reprod Suppl. 2003;61:239–51. [PubMed] [Google Scholar]
- [35].Ezashi T, Roberts RM. Colony stimulating factor-1 enhances bovine interferon-tau gene transcription via a Ras-responsive enhancer. Biol. Reprod. 2000;62:289. Abstr.464. [Google Scholar]
- [36].Chen Y, Green JA, Antoniou E, Ealy AD, Mathialagan N, Walker AM, et al. Effect of interferon-tau administration on endometrium of nonpregnant ewes: a comparison with pregnant ewes. Endocrinology. 2006;147:2127–37. doi: 10.1210/en.2005-1310. [DOI] [PubMed] [Google Scholar]
- [37].Bazer FW, Thatcher WW, Hansen PJ, Mirando MA, Ott TL, Plante C. Physiological mechanisms of pregnancy recognition in ruminants. J Reprod Fertil Suppl. 1991;43:39–47. [PubMed] [Google Scholar]
- [38].Robinson RS, Mann GE, Lamming GE, Wathes DC. Expression of oxytocin, oestrogen and progesterone receptors in uterine biopsy samples throughout the oestrous cycle and early pregnancy in cows. Reproduction. 2001;122:965–79. [PubMed] [Google Scholar]
- [39].Asselin E, Bazer FW, Fortier MA. Recombinant ovine and bovine interferons tau regulate prostaglandin production and oxytocin response in cultured bovine endometrial cells. Biol Reprod. 1997;56:402–8. doi: 10.1095/biolreprod56.2.402. [DOI] [PubMed] [Google Scholar]
- [40].Xiao CW, Murphy BD, Sirois J, Goff AK. Down-regulation of oxytocin-induced cyclooxygenase-2 and prostaglandin F synthase expression by interferon-tau in bovine endometrial cells. Biol Reprod. 1999;60:656–63. doi: 10.1095/biolreprod60.3.656. [DOI] [PubMed] [Google Scholar]
- [41].Magness RR, Huie JM, Hoyer GL, Huecksteadt TP, Reynolds LP, Seperich GJ, et al. Effect of chronic ipsilateral or contralateral intrauterine infusion of prostaglandin E2 (PGE2) on luteal function of unilaterally ovariectomized ewes. Prostaglandins Med. 1981;6:389–401. doi: 10.1016/0161-4630(81)90071-9. [DOI] [PubMed] [Google Scholar]
- [42].Chen Y, Antoniou E, Liu Z, Hearne LB, Roberts RM. A microarray analysis for genes regulated by interferon-{tau} in ovine luminal epithelial cells. Reproduction. 2007;134:123–35. doi: 10.1530/REP-07-0387. [DOI] [PubMed] [Google Scholar]
- [43].Gray CA, Abbey CA, Beremand PD, Choi Y, Farmer JL, Adelson DL, et al. Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol Reprod. 2006;74:383–94. doi: 10.1095/biolreprod.105.046656. [DOI] [PubMed] [Google Scholar]
- [44].Ishiwata H, Katsuma S, Kizaki K, Patel OV, Nakano H, Takahashi T, et al. Characterization of gene expression profiles in early bovine pregnancy using a custom cDNA microarray. Mol Reprod Dev. 2003;65:9–18. doi: 10.1002/mrd.10292. [DOI] [PubMed] [Google Scholar]
- [45].Klein C, Bauersachs S, Ulbrich SE, Einspanier R, Meyer HH, Schmidt SE, et al. Monozygotic twin model reveals novel embryo-induced transcriptome changes of bovine endometrium in the preattachment period. Biol Reprod. 2006;74:253–64. doi: 10.1095/biolreprod.105.046748. [DOI] [PubMed] [Google Scholar]
- [46].Bauersachs S, Ulbrich SE, Gross K, Schmidt SE, Meyer HH, Wenigerkind H, et al. Embryo-induced transcriptome changes in bovine endometrium reveal species-specific and common molecular markers of uterine receptivity. Reproduction. 2006;132:319–31. doi: 10.1530/rep.1.00996. [DOI] [PubMed] [Google Scholar]
- [47].Cross JC, Roberts RM. Porcine conceptuses secrete an interferon during the preattachment period of early pregnancy. Biol Reprod. 1989;40:1109–18. doi: 10.1095/biolreprod40.5.1109. [DOI] [PubMed] [Google Scholar]
- [48].Cross JC, Farin CE, Sharif SF, Roberts RM. Characterization of the antiviral activity constitutively produced by murine conceptuses: absence of placental mRNAs for interferon alpha and beta. Mol Reprod Dev. 1990;26:122–8. doi: 10.1002/mrd.1080260205. [DOI] [PubMed] [Google Scholar]
- [49].La Bonnardiere C. Nature and possible functions of interferons secreted by the preimplantation pig blastocyst. J Reprod Fertil Suppl. 1993;48:157–70. [PubMed] [Google Scholar]
- [50].Lefevre F, Boulay V. A novel and atypical type one interferon gene expressed by trophoblast during early pregnancy. J Biol Chem. 1993;268:19760–8. [PubMed] [Google Scholar]
- [51].Lefevre F, Martinat-Botte F, Guillomot M, Zouari K, Charley B, La Bonnardiere C. Interferon-gamma gene and protein are spontaneously expressed by the porcine trophectoderm early in gestation. Eur J Immunol. 1990;20:2485–90. doi: 10.1002/eji.1830201119. [DOI] [PubMed] [Google Scholar]
- [52].Krause CD, Pestka S. Evolution of the Class 2 cytokines and receptors, and discovery of new friends and relatives. Pharmacol Ther. 2005;106:299–346. doi: 10.1016/j.pharmthera.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [53].Lefevre F, Guillomot M, D’Andrea S, Battegay S, La Bonnardiere C. Interferon-delta: the first member of a novel type I interferon family. Biochimie. 1998;80:779–88. doi: 10.1016/s0300-9084(99)80030-3. [DOI] [PubMed] [Google Scholar]
- [54].Cencic A, La Bonnardiere C. Trophoblastic interferon-gamma: current knowledge and possible role(s) in early pig pregnancy. Vet Res. 2002;33:139–57. doi: 10.1051/vetres:2002003. [DOI] [PubMed] [Google Scholar]
- [55].Lefevre F, Martinat-Botte F, Locatelli A, De Niu P, Terqui M, La Bonnardiere C. Intrauterine infusion of high doses of pig trophoblast interferons has no antiluteolytic effect in cyclic gilts. Biol Reprod. 1998;58:1026–31. doi: 10.1095/biolreprod58.4.1026. [DOI] [PubMed] [Google Scholar]
- [56].Geisert RD, Ross JW, Ashworth MD, White FJ, Johnson GA, DeSilva U. Maternal recognition of pregnancy signal or endocrine disruptor: the two faces of oestrogen during establishment of pregnancy in the pig. Soc Reprod Fertil Suppl. 2006;62:131–45. [PubMed] [Google Scholar]
- [57].Barlow DP, Randle BJ, Burke DC. Interferon synthesis in the early post-implantation mouse embryo. Differentiation. 1984;27:229–35. doi: 10.1111/j.1432-0436.1984.tb01433.x. [DOI] [PubMed] [Google Scholar]
- [58].Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- [59].Hwang SY, Hertzog PJ, Holland KA, Sumarsono SH, Tymms MJ, Hamilton JA, et al. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci U S A. 1995;92:11284–8. doi: 10.1073/pnas.92.24.11284. [published erratum appears in Proc Natl Acad Sci U S A 1996 Apr 30;93(9):4519] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Paulesu L. Cytokines in mammalian reproduction and speculation about their possible involvement in nonmammalian viviparity. Microsc Res Tech. 1997;38:188–94. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<188::AID-JEMT19>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [61].Roberts R, Cross J, Farin C, Kramer K, Schalue-Francis T, Hansen TR. Trophoblast interferons as antiluteolysins. In: Edwards RG, editor. Establishing a successful human pregnancy; Serono Symposia Vol. 66; Serono Symposia Publications; New York:NY; Raven Press. 1990.pp. 257–268. [Google Scholar]
- [62].Paulesu L, Bocci V, King A, Loke YM. Immunocytochemical localization of interferons in human trophoblast populations. J Biol Regul Homeost Agents. 1991;5:81–5. [PubMed] [Google Scholar]
- [63].Paulesu L, Romagnoli R, Cintorino M, Ricci MG, Garotta G. First trimester human trophoblast expresses both interferon-gamma and interferon-gamma-receptor. J Reprod Immunol. 1994;27:37–48. doi: 10.1016/0165-0378(94)90013-2. [DOI] [PubMed] [Google Scholar]
- [64].Aboagye-Mathiesen G, Toth FD, Zdravkovic M, Ebbesen P. Functional characteristics of human trophoblast interferons. Am J Reprod Immunol. 1996;35:309–17. doi: 10.1111/j.1600-0897.1996.tb00486.x. [DOI] [PubMed] [Google Scholar]
- [65].Whaley AE, Meka CS, Harbison LA, Hunt JS, Imakawa K. Identification and cellular localization of unique interferon mRNA from human placenta. J Biol Chem. 1994;269:10864–8. [PubMed] [Google Scholar]
- [66].Roberts RM, Ealy AD, Alexenko AP, Han CS, Ezashi T. Trophoblast interferons. Placenta. 1999;20:259–64. doi: 10.1053/plac.1998.0381. [DOI] [PubMed] [Google Scholar]
- [67].Platt JS, Hunt JS. Interferon-gamma gene expression in cycling and pregnant mouse uterus: temporal aspects and cellular localization. J Leukoc Biol. 1998;64:393–400. doi: 10.1002/jlb.64.3.393. [DOI] [PubMed] [Google Scholar]
- [68].Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61:493–502. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- [69].Monk JM, Makinen K, Shrum B, Woodward B. A brief communication: Blood corticosterone concentration reaches critical illness levels early during acute malnutrition in the weanling mouse. Experimental Biology and Medicine. 2006;231:264–268. doi: 10.1177/153537020623100304. [DOI] [PubMed] [Google Scholar]
- [70].Leonard S, Murrant C, Tayade C, van den Heuvel M, Watering R, Croy BA. Mechanisms regulating immune cell contributions to spiral artery modification -- facts and hypotheses -- a review. Placenta. 2006;27(Suppl A):S40–6. doi: 10.1016/j.placenta.2005.11.007. [DOI] [PubMed] [Google Scholar]
- [71].Choi JC, Holtz R, Petroff MG, Alfaidy N, Murphy SP. Dampening of IFN-gamma-inducible gene expression in human choriocarcinoma cells is due to phosphatase-mediated inhibition of the JAK/STAT-1 pathway. J Immunol. 2007;178:1598–607. doi: 10.4049/jimmunol.178.3.1598. [DOI] [PubMed] [Google Scholar]
- [72].Austin KJ, Bany BM, Belden EL, Rempel LA, Cross JC, Hansen TR. Interferon-stimulated gene-15 (Isg15) expression is up-regulated in the mouse uterus in response to the implanting conceptus. Endocrinology. 2003;144:3107–13. doi: 10.1210/en.2002-0031. [DOI] [PubMed] [Google Scholar]
- [73].Bany BM, Cross JC. Post-implantation mouse conceptuses produce paracrine signals that regulate the uterine endometrium undergoing decidualization. Dev Biol. 2006;294:445–56. doi: 10.1016/j.ydbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- [74].Kashiwagi A, Digirolamo CM, Kanda Y, Niikura Y, Esmon CT, Hansen TR, et al. The Post-Implantation Embryo Differentially Regulates Endometrial Gene Expression and Decidualization. Endocrinology. 2007 doi: 10.1210/en.2007-0268. [DOI] [PubMed] [Google Scholar]
- [75].Li Q, Zhang M, Kumar S, Zhu LJ, Chen D, Bagchi MK, et al. Identification and implantation stage-specific expression of an interferon-alpha-regulated gene in human and rat endometrium. Endocrinology. 2001;142:2390–400. doi: 10.1210/endo.142.6.8101. [DOI] [PubMed] [Google Scholar]
- [76].Bebington C, Bell SC, Doherty FJ, Fazleabas AT, Fleming SD. Localization of ubiquitin and ubiquitin cross-reactive protein in human and baboon endometrium and decidua during the menstrual cycle and early pregnancy. Biol Reprod. 1999;60:920–8. doi: 10.1095/biolreprod60.4.920. [DOI] [PubMed] [Google Scholar]
- [77].Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–17. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- [78].Popovici RM, Betzler NK, Krause MS, Luo M, Jauckus J, Germeyer A, et al. Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology. 2006;147:5662–75. doi: 10.1210/en.2006-0916. [DOI] [PubMed] [Google Scholar]
- [79].Hansen TR, Austin KJ, Perry DJ, Pru JK, Teixeira MG, Johnson GA. Mechanism of action of interferon-tau in the uterus during early pregnancy. J Reprod Fertil Suppl. 1999;54:329–39. [PubMed] [Google Scholar]
- [80].Austin KJ, Ward SK, Teixeira MG, Dean VC, Moore DW, Hansen TR. Ubiquitin cross-reactive protein is released by the bovine uterus in response to interferon during early pregnancy. Biol Reprod. 1996;54:600–6. doi: 10.1095/biolreprod54.3.600. [DOI] [PubMed] [Google Scholar]
- [81].Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod. 2005;20:1372–8. doi: 10.1093/humrep/deh775. [DOI] [PubMed] [Google Scholar]
- [82].Jorgenson RL, Young SL, Lesmeister MJ, Lyddon TD, Misfeldt ML. Human endometrial epithelial cells cyclically express Toll-like receptor 3 (TLR3) and exhibit TLR3-dependent responses to dsRNA. Hum Immunol. 2005;66:469–82. doi: 10.1016/j.humimm.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]