Abstract
Internally fertilizing organisms transfer a complex assortment of seminal fluid proteins, a substantial fraction of which are proteolysis regulators. In mammals, some seminal protease inhibitors have been implicated in male infertility and these same molecular classes of protease inhibitors are also found in Drosophila seminal fluid. Here, we tested the reproductive functions of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F by generating a precise deletion of the Acp62F gene. We did not detect a nonredundant function for Acp62F in modulating the egg laying, fertility, remating frequency, or life span of mated females. However, loss of Acp62F did alter a male's defensive sperm competitive ability, consistent with the localization of Acp62F to sperm storage organs. In addition, the processing of at least one seminal protein, the ovulation hormone ovulin, is slower in the absence of Acp62F.
DURING mating, the female reproductive tract becomes an active proteolytic environment with a mixture of male and female proteases and protease inhibitors. Seminal fluid protease inhibitors have been identified in a wide range of organisms from insects to mammals (e.g., Laurell et al. 1992; Ohlsson et al. 1995; Murer et al. 2001; Swanson et al. 2001; Kotlowska et al. 2005), suggesting that protease inhibitors play significant and possibly conserved roles in reproduction. Interestingly, comparative structural modeling has revealed that the mammalian and Drosophila seminal fluid protease inhibitors are similar in predicted protein structures and hence likely to share biochemical functions (Mueller et al. 2004).
In Drosophila melanogaster, proteolysis regulators represent over a quarter of the identified seminal fluid proteins, many of which are male accessory gland proteins (Acps) (reviewed in Ravi Ram and Wolfner 2007b). Seminal fluid proteolysis regulators have been suggested to indirectly mediate postmating functions in the female by regulating the processing of prohormones transferred by the male (Park and Wolfner 1995; Ravi Ram et al. 2006) or the release of peptides from sperm (Peng et al. 2005). Indeed, the D. melanogaster Acp protease, CG11864, has been shown to regulate the processing of the Acp ovulation hormone ovulin (Acp26Aa) and the sperm storage protein Acp36DE (Ravi Ram et al. 2006). CG11864's regulation of ovulin processing, and possibly its own processing, may be modulated by seminal fluid protease inhibitors.
Initial insights into the reproductive roles of D. melanogaster protease inhibitors stem from their localization patterns within the mated female. The five Acp protease inhibitors characterized to date localize predominantly to three regions within the female (Lung et al. 2002; Ravi Ram et al. 2005). Four of these Acp protease inhibitors target to the sperm storage organs, suggesting that they may protect sperm from proteolysis or in some other way “manage” sperm in storage. These D. melanogaster protease inhibitors could function in ways analogous to the mammalian seminal protease inhibitors that regulate sperm motility and sperm capacitation (De Lamirande et al. 2001; Parks et al. 2004). Additionally, all five Acp protease inhibitors are present in the mated female's uterus, where they could potentially regulate the proteolytic processing of seminal fluid proteins such as ovulin (Park and Wolfner 1995). All five also enter the female's circulatory system (Lung et al. 2002; Ravi Ram et al. 2005; K. Ravi Ram, J. L. Mueller and M. F. Wolfner, unpublished data) and thus have the opportunity to regulate extracellular proteolytic cascades within the mated female (reviewed in Theopold et al. 2002; Leclerc and Reichhart 2004). D. melanogaster Acp protease inhibitor proteins may interact with the many proteases identified in the female reproductive tract (Swanson et al. 2004) or with proteases whose transcript levels are modulated by mating (Lawniczak and Begun 2004, 2007; McGraw et al. 2004; Mack et al. 2006) and/or present in the seminal fluid (Mueller et al. 2005).
The biochemical function of only a single D. melanogaster seminal fluid protease inhibitor, Acp62F, has been determined to date. The predicted active site of Acp62F suggests that it is a trypsin inhibitor and in vitro Acp62F is able to inhibit the protease activity of trypsin serine proteases (Lung et al. 2002). In a mated female, Acp62F localizes to the sperm storage organs (spermathecae and seminal receptacle) (Lung et al. 2002), which strongly suggests a sperm-related function. Previous genetic analyses of Acp62F have not yet clearly revealed its postmating functions in the female. Knockdown of Acp62F expression via RNA interference (RNAi) did not reveal any effects of Acp62F on mated female egg laying, fertility, or receptivity (Lung 2000) or indicate any involvement of Acp62F in determining female mating costs (Bangham 2003). However, the residual 2–3% of Acp62F protein in the knockdown flies may be sufficient for Acp62F function (Lung 2000; Bangham 2003), which could therefore have obscured any such effects of Acp62F. Ectopic expression studies of Acp62F have revealed that it is toxic to D. melanogaster upon single or multiple exposures in preadults and adults, respectively (Lung et al. 2002). The potential toxicity of Acp62F suggests that it interferes with essential proteolytic cascades and may thus also contribute to the cost of mating in mated females. Consistent with this hypothesis, QTL analysis in D. sechellia has identified a genomic region containing Acp62F that contributes to the cost of mating (Civetta et al. 2005).
To conclusively dissect the in vivo reproductive role of D. melanogaster Acp62F, a null mutation is necessary. A two-step gene-targeting approach (Xie and Golic 2004) was used here to generate a precise deletion of the Acp62F coding region, which represents the definitive null allele needed for phenotypic characterization. Flies lacking Acp62F were tested for roles in receptivity to mating, egg laying, fertility, proteolytic processing, sperm competition, and the cost of mating. We find that females mated to males lacking Acp62F process ovulin more slowly and resist sperm displacement better than males that transfer Acp62F.
MATERIALS AND METHODS
Creating an Acp62F gene-targeting construct:
An 800-bp deletion of Acp62F, 145 bp upstream of the start codon to 316 bp downstream of its stop codon, was generated in vitro beginning with PCR products amplified from genomic DNA isolated from w1118 flies. Acp62F primers 1–4 (supplemental Table 1 at http://www.genetics.org/supplemental/) were used to amplify 4.4-kb (5′ flanking region) and 4-kb (3′ flanking region) PCR products under conditions specified by Expand High Fidelity PCR (Roche). The products were ligated together and inserted into the pCR2.1 TOPO vector (Invitrogen, San Diego) in an orientation that matched the native D. melanogaster genome order. An I-SceI recognition sequence was synthesized as two oligonucleotides with NcoI sites at each end. These were annealed and inserted into an NcoI site 1.8 kb into the 3′ flanking region. NotI sites at the ends of the Acp62F deletion allele (primers Acp62F-1 and Acp62F-4) were used to clone the donor element into the P-element targeting vector pTV2 (Rong and Golic 2000), which generated the construct pAcp62Fdel.
The Acp62Fdel construct was inserted into the genome of w1118 flies by P-element-mediated transformation (Rubin and Spradling 1982) and subsequently made homozygous. Two second chromosome P-element transpositions were used as the source for transpositions to generate targeting events.
Acp62F gene-targeting cross scheme:
Gene targeting was performed as in Rong and Golic (2001) (Figure 1). Crosses were carried out in standard fly vials containing yeast–glucose medium with four to five virgin females and males per vial. Flies with a second chromosome Acp62Fdel insert were crossed to hsp70-FLP and hsp70-SceI transgenic lines (Rong and Golic 2000) and heat-shocked as in Rong and Golic (2000) to generate targeting events (Figure 1, A and C). Nineteen putative gene-targeted lines were identified with a stable Acp62Fdel construct and a single FRT site (nonmosaic eyes when crossed to eyeless-FLP flies; Figure 1, A and C). Using the whs as a marker, 14 of the 19 targeting events were mapped to the third chromosome, the location of Acp62F. These 14 targeting events likely resulted in a tandem duplication of Acp62F with the Acp62Fdel targeting allele adjacent to the endogenous Acp62F allele (Figure 1A). Southern blot analysis was used to confirm the expected targeted tandem duplication for 5 of the 14 lines (data not shown), 1 of which was used for the reduction step. A reduction step, as in Xie and Golic (2004), was then executed to leave a single copy of the Acp62F gene (Figure 1B). One of the 14 third chromosome targeting events was used to screen for recombination events within the flanking regions of wild-type Acp62F and the adjacent Acp62Fdel. Males with the targeted allele were crossed to females carrying a hsp70-CreI transgene (Xie and Golic 2004) and heat-shocked to induce Cre-mediated recombination between flanking regions of the Acp62F tandem copies (Figure 1C). Four reduction events leaving only the deleted copy of Acp62F were identified (Acp62F1a, Acp62F1b, Acp62F1c, and Acp62F1d), which are expected to be equivalent in their removal of Acp62F. Descriptions of all fly strains used for Acp62F targeting can be found at http://flybase.bio.indiana.edu. Heat shocks were performed in a water bath as previously described by Golic and Lindquist (1989).
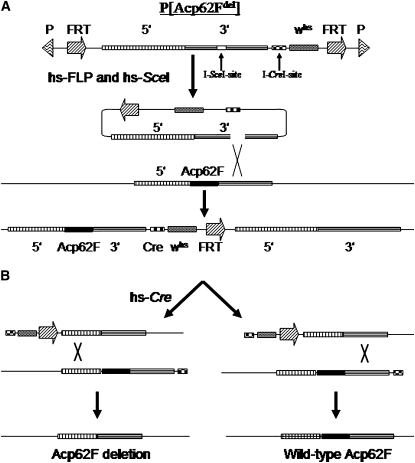
Figure 1.—
(A) Targeting an Acp62F null allele to the Acp62F locus. FLP and I-SceI induction mediates excision of the donor construct and introduces a double-strand break. Homologous recombination within the 3′ flanking region results in a tandem duplication of endogenous Acp62F adjacent to the targeted null Acp62F allele. The targeting strategy presented here is based upon the gene-targeting technique of Rong and Golic (2000). (B) Reduction of Acp62F tandem duplicates to a single copy. (Left) Recombination events between the 5′ flanking regions of the duplicates leaves only the Acp62F null allele. (Right) Recombination between the 3′ flanking regions leaves only the wild-type Acp62F. The two reduction events can be differentiated with primers Acp62Fscreen-1 and Acp62Fscreen-2 (arrows in 5′ and 3′ flanking regions and supplemental Table 1). The reduction strategy presented here is based upon the previous technique of Xie and Golic (2004). (C) Crossing scheme used to generate a deletion of the Acp62F coding region. Targeted donor constructs that map the whs marker to the third chromosome were used for targeting events. Progeny of males carrying the donor construct P[Acp62Fdel] crossed to females carrying hsp70-FLP and hsp70-SceI transgenes were heat-shocked to excise the donor construct. Stably integrated targeting events at the Acp62F locus were identified by the whs marker segregating with the third chromosome and nonmosaics when crossed to eyeless-FLP. Females carrying the duplicated Acp62F alleles were crossed to hsp70-Cre transgenic males to induce Cre-mediated double-strand-break recombinase and thus reduction events. Lines that lost the whs marker were candidate Acp62F null alleles.
Diagnostic PCRs and Western blotting:
The reduction events could leave either a wild-type Acp62F allele or a mutant allele. To screen for reduction events with only the mutant Acp62F allele, genomic DNA isolated from heterozygous mutant flies for 109 candidate reduction event lines were PCR screened using standard PCR conditions (Promega, Madison, WI). Acp62F-screen1 and Acp62F-screen2 primers were used to identify reduction events, leaving only an Acp62Fdel allele (PCR product of 825 bp) or wild-type Acp62F allele (PCR product of 1635 bp).
For Western blots, extracts were prepared from animals homozygous for a given Acp62F knockout allele (Acp62F1a,b,c,d/Acp62F1a,b,c,d) or heterozygous for a knockout allele and the balancer (Acp62F1a,b,c,d/TM3, Sb). Protein was extracted from male accessory glands, whole males, and mated females as previously described in Monsma and Wolfner (1988). Protein from four Canton-S females mated with either homozygous or heterozygous Acp62F1a,b,c,d males was extracted 30 min after copulation ended to enable examination of ovulin processing. One fly equivalent of protein was loaded on a 15% polyacrylamide gel and blotted using standard Western blot techniques as performed in Park and Wolfner (1995). Affinity-purified antibodies against ovulin and Acp62F (Monsma and Wolfner 1988; Lung and Wolfner 1999) were used as primary antibodies at a 1:1000 dilution, and anti-rabbit secondary antibody with conjugated horseradish peroxidase (Amersham, Piscataway, NJ) was used at a dilution of 1:2000. Secondary antibody was visualized using enhanced chemiluminescence (Amersham) and exposure to X-ray film.
Receptivity, fertility, and egg laying of females mated to males lacking Acp62F:
To determine the effects of Acp62F on female receptivity to remating, we compared the remating frequencies of females that had previously mated to males lacking Acp62F to those of females that had previously mated to control males. Thirty-six virgin wild-type females (Canton-S) of 3–5 days of age were placed individually in vials. Two 3- to 5-day-old null (Acp62F1b/Acp62F1b) or control (Acp62F1b/TM3, Sb) males were placed with each virgin female, and matings were observed for 1 hr, as in Kalb et al. (1993). Twenty-one females mated with Acp62F1b/Acp62F1b homozygous males, and 15 mated with Acp62F1b/TM3, Sb heterozygous males. The mating durations for Acp62F1b heterozygous or homozygous males were similar to those of wild-type males (data not shown). Immediately following the matings, all males were removed from each vial. The following day, single Canton-S males were placed into each vial containing a mated female and the percentage of those females that remated during a 1-hr observation period was scored as in Aigaki et al. (1991) and Kalb et al. (1993).
To identify any effects of Acp62F on egg production, 36 Canton-S virgin females (3–5 days old) were singly mated to Acp62F1b or Acp62F1c homozygous males and 35 virgin females were singly mated to heterozygous males. Males were discarded after mating. Each morning thereafter, for the next 8 days, females were transferred individually to new vials. The total number of eggs laid in each vial was counted daily. Fertility levels were determined by counting the total number of progeny from each vial over the 9-day period.
Male sperm competitive ability of Acp62F null males:
The possible role of Acp62F in sperm competition was determined by performing sperm competition assays similar to those in Clark et al. (1995). For “offense” sperm competition tests, 42 single-pair matings of cn bw males mated to cn bw virgin females were first performed. Flies were observed to ensure that no double matings had occurred. The cn bw males were removed from these vials after mating (vial 1). Cases in which vial 1 had no progeny were discarded and were not incorporated into the analysis, because they likely reflected unsuccessful matings. On day 3, single-pair matings of 21 null homozygous Acp62F1b (experimental) or 21 w1118 (control) males were mated to cn bw females and watched to ensure that mating occurred. Immediately following the second mating, the females were transferred individually to new vials (vial 2). On day 8, females were transferred to new vials (vial 3). All progeny were scored 17 days after transfer to vial 3.
To determine “defense” sperm competitive ability, the order of matings was reversed. Seventeen experimental and 20 control crosses were performed, and to determine whether the number of offspring sired by the second male (P2′) or the first male (P1′) was significantly different between the control and experimental lines, the number of cn bw (white-eyed) vs. cn+ bw+ (red-eyed) progeny were compared. For defense, P1′ represents the percentage of progeny sired by the first male, while for offense P2′ represents the percentage of progeny sired by the second male. Two replicate experiments for both offensive and defensive sperm competitive ability yielded similar results.
To test for differences in sperm storage, we counted the number of sperm stored in the seminal receptacles of females mated to either Acp62F knockout males or control males at 90 min after the start of mating (ASM) and 3 days ASM. Sperm counts were carried out as in Neubaum and Wolfner (1999). Briefly, reproductive tracts were dissected from mated females, fixed in 50% acetic acid, and then stained with 2% orcein for 2–3 hr. Each tissue was transferred to a slide containing 50% acetic acid. The seminal receptacle was uncoiled, covered with a cover glass, and the slide was sealed with nail polish. Slides were coded to avoid any counting biases. The number of sperm stored in each seminal receptacle was counted under ×100 magnification using bright field. A minimum of 15–20 replicates were counted for each time point, per treatment, and were analyzed using a two-tailed Student's t-test.
ANOVA and t-test statistical analyses were used to determine whether significant differences between control and experimental levels of egg laying, fertility, and sperm competitive ability could be detected. The Statview (version 5.0.1) and JMP (version 5.1.2) software programs were used to perform these analyses.
Life-span assays of females continually mated to Acp62F null and control males:
To test the effects of Acp62F on continually mated female life span, the Acp62F strains were further manipulated using a balancer crossing scheme to produce null (Acp62F1bB and Acp62F1c) and control (Acp62FTandem and Acp62F1cCtrl) line males with wild-type eye color in an isogenized genetic background. All null and control lines underwent the same isogenization scheme, and the resulting null and control lines were verified by Western blotting (data not shown). The Acp62F1bB line was a subline derived from the Acp62F1b allele, and the Acp62FTandem control was derived from a strain carrying a wild-type Acp62F adjacent to Acp62Fdel. Having isogenic genetic backgrounds was important for the continual male exposure treatments used in the life-span assays because, unlike for the single-mating assays used earlier, subtle differences in background (e.g., courtship intensity) could become confounding unless controlled. For the life-span assays, 120 Dahomey virgin wild-type females per treatment were continuously housed in groups of five with either five Acp62F null (Acp62F1bB or Acp62F1c) or control (Acp62FTandem or Acp62F1cCtrl) males in sugar–yeast vials [100 g autolyzed yeast powder, 100 g sugar, 20 g agar, 30 ml nipagin (10% solution) and 3 ml propionic acid/liter distilled water] seeded with live yeast. Female deaths were scored every 1–2 days and females were transferred to fresh vials every 2–3 days until day 44 when the experiment was terminated. During the experiment, dead males were replaced to maintain a 1:1 sex ratio. All males were replaced on days 11 and 25 with new 2- to 3-day-old males to encourage mating throughout the experiment.
We also measured the effect of Acp62F on egg and progeny production, and egg–adult viability, under these conditions to test for effects of Acp62F throughout the life span and to test whether these lines showed similar effects on egg production and fertility as compared to the non-isogenized Acp62F null males used in the single-mating tests above. Samples of the fecundity and fertility of females in the life-span assays were taken on days 2, 5, 7, 9, 13, 15, 18, 21, 24, 27, 31, and 34. Females from the main experiment were transferred to fresh sugar–yeast charcoal vials (4 g of charcoal powder added to each liter of the sugar–yeast medium described above) for a period between 4 and 24 hr before transfer back into fresh sugar–yeast vials. The sampling time was the same across all treatments on each sampling day, but increased as the experiment progressed and fecundity levels decreased. Fecundity and egg–adult viability (number of progeny/number of eggs laid × 100) in each of these vials was then recorded by counting eggs within 24 hr of the latter transfer and by counting the emerging progeny. These measures were then corrected for the number of laying females and the sampling time.
RESULTS
Generation of an Acp62F null allele:
We used a two-step ends-in gene-targeting approach, based upon Xie and Golic (2004), to delete the entire Acp62F coding region. An in vitro donor construct carrying large Acp62F flanking regions and an 800-bp deletion of the Acp62F coding region were inserted into a P-element vector (P[Acp62Fdel]; Figure 1A). FLP recombinase and I-SceI endonuclease were ectopically expressed in flies carrying the donor construct on the second chromosome to excise the donor construct and generate a double-strand break within the 3′ 4-kb region to produce a tandem duplicate of Acp62Fdel adjacent to the endogenous wild-type Acp62F (Figure 1A). For the reduction event, the Cre endonuclease recognition site, between Acp62Fdel and the endogenous wild-type Acp62F, was the double-strand-break target of the heat-shock-induced Cre recombinase. Recombination in the 5′ flanking region results in recovery of the Acp62Fdel allele (Figure 1B), while recombination in the 3′ flanking region results in recovery of the wild-type allele (Figure 1B). All reduction events were detected by loss of the whs marker (this produces white-eyed males). Single heterozygous males without the whs marker were screened via PCR for the desired deletion (825 bp) or the wild-type allele (1635-bp product) of Acp62F (supplemental Figure 1, lane 3, at http://www.genetics.org/supplemental/). Of the 109 reduction-event heterozygous lines identified, 4 carried the Acp62F deletion allele.
The targeted knockout of Acp62F generates male flies lacking Acp62F protein:
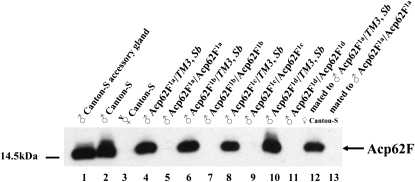
Western blot analyses were performed to confirm, at the protein level, the successful targeted knockout of Acp62F. The removal of the entire coding region of Acp62F resulted in the absence of the 14-kDa wild-type Acp62F protein in whole-fly protein extracts of homozygous null Acp62F1a, Acp62F1b, Acp62F1c, and Acp62F1d males, but its presence in heterozygous control males of the Acp62F1a–d/TM3, Sb deletion lines (Figure 2). Acp62F1a–d homozygous null males produced and transferred normal amounts of other Acps tested (ovulin, Acp36DE, CG6289, CG8137, CG9334, and CG11864; supplemental Figure 2 (http://www.genetics.org/supplemental/) and data not shown), signifying that all phenotypes detected by males with a deleted Acp62F protein are due to the lack of only Acp62F.
Figure 2.—
Western blot analyses of Acp62F1a–d null lines of whole male flies, dissected male accessory glands, and mated female flies. The solid arrow at the right marks Acp62F, which runs at ∼14 kDa (Lung and Wolfner 1999). Acp62F protein is produced in Acp62F1a–d/TM3, Sb males and is not produced in Acp62F1a–d/Acp62F1a–d males (lanes 4–11). Acp62F is transferred in Canton-S females mated to Acp62F1a/TM3, Sb males (lane 12), but not in Canton-S females mated to Acp62F1a/Acp62F1a males.
Acp62F affects ovulin processing within the mated female:
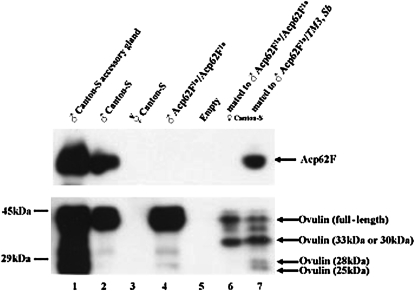
Three Acps (Acp36DE, CG11864 and ovulin) are known to be processed during mating (Park and Wolfner 1995; Bertram et al. 1996; Ravi Ram et al. 2005). Since Acp62F is a protease inhibitor, it could potentially regulate the processing of other Acps within the accessory gland during mating or in the mated female. To determine if the Acp62F knockout solely targets the production of Acp62F protein, or whether the processing of other Acps is potentially altered, we also tested the expression and transfer of a processed Acp, ovulin, in Acp62F null males. Full-length ovulin is normally detected as a protein doublet (41 and 37 kDa) in males because of differences in the addition of glycosyl moieties (Park and Wolfner 1995). In Acp62F1a/Acp62F1a males, the ovulin doublet is produced at normal levels (Figure 3, lane 4); thus, production of ovulin appears similar to normal in Acp62F null males. In addition, ovulin is transferred at normal levels to the female in Acp62F1a/Acp62F1a males (Figure 3, lanes 6 and 7).
Figure 3.—
Expression, transfer, and cleavage of ovulin in null Acp62F males. Lane 1, male accessory gland. Lane 2, Canton-S males. Lane 3, virgin Canton-S females. Lane 4, Acp62F1a/Acp62F1a males. Lane 6, Canton-S females mated to Acp62F1a/Acp62F1a males, 10 min after mating, receive normal levels of ovulin. Ovulin full-length and processed products can be detected in mates of Acp62F1a/TM3, Sb and Acp62F1a/Acp62F1a males. Ovulin processing appears to be differentially regulated in mates of Acp62F1a/TM3, Sb (lane 7) and Acp62F1a/Acp62F1a males (lane 6).
However, after mating, the 41- and 37-kDa forms of ovulin are processed into 33- and 30-kDa products and then later into 28- and 25-kDa products (Park and Wolfner 1995), and in mates of Acp62F1a/Acp62F1a null males, the rate of processing of ovulin differs from controls. At 30 min ASM, only the 28- and 25-kDa products are visible in mates of the Acp62F1a/TM3, Sb control but not of Acp62F1a/Acp62F1a males (Figure 3, lanes 6 and 7). Time-course analysis of the last two ovulin-processing products (28 and 25 kDa) at 20 min ASM reveals that the two products are not observed as quickly in the mates of Acp62F1a/Acp62F1a as in the mates of Acp62F1a/TM3, Sb males (supplemental Figure 3 at http://www.genetics.org/supplemental/). However, by 50 min ASM, ovulin processing is complete in mates of both controls and knockout males (supplemental Figure 3). These data suggest that Acp62F affects the rate of ovulin processing. We did not detect differences in processing of Acp36DE or CG11864 in mates of Acp62F null males relative to controls (supplemental Figure 2). Thus, Acp62F may affect only the processing rate of a specific subset of Acps.
Males lacking Acp62F have normal effects on female receptivity to remating, egg-laying rate, and fertility:
Females mated to males lacking Acp62F do not show any significant difference in remating frequency 24 hr postmating, relative to mates of control males expressing Acp62F (P = 0.471; two-tailed Fisher's exact test). Of the 21 Canton-S females mated to males lacking Acp62F on the first day and Canton-S males the following day, only 3 remated (86% nonreceptivity). Of the 15 Canton-S females mated to heterozygous Acp62F1b/TM3, Sb males the first day, only 1 mated to a Canton-S male on the following day (93% nonreceptivity). The average rate of remating receptivity in wild-type crosses is ∼5% (Aigaki et al. 1991), which is similar to what was observed in our tests of the mates of Acp62F1b/Acp62F1b and Acp62F1b/TM3, Sb males.
Acp-dependent regulation of the female's fertility and egg laying occurs largely within the first few days after mating, while the presence of sperm (the sperm effect) allows some effects of Acps to persist for many days past the Acp-regulated phase (Kalb et al. 1993; Peng et al. 2005). Our egg-laying and fertility experiments tested the short term (Acp phase) and long term (sperm-effect phase) to determine if Acp62F regulated either of these processes. Females were singly mated to homozygous Acp62F1b or Acp62F1c males or to control males, which were heterozygous for the knockout allele and the balancer. The total number of eggs laid and the progeny for each day were counted for 9 days (Table 1). Total numbers of eggs and progeny from both the mutant and control lines did not differ significantly across all 9 days (Table 1) or for any single day within the 9 days (supplemental Table 2 at http://www.genetics.org/supplemental/), when compared by t-tests. Thus, females mated to males who lack Acp62F protein do not exhibit abnormalities in fertility or egg laying. Although a lack of Acp62F did not detectably affect fertility or egg laying from a single mating, it was possible that comparisons of fertility and egg laying from multiple matings could reveal a phenotype. However, we also did not detect any age-specific change in egg laying or egg–adult viability of progeny in continually mated females in the life-span assays (supplemental Figure 4 at http://www.genetics.org/supplemental/). Thus, it appears that the lack of Acp62F, upon transfer to the female, does not detectably alter either short-term or long-term postmating effects on egg laying or on fertility.
TABLE 1.
Average number of eggs and progeny of females mated to Acp62F1b or cvs. Acp62F+ males over 9 days
| Females mated to:
|
|||
|---|---|---|---|
| Control males (n) | Knockout males (n) | P-value | |
| Egg laying (Acp62F1b) | 25.79 ± 2.91 (18) | 27.42 ± 3.64 (17) | 0.27 |
| Egg laying (Acp62F1c) | 28.32 ± 4.05 (17) | 25.80 ± 2.12 (19) | 0.35 |
| Fertility (Acp62F1b) | 24.68 ± 2.11 (18) | 25.58 ± 1.85 (17) | 0.41 |
| Fertility (Acp62F1c) | 26.73 ± 1.93 (17) | 24.14 ± 2.84 (19) | 0.21 |
Mean values ± SE are given for each test. Average daily number of eggs laid and progeny produced by a single-pair mating were determined for 9 days after the start of mating. P-values for unpaired t-tests were performed on egg laying and progeny counts between mates of males wild type or mutant for Acp62F. Knockout males were homozygous Acp62F1b or c and control males were Acp62F1b or c/TM3, Sb. Egg laying or fertility tests for the respective line are given in the parentheses adjacent to their respective assay.
Sperm from Acp62F null males are better able to resist displacement than sperm from control males:
Acp62F localizes to the sperm storage organs (paired spermathecae and seminal receptacle) in the mated female (Lung et al. 2002), and thus Acp62F has the potential to interact with sperm and possibly regulate their storage. One means of addressing Acp62F's function in “sperm management” is by examining the sperm competitive ability of Acp62F null males. To measure the ability of sperm displacement of males lacking Acp62F, the number of offspring produced by mated cn bw females after a second mating to either Acp62F1b/Acp62F1b or w1118; Acp62F+/Acp62F+ males was compared. In both cases, Acp62F1b/Acp62F1b and w1118; Acp62F+/Acp62F+ males sired the majority of the progeny; Acp62F1b/Acp62F1b males fathered 80% of the progeny and w1118; Acp62F+/Acp62F+ males 78% of the progeny (Table 2). This suggests that Acp62F is not essential (or is functionally redundant with another Acp) for “offense” components of sperm competitive ability.
TABLE 2.
Sperm competitive ability of Acp62F null males
| Male genotype | Mean P2′ | Count | Standard deviation | Standard error | |
|---|---|---|---|---|---|
| Offense | |||||
| Acp62F1b/Acp62F1b | 0.800 | 21 | 0.076 | 0.017 | |
| Acp62F+/Acp62F+ | 0.776 | 21 | 0.035 | 0.008 | P = 0.197 |
| Male genotype | Mean P1′ | Count | Standard deviation | Standard error | |
| Defense | |||||
| Acp62F1b/Acp62F1b | 0.705 | 17 | 0.191 | 0.046 | |
| Acp62F+/Acp62F+ | 0.390 | 20 | 0.299 | 0.080 | P = 0.0013 |
Offensive and defensive sperm competitive ability of Acp62F null males. Mean P2′ represents the percentage of offspring sired by the second male and the mean P1′ represents the average number of offspring sired by the first male. ANOVA statistical analysis was performed to determine significance values. Acp62F+/Acp62F+ flies have a w1118 genetic background, which is the same genetic background in which Acp62F1 targeting P-element transformations were performed.
To test for the ability of a first male's sperm to resist displacement by a second male's sperm, cn bw females were first mated to either Acp62F1b/Acp62F1b or w1118; Acp62F+/Acp62F+ males and subsequently to cn bw males. Surprisingly, sperm from males lacking Acp62F could resist displacement significantly better than males with Acp62F (ANOVA, P = 0.0013, Table 2). Acp62F1b/Acp62F1b null males fathered close to 70% of the total progeny after the second mating (Table 2), while w1118; Acp62F+/Acp62F+ males fathered only 39% (Table 2). The average numbers of offspring sired by both Acp62F1b/Acp62F1b (70%) and w1118; Acp62F+/Acp62F+ (39%) first males when competed against cn bw second males were higher than the typical level of P1′ found in natural populations (∼20%) (Clark 2002). This difference could be due to genetic relatedness (which is higher between cn bw females and males than between cn bw females and Acp62F null and control males) (Clark et al. 1999), since sperm competitive ability is negatively correlated with reproductive success (Mack et al. 2002). Additionally, association tests between different third chromosome substitution lines have revealed epistatic interactions, which can affect sperm competitive ability (Fiumera et al. 2007). Nonetheless, the effect of Acp62F on defensive sperm competitive ability is probably not due to genetic background differences alone, because the Acp62F1b/Acp62F1b males are derived from a w1118 genetic background. Acp62F appears to interfere with the ability to resist sperm displacement (the “defense” component of sperm competition) by sperm from a subsequent mating male.
Acp-mediated sperm storage has been suggested to be one of the factors that contribute to sperm competition (Chapman et al. 2000). To see if Acp62F's effect on sperm competition reflects an effect on sperm storage, we counted the number of sperm stored in seminal receptacles of females mated to Acp62F control or knockout males. Our focus on seminal receptacles reflects the disproportionate effects on displacement of sperm stored in this organ as opposed to spermathecae (Gilchrist and Partridge 1995; Civetta 1999; Price et al. 1999). The average number (mean ± SE) of sperm stored in seminal receptacles of females mated to Acp62F null males (90 min ASM: 297.65 ± 10.55; 3 days ASM: 175.75 ± 30.63) did not significantly differ from those in females mated to control males (90 min ASM: 290.70 ± 15.83; 3 days ASM: 180.55 ± 24.32) either at 90 min ASM (P = 0.71) or at 3 days ASM (P = 0.91). This suggests that Acp62F's effect on defensive sperm competition is not due to differences in seminal receptacle sperm storage, but rather to other aspects of sperm competitive ability.
Absence of Acp62F does not affect survival, age-specific fecundity, or fertility of mated females:
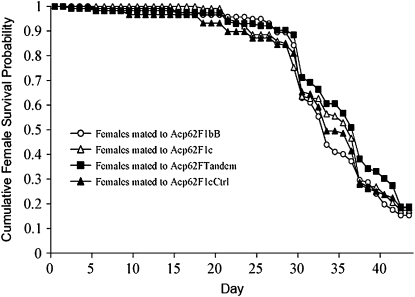
To determine whether Acp62F contributes to the decreased life span seen in multiply mated females, we examined the survival of females exposed throughout their lives to Acp62F null or control males. We found no significant differences in survivability between females mated to males that transferred or did not transfer Acp62F (log-rank test χ2 = 1.90, d.f. = 3, P = 0.5942; Figure 4). This result is not confounded by mating frequency, which did not differ significantly between the treatments (data not shown). Consistent with the single-mating assays, there were no differences in the age-specific fecundity of females exposed to Acp62F null or control males (supplemental Figure 4). There was also no consistent effect of Acp62F on egg–adult viability (supplemental Figure 4).
Figure 4.—
Effect of Acp62F on female life span. Survival of females continuously mated to null (Acp62F1bB and Acp62F1c) or control (Acp62FTandem and Acp62F1cCtrl) males against time (days).
DISCUSSION
We used ends-in gene targeting to precisely delete the gene encoding the known seminal fluid serine protease inhibitor Acp62F (Lung et al. 2002) and tested the role of Acp62F in postmating phenomena. As a protease inhibitor, Acp62F could regulate processing of other seminal fluid proteins upon transfer to the female. Ovulin, which stimulates ovulation (Herndon and Wolfner 1995; Heifetz et al. 2000), is a prohormone that requires the presence of both male and female factors for it to be proteolytically cleaved upon entry into the mated female's reproductive tract (Park and Wolfner 1995). Recent results suggest that seminal proteolysis occurs as a stepwise process, beginning in the male and continuing in the female (Ravi Ram et al. 2006). Here, we show that Acp62F contributes to this proteolytic cascade by modulating the rate of ovulin processing. Although Acp62F is a protease inhibitor, ovulin processing appears to be slower if Acp62F is not present. Lack of Acp62F does not alter the synthesis or processing of CG11864, which is a male-derived Acp metalloprotease required for the normal processing of ovulin (Ravi Ram et al. 2006). Acp62F may therefore be responsible for triggering the activation of a protease other than CG11864 or the activation of a cascade that induces a protease's expression within the female reproductive tract. Lack of such an active protease could lead to a lower efficiency of ovulin processing.
Although Acp62F participates in regulating the processing of ovulin, Acp62F null males stimulate egg laying, both following single and multiple matings, to levels comparable to those by control males that transfer Acp62F. That Acp62F null males are not defective in stimulating a mated female's egg-laying rate, even though ovulin processing is differentially regulated, could be because full-length ovulin is sufficient to stimulate ovulation (Heifetz et al. 2005) or because the measures of egg laying that we used do not detect ovulation differences with sufficient sensitivity. Mates of males deficient in another Acp, CG11864, which is also necessary for complete ovulin processing, also do not show any detectable abnormality in egg-laying rate (Ravi Ram et al. 2006). The apparent lack of a significant effect of Acp62F on mated female egg-laying rate (as well receptivity and fertility) may also be due to genetic redundancy. Six other Acps (Acp76A, CG6289, CG8137, CG9334, CG10956, and CG32203) are predicted serine protease inhibitors, and some of these protease inhibitor Acps target to similar regions within the female as Acp62F (Ravi Ram et al. 2005). The transfer and presence of any of the six Acp protease inhibitors could mask the Acp62F null male phenotype, assuming that all of the other protease inhibitors are transferred at normal levels in Acp62F null males, which seems likely, as at least CG8137, CG9334, and CG6289 are transferred at normal levels in Acp62F null males (K. Ravi Ram and M. F. Wolfner, unpublished data). Individual RNAi knockdowns of the six Acp protease inhibitors other than Acp62F did not uncover any effects on reproduction (Ravi Ram et al. 2006; Ravi Ram and Wolfner 2007a), which is also consistent with redundancy among Acp protease inhibitors. The full repertoire of Acp62F reproductive functions may thus be revealed only when its null allele is combined with knockouts of other Acp or female-induced serine protease inhibitor.
Alleles of several Acp genes correlate with sperm competition and Acps in general are known to influence sperm competition (Clark et al. 1995; Fiumera et al. 2005, 2007). Only one other Acp, Acp36DE, has been shown to be directly involved in sperm competitive ability (Chapman et al. 2000). Sperm of Acp36DE-deficient males do not compete well against second males' sperm, because the mates of Acp36DE-deficient males store fewer sperm (Neubaum and Wolfner 1999; Chapman et al. 2000). In contrast, Acp62F's effects on sperm competition do not appear to reflect effects on the number of sperm stored within seminal receptacles, because the number of such sperm stored by females mated to Acp62F null males is similar to that stored by mates of control males. Nevertheless, sperm from males who do not transfer Acp62F are able to resist displacement significantly better than those from males that transfer Acp62F. This paradoxical result might suggest that the ability of Acp62F null males to “defend” their sperm from sperm of a second male may be due to differences in the management/organization or modifications of sperm in storage, such as sperm apportionment among different types of storage organs (e.g., see Iida and Cavener 2004), or to effects on sperm storage efficiency within spermathecae, which were not examined here.
There is a cost of mating to females in terms of reduced longevity and lifetime reproductive success. A portion of this cost is mediated by the action of Acps that are transferred along with sperm by males at mating (Chapman et al. 1995). Recently, the sex peptide has been shown to mediate part of the cost (Wigby and Chapman 2005). Sex peptide is also toxic to D. melanogaster upon ectopic expression (Mueller et al. 2007), suggesting that such toxicity could be a useful tool in identifying other contributors to the cost of mating. Acp62F is one of the other three Acps that are toxic in the ectopic expression assays (Lung et al. 2002; Mueller et al. 2007); 20 other Acps tested were not toxic. This suggested that it was important to test directly whether Acp62F contributes to the cost of mating. However, we found that Acp62F null males do not differ from controls in their ability to exact female life-span costs. There are at least two possible explanations for this finding. First, despite its toxicity upon ectopic expression, Acp62F might not contribute to the cost of mating and thus the toxicity of an Acp might not enable predictions of the Acp cost-of-mating contributors. Second, as mentioned previously for other functions of Acp62F, another Acp protease inhibitor may mask Acp62F's cost-of-mating phenotype because of functional redundancy. If another Acp protease inhibitor disrupts the same pathways responsible for the cost-of-mating phenotype, we would be unable to detect such cost-of-mating effects in Acp62F null males.
Of seven known or predicted Acp protease inhibitors, only one is conserved in the distantly related D. pseudoobscura genome, which strongly suggests that positive selection is driving the rapid loss/gain of these seminal fluid protease inhibitors in Drosophila (Mueller et al. 2005). Acp62F is the one D. melanogaster Acp protease inhibitor that has a detectable ortholog in D. pseudoobscura, and its conservation extends out even to Anopheles gambiae (Dottorini et al. 2007). Since its conservation suggests that maintenance of Acp62F in the genome is important for reproductive success in many insects, the increased ability of sperm from males that do not transfer Acp62F to resist displacement begs the question of why Acp62F has been maintained and how its apparently deleterious effect on sperm displacement evolved. One possibility is that Acp62F may perform some other crucial positive postmating function during mating, which we have yet to detect and which could counteract any deleterious effect it has on sperm displacement. For example, Acp62F's presence may ensure the optimal rate or amount of processing of ovulin, which in turn could ensure the optimal rate of ovulation (Heifetz et al. 2005). Effects of such differences in Acp62F's processing of ovulin may be undetectable in the assays performed here, but could be of greater importance in natural settings where the nutrients necessary for high egg-laying rates are likely to be limited in supply. Another reason to maintain Acp62F could be to optimize the coagulation of the seminal fluid after mating. For example, male mice deficient in the seminal fluid protease inhibitor protease nexin-1 do not properly coagulate their copulatory plug postmating (Murer et al. 2001). Although plug formation in the mates of Acp62F null males appears normal (J. L. Mueller and M. F. Wolfner, unpublished data), it is possible that the absence of Acp62F could result in a more viscous seminal fluid in the sperm storage organs where Acp62F localizes, which could be preventing sperm displacement by subsequent males. The evolution of multiple Acp62F functions may therefore have been constrained by pleiotropy; however, the mechanistic basis of the links between the different processes potentially involved (sperm competition, Acp processing, and mating plug formation) is not yet known.
Acknowledgments
We thank C. Aquadro, J. Ewer, A. Fiumera, M. Goldberg, L. McGraw, and two anonymous reviewers for constructive comments; K. Golic for the hsp70-FLP, hsp70-ISceI, and hsp70-Cre transgenic flies and advice; E. Alani, J. Ewer, E. Kubli, and H. Liu for suggestions and advice on generating the knockout; and A. Fiumera for suggestions on the sperm competition assays. This work was supported by National Institutes of Health (NIH) grant HD38921 (to M.F.W.). During part of this work, J.L.M. was supported by a traineeship from NIH training grant T32GM007617. T.C. thanks the Royal Society (University Research Fellowship to T.C.) and Natural Environment Research Council (Ph.D. studentship award to J.L.) for funding.
References
- Aigaki, T., I. Fleischmann, P. S. Chen and E. Kubli, 1991. Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron 7 557–563. [DOI] [PubMed] [Google Scholar]
- Bangham, J., 2003. The evolutionary significance of reproductive traits in Drosophila melanogaster. Ph.D. Thesis, University College of London, London.
- Bertram, M. J., D. M. Neubaum and M. F. Wolfner, 1996. Localization of the Drosophila male accessory gland protein Acp36DE in the mated female suggests a role in sperm storage. Insect Biochem. Mol. Biol. 26 971–980. [DOI] [PubMed] [Google Scholar]
- Chapman, T., L. F. Liddle, J. M. Kalb, M. F. Wolfner and L. Partridge, 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373 241–244. [DOI] [PubMed] [Google Scholar]
- Chapman, T., D. M. Neubaum, M. F. Wolfner and L. Partridge, 2000. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc. R. Soc. Lond. B Biol. Sci. 267 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta, A., 1999. Direct visualization of sperm competition and sperm storage in Drosophila. Curr. Biol. 9 841–844. [DOI] [PubMed] [Google Scholar]
- Civetta, A., K. L. Montooth and M. Mendelson, 2005. Quantitative trait loci and interaction effects responsible for variation in female postmating mortality in Drosophila simulans and D. sechellia introgression lines. Heredity 94 94–100. [DOI] [PubMed] [Google Scholar]
- Clark, A. G., 2002. Sperm competition and the maintenance of polymorphism. Heredity 88 148–153. [DOI] [PubMed] [Google Scholar]
- Clark, A. G., M. Aguadé, T. Prout, L. G. Harshman and C. H. Langley, 1995. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics 139 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, A. G., D. J. Begun and T. Prout, 1999. Female × male interactions in Drosophila sperm competition. Science 283 217–220. [DOI] [PubMed] [Google Scholar]
- de Lamirande, E., K. Yoshida, T. M. Yoshiike, T. Iwamoto and C. Gagnon, 2001. Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. J. Androl. 22 672–679. [PubMed] [Google Scholar]
- Dottorini, T., L. Nicolaides, H. Ranson, D. W. Rogers, A. Crisanti et al., 2007. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc. Natl. Acad. Sci. USA 104 16215–16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera, A. C., B. L. Dumont and A. G. Clark, 2005. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics 169 243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera, A. C., B. L. Dumont and A. G. Clark, 2007. Associations between sperm competition and natural variation in male reproductive genes on the third chromosome of Drosophila melanogaster. Genetics 176 1245–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist, A. S., and L. Partridge, 1995. Male identity and sperm displacement in Drosophila melanogaster. J. Insect Physiol. 41 1087–1092. [Google Scholar]
- Golic, K. G., and S. Lindquist, 1989. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59 499–509. [DOI] [PubMed] [Google Scholar]
- Heifetz, Y., O. Lung, E. A. Frongillo, Jr. and M. F. Wolfner, 2000. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 10 99–102. [DOI] [PubMed] [Google Scholar]
- Heifetz, Y., L. N. Vandenberg, H. I. Cohn and M. F. Wolfner, 2005. Two cleavage products of the Drosophila accessory gland protein ovulin can independently induce ovulation. Proc. Natl. Acad. Sci. USA 102 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon, L. A., and M. F. Wolfner, 1995. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc. Natl. Acad. Sci. USA 92 10114–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida, K., and D. R. Cavener, 2004. Glucose dehydrogenase is required for normal sperm storage and utilization in female Drosophila melanogaster. J. Exp. Biol. 207 675–681. [DOI] [PubMed] [Google Scholar]
- Kalb, J. M., A. J. DiBenedetto and M. F. Wolfner, 1993. Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proc. Natl. Acad. Sci. USA 90 8093–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlowska, M., R. Kowalski, J. Glogowski, J. Jankowski and A. Ciereszko, 2005. Gelatinases and serine proteinase inhibitors of seminal plasma and the reproductive tract of turkey (Meleagris gallopavo). Theriogenology 63 1667–1681. [DOI] [PubMed] [Google Scholar]
- Laurell, M., A. Christensson, P. A. Abrahamsson, J. Stenflo and H. Lilja, 1992. Protein C inhibitor in human body fluids. Seminal plasma is rich in inhibitor antigen deriving from cells throughout the male reproductive system. J. Clin. Invest. 89 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawniczak, M. K., and D. J. Begun, 2004. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome 47 900–910. [DOI] [PubMed] [Google Scholar]
- Lawniczak, M. K., and D. J. Begun, 2007. Molecular population genetics of female-expressed mating-induced serine proteases in Drosophila melanogaster. Mol. Biol. Evol. 24 1944–1951. [DOI] [PubMed] [Google Scholar]
- Leclerc, V., and J. M. Reichhart, 2004. The immune response of Drosophila melanogaster. Immunol. Rev. 198 59–71. [DOI] [PubMed] [Google Scholar]
- Lung, O., 2000. Fate and function of seminal fluid proteins in the mated Drosophila melanogaster female. Ph.D. Thesis, Cornell University, Ithaca, NY.
- Lung, O., and M. F. Wolfner, 1999. Drosophila seminal fluid proteins enter the circulatory system of the mated female fly by crossing the posterior vaginal wall. Insect Biochem. Mol. Biol. 29 1043–1052. [DOI] [PubMed] [Google Scholar]
- Lung, O., U. Tram, C. M. Finnerty, M. A. Eipper-Mains, J. M. Kalb et al., 2002. The Drosophila melanogaster seminal fluid protein Acp62F is a protease inhibitor that is toxic upon ectopic expression. Genetics 160 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack, P. D., B. A. Hammock and D. E. Promislow, 2002. Sperm competitive ability and genetic relatedness in Drosophila melanogaster: similarity breeds contempt. Evolution 56 1789–1795. [DOI] [PubMed] [Google Scholar]
- Mack, P. D., A. Kapelnikov, Y. Heifetz and M. Bender, 2006. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 103 10358–10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw, L. A., G. Gibson, A. G. Clark and M. F. Wolfner, 2004. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr. Biol. 14 1509–1514. [DOI] [PubMed] [Google Scholar]
- Monsma, S. A., and M. F. Wolfner, 1988. Structure and expression of a Drosophila male accessory gland gene whose product resembles a peptide pheromone precursor. Genes Dev. 2 1063–1073. [DOI] [PubMed] [Google Scholar]
- Mueller, J. L., D. R. Ripoll, C. F. Aquadro and M. F. Wolfner, 2004. Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc. Natl. Acad. Sci. USA 101 13542–13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, J. L., K. Ravi Ram, L. A. McGraw, M. C. Bloch Qazi, E. D. Siggia et al., 2005. Cross-species comparison of Drosophila male accessory gland protein genes. Genetics 171 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, J. L., J. L. Page and M. F. Wolfner, 2007. An ectopic expression screen reveals the protective and toxic effects of Drosophila seminal fluid proteins. Genetics 175 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer, V., J. F. Spetz, U. Hengst, L. M. Altrogge, A. de Agostini et al., 2001. Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proc. Natl. Acad. Sci. USA 98 3029–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum, D. M., and M. F. Wolfner, 1999. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 153 845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson, K., A. Bjartell and H. Lilja, 1995. Secretory leucocyte protease inhibitor in the male genital tract: PSA-induced proteolytic processing in human semen and tissue localization. J. Androl. 16 64–74. [PubMed] [Google Scholar]
- Park, M., and M. F. Wolfner, 1995. Male and female cooperate in the prohormone-like processing of a Drosophila melanogaster seminal fluid protein. Dev. Biol. 171 694–702. [DOI] [PubMed] [Google Scholar]
- Parks, A. L., K. R. Cook, M. Belvin, N. A. Dompe, R. Fawcett et al., 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36 288–292. [DOI] [PubMed] [Google Scholar]
- Peng, J., S. Chen, S. Busser, H. Liu, T. Honegger et al., 2005. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 15 207–213. [DOI] [PubMed] [Google Scholar]
- Price, C. S., K. A. Dyer and J. A. Coyne, 1999. Sperm competition between Drosophila males involves both displacement and incapacitation. Nature 400 449–452. [DOI] [PubMed] [Google Scholar]
- Ravi Ram, K., and M. F. Wolfner, 2007. a Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 3 e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram, K., and M. F. Wolfner, 2007. b Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 47 427–445. [DOI] [PubMed] [Google Scholar]
- Ravi Ram, K., S. Ji and M. F. Wolfner, 2005. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem. Mol. Biol. 35 1059–1071. [DOI] [PubMed] [Google Scholar]
- Ravi Ram, K., L. K. Sirot and M. F. Wolfner, 2006. Predicted seminal astacin-like protease is required for processing of reproductive proteins in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 103 18674–18679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2000. Gene targeting by homologous recombination in Drosophila. Science 288 2013–2018. [DOI] [PubMed] [Google Scholar]
- Rong, Y. S., and K. G. Golic, 2001. A targeted gene knockout in Drosophila. Genetics 157 1307–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218 348–353. [DOI] [PubMed] [Google Scholar]
- Swanson, W. J., A. G. Clark, H. M. Waldrip-Dail, M. F. Wolfner and C. F. Aquadro, 2001. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc. Natl. Acad. Sci. USA 98 7375–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson, W. J., A. Wong, M. F. Wolfner and C. F. Aquadro, 2004. Evolutionary expressed sequence tag analysis of Drosophila female reproductive tracts identifies genes subjected to positive selection. Genetics 168 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theopold, U., D. Li, M. Fabbri, C. Scherfer and O. Schmidt, 2002. The coagulation of insect hemolymph. Cell. Mol. Life Sci. 59 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby, S., and T. Chapman, 2005. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 15 316–321. [DOI] [PubMed] [Google Scholar]
- Xie, H. B., and K. G. Golic, 2004. Gene deletions by ends-in targeting in Drosophila melanogaster. Genetics 168 1477–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]