Abstract
The cottontail rabbit papillomavirus (CRPV) / rabbit model has proved useful for the investigation of prophylactic and therapeutic vaccines and for the study of the pathogenesis of papillomavirus infection. It is currently the only animal model in which the entire viral program can be recapitulated, including progression to cancer. CRPV DNA is infectious in domestic rabbits and therefore mutants can be studied without the need to generate corresponding viruses. Although the CRPV animal model is used widely in various laboratories, no optimized or standardized method is used for creating CRPV viral and especially DNA infections. These different methods have made it difficult for investigators to compare results from laboratory to laboratory. A simple and highly efficient method is reported here; it has been refined based on previous methodology for the production of CRPV infections from both virus and plasmid DNA. This method can be adapted easily by other investigators in the field. The resulting standardization will aid in the evaluation of data from different laboratories.
Keywords: wounding, infectivity, challenge, CRPV, viral DNA
1. Introduction
The cottontail rabbit papillomavirus was one of the first viruses documented to be associated with the etiology of cancer. Since then, it has been investigated extensively(Brandsma 2005;Campo 2002;Christensen et al. 2000). CRPV studies provided some of the proof of concept data(Christensen et al. 1996;Lowy and Schiller 2006) that lead to the Gardasil™ vaccine recently released as a prophylactic vaccine against a few of the most troublesome human papillomaviruses. The model has been used to study fundamental questions of papillomavirus biology, cell mediated immune responses, and cancer progression (Breitburd, et. al., 2006). It is also a useful model for the testing of drug candidates against virus-induced tumors(Christensen, 2005).
Different laboratories have developed different methods for infecting the animals and this has led to difficulties in the interpretation and comparison of results. In the past, this laboratory has used a mixture of turpentine and acetone to create hyperplastic skin prior to viral DNA delivery(Kreider et al. 1995). While this technique was effective, the potential toxicity of the chemicals argued for a better challenge method. The question was asked whether simple wounding of the skin prior to infection would be adequate. For many years, investigators have shown that wounding confers an advantage to the virus (Friedewald, WF, 1942). Other studies have led to the hypothesis that wounding increases epidermal cells that are the likely targets of CRPV infection (Nonnenmacher et al. 2006). Indeed, it was found that a more consistent outcome could be achieved using this simple technique(Hu et al. 2006). In addition, when the same treatment was applied prior to virus infection, the papilloma outgrowth was significantly improved. Because the method is simple and effective, the procedure is now the standard infection method for virus and viral DNA challenge in this laboratory.
2. Materials and methods
2.1 Hershey CRPV virus and CRPV DNA
Hershey CRPV (H.CRPV) virus stock was generated in immune compromised nude mice and titrated to determine a suitable infectious dose. The virus was found to be reliably infectious at dilutions of 10−2 in previous studies(Han et al. 2000). H.CRPV DNA cloned at SalI was used for this study(Hu et al. 2002b). The Hershey strain differs minimally from the reference clone (GenBank accession number is KO2708 for reference clone), primarily in the putative E5 region but also in other regions throughout the genome. Percent nucleotide difference between the reference clone and H.CRPV is 0.6%.
2.2 Rabbit pre- treatment
New Zealand White (NZW) rabbits were maintained in the animal facility of the Pennsylvania State University College of Medicine. The studies were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine.
Before viral or DNA challenge, rabbits were anaesthetized with a mixture of 40mg/kg Ketamine and 5mg/kg Xylazine. Rabbit backs were shaved and scarified using a scalpel blade, the number of sites depending upon the experiment. The scarified sites were about one cm in diameter and were created by scraping the scalpel blade across the skin to create a “brush burn”-like lesion sufficient to produce a serous fluid with minimal bleeding. For the initial study to test the ability of wounding to promote papilloma growth, animals were scarified and DNA was applied at day 3, 5, 6 or 10 following the scarification. In this experiment, a zero time point was not included as earlier work had shown infections were unreliable when initiated at this time point. Based on the results of this preliminary study, scarification at day 0 (the day of the infection) or day −3 (three days prior to infection) was chosen for the remainder of the studies.
A Dermo-jet (Robbins Instruments, Passaic, New Jersey) was also used for wounding for comparison. The Dermo-jet is available with two heads, one with five injectors and one with a single injector.
2.3 Virus infection
Different dilutions of H.CRPV stock virus were applied to sites on the anaesthetized rabbits in a total volume of 50µl of PBS at day 0 or three days following scarification. Animals were carefully placed on their abdomens to allow virus to dry before being returned to their cages.
2.4 DNA infection
Viral DNA was prepared as described previously and delivered by a traditional pipette or by a Helios Gene Gun (Bio-Rad, Hercules, CA).
For pipette delivery, the appropriate amount of DNA in 1×TE buffer (50 µl total volume) was used. The DNA was applied to the previously scarified site and a tuberculin syringe with 25G needle was used to scratch the DNA into the wound; a total of 20 scratches were used per site, five scratches in each of four directions. The scratches were of such intensity as to generate micro abrasions but not to remove the scab. Challenge sites were marked at a location midway between each left and right site with a small volume of Pelican ink delivered intradermally. These markings remained visible throughout the course of the experiments.
For gene gun delivery, the bullets were prepared as described previously(Hu et al. 2002b). In brief, the DNA was concentrated to 1µg/µl and coated onto 1.6 micron gold particles following the protocol supplied by the manufacturer. The DNA-coated gold was precipitated onto Tefzel tubing according to the manufacturer’s protocol. The tubing was cut into 1.2cm lengths for the gene gun cartridges. Bullets were prepared just prior to use to assure integrity of the DNA. 10 shots (theoretically 10µg) were delivered to each pre-scarified site at a pressure of 400 psi. The gun was moved slightly between shots so that the shots were not entirely co-localized.
2.5 Statistics
Monitoring of papilloma outgrowth normally began three weeks after DNA or virus delivery and continued until week 12. Experience has shown that the data obtained from the first three months of growth, before the animals begin to gnaw the lesions, is more consistent. Papilloma size was determined by calculating the cubic root of the product of length × width × height of individual papillomas in millimeters to obtain a geometric mean diameter (GMD). Data were represented as the means (± SEMs) of the GMDs for each test group. Typically, each site developed numerous tiny papillomas by week 3, which then become confluent by week 5 or 6. New papillomas did not appear to develop after week 3.
3. Results
3.1 Wounding prior to plasmid DNA infection resulted in robust papilloma growth
The previous DNA challenge method utilized turpentine-acetone pre-treatment to create a hyperproliferative state in the skin(Kreider et al. 1995). Because of the concern about potential toxicity of both turpentine and acetone and also because of the odor from treatment with these chemicals, it was hypothesized that an alternative method of simple wounding prior to infection could be equally effective. The back skin of the rabbits was scarified and DNA was applied at day three, five, six or ten following this pre-scarification. In this pilot experiment, two rabbits were used and there were two sites for each time point. The papilloma outgrowth was monitored weekly from 3 weeks post challenge.
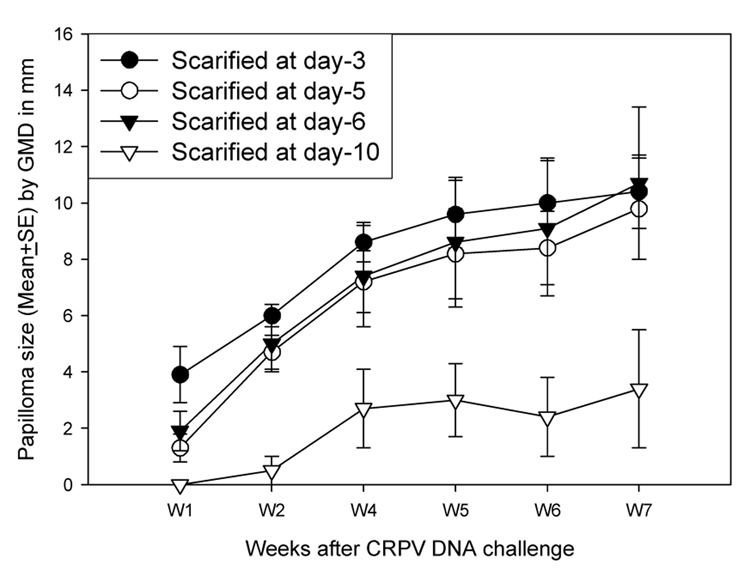
The data showed that infection at three, five and six days following wounding was efficient (Figure 1). However, infectivity in rabbits challenged at day ten was at a lower efficiency than for the earlier time points.
Figure 1.
Papilloma outgrowth on sites pre-scarified at day −3 to −10 before DNA challenge. Two sites on each of two rabbits scarified at day −3 to −10 were challenged with 10µg of CRPV DNA. All the pre-scarification time points except day −10 showed consistent papilloma growth as early as one week after DNA challenge. No significant difference in papilloma size was found among these treatments (P>0.05, unpaired student t test)‥
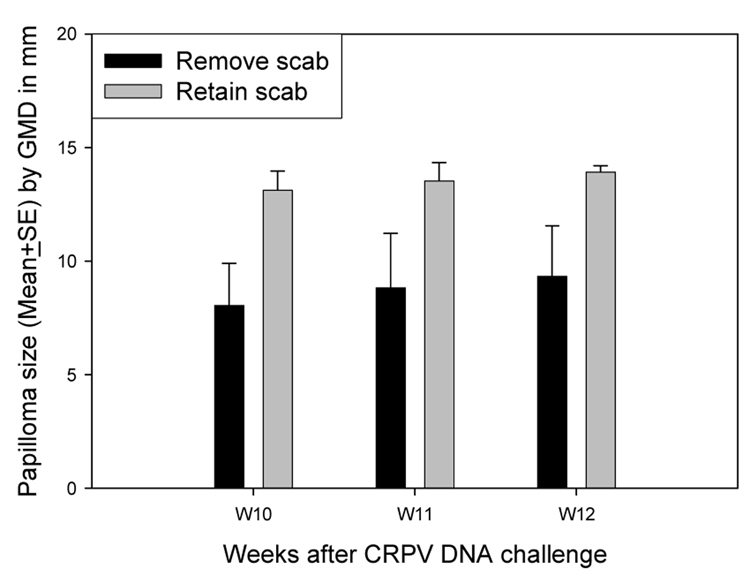
In another pilot study using pre-scarification at day - 3, the papilloma outgrowth in animals challenged with viral DNA with or without removal of the scab was compared. Papillomas on sites from which the scab had been removed were significantly smaller than those on sites on which the scab had been retained. (P<0.05, unpaired student t test, Figure 2). Therefore, removal of scabs prior to infection reduced efficiency of infection.
Figure 2.
Papilloma outgrowth on sites retaining scab or removing scab. Two sites on each of two rabbits scarified at day −3 were challenged on day 0 with 10µg of CRPV DNA with or without removing the scab. Papillomas on sites from which the scab had been removed were significantly smaller than those on sites on which the scab had been retained (P<0.05, unpaired student t test).
3.2 5µg DNA was sufficient to induce papillomas more consistently
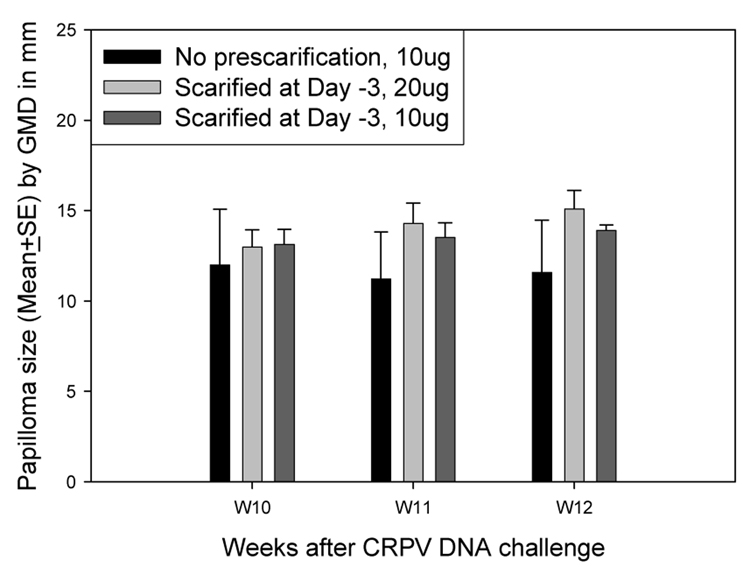
The results in the pilot study indicated that wounding 3–6 days prior to DNA application was effective. The amount of DNA sufficient to induce uniform tumors in rabbits was next determined. First, two animals were challenged with 10µg (the normal dose for DNA infection) and 20µg CRPV DNA at two sites per animal following pre-scarification at day −3. Control sites were challenged with 10 µg plasmid DNA without pre-scarification. The 10µg DNA challenge sites induced tumors comparable to the 20µg DNA challenge sites and the standard error of the tumor sizes was smaller (Figure 3). Therefore, 10µg DNA was preferable to 20 micrograms.
Figure 3.
Papilloma outgrowth on sites challenged with different doses of CRPV DNA. Two sites on each of two rabbits were challenged with 10µg and 20µg of CRPV 3 days after scarification. Control sites received 10µg DNA with no pre-scarification. Papillomas on sites challenged with 10µg of CRPV plasmid were comparable to those on sites challenged with 20µg of CRPV plasmid (P>0.05, unpaired student t test).
3.3 Wounding three days prior to infection with viral DNA increased infection efficiency and papilloma size
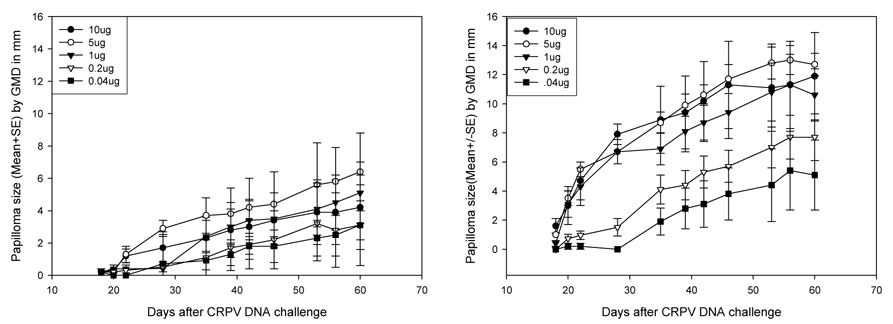
To explore further the optimal dose for DNA challenge, 10µg, 5µg, 1µg, 200ng and 40 ng aliquots of plasmid DNA were prepared in 50µl 1×TE and delivered at two sites each to five NZW rabbits. In this experiment, one site per dose was scarified at day −3 and one site was scarified just prior to infection. All sites scarified at day −3 and then challenged with 10µg, 5µg, 1µg and 200ng of DNA grew papillomas, as did three of the five sites challenged with 40 ng DNA (Figure 4B). A number of the sites that were not pre-scarified also developed papillomas although infection was variable (Figure 4A). These results demonstrated that pre-scarification at day −3 yielded reliable infections with DNA loads as low as 200ng. The results also showed that 5µg DNA produced more consistent papillomas than did 10µg (Figures 5). The new wounding technique will enable DNA use to be reduced thereby resulting in cost savings while at the same time yielding results that are more consistent.
Figure 4.
Papilloma outgrowth on sites challenged with different doses of CRPV DNA with (B) or without (A) pre-scarification. Two sites on each of five rabbits were challenged with from 0.04µg to 10µg of CRPV either 3 days after scarification (B) or without prior scarification (A). Some sites challenged with low amounts of CRPV DNA (0.04µg) grew papillomas following pre-scarification while scattered papillomas were found on sites challenged without pre-scarification (A).
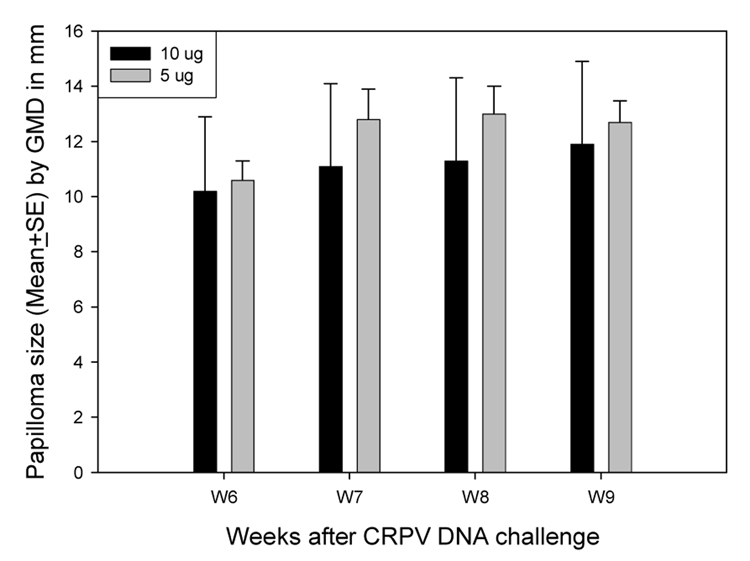
Figure 5.
Papilloma outgrowth on sites challenged with 5µg of CRPV was comparable to that on sites challenged with 10µg of CRPV. Two sites on each of four rabbits were challenged with 5µg and 10µg of CRPV three days after scarification. 5µg of CRPV was as efficient as 10 micrograms in inducing papillomas (P>0.05, unpaired student t test).
3.4 Wounding three days prior to virus infection resulted in infections that were more efficient
Previous studies with infectious CRPV have been carried out without pre-scarification. Following the observations that significant improvements in viral DNA infectivity could be achieved with pre-challenge wounding, it was important to determine whether this method could also improve the efficiency of virus infection. Sequential 1:10 dilutions of a stock of H.CRPV were made to yield dilutions of 10−2, 10−3, 10−4, 10−5, and 10−6.
Five animals were challenged at two sites with each of the dilutions. One site of these two sites was scarified at day −3 and the other site was not. Animals were monitored daily for papilloma outgrowth for the first three weeks and papillomas were measured each day. Monitoring and measuring were then continued on a weekly basis.
Day −3 pre-scarification (Figure 6B) reliably produced two logs of improvement in infectivity of the virus when compared with no pre-scarification (Figure 6A). Papillomas appeared on some sites challenged with 10−5 virus. This dilution is three logs below the normal dose used in this laboratory, the dose found to reliably produce papillomas in the absence of prior scarification. Therefore pre-scarification three days prior to viral infection greatly enhanced viral infectivity.
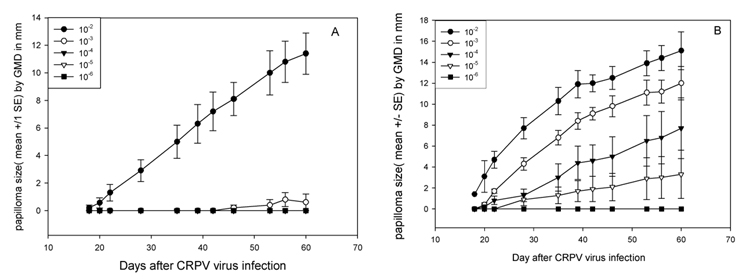
Figure 6.
Papilloma outgrowth on sites challenged with different doses of CRPV infectious virus with (B) or without (A) pre-scarification. Two sites on each of five NZW rabbits were challenged with from 10−2 to 10−6 dilutions of H.CRPV virus stock at 3 day after scarification (B) or with no pre-scarification (A). No sites challenged with a low dose (lower than 10−3) grew papillomas without pre-scarification treatment (A) while papillomas were found on sites challenged with doses of CRPV as low as 10−5 with pre-scarification (B).
3.5 Efficiency of DNA delivery by gene gun was improved by pre-scarification
The gene gun and related delivery systems have been used by investigators in other laboratories to produce CRPV DNA infections. It was important therefore to test if pre-scarification could improve the efficiency of CRPV DNA infection by gene gun delivery.
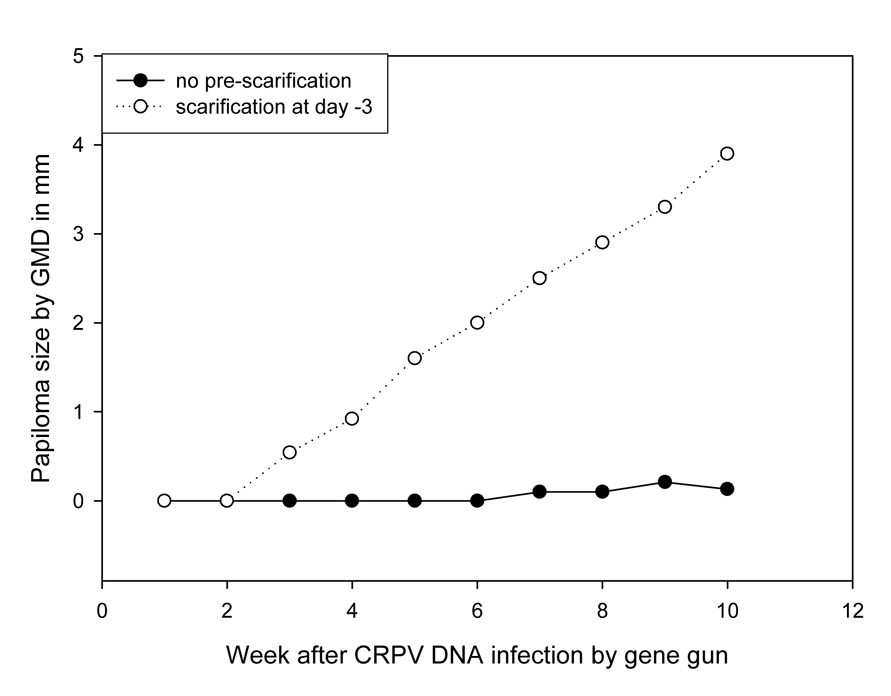
Five rabbits were used for this study. Pre-scarification three days prior to gene gun infection resulted in more efficient production of papillomas (Figure 7, P<0.05, unpaired student t test).
Figure 7.
Papilloma outgrowth on sites challenged with 10µg of CRPV DNA delivered by gene-gun with or without pre-scarification. Two sites on each of five rabbits were challenged with 10µg of CRPV DNA 3 days after scarification or with no pre-scarification. A few sites without pre-scarification treatment grew tiny papillomas whereas most sites with pre-scarification grew significantly larger papillomas (P<0.05, unpaired student t test).
3.6 The Dermo-jet did not produce a wounding environment capable of improving efficiency of DNA infection
The scarification method requires anaesthetizing the animals. The Dermo-jet can be used without anaesthetization and creates wounding with little discomfort to the animal. An experiment was conducted to determine whether this instrument could be used to generate wounding sufficient for DNA infection.
Two rabbits were used for this pilot study. Five sites on the left and right sides were wounded at day −3 using the head with five injectors on one side and the head with a single injector on the other. The rabbits were then challenged with 10µg CRPV DNA on day 0.
Wounding via the Dermo-jet three days prior to infection, either with the single injector head or the multiple injector head, did not result in subsequent reliable papilloma growth (Data not shown). Only 50% of the challenge sites grew papillomas. Thus, Dermo-jet wounding is not an adequate substitute for scarification via scalpel blade.
4. Discussion
The CRPV model is the only animal model capable of recapitulating the full range of papillomavirus pathology from initiation of infection to malignant progression(Brandsma 2005;Campo 2002;Christensen et al. 2000). The model is also valuable for the investigation of immune responses to papillomavirus antigens(Christensen 2005). A significant benefit of the model is that CRPV DNA containing the cloning vector PUC19 is infectious(Hu et al. 2002a;Hu et al. 2007). This characteristic, therefore, allows papillomavirus DNA mutants to be studied in vivo, something that cannot be done in other animal models to date. However, different CRPV DNA challenge methods have been used in different laboratories(Brandsma et al. 1991;Jeckel et al. 2002;Kreider et al. 1995;Lin et al. 1993;Nonnenmacher et al. 2006;Salmon et al. 1997;Salmon et al. 2000). Some delivery methods are more efficient than others and these outcomes complicate the interpretation of results obtained in the different laboratories. A panel of CRPV DNA mutants has been tested for infectivity in this laboratory and there has always been concern about the efficiency of the challenge method(Hu et al. 2006). After several years of comparative studies, the turpentine-acetone pretreatment protocol was replaced with an improved new method that is simple to use and consistent from experiment to experiment. This new method can be adapted by other laboratories using the CRPV/rabbit model.
In this work, the influence of wounding on the ability of plasmid DNA and infectious virus to create infections on the back skin of rabbits was examined. Papillomavirus infections are associated with abrasions and it is commonly believed that wounding is necessary to initiate infection (Kreider et al. 1995). In this study, the wounding created three days prior to DNA or virus infection significantly enhanced the efficiency of infection implying that the wound-healing environment is of importance to the infection. In past studies, wounding/proliferation was achieved by the application of turpentine and acetone prior to infection with viral DNA (Kreider et al. 1995). The turpentine and acetone technique was unpleasant for the animals and unhealthy for the technicians. In this study, a more benign alternative for wounding was developed; this alternative strategy allowed for efficient and consistent papilloma production.
Different time points of wounding prior to viral DNA application were examined. Pre-scarification three to six days prior to application of the viral DNA improved infectivity significantly when compared to no pre-scarification. Pre-scarification three days prior to viral DNA challenge yielded papilloma outgrowth with as little as 40ng DNA infection. In addition, a lower amount of viral DNA (half of the usual dose) was sufficient for an effective and consistent infection. These data demonstrate that the new challenge method is safer, more reliable and more cost-effective than the previous technique. Furthermore, this wounding method also resulted in more than two logs of improvement in infectivity of virus. These findings will be of special importance to laboratories that utilize virus for their in vivo infection experiments but do not have production capabilities.
Wounding with the Dermo-jet was tested in the study as well. The Dermo-jet induces very small surface abrasions and it is not necessary to anaesthetize the rabbits when using this device. It would be a preferable way to create a wound in comparison with the scalpel blade, the use of which requires prior anaesthetization. The data showed convincingly, however, that wounding by the Dermo-jet was not equivalent to wounding by scalpel blade. In work not reported here, Dermojet delivery of DNA to pre-scarified sites was an effective method to induce papillomas indicating that the proper cells are targeted by the device once the wounding environment has been established.
The gene gun and the Biojector were reported to deliver viral DNA into rabbit skin by other investigators (Brandsma et al. 1991, Nonnenmacher, et. al, 2006). The gene gun has been used in this laboratory for vaccination and has been shown to be very effective(Han et al. 2000). However, the gene-gun delivery system is expensive and this laboratory has not made use of this technology for DNA infections to any significant extent. Limited attempts in the past to create infections resulted in variable papilloma growth (data not shown). In the present study, pre-scarification treatment was coupled with the gene gun delivery system for viral DNA challenge; significant improvement in infection was achieved with gene-gun delivered DNA when sites were scarified three days prior to infection.
In summary, the improved challenge method utilizing simple wounding via scalpel blade three days prior to infection set the stage for productive DNA and virus infections. Based on the results of this study, it is recommended that the following methodology be used for establishing DNA and virus infections:
Scarify sites on the backs of the rabbits at day - 3;
Apply either DNA (5µg) or virus (dilution dependent upon titration) three days later (day 0) in a volume of 50µl using a laboratory pipettor for delivery. Scratch the DNA or virus into the wound with the edge of a fine needle, e.g. 25G. Take care not to remove the scab.
Acknowledgements
This work was supported by NCI grant CA47622 and NIAID grant AI15435 (Utah State University; subcontract to Penn State University, College of Medicine), and the Jake Gittlen Memorial Golf Tournament.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Brandsma JL. The cottontail rabbit papillomavirus model of high-risk HPV-induced disease. Methods Mol. Med. 2005;119:217–235. doi: 10.1385/1-59259-982-6:217. [DOI] [PubMed] [Google Scholar]
- Brandsma JL, Yang ZH, Barthold SW, Johnson EA. Use of a rapid, efficient inoculation method to induce papillomas by cottontail rabbit papillomavirus DNA shows that the E7 gene is required. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4816–4820. doi: 10.1073/pnas.88.11.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitburd F, Nonnenmacher M, Salmon J, Orth G. Rabbit viral papillomas and carcinomas: model diseases for human papillomavirus infections and carcinogenesis. In: Campo S, editor. Papillomavirus research: from natural history to vaccines and beyond. Norwich, UK: caister Academic Press; 2006. pp. 339–420. [Google Scholar]
- Campo MS. Animal models of papillomavirus pathogenesis. Virus Res. 2002;89:249–261. doi: 10.1016/s0168-1702(02)00193-4. [DOI] [PubMed] [Google Scholar]
- Christensen ND. Cottontail rabbit papillomavirus (CRPV) model system to test antiviral and immunotherapeutic strategies. Antivir. Chem. Chemother. 2005;16:355–362. doi: 10.1177/095632020501600602. [DOI] [PubMed] [Google Scholar]
- Christensen ND, Pickel MD, Budgeon LR, Kreider JW. In vivo anti-papillomavirus activity of nucleoside analogues including cidofovir on CRPV-induced rabbit papillomas. Antiviral Research. 2000;48:131–142. doi: 10.1016/s0166-3542(00)00124-8. [DOI] [PubMed] [Google Scholar]
- Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 1996;70:960–965. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WF. Cell state as affecting susceptibility to a virus. Enhanced effectiveness of the rabbit papillomavirus on hyperplastic epidermis. J. Exp. Med. 1942;75:197–219. doi: 10.1084/jem.75.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R, Reed CA, Cladel NM, Christensen ND. Immunization of rabbits with cottontail rabbit papillomavirus E1 and E2 genes: protective immunity induced by gene gun-mediated intracutaneous delivery but not by intramuscular injection. Vaccine. 2000;18:2937–2944. doi: 10.1016/s0264-410x(00)00110-9. [DOI] [PubMed] [Google Scholar]
- Hu J, Cladel NM, Balogh K, Budgeon L, Christensen ND. Impact of genetic changes to the CRPV genome and their application to the study of pathogenesis in vivo. Virology. 2007;358:384–390. doi: 10.1016/j.virol.2006.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Cladel NM, Pickel MD, Christensen ND. Amino acid residues in the carboxy-terminal region of cottontail rabbit papillomavirus E6 influence spontaneous regression of cutaneous papillomas. J. Virol. 2002a;76:11801–11808. doi: 10.1128/JVI.76.23.11801-11808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Han R, Cladel NM, Pickel MD, Christensen ND. Intracutaneous DNA vaccination with the E8 gene of cottontail rabbit papillomavirus induces protective immunity against virus challenge in rabbits. J. Virol. 2002b;76:6453–6459. doi: 10.1128/JVI.76.13.6453-6459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeckel S, Huber E, Stubenrauch F, Iftner T. A transactivator function of cottontail rabbit papillomavirus e2 is essential for tumor induction in rabbits. J. Virol. 2002;76:11209–11215. doi: 10.1128/JVI.76.22.11209-11215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider JW, Cladel NM, Patrick SD, Welsh PA, DiAngelo SL, Bower JM, Christensen ND. High efficiency induction of papillomas in vivo using recombinant cottontail rabbit papillomavirus DNA. J. Virol. Methods. 1995;55:233–244. doi: 10.1016/0166-0934(95)00062-y. [DOI] [PubMed] [Google Scholar]
- Lin YL, Borenstein LA, Ahmed R, Wettstein FO. Cottontail rabbit papillomavirus L1 protein-based vaccines: protection is achieved only with a full-length, nondenatured product. J. Virol. 1993;67:4154–4162. doi: 10.1128/jvi.67.7.4154-4162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J. Clin. Invest. 2006;116:1167–1173. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnenmacher M, Salmon J, Jacob Y, Orth G, Breitburd F. Cottontail rabbit papillomavirus E8 protein is essential for wart formation and provides new insights into viral pathogenesis. Journal of Virology. 2006;80:4890–4900. doi: 10.1128/JVI.80.10.4890-4900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J, Nonnenmacher M, Caze S, Flamant P, Croissant O, Orth G, Breitburd F. Variation in the nucleotide sequence of cottontail rabbit papillomavirus a and b subtypes affects wart regression and malignant transformation and level of viral replication in domestic rabbits. J. Virol. 2000;74:10766–10777. doi: 10.1128/jvi.74.22.10766-10777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J, Ramoz N, Cassonnet P, Orth G, Breitburd F. A cottontail rabbit papillomavirus strain (CRPVb) with strikingly divergent E6 and E7 oncoproteins: An insight in the evolution of papillomaviruses. Virology. 1997;235:228–234. doi: 10.1006/viro.1997.8680. [DOI] [PubMed] [Google Scholar]