IN 1974, two articles regarding meiotic recombination in fungi were submitted for publication: one to the proceedings of a meeting held in Gatlinburg, Tennessee (Mortimer and Fogel 1974), and the other, which remained unpublished for 4 years, to Genetics (Kitani 1978). The two articles dealt with relationships among gene conversion, crossing over, and crossover interference, and they appeared to flatly contradict each other. Mortimer and Fogel claimed that crossovers accompanied by conversion interfered with additional, nearby crossing over; Kitani claimed that such crossovers did not interfere.
Mortimer and Fogel's (1974) article was based on data from the ARG4 and HIS1 loci of yeast (Saccharomyces cerevisiae); Kitani's was based on data from the g locus of Sordaria fimicola. Was one of them wrong, or is recombination in Sordaria truly so different from that in yeast? And why did it take 4 years to move Kitani's article through the Genetics editorial process?
SOME BACKGROUND
The 1960s and 1970s featured efforts to understand recombination at the Watson–Crick level. The fungi, with their meiotic tetrads, played a central role in these efforts. They provided the key observation that “gene conversion”—defined in this review to mean 5:3, 6:2, or aberrant 4:4 segregation of markers (Table 1)—was often (about half the time) accompanied by crossing over of closely linked markers bracketing the converted site. Moreover, crossovers for closely linked markers frequently manifested conversion for a marker lying between them. This strong association between conversion and crossing over inspired models that described these processes as consequences of a single event that could, but need not, lead to crossing over and could, but need not, lead to conversion. Some simple arithmetic based on data from yeast (Fogel et al. 1971) encouraged the assumption that all crossing over would be accompanied by conversion, if there were markers present to reveal it. Thus, since crossing over scored without regard to conversion is characterized by interference in Sordaria (Perkins et al. 1963) as well as in yeast (e.g., Fogel and Mortimer 1971), crossing over that accompanies conversion was expected to similarly interfere with neighboring crossovers. In fact, Stadler (1959) had earlier presented evidence from Neurospora supporting that position, as had Fogel and Mortimer (1971) for yeast.
TABLE 1.
Crossover tetrad types from the cross A m B × a + b
| No conversion:a | Conversion at ma
|
||
|---|---|---|---|
| Normal 4:4 | Aberrant 4:4 | 5:3 | 6:2 |
| A m/m B | A m/m B | A m/m B | A m/m B |
| A m/m b | A m/+ b | A m/m b | A m/m b |
| a +/+ B | a +/m B | a m/+ B | a m/m B |
| a +/+ b | a +/+ b | a +/+ b | a +/+ b |
Tetrads are monitored for crossing over between markers A and B, closely bracketing site m.
The Watson–Crick duplex nature of the haploid products of meiosis is indicated only for site m. In the 5:3 and 6:2 tetrads, conversion has been arbitrarily indicated in favor of the mutant marker. In the text, 5:3 and 6:2 indicate combined frequencies of conversion without regard to whether the conversion favored m or favored +.
Clearly, Kitani (1978) was swimming against a heavy tide when, first, he presented data showing that, in Sordaria at least, conversion and interference were mutually exclusive and then explained his observation with the hypothesis that interfering crossovers occur only where there are no markers, i.e., in spaces between genes. Developments reported in this issue of Genetics by Getz et al. (2008) make that interpretation untenable, but they reveal that Kitani was right when he proposed (1) that meiotic recombination probably occurs at two different stages (or phases, in the language of Getz et al. 2008), (2) that crossovers in the two stages of recombination have different relationships to gene conversion, (3) that crossovers in one stage do not positively interfere with those in the other, and (4) that the wide variation among organisms in their reported interference indices probably represents varying proportions of the two stages of crossing over.
WHY DID IT TAKE 4 YEARS?
Documents relating to the publication of Kitani (1978), kindly supplied by Kitani, provide support for each of several possibilities for the delay in publication.
More experiments were needed:
The first version of the manuscript reported convincing evidence that conversion crossovers (in Sordaria) lacked interference. However, the editor (a Neurospora geneticist) agreed with the referees that Kitani needed better evidence that “ordinary” crossovers in Sordaria did have interference. Kitani promptly collected control data and found that crossovers selected for being nonconversions showed robust interference.
The manuscript was unclear:
Two editors (the second being a yeast geneticist) and several referees declared the writing to be poor and the arguments unclear. The author and editors did a lot of rewriting for clarification.
One editor was slow:
A turnaround interval of 6 months was too long, even by the standards of Genetics. The editor put the blame, in part, on the “complexities of the manuscript.” The issues dealt with by Kitani are complex, and the field was (and still is) burdened with inconsistent, model-dependent, and otherwise poorly defined vocabulary. However, part of the delay may well be attributable to the editor's move from her home lab in the United States to a sabbatical in France.
There were disagreements regarding content and style:
The largest single reason for the 4-year delay seems to have been Kitani's inability to accept the editors' advice regarding the discussion and conclusions. The discussion, as published, has >500 words devoted to cytological observations supporting the view that chromosomes appear to have many opportunities for recombination. The editors repeatedly tried, but failed, to convince Kitani that he would be better served by a simple statement that recombination may occur at more than one stage in meiotic prophase. (On this point, Kitani suspected a conspiracy to suppress his ideas. As a result of our own experiences, we deeply sympathize with Kitani, while acknowledging the validity of the editors' position.)
The concluding statement in the article, as published, makes no mention of the contradictory yeast and Neurospora data despite the editors' insistence that it do so. The history of this outcome is, we suspect, a key to understanding the protracted exchanges between author and editors. In exchanges with the second editor, Kitani agreed to mention in the conclusion that the data from Neurospora and yeast contradicted his Sordaria data, saying he would do so “in a gentle manner.” He offered the following sentence, which appears to have been editorially deleted from the published article: “This conclusion does not conflict with the observations in Neurospora and Saccharomyces as far as the structures of gene loci are reconsidered.” We surmise that Kitani meant this puzzling sentence to imply that, with respect to other considerations, his data were, in fact, in direct conflict with those of Stadler and of Fogel. Could a cultural imperative to avoid direct confrontation account for this and other apparent miscommunications?
THE POST-PUBLICATION RECEPTION
Did the article fare better once it was published? Examination of the post-1978 literature reveals few citations to Kitani (1978), indicating that the genetics community was, in fact, reluctant to take his data and/or proposals seriously. The several citations to Kitani (1978), other than by Kitani himself, are of three sorts: (1) those that do not relate to the main conclusion of the article (e.g., Holm and Rasmussen 1980); (2) those that somewhat dismissively say “… but see Kitani (1978)” (e.g., Carpenter 1979; Foss et al. 1993); and (3) one (Roman 1985, p. 927) that seriously misrepresents the article when it states that “Kitani … found two classes of convertants in Sordaria, one that interfered with an adjacent crossover and one that did not.”
Why did Kitani (1978) receive so little attention? Although, like most tetrad analysis articles, Kitani (1978) is difficult to read, it does contain remarkable data and conclusions. We suspect that part of its neglect was a result of “Saccharomycelial imperialism.” The relevant history is related below.
Symmetry and asymmetry:
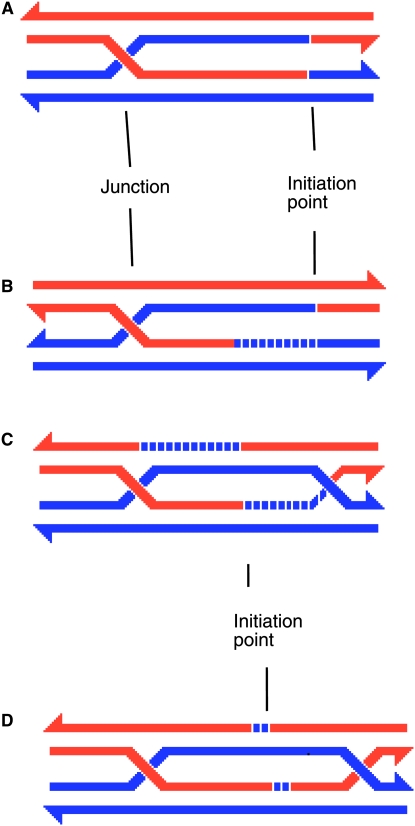
The influential models for recombination, from the 1960s through today, feature hybrid DNA, segments of duplex in which the two strands come from different parents. The models differ, however, in the degree to which this swapping of strands is reciprocal (Figure 1). The Sordaria data (Kitani et al. 1962), which were rich in aberrant 4:4 conversions (Table 1), argued that strand swapping was, for the most part, reciprocal, creating “symmetric heteroduplex.” Holliday (1964) embodied this reciprocality in a simple model in which a bimolecular intermediate (joint molecule) featured such a symmetric heteroduplex (Figure 1A) (see Stahl 1994). Some Ascobolus data (Leblon and Rossignol 1973) supported the symmetric heteroduplex view. On the other hand, Ascobolus data reported by Stadler and Towe (1971) and yeast data acquired soon thereafter by Sy Fogel and Bob Mortimer implied that such reciprocality was not general and was probably rare in yeast. In an effort to achieve a general model, Meselson and Radding (1975) proposed that there be two steps in heteroduplex formation. The first step would feature an asymmetric heteroduplex arising from nonreciprocal strand exchange near the point of initiation of the recombination event. The second step would create a symmetric heteroduplex, arising from “branch migration,” located at a distance from the initiation point (see Figure 1B). This model was attractive for its ability to deal with both the Sordaria and the yeast data simply by varying the relative lengths of the two steps. When experiments at the b2 locus in Ascobolus yielded data supporting asymmetric heteroduplex near a putative initiation point changing to symmetric heteroduplex farther away (Paquette and Rossignol 1978), the Meselson–Radding model became the dominating paradigm.
Figure 1.—
Hypothetical bimolecular recombination intermediates (joint molecules). Material contributions from the two interacting chromatids are in red and blue. Newly synthesized DNA is in broken lines, with the color indicating the chromatid on which it was templated. The arrowheads indicate the 3′-ends of strands. (A) The intermediate proposed by Holliday (1964) features a reciprocal exchange of single strands of DNA, resulting in symmetric heteroduplex. (B) The intermediate proposed by Meselson and Radding (1975) features a stretch of asymmetric heteroduplex, near the point of initiation of recombination, followed by a stretch of symmetric heteroduplex. (C) The meiotic double-strand-break-repair intermediate as envisioned for yeast (Sun et al. 1991). Heteroduplex is (at least mostly) asymmetric. (D) The meiotic double-strand-break-repair intermediate as envisioned for the Sordaria g locus. Heteroduplex is predominantly symmetric.
At Cold Spring Harbor that year (1978), Sy Fogel, a formidable figure in the yeast field, declared allegiance to the Meselson–Radding model in a magnum opus entitled “Meiotic gene conversion: a signal of the basic recombination event in yeast” (Fogel et al. 1979). These developments, combined with a wave of budding-yeast chauvinism, created a bandwagon for simple models, such as that of Meselson and Radding, with the result that deviant data from “weird” organisms tended to be ignored. Consequently, Kitani's (1978) demonstration that, in Sordaria, conversion crossovers lack interference while interfering crossovers lack conversion did not trigger investigations by others. No one appears to have asked whether features characteristic of Sordaria, but not of yeast, might underlie Kitani's observations. Instead, his discovery was allowed to die and could not be reborn until yeast caught up with Sordaria.
Rediscovering Kitani (1978):
Kitani's proposal for Sordaria—that meiosis generates both interfering and noninterfering crossovers, which probably occur at different stages—was newly suggested to apply to yeast by Zalevsky et al. (1999). However, Zalevsky did not challenge the popular assumption that all crossing over is associated with conversion. Stahl et al. (2004) embraced Zalevsky's (1999) proposal and made some predictions regarding crossing over in the noninterference and the interference recombinational stage in yeast. Our co-workers (Getz et al. 2008, this issue) set out to test those predictions. Working overtime, under pressure imposed by the impending closure of our laboratory, they dissected and analyzed the thousands of tetrads required. As the deadline drew near, they presented their data and conclusions: (1) like those of Kitani in Sordaria, the crossovers among our 5:3's failed to manifest crossover interference!, and (2) unlike those of Kitani, but like those of Malkova et al. (2004) and presumably like those of Mortimer and Fogel (1974; see below), our 6:2 crossovers, as a class, did manifest interference. These results were not what we had expected.
Reconciliation:
When we recovered, we recognized convergence among Kitani (1978), Mortimer and Fogel (1974), and ourselves. Full reconciliation depended upon clarification of three issues.
First, why did Mortimer and Fogel (1974) not see any noninterfering 5:3 crossovers? The answer lies in the fact that the mismatches made by most yeast markers are always rectified by meiotic mismatch repair (MMR). Thus, Mortimer and Fogel's conversions, like those of Malkova et al. (2004), probably did not include any 5:3's, the only class in which noninterfering crossovers would be detectable. By contrast, in the work of Getz et al. (2008) the incorporation of “poorly repairable mismatches,” arising from the intentional use of small palindromes (Nag and Petes 1991), enabled the authors to recover and analyze 5:3 tetrads.
Second, did the discovery that, in yeast, noninterfering crossovers were identified through the use of palindromic markers imply that Kitani must have used special markers? An overdue rereading of Kitani (1978) reminded us that MMR is less efficient in Sordaria than in yeast. In fact, it appears that all mismatches in Sordaria are poorly repairable.
Third, the work by Getz et al. (2008, this issue) offers a clue to the remaining question: Why would Kitani's (1978) 6:2 crossover data lack interference while our 6:2 yeast crossovers, as a class, do manifest interference? As elaborated below, the analysis by Getz et al. (2008) explains how two sets of rules for MMR—one for products of the noninterference stage and the other for products of the interference stage—would apply to the relations observed among crossing over, interference, and conversion in Sordaria as well as in yeast.
The rules were developed within the framework of the currently favored double-strand-break-repair model for meiotic recombination (Figure 1, C and D), building on the notion that MMR is guided by strand interruptions, as it is when operating at replication forks to diminish mutation rates. In this model there are two occasions when strand interruptions can guide MMR: (1) during the strand invasion that leads to the pictured intermediates (Figure 1, C and D), resulting in 6:2 conversion (Szostak et al. 1983; Haber et al. 1993), and (2) following or during resolution of the bimolecular intermediate (Foss et al. 1999), allowing the possibility of restoration of normal 4:4 segregation. Accordingly, Getz et al. (2008), working with yeast, proposed that a poorly repairable mismatch arising in the noninterference stage enjoys some MMR, but only at invasion. Such MMR would generate noninterfering 6:2 crossovers, as reported by Kitani (1978) for Sordaria. In contrast, MMR of poorly repairable mismatches in the interference stage was proposed by Getz et al. (2008) to occur invariably, but only at junction resolution. In an asymmetric heteroduplex, typical of yeast (Figure 1C), resolution-directed repair will yield interfering crossovers with either 6:2 or normal 4:4 segregation, depending on which of the two resolved junctions directs the MMR. In yeast, the interference in such 6:2 tetrads would mask the lack of interference of 6:2 tetrads derived from the noninterference pathway. By contrast, in the symmetric heteroduplex characteristic of Sordaria (Figure 1D), MMR directed by either of the two resolved junctions would restore normal segregation. Consequently, in Kitani's (1978) experiments, there would be no 6:2 interfering crossovers to mask the lack of interference in the 6:2 crossovers of the noninterference stage. At the same time, Kitani's normal 4:4 interfering crossovers would give no hint of their origin from intermediates that were heteroduplex for intragenic DNA. Thus, there seems to be no need to suppose, as Kitani (1978) did, that interfering crossovers occur only in spaces between genes.
Conclusion:
The apparently conflicting data of Kitani (1978), Mortimer and Fogel (1974), and Getz et al. (2008, this issue) are reconciled as follows: (1) Mortimer and Fogel's (1974) use of well-repairable mismatches prevented them from seeing the noninterfering 5:3 crossovers identified by Kitani (1978) in Sordaria and by Getz et al. (2008) in yeast, and (2) junction-directed MMR in symmetric heteroduplexes (Figure 1D), as manifested in Sordaria by abundant aberrant 4:4 tetrads, would fail to generate the interfering 6:2 crossovers seen in yeast (Getz et al. 2008).
Kitani's remarkable 1974/1978 contribution appears to have been a casualty of poor timing. It could be reconciled with Mortimer and Fogel (1974) and with Fogel et al. (1979) only with the realization that both yeast and Sordaria have two meiotic recombination stages, which differ from each other with respect to both crossover interference and MMR.
Readers can consult Kitani (1978) as well as Kitani's (1989) review article for a full treatment of his views as held at that time. The 1978 article is available in the online Genetics archives. The 1989 article, available through PubMed, appears to be the last published word by Kitani on Sordaria genetics. Since losing his laboratory and cultures upon closure of the Kihara Institute for Biological Research at Matsukawa, Yokohama, Japan, Kitani has been exercising his interests in Chinese and English poetry and in gardening (Y. Kitani, personal communication).
Acknowledgments
In his last days, David Perkins put us in touch with Y. Kitani and encouraged us to record this episode in the history of fungal genetics. We are deeply grateful to Kitani, who made this effort possible by giving us carefully assembled copies of 76 documents related to the publication of Kitani (1978). Jim Haber and John Cairns improved the accuracy and clarity of our text.
This article is dedicated to the memory of David Perkins and Dorothy Newmeyer Perkins, whose own perspectives were broad, intelligent, and generous.
References
- Carpenter, A. T. C, 1979. Recombination nodules and synaptonemal complex in recombination-defective females of Drosophila melanogaster. Chromosoma 75 259–292. [DOI] [PubMed] [Google Scholar]
- Fogel, S., and R. K. Mortimer, 1971. Recombination in yeast. Annu. Rev. Genet. 5 219–236. [DOI] [PubMed] [Google Scholar]
- Fogel, S., D. D. Hurst and R. K. Mortimer, 1971. Gene conversion in unselected tetrads from multipoint crosses. Stadler Symp. 1/2 89–110. [Google Scholar]
- Fogel, S., R. Mortimer, K. Lusnak and F. Tavares, 1979. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harbor Symp. Quant. Biol. 43 1325–1341. [DOI] [PubMed] [Google Scholar]
- Foss, E., R. Lande, F. W. Stahl and C. M. Steinberg, 1993. Chiasma interference as a function of genetic distance. Genetics 133 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss, H. M., K. J. Hillers and F. W. Stahl, 1999. A role for mismatch repair directed by biased resolution of the recombinational intermediate. Genetics 153 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz, T. J., S. A. Banse, L. S. Young, A. V. Banse, J. Swanson et al., 2008. Reduced mismatch repair of heteroduplexes reveals “non”-interfering crossing over in wild-type Saccharomyces cerevisiae. Genetics 178 1251–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, J. E., B. L. Ray, J. M. Kolb and C. I. White, 1993. Rapid kinetics of mismatch repair of heteroduplex DNA that is formed during recombination in yeast. Proc. Natl. Acad. Sci. USA 90 3363–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday, R., 1964. A mechanism for gene conversion in fungi. Genet. Res. 5 282–304. [DOI] [PubMed] [Google Scholar]
- Holm, P. B., and S. W. Rasmussen, 1980. Chromosome pairing, recombination nodules and chiasma formation in diploid Bombyx males. Carlsberg Res. Commun. 45 483–548. [Google Scholar]
- Kitani, Y., 1978. Absence of interference in association with gene conversion in Sordaria fimicola, and presence of interference in association with ordinary recombination. Genetics 89 467–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani, Y., 1989. Composition of eukaryotic loci regarding gene conversion units and the presence or the absence of intralocus reciprocal recombination. Jpn. J. Genet. 64 295–313. [DOI] [PubMed] [Google Scholar]
- Kitani, Y., L. S. Olive and A. S. El-Ani, 1962. Genetics of Sordaria fimicola. V. Aberrant segregation at the g locus. Am. J. Bot. 49 697–706. [Google Scholar]
- Leblon, G., and J.-L. Rossignol, 1973. Mechanism of gene conversion in Ascobolus immersus III. The interaction of heteroalleles in the conversion process. Mol. Gen. Genet. 122 165–182. [DOI] [PubMed] [Google Scholar]
- Malkova, A., J. Swanson, M. German, J. H. McCusker, E. A. Housworth et al., 2004. Gene conversion and crossing over along the 405-kb left arm of Saccharomyces cerevisiae chromosome VII. Genetics 168 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson, M. S., and C. M. Radding, 1975. A general model for genetic recombination. Proc. Natl. Acad. Sci. USA 72 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer, R. K., and S. Fogel, 1974. Genetical interference and gene conversion, pp. 263–275 in Mechanisms in Recombination, edited by R. F. Grell. Plenum, New York/London.
- Nag, D. K., and T. D. Petes, 1991. Seven-base-pair inverted repeats in DNA form stable hairpins in vivo in Saccharomyces cerevisiae. Genetics 129 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette, N., and J.-L. Rossignol, 1978. Gene conversion spectrum of 15 mutants giving post-meiotic segregation in the b2 locus of Ascololus immersus. Mol. Gen. Genet. 163 313–326. [Google Scholar]
- Perkins, D. D., A. S. El-Ani, L. S. Olive and Y. Kitani, 1963. Interference between exchanges in tetrads of Sordaria fimicola. Am. Nat. 97 249–252. [Google Scholar]
- Roman, H., 1985. Gene conversion and crossing over. Environ. Mutagen. 7 923–932. [DOI] [PubMed] [Google Scholar]
- Stadler, D. R., 1959. The relationship of gene conversion to crossing over in Neurospora. Proc. Natl. Acad. Sci. USA 45 1625–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler, D. R., and A. M. Towe, 1971. Evidence for meiotic recombination in Ascobolus involving only one member of a tetrad. Genetics 68 401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, F. W., 1994. The Holliday junction on its thirtieth anniversary. Genetics 138 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, F. W., H. M. Foss, L. S. Young, R. H. Borts, M. F. F. Abdullah et al., 2004. Does crossover interference count in Saccharomyces cerevisiae? Genetics 168 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H., D. Treco and J. W. Szostak, 1991. Extensive 3′-overhanging, single-stranded DNA associated with the meiosis-specific double-strand breaks at ARG4 recombination initiation site. Cell 64 1155–1161. [DOI] [PubMed] [Google Scholar]
- Szostak, J., T. L. Orr-Weaver, R. J. Rothstein and F. W. Stahl, 1983. The double-strand-break repair model for recombination. Cell 33 25–35. [DOI] [PubMed] [Google Scholar]
- Zalevsky, J., A. J. MacQueen, J. B. Duffy, K. J. Kemphues and A. M. Villeneuve, 1999. Crossing over during Caenorhabditis elegans meiosis requires a conserved MutS-based pathway that is partially dispensable in budding yeast. Genetics 153 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]