Abstract
How spatial, temporal, and sexual specific cues are integrated to specify distinct cell fates during multicellular organism development is largely unknown. Here we demonstrate that the Caenorhabditis elegans PcG-like gene sop-2 determines the temporal and sexual specificities of a row of hypodermal seam cells, in addition to specifying their positional identities. Loss-of-function of sop-2 causes premature expression of adult fates at larval stages. sop-2 acts upstream of lin-29 in the heterochronic pathway and genetically interacts with other heterochronic genes in specifying the temporal fates of seam cells at different larval stages. We show that the number of ALG-1-containing P bodies is increased in seam cells in sop-2 mutants. Furthermore, the microRNA-mediated repression of a heterochronic gene reporter is enhanced in sop-2 mutants. Mutations in sop-2 also cause partial hermaphrodite-to-male sexual transformations. The homeotic transformations, heterochronic defects, and sexual transformations can occur concomitantly in sop-2 mutants. In summary, our studies reveal that sop-2 integrates spatial, temporal, and sexual cues during C. elegans development.
IN multicellular organisms cell fates are determined by a combination of spatial, temporal, and sex-specific cues. Studies in model systems, including Drosophila and Caenorhabditis elegans, have revealed that distinct classes of genes are involved in specifying the positional, temporal, and sexual specificities of cell fates. The positional identities of cells along the anterior–posterior axis are specified by Hox proteins (for review, see Gellon and McGinnis 1998). The sex-determination pathway specifies sexual fate and the correct mode of dosage compensation in accordance with the genotypic sex (for review, see Cline and Meyer 1996). Temporal developmental events are regulated by heterochronic genes (for review, see Ambros 2000). The mechanism by which these regulatory factors synchronize to generate an elaborate developmental program for each cell is poorly understood.
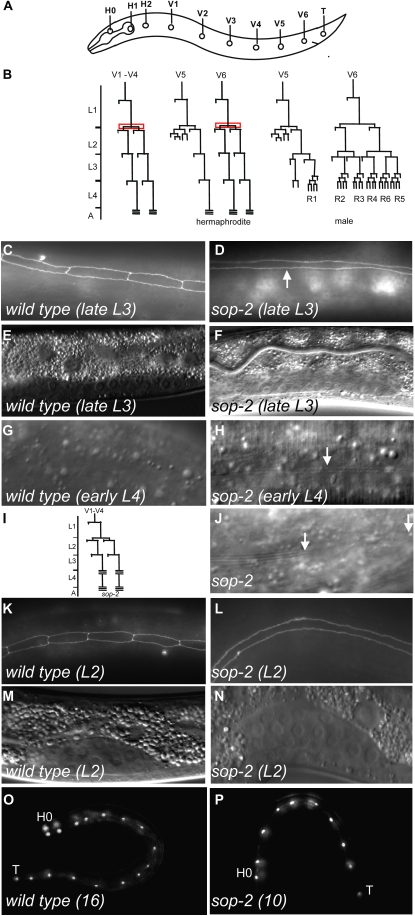
In C. elegans, the postembryonic development of hypodermal seam cells provides a useful model for examining how cells adopt the correct spatial, temporal, and sex-specific cell fates. Ten seam cells, H0–H2, V1–V6, and T, aligned on each side from anterior to posterior, undergo stage-specific division patterns at each of four (L1–L4) postembryonic larval stages (Figure 1A). At the middle L4 stage, these seam cells begin to terminally differentiate, which involves cessation of cell divisions, fusion with neighboring seam cells, and synthesis of adult-specific cuticular structure, called alae (Ambros and Horvitz 1984).
Figure 1.—
A sop-2 mutation causes premature terminal differentiation of seam cells. (A) Schematic arrangement of the nuclei of seam cells H0–H2, V1–V6, and T on each side of a wild-type L1 larva. (B) The postembryonic division pattern of a subset of seam cells in a wild-type animal. The proliferative S2 seam cell division is highlighted in red. In males, the V1–V4 seam cells adopt the same fate as in hermaphrodites, but V5, V6, and T (not shown here) generate rays. The postembryonic stages are indicated along the vertical axis, separated by molting. (C–F) Precocious fusion of seam cells at the late L3 stage in sop-2 mutants. In wild-type late L3 larvae, seam cells remain unfused, as shown by an ajm-1∷gfp reporter (C). In sop-2 mutants, seam cells fuse together at the late L3 stage (D, arrow). (E and F) Nomarski images of the gonads of the animals in C and D, respectively. The sop-2(bx91ts) mutants are shifted at the hatching stage from the permissive 15° to the nonpermissive 23° unless otherwise noted. (G and H) Precocious formation of adult-specific alae in sop-2 mutants. No alae are formed in early L4 wild-type larvae (G). In sop-2 mutant animals, adult-specific alae (arrow) are formed at the early L4 stage (H). Note that the alae are not well formed in the L4 sop-2 mutant animal shown. (I) Cell lineage of the lateral hypodermal V cells in sop-2 mutants. The division patterns of seam cells are followed in six animals from the L2-to-adult stage. The expected 16 seam cells are found in all six animals. As in lin-41 and hbl-1 mutants (Slack et al. 2000; Abrahante et al. 2003), sop-2 mutants still undergo the L4 molting. (J) One or several gaps in alae are present in 26% of sop-2 mutant hermaphrodites (n = 42) (between arrows), suggesting that the cuticle synthesis is defective in sop-2 mutant adults. (K–N) Premature fusion of seam cells at the L2 stage in sop-2(bx91ts) mutants, which are shifted from the permissive 15° to the nonpermissive 23° at early embryonic stages (L). The seam cells at the L2 stage in a wild-type animal are shown in K. (M and N) Nomarski images of the gonads of the animals in K and L, respectively. (O and P) Defects in the proliferative S2 seam cell division in sop-2 mutants, which are shifted from the permissive 15° to the nonpermissive 23° at early embryonic stages. In a wild-type animal, there are 16 seam cells from the late L2 larval stage onward (O). In the late L2 sop-2 mutant larva shown, there are only 10 seam cells (P).
The temporal fates of seam cells are regulated by a cascade of heterochronic genes that form a complex regulatory network (for review, see Rougvie 2005). Mutations in heterochronic genes cause the omission or reiteration of stage-specific division patterns. The expression of many heterochronic genes is regulated by microRNAs (miRNAs), which inhibit gene translation through complementary sequences in the 3′-UTR of target genes (for review, see Banerjee and Slack 2002). For example, the expression of heterochronic genes lin-28 and lin-14 is regulated by miRNA lin-4 and the expression of lin-41 is repressed by miRNA let-7 (Lee et al. 1993; Wightman et al. 1993; Moss et al. 1997; Slack et al. 2000; Bagga et al. 2005). miRNA-mediated gene repression requires the C. elegans Argonaute homologs ALG-1 and ALG-2 (Grishok et al. 2001). Argonaute and other key components in the miRNA-mediated gene repression are localized to cytoplasmic processing bodies, called P bodies, which have been shown to play a role in miRNA-mediated gene repression (Ding et al. 2005; Liu et al. 2005; Sen and Blau 2005).
The anterior seam cells follow the same developmental program in both hermaphrodites and males: generation of alae. The posterior seam cells, V5, V6, and T, however, exhibit sexual dimorphism. Instead of generation of alae, they give rise to nine pairs of sensory organs, called rays, in males at the L4 larval-to-adult switch. The sexual specific fates adopted by V5, V6, and T are under the control of the sex-determination signaling pathway (for review, see Stothard and Pilgrim 2003). The distinct fates adopted by the anterior and posterior seam cells in males require the activity of Hox genes mab-5 and egl-5 (for review, see Kenyon et al. 1997). In mab-5 mutant males, V5 and V6 produce alae instead of rays, while ectopic expression of mab-5 or egl-5 leads to the generation of rays from the anterior seam cells. Thus, the development of seam cells requires the concerted action of Hox genes, heterochronic genes, and sex-determination genes to control their positional, temporal, and sexual specificities, respectively.
The proper expression domains of Hox genes in C. elegans are maintained by the PcG-like gene sop-2, which shares many characteristic properties and structures with the PcG protein Polyhomeotic (PH) in other organisms (Zhang et al. 2003, 2004). In sop-2 mutants, Hox genes mab-5 and egl-5 are globally derepressed, leading to homeotic transformations. For example, in sop-2 mutant males, the anterior seam cells generate rays instead of alae. Thus, sop-2 specifies the positional identities of seam cells by transcriptionally repressing the expression of Hox genes (Zhang et al. 2003).
In this study we demonstrate that sop-2 also controls the temporal and sexual fates of seam cells. Mutations in sop-2 cause precocious terminal differentiation of seam cells and the expression of male-specific fates by hermaphrodite seam cells. We further show that the number of ALG-1-containing P bodies is increased and the miRNA-mediated gene repression is enhanced in sop-2 mutants. Our studies reveal that the C. elegans PcG-like gene sop-2 controls the positional, temporal, and sexual specificities of cell fates.
MATERIALS AND METHODS
Strains:
Most strains carry the him-5(e1490) mutation, which gives rise to a high frequency of male self-progeny. The following mutant alleles were used in this work: LGI, lin-41(ma104) and lin-28(n719); LGII, sop-2(bx91ts), sop-2(bp186), lin-4(e912)/dpy-10(e128), lin-29(n333), and bpIs56(alg-1∷:dsRed); LGIII, mab-5(e1239) egl-5(n945); LGIV, jcIs1(ajm-1∷GFP); LGV, lin-46(bp312), wIs51(scm-1∷GFP), bxIs14(pkd-2∷gfp, and pha-1(+)); and LGX, hbl-1(ve18), let-7(n2853), daf-12(rh257), alg-1(bp308), and lin-14(n179ts). The genomic location for bpIs33(col-10∷gfp∷lin-41(3′-UTR)) has not been determined. In sop-2 mutants, migration and elongation of the gonad arms occur normally. The development of vulva is also normal in sop-2 mutants. None of the sop-2(bx91ts) mutant animals (n = 22), which exhibit precocious seam cell fusion, display precocious vulval formation.
Temperature-shift assays:
sop-2(bx91ts) is a temperature-sensitive (ts) mutation. At 15°, it does not cause obvious defects. At 23°, it causes animals to arrest at the L1–L2 larval stages. To study the role of sop-2 at late larval stages, embryos are grown at 15° and then are shifted to 23° at the hatching stage. To study the role of sop-2 at early larval stages, embryos are directly shifted to 23°.
Reporter gene construction:
The col-10∷gfp∷lin-41 reporter contains the lin-41 3′-UTR (C12C8, nt 15,170–16,321) and the col-10 promoter (B0222, nt 32,747–33,976). The construct was co-injected with pRF4 (Mello et al. 1991) and stable transgenic lines were obtained by γ-ray irradiation. The integrated lines were backcrossed three times to N2.
Northern blot:
For analysis the expression level of let-7 and lin-4, total RNA isolation, and Northern analyses were performed as described in Grishok et al. (2001).
RNA interference:
Single-stranded RNA (ssRNA) was transcribed from the T7- and T3-flanked PCR template with MEGAscript T3 and T7 kits (Ambion, Austin, TX). The ssRNAs were then annealed and injected into animals. F1 progeny generated 4 hr after injection were analyzed. The PCR template used for RNAi was as follows: lin-29 (Y17G7A, nt 3457–4182), alg-1 and alg-2 (Grishok et al. 2001), fem-3 (C01F6, nt 10,238–10,962), xol-1 (cDNA, nt 29–526), and her-1 (cDNA, nt 27–1166).
RESULTS
Mutations in sop-2 cause premature terminal differentiation of seam cells:
Seam cells divide in a characteristic pattern at each larval stage and at the L4 molt they exit the cell cycle permanently, fuse together, and synthesize adult-specific alae (Figure 1, A and B) (Sulston and Horvitz 1977). Since sop-2 null mutants arrest at the early L1 larval stage, a temperature-sensitive mutation of sop-2, sop-2(bx91ts), is used to study the function of sop-2 at later developmental stages. The sop-2(bx91ts) mutants grow to adulthood when the animals are shifted from the permissive temperature (15°) to the nonpermissive temperature (23°) at the hatching stage. We found that fusion of seam cells, as visualized by the adherens junction reporter ajm-1∷gfp, occurred prematurely at the late L3 larval stage in sop-2 mutants (Figure 1, C–F); 71% of sop-2 mutants (n = 21) showed fusion of all seam cells (referred to as full fusion), while the rest displayed fusion of some neighboring seam cells (referred to partial fusion). The adult-specific alae were formed at the early L4 stage in 25% of sop-2 mutants (n = 20) (Figure 1, G and H). Aided by the seam-cell-specific marker scm∷gfp, we followed division patterns of seam cells in sop-2 mutants from the L2 stage to young adult and found that the seam cells exited the cell cycle at the early L4 stage in sop-2 mutants (Figure 1I). Taken together, seam cells in sop-2 mutants undergo precocious terminal differentiation at the L3 molt.
The precocious terminal differentiation of seam cells also occurs at the early larval stages in sop-2(bx91ts) mutants. When sop-2 mutants are shifted from 15° to 23° at early embryonic stages, mutant animals grow to the L3 larval stage. Under this experimental condition, 24% of sop-2 mutant animals (n = 50) showed fusion of seam cells at the L2 molt (Figure 1, K–N). Moreover, the proliferative “S2” seam cell division (the division with both daughter cells maintaining seam cell fate) was found to be defective in sop-2 mutants. In wild-type animals (n = 30), the number of seam cells increases from 10 at the hatching stage to 16 at the late L2 stage due to the proliferative S2 division of seam cells H1, V1–V4, and V6 (Figure 1, B and O). When sop-2 mutants were shifted at early embryonic stages, the average number of seam cells was reduced to 11 (n = 35, ranging from 10 to 14) in L2 larvae (Figure 1P). Thus, sop-2 plays a role in timing the early larval stage-specific events of seam cells. For the rest of the study, the sop-2 mutant animals were shifted at the hatching stage unless otherwise noted.
The precocious fusion of seam cells at the late L3 stage also occurred in 35% of weak loss-of-function sop-2(bp186) mutant animals (n = 34), further confirming that the precocious heterochronic defects are due to loss-of-function of sop-2.
sop-2 acts upstream of lin-29 in the heterochronic pathway:
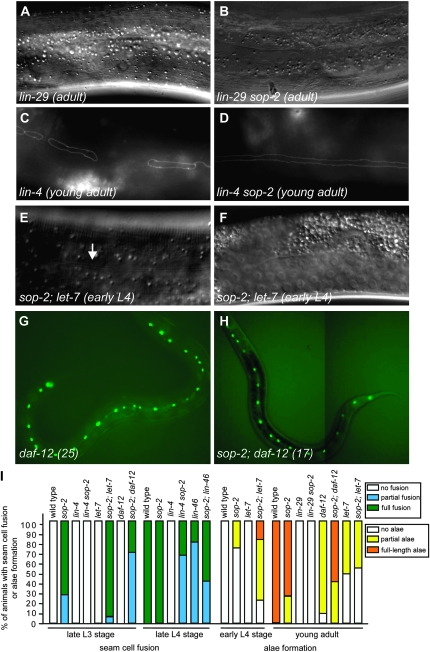
To determine where in the heterochronic pathway sop-2 acts in regulating the seam cell fate, we analyzed the genetic interactions between sop-2 and other heterochronic genes. The terminal differentiation of seam cells is directly controlled by the zinc-finger transcription factor LIN-29 (Rougvie and Ambros 1995). In lin-29 mutants, the larval-to-adult switch is blocked; seam cells remain unfused and no alae are synthesized (Figure 2A). We found that, as in lin-29 single mutants (n = 30), alae were not formed in lin-29 sop-2 young adults (n = 63) (Figure 2, B and I). Mutations in lin-29 also completely suppressed the precocious seam cell fusion at the L2 stage in sop-2 mutants that was observed when the mutant animals were shifted from 15° to 23° at early embryonic stages. Thus, the precocious terminal differentiation of seam cells observed in sop-2(bx91ts) mutants requires wild-type lin-29 function. Because LIN-29 protein expression is not observed prior to the L4 stage in seam cells (Bettinger et al. 1996), sop-2 seems likely to act upstream of lin-29.
Figure 2.—
The genetic interaction between sop-2 and retarded heterochronic mutants. (A and B) Alae are not formed in lin-29 sop-2 mutant adults, as in lin-29 single mutants (A). (C and D) Partial suppression of the retarded phenotypes in lin-4 mutants by a sop-2 mutation. Seam cells remain unfused in lin-4 young adult animals (C). In lin-4 sop-2 double-mutant young adults, seam cells are fused (D). Note that the adult-specific alae could not been observed in the double mutants. (E and F) Suppression of the retarded heterochronic defects in let-7 mutants by a sop-2 mutation. In sop-2; let-7 double mutants, the alae are formed at the early L4 stage (arrow). (F) Nomarski image of the gonad of the same animal in E. (G and H) Suppression of the retarded heterochronic defects in daf-12 mutants by a sop-2 mutation. The proliferative S2 division is reiterated in a daf-12 mutant (G), and this defect is suppressed by sop-2(bx91ts) (H). (I) Genetic interactions between sop-2 and lin-4, let-7, daf-12, lin-46, or lin-29 mutants. The frequency of animals displaying precocious fusion of seam cells (N, no fusion; F, full fusion; P, partial fusion) is shown below. Wild type: late L3 stage (n = 30), N, 100%; late L4 stage (n = 30), F, 100%. sop-2: late L3 stage (n = 21), P, 29%; F, 71%; late L4 stage (n = 30), F, 100%. lin-4: late L3 stage (n = 30), N, 100%; late L4 stage (n = 30), N, 100%. let-7: late L3 stage (n = 9), N, 100%. lin-4 sop-2: late L3 stage (n = 14), N, 100%; late L4 stage (n = 22), P, 68%; F, 32%. sop-2; let-7: late L3 stage (n = 51), P, 6%; F, 94%. daf-12: late L3 stage (n = 13), N, 100%. sop-2; daf-12: late L3 stage (n = 39), P, 72%; F, 28%. lin-46: late L4 stage (n = 22), P, 82%; F, 18%. sop-2; lin-46: late L4 stage (n = 22), P, 45%; F, 55%. The frequency of animals having precocious formation of alae (N, no alae; F, full-length alae; P, partial alae) is shown below. Wild type, early L4 stage (n = 30), N, 100%; young adult (n = 30), F, 100%. sop-2: early L4 stage (n = 20), N, 75%; P, 25%; young adult (n = 42), P, 26%; F, 74%. let-7: early L4 stage (n = 20), N, 100%; young adult (n = 44), N, 50%; P, 50%. daf-12: young adult (n = 30), N, 10%; P, 90%. sop-2; let-7: early L4 stage (n = 18), N, 22%; P, 61%; F, 17%; young adult (n = 25), N, 56%; P, 44%. sop-2; daf-12: young adult (n = 32), P, 41%; F, 59%. lin-29: young adult (n = 30), N, 100%. lin-29 sop-2: young adult (n = 63), N, 100%.
Mutations in sop-2 partially suppress the retarded heterochronic defects associated with lin-4, let-7, daf-12, and lin-46 mutants:
Next we examined the relationship between sop-2 and retarded heterochronic mutants, including lin-4, let-7, daf-12, and lin-46. Mutations in lin-4 lead to the reiteration of the L1 division pattern at subsequent larval stages. Therefore, seam cells remain unfused and alae are not generated in lin-4 null mutants (Ambros and Horvitz, 1984) (Figure 2C). We found that 100% of the lin-4 sop-2 double mutants (n = 22) displayed fusion of seam cells at the late L4 larval stage, compared to 0% in lin-4 single mutants (n = 30) (Figure 2, D and I). Mutations in lin-4 also suppressed the precocious fusion of seam cells in the sop-2 mutants. None of the lin-4 sop-2 double mutants (n = 14) showed fusion of seam cells at the L3 molt (Figure 2I). The mutual suppression of lin-4 and sop-2 suggests that sop-2 and lin-4 affect a common process, but do not function in a simple linear pathway.
Mutations in late-acting miRNA, let-7, cause a delay in the terminal differentiation of seam cells (Reinhart et al. 2000). We found that the retarded defects in strong loss-of-function let-7(n2853) mutants were suppressed by sop-2(bx91ts). In sop-2; let-7 double mutants, the fusion of seam cells and the formation of adult alae still occurred prematurely at the late L3 and early L4 stages, respectively (Figure 2, E, F, and I), suggesting that sop-2 is epistatic to let-7 in controlling the precocious fusion of seam cells and formation of alae. However, sop-2 and let-7 do not form a linear regulatory pathway with respect to the terminal differentiation of seam cells. The let-7 mutation weakly enhanced the precocious defects in sop-2 mutants at the L3 and early L4 larval stages. Moreover, the let-7 activity influences the formation of alae in sop-2 mutant adults. Fifty-six percent of sop-2; let-7 double mutants (n = 25) did not contain well-formed alae at the young-adult stage, whereas alae were present in all of the sop-2 single mutants (Figure 2I). Perhaps let-7 may have different targets that play opposite roles in controlling the differentiation of seam cells at different developmental stages.
Mutations in sop-2 also partially suppress the retarded heterochronic defects associated with a recessive gain-of-function daf-12(rh61) mutation (Antebi et al. 1998). In daf-12 mutant young adults, alae are not fully formed due to the retarded differentiation of seam cells. Furthermore, the proliferative S2 division is repeated at the L3 stage in daf-12 mutants (Antebi et al. 1998), resulting in an increased number of seam cells from 16 in wild-type animals (n = 30) to an average of 26 in daf-12 mutants (n = 12, ranging from 23 to 29) (Figure 2G) (in this study). In 100% of sop-2; daf-12 mutants, seam cells were precociously fused at the late L3 stage (Figure 2I). Furthermore, 59% of sop-2; daf-12 mutants (n = 32) had full-length alae in young adulthood, compared to 0% in daf-12 single mutants (n = 30) (Figure 2I). As in sop-2 mutants, sop-2; daf-12 double mutants had an average number of 16 (n = 62, ranging from 15 to 17) and 11 (n = 16, ranging from 10 to 12) seam cells when the mutants were shifted from 15° to 23° at the hatching and early embryonic stages, respectively (Figure 2H), indicating that sop-2 has a function in regulating the seam cell division pattern in daf-12 mutants.
The retarded heterochronic defects and the reiteration of the S2 division in lin-46 mutants are also partially suppressed by a sop-2 mutation (Pepper et al. 2004). Fifty-five percent of sop-2; lin-46 mutants (n = 22) displayed full fusion of seam cells at the late L4 stage, compared to 18% in lin-46 single mutants (n = 22). Furthermore, the average number of seam cells was 16 (n = 20, ranging from 14 to 22) in sop-2; lin-46 mutants, compared to 20 (n = 11, ranging from 16 to 23) in lin-46 single mutants. Although the daf-12, lin-46, and sop-2 mutations analyzed in these experiments are not null, the genetic interactions are consistent with the possibility that sop-2 functions in specifying the temporal specificities of seam cells.
sop-2 genetically interacts with other heterochronic genes to prevent the premature terminal differentiation of seam cells:
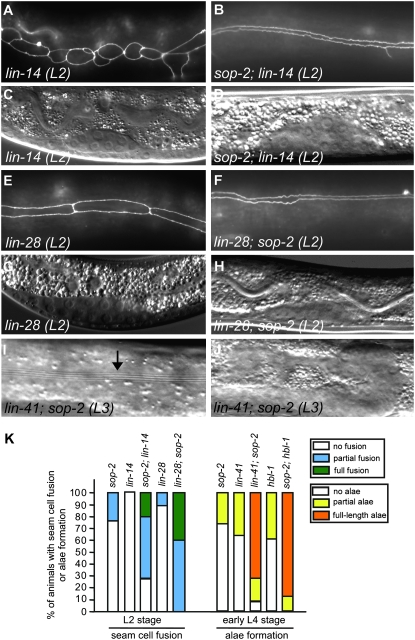
We further examined whether sop-2(bx91ts) could enhance the defects conferred by precocious mutations in the heterochronic gene pathway. Mutations in the early-acting heterochronic genes lin-14 and lin-28 cause the omission of the L1 and L2 larval stages, respectively, resulting in the adoption of adult-specific seam cell fates one stage earlier (Ambros and Horvitz 1984). Animals that are homozygous for the reduction-of-function lin-14(n179ts) mutation do not show fusion of seam cells at the L2 molt (Figure 3, A and C). By contrast, 71% of sop-2(bx91ts); lin-14(n179ts) double mutants (n = 17) exhibited fusion of seam cells in L2 larvae at 23° (Figure 3, B, D, and K). An increase in premature seam cell fusion was also observed when the sop-2(bx91ts) mutation was combined with the lin-28 (n719) null mutation (Figure 3, E–H and K). These genetic interactions suggest that sop-2 acts with or in parallel to lin-14 and lin-28 in preventing the fusion of seam cells at the L2 larval stage.
Figure 3.—
Mutations in sop-2 enhance the precocious heterochronic defects in other mutants. (A–H) The synergistic interaction between sop-2(bx91ts) and lin-14 or lin-28 in causing premature fusion of seam cells. In lin-14 or lin-28 mutants, seam cells remain unfused at the L2 larval stage (A and E). However, in sop-2; lin-14 or lin-28; sop-2 double mutants, seam cells are fused together at the L2 molt (B and F). (C, D, G, and H) Nomarski images of the gonads of the animals in A, B, E, and F, respectively. In these experiments, sop-2(bx91ts) animals are shifted from 15° to 23° at the hatching stage and thus do not display premature fusion of seam cells at the L2 larval stage. (I and J) The synergistic interaction between sop-2 and a lin-41 mutation in causing the precocious formation of adult alae. Full-length well-formed alae are present in 72% of lin-41; sop-2 double mutants at the early L4 larval stage (arrow). (J) The Nomarski image of the gonad of the animal in I. (K) Enhancement of the precocious heterochronic defects between sop-2 and lin-14, lin-28, lin-41, or hbl-1 mutants. The frequency of animals displaying precocious fusion of seam cells at the L2 larval stage is as follows. sop-2 (n = 50): N, 76%; P, 24%. lin-14 (n = 14): N, 100%. sop-2; lin-14 (n = 17): N, 29%; P, 53%; F, 18%. lin-28 (n = 33): N, 90%; P, 10%. lin-28; sop-2 (n = 18): P, 61%; F, 39%. The frequency of animals having precocious formation of alae at the early L4 larval stage is as follows. sop-2 (n = 20): N, 75%; P, 25%. lin-41 (n = 12): N, 67%; P, 33%. lin-41; sop-2 (n = 25): N, 8%; P, 20%; F, 72%. hbl-1 (n = 22): N, 64%; P, 36%. sop-2; hbl-1 (n = 26): P, 12%; F, 88%.
We next investigated whether sop-2(bx91ts) genetically interacts with lin-41 and hbl-1 in repressing adult-specific seam cell fates at the L3 larval stage. Nonnull reduction-of-function mutations in lin-41(ma104) and hbl-1(ve18), like sop-2(bx91ts), cause precocious terminal differentiation of seam cells at the L3 molt (Slack et al. 2000; Abrahante et al. 2003; Lin et al. 2003). We found that the formation of precocious alae was increased in lin-41(ma104); sop-2(bx91ts) mutants. Full-length, well-formed alae were present in 72% of lin-41(ma104); sop-2(bx91ts) double mutants (n = 25) at the early L4 stage, a phenotype that has not been observed in either single mutant (Figure 3, I, J, and K). Similarly, formation of full-length alae was increased from 0% in hbl-1 single mutants (n = 22) to 88% in sop-2(bx91ts); hbl-1(ve18) double mutants (n = 26) (Figure 3K). These genetic interactions suggest that sop-2 could function with and/or in parallel to lin-41 and hbl-1 in regulating the larval-to-adult switch.
Formation of ALG-1-containing P bodies is enhanced in the seam cells in sop-2 mutants:
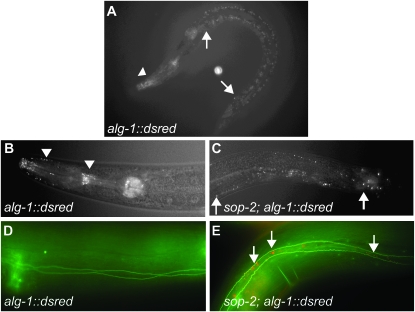
To investigate how sop-2 might specify the temporal fates of seam cells, we examined the expression of alg-1 in sop-2 mutants by using the functional alg-1∷dsRed reporter (Ding et al. 2005). alg-1 encodes a C. elegans Argonaute homolog, which is required for miRNA-mediated repression of heterochronic genes (Grishok et al. 2001). In tissue culture cells, Argonaute proteins localize to cytoplasmic processing bodies (P bodies) (Liu et al. 2005; Sen and Blau 2005). In C. elegans, the ALG-1-containing P bodies are mainly restricted in the head and tail regions (Ding et al. 2005 and Figure 4, A and B). However, in sop-2 mutants, the number of ALG-1-containing P bodies was increased and they were distributed throughout the body (Figure 4C).
Figure 4.—
Enhanced formation of ALG-1-containing P bodies in sop-2 mutants. (A and B) The expression of alg-1∷dsred in a wild-type animal. ALG-1-containing bodies are mainly restricted in the head and tail regions (arrowheads). (C and D) Irregular fluorescence particles between arrows (C) are gut autofluorescence. (C) The formation of ALG-1-containing P bodies is greatly enhanced in sop-2 mutants. In addition to the head and tail regions, P bodies are also formed in other body regions, such as in the region shown (between arrows). (D) No or very few ALG-1-containing P bodies are formed in the seam cells in wild-type animals. (E) The formation of ALG-1-containing P bodies in the seam cells is greatly enhanced in sop-2 mutants (arrows). The seam cells are labeled with ajm-1∷gfp (green), and ALG-1-containing bodies are shown in red.
To assess the number of ALG-1-containing P bodies in seam cells in sop-2 mutants, an ajm-1∷gfp reporter was used, which marks seam cell boundaries. In wild-type animals, we observed very few ALG-1-containing P bodies in seam cells (Figure 4D), whereas in sop-2 mutants, the formation of ALG-1-containing P bodies in seam cells was enhanced (Figure 4E).
let-7 miRNA-mediated post-transcriptional gene repression may be enhanced in sop-2 mutants:
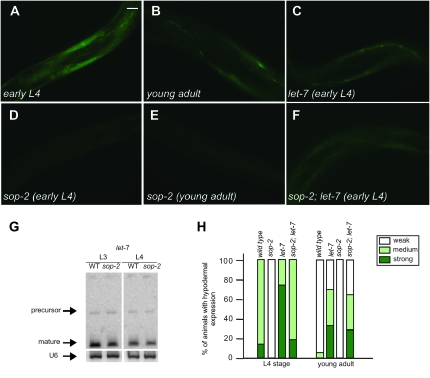
To investigate whether sop-2 mutants might show enhanced miRNA-mediated gene repression, we examined the expression of a hypodermally expressed reporter, col-10∷gfp∷lin-41(3′-UTR), which contains the lin-41 3′-UTR. Several let-7 binding sites are present in the lin-41 3′-UTR and they are sufficient to mediate the downregulation of lin-41 by let-7 (Vella et al. 2004). Similar to the temporal expression of the endogenous lin-41 gene, this reporter is strongly expressed at larval stages, while its expression is significantly decreased in young adults (Slack et al. 2000 and Figure 5, A, B, and H). In let-7 mutants, col-10∷gfp∷lin-41(3′-UTR) is still strongly expressed in adult animals. We found that sop-2 mutant animals bearing the same transgene displayed much weaker expression in L4 larvae (Figure 5D). The expression of the reporter was almost undetectable in sop-2 mutant young adults (Figure 5, E and H), indicating that a sop-2 mutation caused a markedly enhanced downregulation of the reporter. The expression level of let-7 was not significantly altered in sop-2 mutant animals (Figure 5G). In sop-2; let-7 mutants, the col-10∷gfp∷lin-41(3′-UTR) reporter was still strongly expressed (Figure 5, C and F). A control reporter gene bearing the unc-54 3′-UTR, which is not regulated by let-7, was strongly expressed at all larval and adult stages in both wild-type and sop-2 mutant animals (data not shown). These results are consistent with the possibility that let-7 miRNA-mediated repression is enhanced in sop-2 mutants.
Figure 5.—
Enhanced miRNA-mediated post-transcriptional gene repression in sop-2 mutants. (A and B) Expression of a col-10∷gfp∷lin-41 3′-UTR reporter in wild-type animals. The reporter is strongly expressed in hypodermal nuclei and seam cells in an L4 larva (A), while its expression is decreased at the young adult stage (B). (C) The reporter is still strongly expressed in let-7 mutant young adults. (D and E) Greatly reduced expression level of col-10∷gfp∷lin-41 3′-UTR reporter in sop-2 mutant animals at both the L4 (D) and young adult stages (E). (F) The reporter is strongly expressed in sop-2; let-7 young adults. (G) The expression of let-7 is not significantly altered in sop-2 mutants at the L3 and L4 larval stages. The loading control is U6. (H) Summary of the expression of the col-10∷gfp∷lin-41 3′-UTR reporter in wild-type and sop-2 mutant animals. The frequency of animals showing the expression level of the reporter (S, strong; M, medium; W, weak) is as follows. Wild type: early L4 stage (n = 38), S, 16%; M, 84%; young adult (n = 18), M, 5%; W, 95%. let-7: early L4 stage (n = 32), S, 75%; M, 25%; young adult (n = 28), S, 36%; M, 36%; W, 28%. sop-2: early L4 stage (n = 28), W, 100%; young adult (n = 27), W, 100%. sop-2; let-7: early L4 stage (n = 17), S, 18%; M, 82%; young adult (n = 20), S, 30%; M, 35%; W, 35%. Pictures were taken using the same exposure time.
Loss-of-function of alg-1 suppresses the precocious heterochronic defects in sop-2 mutants:
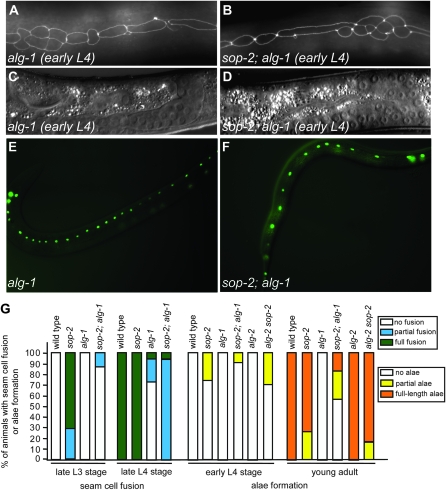
Next we examined the genetic interaction between sop-2 and alg-1. alg-1(RNAi) causes a retarded heterochronic defect. The seam cells fail to undergo terminal differentiation at the young adult stage in alg-1(RNAi) mutants (Figure 6, A and C) (Grishok et al. 2001). We found that the seam cells still underwent division at the late L4 stage in sop-2; alg-1(RNAi) mutants. In addition, the frequency of animals with precocious fusion of seam cells at the late L3 was reduced from 71% (full fusion, n = 21) in sop-2 mutants to 0% (full fusion, n = 25) in sop-2; alg-1(RNAi) mutants, respectively (Figure 6, A, B, and G). Moreover, in sop-2; alg-1(RNAi) mutants, only 4% (n = 26) of the animals displayed full fusion of seam cells at the late L4 stage and 15% (n = 33) of the animals had full-length well-formed alae at the young adult stage, compared to 100% (n = 30) and 74% (n = 42) of sop-2 single mutants, respectively (Figure 6G). The incomplete suppression of sop-2 by alg-1 could be due to the activity of alg-2, which functions redundantly to alg-1 in miRNA-mediated gene repression (Grishok et al. 2001). We found that depletion of alg-2 did not cause heterochronic defects (Figure 6G). Furthermore, the precocious defects in sop-2 mutants were not suppressed by alg-2(RNAi) (Figure 6G). Disruption of both alg-1 and alg-2 caused animals to arrest at embryonic stages, preventing us from analyzing their role during later larval development. sop-2 might specify the temporal fates of seam cells by regulating the expression of alg-1. It is possible that alg-1 affects the expression of heterochronic genes that interact genetically with sop-2.
Figure 6.—
Suppression of the precocious heterochronic defects in sop-2 mutants by alg-1(RNAi). (A–D) The premature fusion of seam cells in sop-2 mutants is suppressed by alg-1(RNAi). As in alg-1(RNAi) animals (A), the seam cells remain unfused at the early L4 stage in sop-2; alg-1(RNAi) mutants (B). (C and D) Nomarski images of the gonads of the animals in A and B, respectively. (E and F) The reiteration of the proliferative S2 division in alg-1(RNAi) animals is suppressed by sop-2(bx91ts). (G) Fusion of seam cells and formation of alae in sop-2 mutants is suppressed by an alg-1 mutation. The frequency of animals displaying precocious fusion of seam cells is as follows (F, full fusion; P, partial fusion; N, no fusion). sop-2: late L3 stage (n = 21), P, 29%; F, 71%; late L4 stage (n = 30), F, 100%. alg-1: late L3 stage (n = 16), N, 100%; late L4 stage (n = 20), N, 75%; P, 20%; F, 5%. sop-2; alg-1: late L3 stage (n = 25), N, 88%; P, 12%; late L4 stage (n = 26), P, 96%; F, 4%. The frequency of animals having precocious formation of alae is as follows. sop-2: early L4 stage (n = 20), N, 75%; P, 25%; young adult (n = 42), P, 26%; F, 74%. alg-1: no alae are formed at both early and young adult stages (n = 29). alg-2: early L4 stage (n = 25), N, 100%; young adult (n = 28), F, 100%. sop-2; alg-1: early L4 stage (n = 24), N, 92%; P, 8%; young adult (n = 33), N, 58%; P, 27%; F, 15%. alg-2 sop-2: early L4 stage (n = 14), N, 72%; P, 28%; young adult (n = 21), F, 86%; P, 14%.
Disruption of alg-1 causes the reiteration of the proliferative S2 seam cell division (Grishok et al. 2001), resulting in an increased number of seam cells from 16 in wild-type animals (n = 30) to an average number of 25 in alg-1(RNAi) animals (n = 15, ranging from 20 to 27) (Figure 6E). In sop-2; alg-1(RNAi) double mutants, however, the average number of seam cells was 14 (n = 23, ranging from 10 to 19) (Figure 6F), indicating that as in daf-12 and lin-46 mutants, the sop-2 activity is required for the reiteration of the proliferative S2 division in alg-1 mutants.
The seam cells in sop-2 hermaphrodites generate male-specific rays:
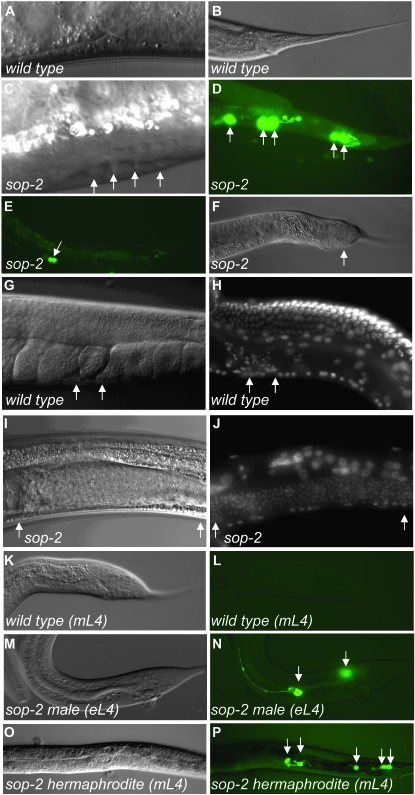
In addition to homeotic transformations and precocious heterochronic defects, mutations in sop-2 result in partial hermaphrodite-to-male sexual transformations. In wild-type animals, rays are generated from the posterior seam cells V5, V6, and T only in males. However, we found that rays as well as fan-like cuticular structures were also formed in the midbody and tail regions in sop-2 mutant hermaphrodites (Figure 7, A and C). We used a pkd-2∷gfp reporter, which specifically labels male ray neurons, to examine ray formation and found that pkd-2∷gfp was expressed in 75% (n = 51) of sop-2 mutant hermaphrodites (Figure 7D) (10% in the tail, 16% in the midbody cells that were likely derived from V3 and V4, and 49% in both tail and midbody cells). This result suggests that both anterior and posterior seam cells in sop-2 mutant hermaphrodites are masculinized.
Figure 7.—
Hermaphrodite-to-male sexual transformations in sop-2 mutants. (A) The cuticle structure of a wild-type adult hermaphrodite. (B) A wild-type hermaphrodite has a tapered tail spike. (C) Generation of rays in sop-2 hermaphrodites. In the animal shown, rays as well as fan-like cuticular structure are formed in the middle body region (arrows). The sop-2 mutant animals are shifted at the hatching stage. (D) Expression of a ray neuron-specific marker, pkd-2∷gfp, in sop-2 hermaphrodites. In the animal shown, five pkd-2∷gfp-positive neurons are formed (arrows). pkd-2∷gfp marks both neuron bodies and axons. pkd-2∷gfp is not expressed in wild-type hermaphrodites. (E) Generation of male-specific serotonergic CP neurons in the sop-2 hermaphrodite ventral cord (arrow). No serotonergic CP neurons are formed in wild-type hermaphrodites. (F) A sop-2 hermaphrodite has a blunt-shaped tail instead of a tail spike (arrow). (G and H) Nomarski micrograph showing the spermatheca in a wild-type adult hermaphrodite (between arrows). DAPI staining shows that the spermatheca contains characteristic small, compact nuclei (between arrows) (H). (I and J) Mutations in sop-2 cause defects in the switch from spermatogenesis to oogenesis. The expanded spermatheca in a sop-2 mutant hermaphrodite is indicated between two arrows (I). DAPI staining shows a dramatically increased number of sperms (between arrows) in a sop-2 hermaphrodite (J). (K and L) In wild-type males, the expression of pkd-2∷gfp is absent in a middle L4 stage male. The stage of animals is determined by several criteria, including the shape of the tail, the gonad, and the presence of mature sperm. (M and N) Expression of pkd-2∷gfp is observed in early L4 sop-2 males. In the animal shown, pkd-2∷gfp is expressed in both the midbody and tail regions (arrows). (O and P) Expression of pkd-2∷gfp in both the midbody and tail regions (arrows) in a middle L4-stage sop-2 hermaphrodite. (K, M, and O) Nomarski images of the same animal shown in L, N, and P, respectively.
The sexual transformation in sop-2 mutants is not limited to seam cells. Male-specific serotonergic CP neurons, as shown by a reporter gene for the serotonin biosynthetic enzyme tryptophan hydroxylase tph-1∷cfp, were also formed in the ventral cord in 68% of sop-2 hermaphrodites (n = 50) (Figure 7E). Moreover, 26% of sop-2 hermaphrodites (n = 34) had a male-specific blunt-shaped tail (Figure 7, B and F). In addition to somatic tissues, germ-line sex determination is also defective in sop-2 mutant hermaphrodites. In early L4 wild-type hermaphrodites, the germ line produces ∼150 spermatids in each gonad arm before switching to the production of oocytes. In sop-2 mutants, 69% (n = 42) of gonad arms contained extra sperm and no oocyte was generated (Figure 7, G–J), indicating that the spermatogenesis-to-oogenesis switch was defective in sop-2 hermaphrodites. By contrast, the somatic gonad apparently develops normally in sop-2 mutants. In conclusion, the sop-2(bx91ts) can affect sexual fate specification in both the soma and the germ line, but some somatic cells are apparently unaffected by the mutation used in these studies.
Generation of rays in sop-2 hermaphrodites requires the activities of the general sex-determination pathway and Hox genes:
Generation of rays in sop-2 mutant hermaphrodites could be an indirect effect of heterochronic defects, as observed in lin-28 mutants, or it could result from the misregulation of sex-determination genes (Ambros and Horvitz 1984; V. Ambros, personal communications). To distinguish between these two possibilities, we examined whether xol-1, her-1, and fem-3, which promote male development, are required for the formation of rays in sop-2 mutant hermaphrodites. We found that no pkd-2∷gfp-expressing seam cells were formed after conducting fem-3(RNAi) (n = 37) or her-1(RNAi) (n = 56) in a sop-2(bx91ts) mutant background. However, pkd-2∷gfp was expressed in 80% of sop-2; xol-1(RNAi) animals (n = 30). These data indicate that the formation of ectopic rays in sop-2 mutant hermaphrodites requires the activity of the sex determination genes her-1 and fem-3.
Generation of ectopic rays in sop-2 mutant males requires the activity of Hox genes mab-5 and egl-5 (Zhang et al. 2003). The activity of mab-5 and egl-5 is also required for the formation of rays in sop-2 mutant hermaphrodites. In sop-2; mab-5 egl-5 mutant hermaphrodites (n = 47), no rays were generated. Thus, the formation of rays in sop-2 hermaphrodites requires the concerted action of a set of genes, including Hox genes and sex-determination genes.
We next examined whether formation of rays occurred precociously in sop-2 mutants. In wild-type males, pkd-2∷gfp is not expressed until the late L4 stage (Figure 7, K and L). However, 41% (n = 22) of males and 36% (n = 105) of hermaphrodites showed the expression of pkd-2∷gfp at the early L4 stage in sop-2 mutants (Figure 7, M–P). Hence, the homeotic transformations (e.g., generation of rays by anterior seam cells), precocious heterochronic defects, and the sexual transformations occurred concomitantly in sop-2 mutants at the early L4 larval stage.
We further determined whether mutations in the sex-determination genes or Hox genes affect the temporal fates of seam cells in sop-2 mutants. We found that in sop-2; fem-3(RNAi) mutants precocious fusion of seam cells still occurred in 100% of animals (57% full fusion and 43% partial fusion, n = 35). Precocious fusion of seam cells was also not affected in sop-2; mab-5 egl-5 mutants (65% full fusion and 35% partial fusion, n = 23). Similarly, mutations in lin-29 did not significantly affect the homeotic and sexual transformations in sop-2 mutants. The pkd-2∷gfp reporter was expressed in 88% of hermaphrodites (n = 34) and 93% of males (n = 34) in lin-29 sop-2 mutant animals.
DISCUSSION
Role of sop-2 in specification of the temporal identities of seam cells:
Our study showed that the terminal differentiation of seam cells, including cessation of cell divisions, fusion of cells, and formation of adult alae, prematurely occurs in the L3 molt in sop-2 mutants. The retarded heterochronic defects in let-7 mutants are suppressed by mutations either in lin-41 (Slack et al. 2000) or in sop-2. Downregulating the expression of lin-41 in sop-2 mutants requires the let-7 activity, suggesting that wild-type sop-2 is likely to have additional targets that act in parallel to let-7/lin-41 in specifying the larval-to-adult switch. This is consistent with the observation that the precocious defects in sop-2 mutants are greatly suppressed by alg-1(RNAi), but not by a let-7 mutation, which suggests that other miRNAs may function in parallel to let-7 in specifying the larval-to-adult switch. The expression of lin-41 and hbl-1 is temporally regulated and they are direct targets of let-7 (Slack et al. 2000; Abrahante et al. 2003; Lin et al. 2003). However, sop-2 is strongly expressed in hypodermal syncytium hyp 7 and seam cells at all developmental stages and the expression level of sop-2 is not affected in lin-4 and let-7 mutants. Furthermore, putative let-7 binding sites are not found in the 3′-UTR of sop-2, indicating that sop-2 is unlikely to be a target of let-7.
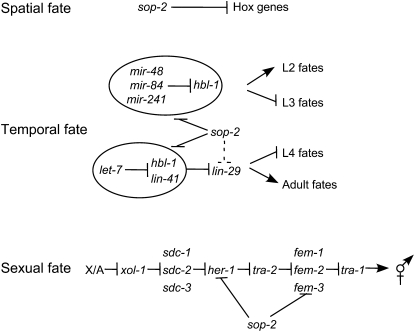
How does sop-2 function at several stages in specifying temporal fates of seam cells? One of the mechanisms could be that mutations in sop-2 enhance the miRNA-mediated gene repression (Figure 8). The expression of many heterochronic genes acting at different larval stages is regulated by microRNAs. For example, the expression of hbl-1 at the L2 larval stage is regulated by mir-48, mir-84, and mir-241 (Abbott et al. 2005; Li et al. 2005). Thus, mutations in mir-48; mir-84; mir-241 cause reiteration of the proliferative S2 division and this defect can be suppressed by an hbl-1 mutation (Abbott et al. 2005; Li et al. 2005). Mutations in sop-2 suppress the proliferative S2 division in daf-12, lin-46, and alg-1, which may be through downregulation of hbl-1. sop-2 could also directly regulate the expression of other heterochronic genes, such as lin-29, in specifying the temporal fates of seam cells. Recently, sop-2 inactivation was identified as an enhancer of let-7(mg279) in a genomewide RNAi screen for miRNA pathway genes (Parry et al. 2007). While the bases for these discrepant conclusions are unclear, it is possible that sop-2(bx91ts) and sop-2(RNAi) might have different impacts on miRNA regulation. Alternatively, reduction of sop-2 function might affect miRNA regulation differently in different cell types.
Figure 8.—
A model for the role of sop-2 in specifying the spatial, temporal, and sexual specificities of seam cells. Mutations in sop-2 enhance the miRNA-mediated translational repression. Additional miRNAs may function in parallel to let-7 in regulating the expression of lin-29 (for details see the discussion). sop-2 acts downstream of xol-1 but upstream of her-1 and fem-3 in the sex-determination pathway in specifying the sexual fates of seam cells. sop-2 might repress the expression of her-1 and/or fem-3.
Role of sop-2 in regulating the sexual specificities of cell fates:
sop-2 mutants display partial hermaphrodite-to-male sexual transformations, including generation of rays by seam cells, production of male-specific serotonergic CP neurons by descendants of P cells in the ventral cord, formation of male-like tail morphology, and defects in the spermatogenesis-to-oogenesis switch. Our RNAi experiments in a sop-2 mutant background suggest that sop-2 acts downstream of xol-1 but upstream of her-1 and fem-3 in the sex-determination pathway. Prior studies of germ-line sex determination showed that the spermatogenesis-to-oogenesis switch requires downregulation of fem-3 at the translational level (Ahringer and Kimble 1991). Possibly, sop-2 might influence the expression of fem-3 through effects on miRNA regulation. It is not clear, however, whether sop-2 functions in the germ line or the somatic gonad to influence germ-line sex determination.
Other genes involved in coordinately regulating the spatial, temporal, and sexual fates of cells:
sop-2 mutants share many heterochronic defects in common with hbl-1 mutants. Interestingly, we found that mutations in hbl-1 also cause spatial and sexual transformations of seam cells. In hbl-1 mutants, ectopic rays are generated by anterior seam cells in males and rays are also formed in hermaphrodites (Q. C. Cai and H. Zhang, unpublished data). Whether sop-2 functions with hbl-1 in regulating the spatial, temporal, and sexual fates of seam cells remains to be investigated. In Drosophila, Hunchback (Hb) (HBL-1 is the C. elegans Hb homolog) controls the spatial identities of cells at early embryonic stages by directly repressing the Hox gene Ubx (Zhang and Bienz 1992). The Hb-interacting protein dMi-2 has been shown to function with PcG proteins in repression of Hox genes, providing a link between Hb and PcG genes (Kehle et al. 1998). Hb also specifies the temporal fates adopted by neuroblasts in the central nervous system (Isshiki et al. 2001). However, it is yet to be determined whether Hb is involved in determining the spatial identities of neurons in the central nervous system or the temporal fates adopted by the cells whose positional fates are altered in Hb mutants. Such detailed analysis and identification of mutants in other organisms that concomitantly affect the spatial, temporal, and sexual specificities of cell fates, as in sop-2(bx91ts) mutants, rely on the availability of specific markers and the techniques for following the cell lineage at the single-cell level. Our findings reveal that the development of multicellular organisms along various developmental axes is tightly controlled by factors acting higher in the hierarchy.
Acknowledgments
We thank Xiaochen Wang, Scott Emmons, Andrea Christoforou, and two anonymous reviewers for their helpful comments on the manuscript. Some strains used in this work were received from the Caenorhabditis Genetics Center, which is supported by a grant from the National Institutes of Health. This work was supported by the National High Technology Projects 863 (2005 AA210910).
References
- Abbott, A. L., E. Alvarez-Saavedra, E. A. Miska, N. C. Lau, D. P. Bartel et al., 2005. The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev. Cell 9 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahante, J. E., A. L. Daul, M. Li, M. L. Volk, J. M. Tennessen et al., 2003. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Dev. Cell 4 625–637. [DOI] [PubMed] [Google Scholar]
- Ahringer, J., and J. Kimble, 1991. Control of the sperm-oocyte switch in Caenorhabditis elegans hermaphrodites by the fem-3 3′ untranslated region. Nature 349 346–348. [DOI] [PubMed] [Google Scholar]
- Ambros, V., 2000. Control of developmental timing in Caenorhabditis elegans. Curr. Opin. Genet. Dev. 10 428–433. [DOI] [PubMed] [Google Scholar]
- Ambros, V., and H. R. Horvitz, 1984. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226 409–416. [DOI] [PubMed] [Google Scholar]
- Antebi, A., J. G. Culotti and E. M. Hedgecock, 1998. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development 125 1191–1205. [DOI] [PubMed] [Google Scholar]
- Bagga, S., J. Bracht, S. Hunter, K. Massirer, J. Holtz et al., 2005. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 122 553–563. [DOI] [PubMed] [Google Scholar]
- Banerjee, D., and F. Slack, 2002. Control of developmental timing by small temporal RNAs: a paradigm for RNA-mediated regulation of gene expression. BioEssays 24 119–129. [DOI] [PubMed] [Google Scholar]
- Bettinger, J. C., K. Lee and A. E. Rougvie, 1996. Stage-specific accumulation of the terminal differentiation factor LIN-29 during Caenorhabditis elegans development. Development 122 2517–2527. [DOI] [PubMed] [Google Scholar]
- Cline, T. W., and B. J. Meyer, 1996. Vive la difference: males vs females in flies vs worms. Annu. Rev. Genet. 30 637–702. [DOI] [PubMed] [Google Scholar]
- Ding, L., A. Spencer, K. Morita and M. Han, 2005. The developmental timing regulator AIN-1 interacts with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Mol. Cell 19 437–447. [DOI] [PubMed] [Google Scholar]
- Gellon, G., and W. McGinnis, 1998. Shaping animal body plans in development and evolution by modulation of Hox expression patterns. BioEssays 20 116–125. [DOI] [PubMed] [Google Scholar]
- Grishok, A., A. E. Pasquinelli, D. Conte, N. Li, S. Parrish et al., 2001. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106 23–34. [DOI] [PubMed] [Google Scholar]
- Isshiki, T., B. Pearson, S. Holbrook and C. Q. Doe, 2001. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell 106 511–521. [DOI] [PubMed] [Google Scholar]
- Kehle, J., D. Beuchle, S. Treuheit, B. Christen, J. A. Kennison et al., 1998. dMi-2, a hunchback-interacting protein that functions in polycomb repression. Science 282 1897–1900. [DOI] [PubMed] [Google Scholar]
- Kenyon, C. J., J. Austin, M. Costa, D. W. Cowing, J. M. Harris et al., 1997. The dance of the Hox genes: patterning the anteroposterior body axis of Caenorhabditis elegans. Cold Spring Harbor Symp. Quant. Biol. 62 293–305. [PubMed] [Google Scholar]
- Lee, R. C., R. L. Feinbaum and V. Ambros, 1993. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75 843–854. [DOI] [PubMed] [Google Scholar]
- Li, M., M. W. Jones-Rhoades, N. C. Lau, D. P. Bartel and A. E. Rougvie, 2005. Regulatory mutations of mir-48, a C. elegans let-7 family MicroRNA, cause developmental timing defects. Dev. Cell 9 415–422. [DOI] [PubMed] [Google Scholar]
- Lin, S. Y., S. M. Johnson, M. Abraham, M. C. Vella, A. Pasquinelli et al., 2003. The C. elegans hunchback homolog, hbl-1, controls temporal patterning and is a probable microRNA target. Dev. Cell 4 639–650. [DOI] [PubMed] [Google Scholar]
- Liu, J., M. A. Valencia-Sanchez, G. J. Hannon and R. Parker, 2005. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 7 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C. C., J. M. Kramer, D. Stinchcomb and V. Ambros, 1991. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, E. G., R. C. Lee and V. Ambros, 1997. The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell 88 637–646. [DOI] [PubMed] [Google Scholar]
- Parry, D. H., J. Xu and G. Ruvkun, 2007. A whole-genome RNAi screen for C. elegans miRNA pathway genes. Curr. Biol. 17 2013–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper, A. S., J. E. McCane, K. Kemper, D. A. Yeung, R. C. Lee et al., 2004. The C. elegans heterochronic gene lin-46 affects developmental timing at two larval stages and encodes a relative of the scaffolding protein gephyrin. Development 131 2049–2059. [DOI] [PubMed] [Google Scholar]
- Reinhart, B. J., F. J. Slack, M. Basson, A. E. Pasquinelli, J. C. Bettinger et al., 2000. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403 901–906. [DOI] [PubMed] [Google Scholar]
- Rougvie, A. E., 2005. Intrinsic and extrinsic regulators of developmental timing: from miRNAs to nutritional cues. Development 132 3787–3798. [DOI] [PubMed] [Google Scholar]
- Rougvie, A. E., and V. Ambros, 1995. The heterochronic gene lin-29 encodes a zinc finger protein that controls a terminal differentiation event in Caenorhabditis elegans. Development 121 2491–2500. [DOI] [PubMed] [Google Scholar]
- Sen, G. L., and H. M. Blau, 2005. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 7 633–636. [DOI] [PubMed] [Google Scholar]
- Slack, F. J., M. Basson, Z. Liu, V. Ambros, H. R. Horvitz et al., 2000. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol. Cell 5 659–669. [DOI] [PubMed] [Google Scholar]
- Stothard, P., and D. Pilgrim, 2003. Sex-determination gene and pathway evolution in nematodes. BioEssays 25 221–231. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., and H. R. Horvitz, 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56 110–156. [DOI] [PubMed] [Google Scholar]
- Vella, M. C., E. Y. Choi, S. Y. Lin, K. Reinert and F. J. Slack, 2004. The C. elegans microRNA let-7 binds to imperfect let-7 complementary sites from the lin-41 3′UTR. Genes Dev. 18 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman, B., I. Ha and G. Ruvkun, 1993. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75 855–862. [DOI] [PubMed] [Google Scholar]
- Zhang, C. C., and M. Bienz, 1992. Segmental determination in Drosophila conferred by hunchback (hb), a repressor of the homeotic gene Ultrabithorax (Ubx). Proc. Natl. Acad. Sci. USA 89 7511–7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., R. B. Azevedo, R. Lints, C. Doyle, Y. Teng et al., 2003. Global regulation of Hox gene expression in C. elegans by a SAM domain protein. Dev. Cell 4 903–915. [DOI] [PubMed] [Google Scholar]
- Zhang, H., A. Christoforou, L. Aravind, S. W. Emmons, S. van den Heuvel et al., 2004. The C. elegans Polycomb gene sop-2 encodes a RNA binding protein. Mol. Cell 14 841–847. [DOI] [PubMed] [Google Scholar]