Abstract
Various surgical procedures, e.g. thoracotomy and inguinal hernia repair, frequently evoke persistent pain lasting for many months following the initial surgery. The essential prolonged tissue retraction required during such surgeries may account for the persistence and high incidence of postoperative pain in these patient populations. This study describes a new rat model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR), akin to a clinical procedure. Under anaesthesia, skin and superficial muscle of the medial thigh was incised and a small pair of retractors inserted. This tissue was retracted for one hour causing potential stretch of the saphenous nerve. SMIR surgery evoked persistent significant mechanical hypersensitivity to von Frey stimulation of the plantar ipsilateral hindpaw, compared to either pre-surgery responses or concurrent responses of sham-operated rats. SMIR-evoked mechanical hypersensitivity was observed by postoperative day 3, most prominent between postoperative days 10–13, persisted until at least postoperative day 22 and had dissipated by postoperative day 32. Overall, mechanical sensitivity of the SMIR contralateral paw and the sham ipsilateral paw did not significantly change from pre-surgery responses. SMIR did not evoke significant heat hyperalgesia or cold allodynia. Light microscopy of saphenous nerve sections did not show degeneration or oedema in the saphenous nerve at, or proximally or distally to, the surgical site. In addition, very little to no degeneration was detected with ATF3 staining in DRG from SMIR-operated rats. These data suggest that prolonged retraction of superficial tissue evokes a persistent pain syndrome that is not driven by neuronal damage.
1. Introduction
Persistent postoperative pain occurs following various common surgical procedures including thoracotomy, inguinal hernia repair, coronary artery bypass surgery and caesarean section. All of these procedures involve essential and often prolonged tissue retraction that could account for the persistent nature and high incidence of pain following such surgeries. Postoperative pain following thoracotomy occurs in 30–60% of patients and is reported to persist for 3–30 months (for review see Perkins and Kehlet 2000). A similar duration of postoperative pain following inguinal hernia repair is observed, although its incidence is more variable (for review see Perkins and Kehlet 2000). A review of published studies between 1987 and 2000 showed that 0–63% of patients had pain one year after surgery (Poobalan et al. 2003). The severity of pain within patient populations one year following inguinal herniorrhaphy also differs. In one study of 315 patients, 62.9% had postoperative pain and 11.9% described moderate to severe pain (Cunningham et al. 1996) whereas in another study of 1,166 patients, 28.7% had postoperative pain and 3% described moderate to severe pain (Bay-Nielsen et al. 2001). These studies, among many others, reveal that persistent postoperative pain is a significant clinical problem that reduces quality of life. Furthermore, postoperative pain is a widespread disorder that has been estimated to affect over 60,000 patients per year in the US from inguinal hernia repair surgery alone (Kehlet et al. 2006).
The pathogenic mechanisms underlying persistent postoperative pain are under debate; whether the origin for these pain syndromes are nociceptive from the skin incision, inflammatory, from muscle damage for example, or neuropathic from surgical injury to peripheral nerves, is unclear. Perhaps persistent postoperative pain could arise from a combination of these three possible etiologies. Laboratory investigations of postoperative pain have largely focussed upon nociceptive mechanisms with two rat models of acute incisional pain (Brennan et al. 1996; Duarte et al. 2005). Brennan et al (1996) used a 1cm incision through the skin, fascia and muscle of the rat plantar hindpaw and Duarte et al (2005) employed a 1cm incision in the hairy back skin of the rat. Both of these models evoke 3–5 days of mechanical hypersensitivity. However, these models do not accurately reflect the clinical scenario of postoperative pain, i.e. prolonged tissue retraction resulting in persistent pain. The thoracotomy procedure has been replicated in rats (Buvanendran et al. 2004), where a 1 hour rib retraction produced a persistent mechanical and cold allodynia around the incision site in 50% of operated rats.
The aim of this study was to develop a rat model of persistent postoperative pain employing both prolonged tissue retraction (akin to clinical procedures) and the evaluation of pain behaviours via hindpaw stimulation. This was achieved by skin/muscle incision and retraction (SMIR) in the thigh of the rat and a preliminary report of this work has previously appeared in abstract form (Flatters and Strichartz 2006). Further studies addressed the extent of neuronal damage evoked by SMIR to nerves that are potentially stretched during surgery and those in neighbouring areas of innervation.
2. Methods
2.1 Animals
Adult male Sprague-Dawley rats (200–300g, Charles River USA, SASCO breeding colony) were housed in pairs on sawdust bedding in plastic cages. Artificial lighting was provided on a fixed 12:12 h light:dark cycle (7am lights on) with food and water available ad libitum. These studies were approved by the Standing Committee on Animals at Harvard Medical School and were conducted in accordance with the ethical guidelines for investigations of experimental pain issued by the International Association for the Study of Pain (Zimmermann 1983).
2.2 Skin/muscle incision and retraction (SMIR) surgery
Rats (230 – 300g) were anaesthetised with i.p. Nembutol® (phenobarbitol 50mg/ml), at doses of 65–75mg/kg, laid on their back and the medial thigh on one side was shaved. The shaved skin was then repeatedly swabbed with sterile alcohol wipes to sterilize the area and to enable visualisation of the saphenous vein. A 1.5 – 2cm incision was made in the skin of the medial thigh approximately 4mm medial to the saphenous vein to reveal the muscle of the thigh. An incision (7 – 10mm long) was then made in the superficial (gracilis) muscle layer of the thigh, approximately 4 mm medial to the saphenous nerve. The superficial muscle was then parted further, by spreading blunt scissors within the muscle incision site, to allow the insertion of a micro dissecting retractor. The retractor had 4 prongs spaced over an 8mm distance and each prong was 4mm deep (Cat. No. 13-1090, Biomedical Research Instruments Inc, USA). The retractor was inserted into the incision site, to position all prongs underneath the superficial layer of thigh muscle. The skin and superficial muscle of the thigh were then retracted by 2cm, revealing the fascia of the underlying adductor muscles and this retraction was maintained for one hour (see Figure 1). During the retraction, the saphenous nerve is displaced and potentially stretched around the retractor, however it is not compressed against a hard surface e.g. bone, due to its superficial position on the top of the thigh muscle. The animals were closely monitored during the retraction period and if required, additional anaesthesia was provided using the rapidly reversible inhalation anaesthetic sevoflurane (Abbott Labs, USA). During the retraction period, animals were also completely covered (apart from the top of the head) with a large absorbent bench underpad (VMR International, Cat. No. 56616-031, has an absorbent layer attached to a non-absorbent backing sheet of embossed polypropylene). This was done to minimise heat loss from the whole animal during anaesthesia and to prevent dehydration of the surgical site. Following the SMIR procedure, drying of the muscle and skin of the surgical site was not observed and these tissues were closed with silk 3.0 and 4.0 Vicryl® sutures, respectively. Sham-operated rats underwent the same procedure with the exception of the skin/muscle retraction. Following recovery from anaesthesia, all animals could ambulate normally and rise up on their hindpaws to reach food and water.
Figure 1.
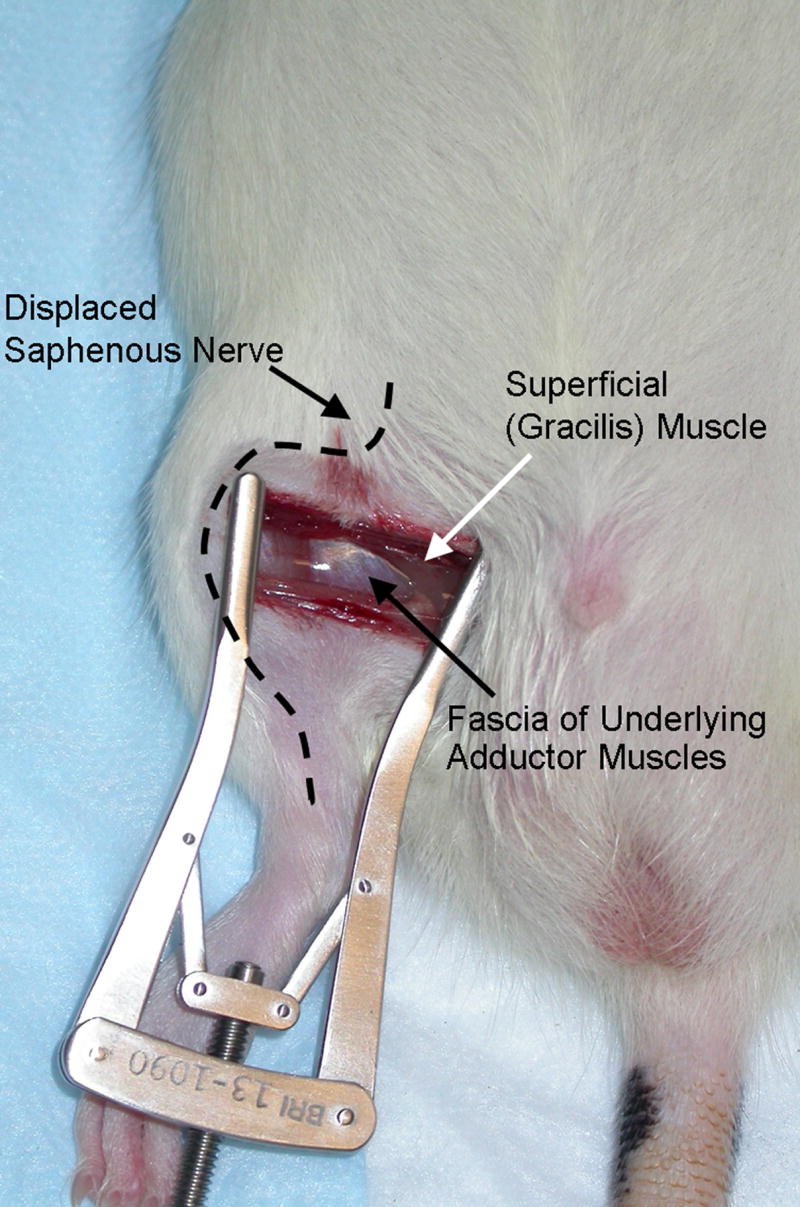
Photograph of injury site during 1-hour retraction period of skin/muscle incision and retraction (SMIR) surgery.
2.3 Behavioural Testing
Prior to any behavioural testing, all animals were habituated three times to the testing environment on three different days. Throughout the behavioural testing time courses, the experimenter was blind to the preceding surgical procedure (SMIR or sham).
2.3.1 Assessment of mechanical sensitivity
Animals were placed on an elevated wire mesh floor and confined underneath individual overturned plastic boxes. Mechanical allodynia/hyperalgesia was assessed using four von Frey filaments with bending forces of 4g, 6g, 10g and 15g (Touch-Test™ Sensory Evaluators, Stoeling Co., USA). In ascending order of force, each filament was applied 10 times, to the mid-plantar area of the hindpaw encircled by tori/footpads. Care was taken to avoid stimulating the same spot repeatedly within this region and to avoid stimulating the tori/footpads themselves. Withdrawal responses to each of the von Frey filaments from both hindpaws were counted and recorded. Prior to the SMIR procedure, three baseline measurements of mechanical sensitivity were taken on separate days and then averaged to provide the pre-surgery baseline response, denoted as postoperative day 0 on Figure 2.
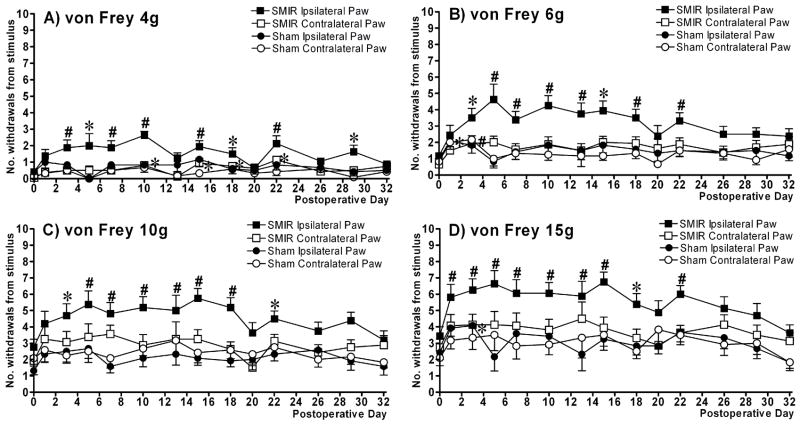
Figure 2.
Behavioural time course of mechanical hypersensitivity evoked by skin/muscle incision and retraction (SMIR) surgery. The graphs show mean ± SEM of the number of hindpaw withdrawals out of 10 stimulations with (A) von Frey 4g, (B) von Frey 6g, (C) von Frey 10g and (D) von Frey 15g. n = 16 for SMIR-operated rats and n = 12 for sham-operated rats for all time points, except on postoperative days 5, 13 & 20 where n = 8 for SMIR-operated rats and n = 6 for sham-operated rats. *p < 0.05, #p < 0.01, one-way repeated measures ANOVA with Dunnett’s post-hoc analysis compared to pre-surgery baseline (day 0). NB: Significance reached for SMIR contralateral paw to von Frey 4g (Fig. 4A) at postoperative days 10, 15, 18 and 22.
Mechanical hyperalgesia was also measured using the pinprick test (Tal and Bennett 1994). The blunt point of a safety pin was applied to the plantar surface of the hindpaw; this indented, but did not pierce the skin. The response to this stimulus was then timed with a stopwatch. The response was taken as the length of time which the hindpaw was lifted upwards from the stimulus until the hindpaw was replaced on the wire mesh platform. This was performed only once on each hindpaw. It was not possible to time the rapid response of a normal rat to pinprick stimulation with a stopwatch, therefore it was assigned a duration of 0.25 sec.
2.3.2 Assessment of heat sensitivity
Animals were placed in individual Perspex boxes on a glass floor. Nociceptive responses to a noxious heat stimulus were examined by measuring the hindpaw withdrawal latency from a focused beam of radiant heat to the plantar surface (Ugo Basile Plantar Test apparatus, Italy). The withdrawal latency to this stimulus was measured in seconds and the apparatus had a build-in cut-off latency of 31.2 seconds. The apparatus was set to give an I.R. intensity of 40 which evoked hindpaw withdrawal latencies of 12–14 seconds. Both hindpaws of each rat were tested three times and then an average of these three readings taken. To avoid sensitisation of the paws, several minutes had elapsed before the same hindpaw was re-tested. Prior to the SMIR procedure, three baseline measurements of heat sensitivity were taken on separate days and then averaged to provide the pre-surgery baseline response, denoted as postoperative day 0 on Figure 3.
Figure 3.
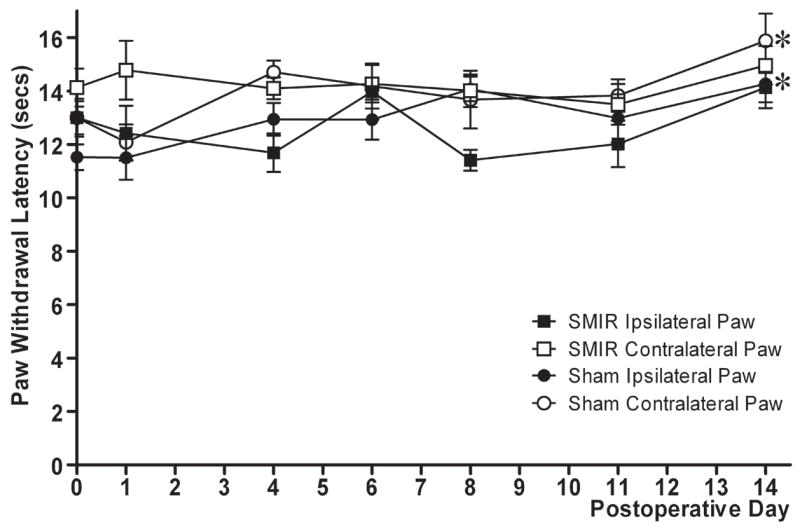
Effect of SMIR surgery on hindpaw withdrawal latencies to noxious heat stimulation. Graph shows mean ± SEM of hindpaw withdrawal latencies in seconds, prior to and following SMIR/sham surgery up to postoperative day 14. n = 8 for SMIR-operated rats, n = 6 for sham-operated rats. *p < 0.05, one-way repeated measures ANOVA with Dunnett’s post-hoc analysis compared to pre-surgery baseline (day 0).
2.3.3 Assessment of cold sensitivity
Sensitivity to cold stimulation was assessed as previously described (Flatters and Bennett 2004). Animals were placed on an elevated wire mesh floor and confined underneath individual overturned plastic boxes. Acetone (0.05 ml) was applied to the centre of the plantar hindpaw using a Gilson micro-pipette, and a stopwatch was started. In the following 20 seconds after acetone application the rat’s response was monitored. If the rat did not withdraw, flick or stamp its hindpaw within this 20-sec period then no response was recorded for that trial (0 - see below). However, if within this 20-sec period the animal responded to the cooling effect of the acetone, then the animal’s response was assessed for an additional 20 seconds, a total of 40 seconds from initial application. Responses to acetone were graded to the following 4-point scale: 0 = no response, 1 = quick withdrawal, flick or stamp of the hindpaw, 2 = prolonged withdrawal or repeated flicking (≥2) of the hindpaw, 3 = repeated flicking of the paw with licking directed at the plantar side of the hindpaw. Acetone was applied alternately three times to each hindpaw and the responses scored categorically. Cumulative scores were then generated for each hindpaw by adding the 3 scores for each hindpaw together, the minimum score being 0 (no response to any trial) and the maximum possible score being 9 (repeated flicking and licking of hindpaw in all trials). Prior to the SMIR procedure, three baseline measurements of cold sensitivity were taken on separate days and then averaged to provide the pre-surgery baseline response, denoted as postoperative day 0 on Figure 4.
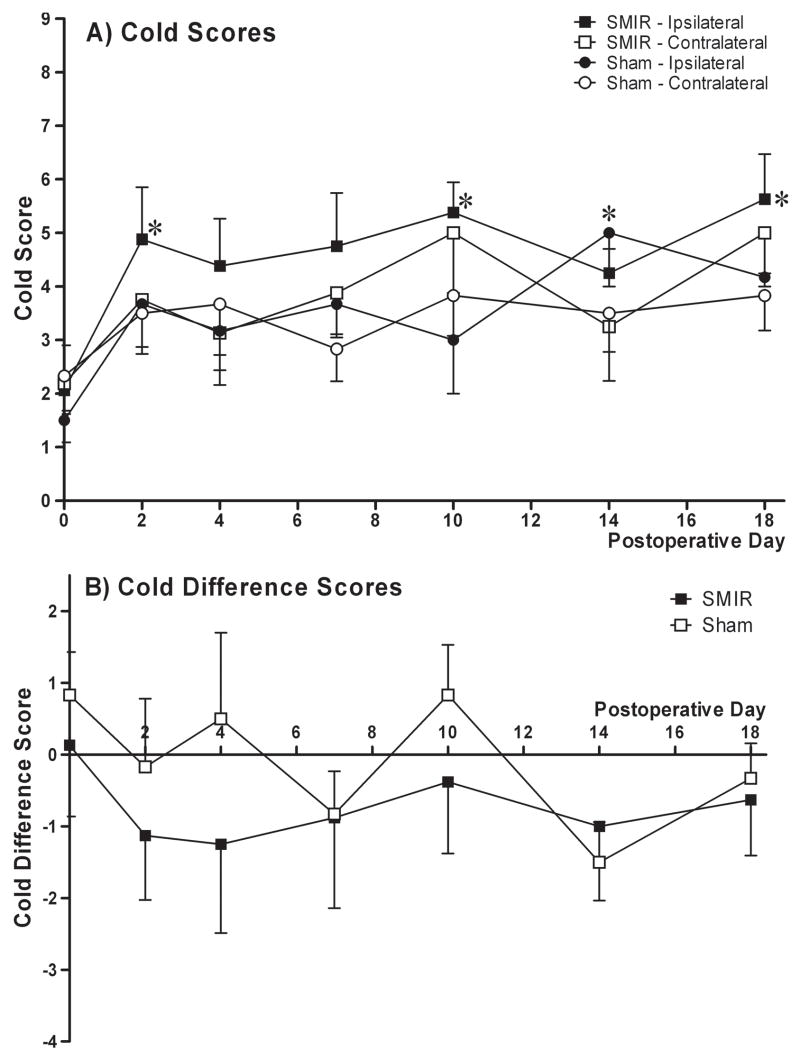
Figure 4.
Effect of SMIR surgery on hindpaw responses to cold (acetone) stimulation. (A) Time course of cold scores, expressed as mean ± SEM, in ipsilateral and contralateral paws of SMIR- (n = 8) and sham-operated (n = 6) rats from pre-surgery baseline (day 0) until postoperative day 18. *p < 0.05, Friedman test with Dunn’s post-hoc analysis compared to pre-surgery baseline (day 0). (B) Time course of cold difference scores (contralateral paw cold score - ipsilateral paw cold score) expressed as mean ± SEM for SMIR- (n = 8) and sham-operated (n = 6) rats from pre-surgery baseline (day 0) until postoperative day 18. No significant difference found between difference score of SMIR and sham group at any time point (one-tailed Mann-Whitney U-tests).
2.4 Light microscopy of saphenous nerve
Rats received an over-dose of sevoflurane anaesthesia and were transcardially perfused, using a motorised pump, with 0.9% saline containing 0.1% sodium nitrite followed by modified Karnovsky’s fixative (2.5% paraformaldehyde and 2% glutaraldehyde in 0.2M cacodylate buffer, pH 7.2). SMIR- and sham-operated rats were perfused at day 12/13 following surgery, i.e. at the maximum of evoked mechanical hypersensitivity. Ten millimetre portions of saphenous nerves were dissected at 3 levels; 1) proximal to retraction site, top of the thigh, 2) adjacent to the retraction site at mid-thigh, and 3) distal to retraction site, just above the ankle. These nerve portions were harvested from the ipsilateral side of SMIR-operated rats, ipsilateral side of sham-operated rats and the contralateral side of sham-operated rats (to act as an uninjured control). Nerve portions were then post-fixed in the fixative described above, incubated in buffered 1% osmium tetroxide, dehydrated in ascending concentrations of alcohol and embedded in blocks of Epon. One micrometre transverse sections of nerves were cut and stained with toluidine blue for examination with light microscopy. Nerve sections were photographed at 1000× and compiled to make a photo-montage of the entire cross-section of the saphenous nerves. One section, per level of harvest, per animal was analysed; n = 5 for SMIR-operated rats and n = 4–7 for sham-operated rats. From these 1000× montages, the total number of myelinated axons was counted in each nerve. The cross-sectional nerve area was calculated through the weight of paper templates of the nerve cross-section. The scale bar produced during photography was measured in centimetres on a photo montage and an exact square of paper with sides of that length was made. Using the weight of this square of known cross-sectional area, the cross-sectional areas of each nerve could be calculated in μm. The density of myelinated axons was calculated by dividing the total number of axons by the cross-sectional nerve area (no./μm2, see Table 1). In distal sections, several separate nerve fascicles were found due to nerve branching. Therefore, the largest fascicle was used for calculation of cross-sectional area and density of myelinated axons. All the quantification and analysis outlined was performed by the same investigator under blind conditions.
Table 1.
Cross-sectional area and density of myelinated axons of saphenous nerves, proximal, adjacent and distal to the surgical site in SMIR- and sham-operated rats. All nerves harvested 12/13 days following SMIR/sham surgery i.e. at the maximum SMIR-evoked mechanical hypersensitivity.
| Proximal to surgical site | Adjacent to surgical site | Distal to surgical site | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SMIR Ipsilateral |
Sham Ipsilateral |
Sham Contralateral |
SMIR Ipsilateral |
Sham Ipsilateral |
Sham Contralateral |
SMIR Ipsilateral |
Sham Ipsilateral |
Sham Contralateral |
|
| No. of animals, n | 5 | 4 | 4 | 5 | 7 | 4 | 5 | 4 | 4 |
| Cross-sectional nerve area (μm2) | 35,651 ± 3148 | 41,162 ± 5200 | 39,320 ± 2809 | 29,825 ± 3846 | 25,975 ± 5384 | 37,131 ± 1880 | 16,214 ± 2554 | 17,605 ± 1520 | 19,873 ± 5549 |
| Density of myelinated axons(no./μm2) | 0.0269 ± 0.0018 | 0.0253 ± 0.0012 | 0.0276 ± 0.0012 | 0.0254 ± 0.0004 | 0.0262 ± 0.001 | 0.0248 ± 0.0008 | 0.0226 ± 0.002 | 0.0233 ± 0.001 | 0.0227 ± 0.0023 |
All nerves harvested 12/13 days following SMIR/sham surgery i.e. at the peak of SMIR-evoked mechanical hypersensitivity. All values expressed as mean ± SEM. One section, per level, per animal was analysed. There was no significant difference in the cross-sectional nerve area or in the density of myelinated axons between the nerve groups at each level (unpaired t-tests).
2.5 Immunohistochemistry
Rats received an over-dose of sevoflurane anaesthesia and were transcardially perfused, using a motorised pump, with 0.9% saline containing 0.1% sodium nitrite followed by 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.2. SMIR- and sham-operated rats were perfused at day 12/13 following surgery, at maximum pain severity. To act as a positive control, nerve-injured rats were perfused 3 days following a saphenous nerve transection (saphenous axotomy) procedure (under anaesthesia the saphenous nerve was exposed, completely transected and a 3mm portion removed to avoid regeneration). The vertebral column was removed and post-fixed for 12 hr in 4% paraformaldehyde, after which the L3, L4 & L5 dorsal root ganglia (DRG) were removed and incubated in 30% sucrose solution at 4°C for at least 6 hours. DRG were frozen and embedded in Optical Cutting Temperature (OCT) compound in groups of 6 (ipsilateral saphenous axotomy, ipsilateral SMIR, contralateral SMIR, ipsilateral sham, contralateral sham, uninjured). 16μm sections of the different DRG groups were cut simultaneously on a cryostat directly onto Superfrost® Plus slides (Fisher Scientific, USA) for concurrent single-label immunofluorescence staining of all section types. Following exposure to phosphate-buffered saline (PBS) with 0.2% Triton-X 100 (PBS+T) containing 10% normal goat serum (NGS) at room temperature (RT) for one hour, sections were exposed to rabbit anti-ATF3 primary antibody (1:500, Santa-Cruz Biotechnology, USA) in PBS+T containing 5% NGS for 24 hours at 4°C. After PBS+T washes, sections were exposed to goat anti-rabbit CY3-labelled secondary antibody (1:200, Jackson ImmunoResearch Laboratories Inc., USA) in PBS+T containing 2% NGS for 90 mins at RT. Following PBS washes, sections were coverslipped with Vectashield® mounting medium (Vector Laboratories Inc., USA). A fluorescent microscope with a rhodamine filter was used to quantify the number of ATF3 positive cells (with intensely fluorescent red nuclei) and the number of ATF3 negative cells (with visible black nuclei) in randomly selected sections. For L3 DRG analysis, 16 sections from 4 rats post-saphenous axotomy, 16 sections from 4 SMIR-operated rats and 16 sections from 4 sham-operated rats were examined (4 sections per animal, per group). For L4 DRG analysis, 16 sections from 4 rats post-saphenous axotomy, 16 sections from 4 SMIR-operated rats, 16 sections from 4 sham-operated rats and 12 sections from 3 uninjured rats were examined (4 sections per animal, per group). For L5 DRG analysis, 12 sections from 3 rats post-saphenous axotomy, 12 sections from 3 SMIR-operated rats, 12 sections from 3 sham-operated rats and 8 sections from 2 uninjured rats were examined (4 sections per animal, per group). For each section, the number of ATF3 positive cells was expressed as a percentage of the total number of cells with visible nuclei present in the section. The mean and SEM of ATF3-like immunoreactivity (ATF3-IR) was then calculated for each of the 6 groups (ipsilateral saphenous axotomy, ipsilateral SMIR, contralateral SMIR, ipsilateral sham, contralateral sham and uninjured) in L3, L4 and L5 DRG, with the exception of ATF3-IR in uninjured L3 DRG which was not examined.
2.6 Statistical analysis
One-way, repeated-measures, analysis of variance (ANOVA) followed by Dunnett’s post hoc analysis was used to compare pre-surgery baseline responses to post-surgery responses in the behavioural time course of responses to mechanical or heat stimulation. One-tailed unpaired t-tests, with Welch correction applied as appropriate, were used to compare the responses to mechanical stimulation of SMIR-operated rats to sham-operated rats at each postoperative day examined. The Friedman test with Dunn’s post hoc analysis was used to compare cold scores following surgery to pre-surgery baseline cold scores. Mann-Whitney U-tests were used to compare the cold difference scores of SMIR-operated rats to those of sham-operated rats at each postoperative day examined. Two-tailed unpaired t-tests were used to compare between nerves from SMIR-operated rats and sham-operated rats for the total number of myelinated axons, density of myelinated axons and cross-sectional nerve area. One-way ANOVA was used to compare these three measurements within surgical groups between the different portions (proximal, adjacent and distal to surgical site) of saphenous nerve. One-tailed Mann Whitney U-tests were applied to compare the proportion of ATF3 expression in the DRG from different surgical groups. Significance was accepted at p < 0.05.
3. Results
No signs of autotomy were evident in any of the SMIR or sham-operated animals. SMIR surgery did not cause any signs of stress or ill-health such as alopecia, diarrhoea or weight loss. All SMIR-operated rats gained weight normally comparable to the sham-operated group.
Skin/muscle incision and retraction (SMIR) surgery evoked a persistent significant mechanical hypersensitivity, compared to pre-surgery baseline responses, which was apparent in the SMIR ipsilateral paw responses to all von Frey forces tested (Fig. 2 A–D, p < 0.05, one-way repeated measures ANOVA, Dunnett’s post-hoc). SMIR-evoked mechanical hypersensitivity in the ipsilateral paw was significant at postoperative day 3 in the responses to von Frey 4g, 6g and 10g (Fig. 2A–C) and significant at postoperative day 1 in the responses to von Frey 15g (Fig. 2D). The maximum SMIR-evoked pain syndrome occurs between postoperative days 10 and 13 (Fig. 2A–D). The endurance of SMIR-evoked mechanical hypersensitivity was similar for responses to all von Frey stimulation, persisting until postoperative day 22 and then returning to pre-surgery levels by postoperative day 32 (Fig. 2A–D). In general, compared to pre-surgery responses no significant mechanical hypersensitivity was observed in the contralateral paw of SMIR-operated rats. However, in the SMIR contralateral paw, responses to von Frey 4g stimulation were elevated compared to baseline at postoperative days 10, 15, 18 and 22 (Fig. 2A, p < 0.05, one-way repeated measures ANOVA, Dunnett’s post-hoc). This could be due to a near-zero response and variability of the SMIR contralateral paw at baseline (0.06 ± 0.04), which was not the case for the SMIR ipsilateral paw (0.41 ± 0.15), sham ipsilateral paw (0.42 ± 0.2) or the sham contralateral paw (0.13± 0.07). The sham surgery (incision of thigh skin and superficial muscle without retraction) did not evoke significant mechanical hypersensitivity compared to baseline, in the ipsilateral and contralateral responses to von Frey stimulation (Figs. 2A–D). There are three data points that are exceptions to this statement; sham contralateral paw responses to von Frey 6g at postoperative days 1 and 3 (Fig. 2B) and sham ipsilateral paw responses to von Frey 15g at postoperative day 3 (Fig. 2D) were significantly elevated from baseline responses (p < 0.05, one-way repeated measures ANOVA, Dunnett’s post-hoc).
To assess the effect of SMIR surgery in relation to the concurrent control group, SMIR ipsilateral paw responses to von Frey stimulation were statistically compared to sham ipsilateral responses during the postoperative behavioural time course. Out of the total (13) postoperative time points assessed, SMIR ipsilateral paw responses were significantly greater than sham ipsilateral paw responses at 7 of 13 time points for von Frey 4g responses, 9 of 13 time points for von Frey 6g responses, 12 of 13 time points for von Frey 10g responses and 13 of 13 time points for von Frey 15g responses (p < 0.05, one-tailed unpaired t-tests, with Welch correction applied as appropriate). Therefore in comparison to both pre-surgery responses and sham-operated control responses, SMIR evokes a persistent mechanical hypersensitivity assessed through a range of von Frey filaments, which lasts at least three weeks post-surgery.
The data shown in Figure 2 are from a combination of two cohorts of 14 animals (n = 8 SMIR, n = 6 sham) and a further three cohorts of SMIR- and sham-operated rats have since been produced for experiments requiring tissue harvesting. The generation and behavioural assessment of these 5 cohorts of animals has been performed over a period of 10 months from May 2006 until March 2007. In all cohorts of animals, mechanical hypersensitivity assessed with von Frey stimulation was clearly evident. A similar time course was observed to that shown in Figure 2, with maximum SMIR-evoked mechanical hypersensitivity occurring between days 10–13 post-surgery. A small proportion of animals did not show significant mechanical hypersensitivity (more than a two-fold increase in response compared to pre-surgery responses) following SMIR procedure. Nonetheless, 83.8% ± 8.2% of animals per cohort developed SMIR-evoked mechanical hypersensitivity and 40 out of a total of 49 SMIR-operated rats generated so far developed mechanical hypersensitivity compared to baseline. None of the 16 SMIR-operated rats whose data is shown in Figure 2 were classed as non-responders.
The pinprick test was also used to examine mechanical hyperalgesia in SMIR-operated animals (n = 9). There was no difference in the response to pinprick stimulation following SMIR surgery compared to pre-surgery baseline responses (data not shown). There was no enhanced response to pinprick stimulation at any time up to and including postoperative day 13. As the maximum of enhanced sensitivity to von Frey stimulation was repeatedly observed by day 13, the behavioural time course to this stimulus was terminated after postoperative day 13.
Attempts were made to assess the behavioural responses to mechanical stimulation at the incision site on the medial thigh. It proved to be unfeasible to gain access to this area with von Frey filaments in the conscious animal without substantial restraint of the whole animal. It was found that a mild anaesthesia could be maintained in rats for a short time using a very low level of inhalation anaesthetic (0.25% isoflurane, Carlo Pancaro, unpublished observations). At this level of anaesthesia, the rat has enough postural tone to stand on all four paws and withdraws from pinprick stimulation to the hindpaw. However, no nocifensive response whatsoever could be evoked by von Frey stimulation of either the plantar hindpaw or the incision site on the thigh under this low level of anaesthesia. Therefore it was not possible to test for primary allodynia/hyperalgesia at the incision site in the SMIR model.
Von Frey stimulation was applied to the mid-plantar part of the hindpaw which is predominantly innervated by the sciatic nerve. As the SMIR procedure involves the potential stretch of the saphenous nerve, von Frey testing was also performed in the most medial region of the plantar hindpaw, reported to be solely innervated by the saphenous nerve (Bajrovic and Sketelj 1998). There was no difference in the responses to von Frey stimulation at this location between the ipsilateral and contralateral paw of SMIR-operated rats at times when maximum SMIR-evoked mechanical hypersensitivity was observed in the mid-plantar hindpaw.
The effect of SMIR surgery on hindpaw responses to hot and cold stimuli was also investigated. Figure 3 illustrates hindpaw withdrawal latencies to a noxious heat stimulus prior to and up to 14 days following SMIR or sham surgery. Withdrawal latencies of ipsilateral and contralateral paws in SMIR-operated rats were not significantly altered up to postoperative day 14 (p < 0.05, one-way repeated measures ANOVA with Dunnett’s post-hoc). Paw withdrawal latencies in the sham-operated group were also predominantly unaltered throughout the time course. Although on postoperative day 14, both ipsilateral and contralateral paw withdrawal latencies of sham-operated rats, were significantly elevated compared to their respective pre-surgery responses (one-way repeated measures ANOVA with Dunnett’s post-hoc). Figure 4 shows the time course of sensitivity to a cold stimulus (acetone) prior to and up to 18 days following SMIR or sham surgery. SMIR ipsilateral paw responses to cold were significantly increased compared to pre-surgery responses at postoperative days 2, 10 and 18 (Fig. 4A, p < 0.05, Friedman test with Dunn’s post-hoc). However, it is clear that the responses of both paws in SMIR- and sham-operated rats were elevated over the behavioural time course compared to baseline responses, reaching significance at postoperative day 14 for the sham ipsilateral paw. To take into account these effects and allow comparison between the SMIR and sham group, difference scores were calculated (contralateral cold score minus ipsilateral cold score) for SMIR- and for sham-operated rats (Fig. 4B). Thus the more negative the difference score, the greater the sensitivity of the ipsilateral paw to cold stimulation. There was no significant difference between the cold difference scores of SMIR-operated rats and those of sham-operated rats at any of the postoperative time points examined (Fig. 4B, one-tailed Mann-Whitney U-tests).
SMIR surgery displaces the saphenous nerve during the retraction of skin and superficial muscle of the thigh. Therefore it was postulated that some degree of damage to the saphenous nerve was caused during this potential stretch causing the resultant SMIR-evoked pain behaviour. To investigate this hypothesis, at the maximum of mechanical hypersensitivity evoked by SMIR surgery, saphenous nerve sections at three different levels; a) proximal to retraction site, top of the thigh, b) adjacent to the retraction site, mid-thigh and c) distal to retraction site, just above the ankle were examined for changes in nerve morphology. Figure 5 shows typical photographs of saphenous nerve sections, proximal, adjacent and distal to retraction site, from a rat 12 days following SMIR surgery. These photographs demonstrate that SMIR surgery has no effect on the gross morphology of the saphenous nerve at the level of SMIR surgery, or at either proximal or distal levels. Examination of the entire nerve cross-section showed that the structure of myelin axonal sheaths and blood capillaries was typical, with no signs of either neurodegeneration or blood flow impairment. The total number of myelinated axons in the saphenous nerve of the SMIR ipsilateral side, sham ipsilateral side and sham contralateral side (to act as an uninjured control) were counted at levels proximal, adjacent and distal to the surgical site (see Figure 6). There was no significant difference, at any of the three levels, between the total number of myelinated axons in SMIR ipsilateral nerves and that in sham ipsilateral nerves (Fig. 6, two-tailed unpaired t-tests). There was also no significant difference between the number of myelinated axons in the ipsilateral and contralateral saphenous nerves of sham-operated animals at any of the three levels (Fig. 6, two-tailed unpaired t-tests).
Figure 5.
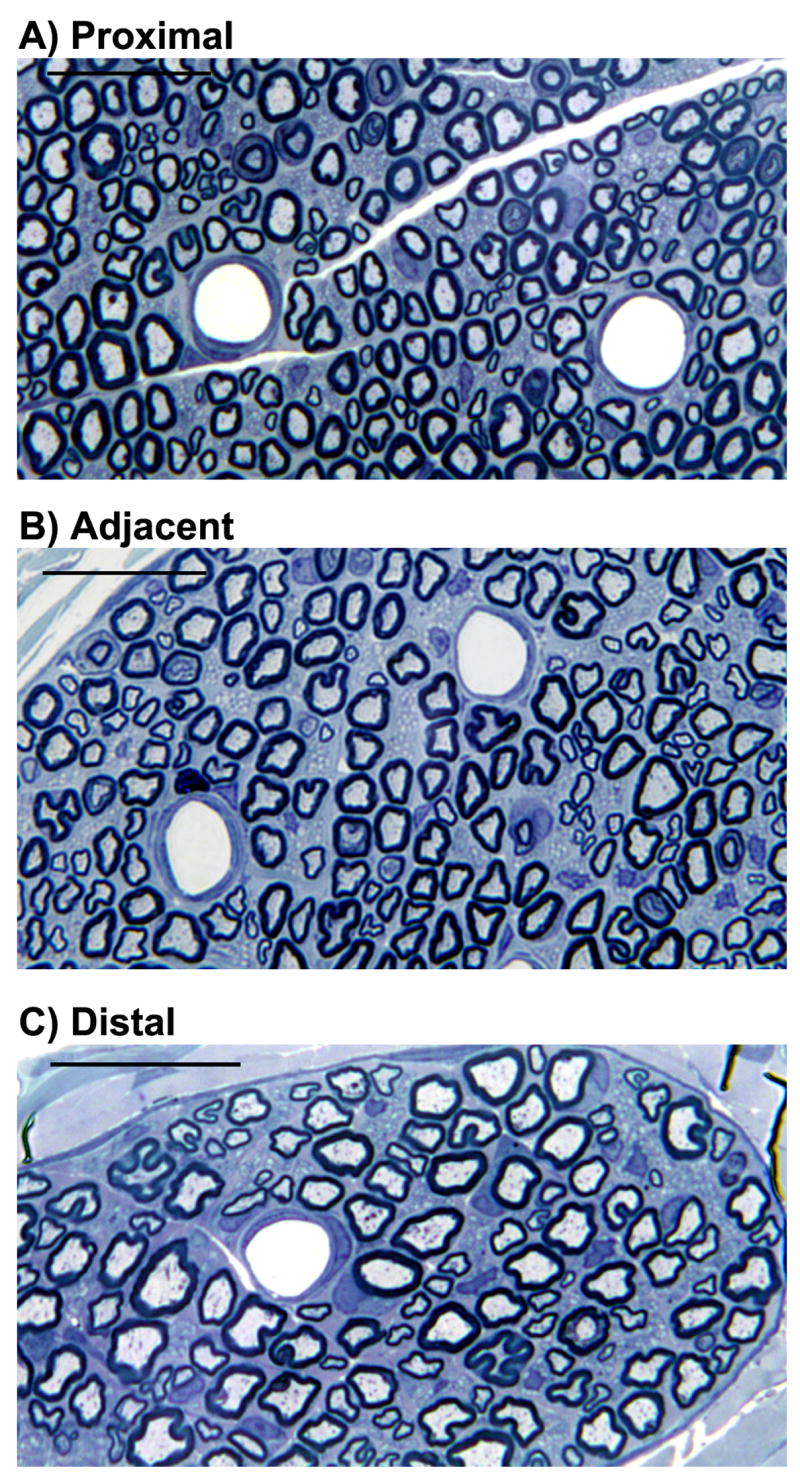
Saphenous nerve sections from a SMIR-operated rat at the maximum SMIR-evoked pain, postoperative day 12 harvested: (A) proximal to retraction site from the top of the thigh, (B) adjacent to the retraction site, mid-thigh and (C) distal to retraction site, just above the ankle. Examination at this magnification (1000×) demonstrates that the overall morphology of the saphenous nerve is unchanged by SMIR surgery. Scale bar = 25μm
Figure 6.
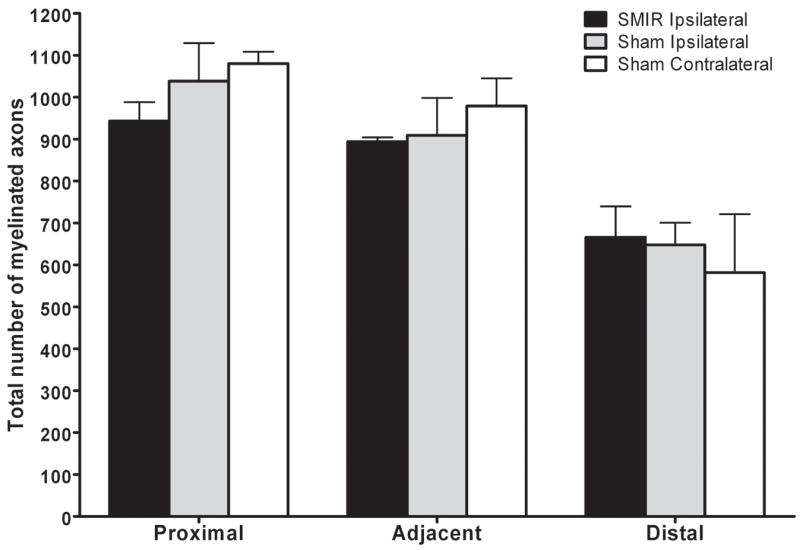
Total number of myelinated axons in saphenous nerve sections proximal, adjacent and distal to the surgical site in SMIR- and sham-operated animals. Graph shows the mean ± SEM of the number of myelinated axons in entire nerve sections, n = 4 – 7 per level, per group. There was no significant difference, at proximal, adjacent or distal levels, in the total number of myelinated axons between SMIR ipsilateral and sham ipsilateral nerves or between sham ipsilateral and contralateral nerves (two-tailed unpaired t-tests).
Table 1 shows the cross-sectional area and density of myelinated axons in the saphenous nerve proximal, adjacent and distal to the surgical site of SMIR- and sham operated rats on postoperative day 12/13, when the maximum SMIR-evoked pain occurs. The cross-sectional area of the saphenous nerve was unaffected by SMIR surgery at any level compared to sham-operated and uninjured controls (Table 1, two-tailed unpaired t-tests) indicating that neural oedema is not present. Similarly, the density of myelinated axons in the saphenous nerve proximal, adjacent and distal to the surgical site was also unaltered by SMIR surgery compared to sham-operated and uninjured controls (Table 1, two-tailed unpaired t-tests). There was a significant decrease in the number of myelinated axons and the cross-sectional nerve area from the proximal level down to the distal level in all nerve groups (p < 0.05, one-way ANOVA). However the density of myelinated axons did not significantly change between the different levels examined in any group (see Table 1). This shows that significant branching of the saphenous nerve occurs from the top of the thigh to the ankle, perhaps as expected, but also that the stretching of the saphenous nerve during SMIR surgery does not significantly affect such branching. In short, these light microscopy studies demonstrate that SMIR surgery does not cause degeneration or oedema in the saphenous nerve at the surgical site, nor proximally nor distally to it. Therefore neither degeneration nor oedema in the saphenous nerve trunk is responsible for the SMIR-evoked pain syndrome.
To further investigate potential nerve damage evoked by SMIR surgery, L3/L4/L5 DRG were harvested and stained for ATF3, a specific marker of neuronal injury (Tsujino et al. 2000). The saphenous nerve is known to have inputs into the spinal cord at L3 & L4 levels (Bajrovic and Sketelj 1998), but not at L5 where the predominant input is from the sciatic nerve. Thus the aim of these immunohistochemical studies was to assess the extent of peripheral neuronal damage caused by SMIR, at conversion points of peripheral afferents from saphenous-innervated territories and from adjacent territories not innervated by the saphenous nerve. L3-L5 DRG were also harvested from rats following complete saphenous nerve transection (saphenous axotomy), to provide a positive control for ATF3 staining and, moreover, to compare the effects of complete saphenous nerve transection to SMIR (a potential stretch of saphenous nerve). Figure 7 shows a typical example of this comparison in L3 DRG; following saphenous axotomy, the majority of (ipsilateral) DRG cells were ATF3-positive, whereas at the maximum SMIR-evoked pain, the majority of (ipsilateral) DRG cells were ATF3-negative. Table 2 shows the proportion of L3/L4/L5 DRG cells that expressed ATF3-IR following saphenous axotomy, SMIR and sham surgeries. ATF3 expression following transection of the saphenous nerve confirmed that the saphenous nerve has a major input into L3 and a lesser input into L4 but no input into L5 DRG (Table 2). In L3 DRG there was a very small (≈1%), but statistically significant, increase in ATF3 expression in SMIR ipsilateral DRG compared to sham ipsilateral DRG and to SMIR contralateral DRG (Table 2, p < 0.05, one-tailed Mann-Whitney U-tests). This represented, on average, 1–4 ATF3 positive cells per section of L3 DRG. In L4 and L5 DRG there was no significant difference in ATF3 expression between SMIR-operated, sham-operated and uninjured animals. A similar low level of ATF3 expression in the DRG of normal animals as observed here, has been previously reported (Tsujino et al. 2000), perhaps reflecting normal cell turnover. In summary, there is very little to no degeneration detectable in the DRG following SMIR that coincided with the maximum of SMIR-evoked pain. Therefore, loss of peripheral afferents is unlikely to be a causal factor in the SMIR-evoked pain syndrome.
Figure 7.
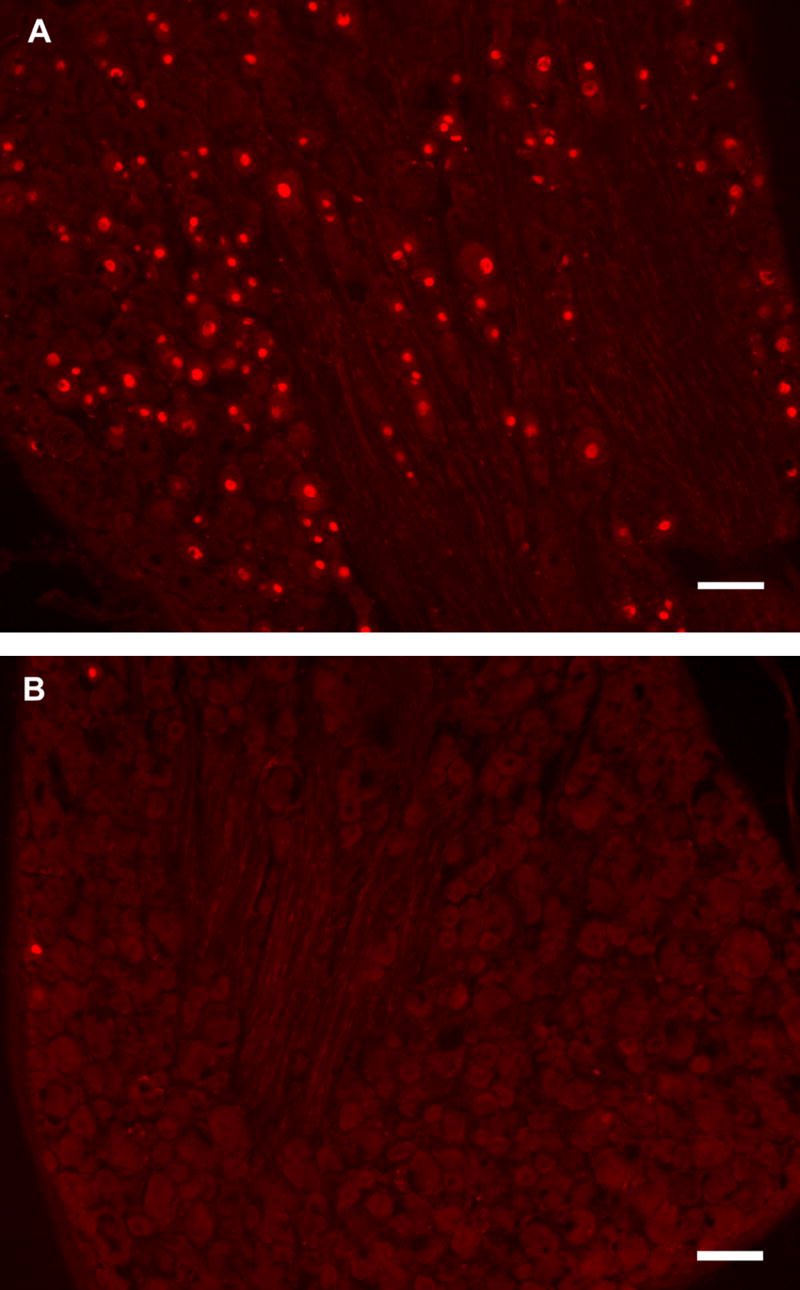
ATF3 expression in L3 DRG cells: (A) Three days following complete saphenous nerve transection, the majority of cells are ATF3-positive with intensely stained red nuclei. (B) 13 days following SMIR surgery, at the maximum of evoked pain syndrome, vast majority of cells are ATF3-negative. NB: On average 1–4 cells per SMIR ipsilateral DRG section were ATF3-positive. Scale bar = 100μm
Table 2.
ATF3 expression in DRG at the maximum of SMIR-evoked pain. Percentage of DRG cells expressing ATF3-IR in the L3, L4 and L5 DRG ipsilateral to saphenous axotomy, SMIR or sham surgeries. DRG contralateral to SMIR or sham surgeries and DRG from uninjured animals are included for control comparisons. DRG were harvested 3 days following saphenous axotomy and 12/13 days following SMIR/sham surgery.
| Saphenous Axotomy | SMIR Ipsilateral | SMIR Contralateral | Sham Ipsilateral | Sham Contralateral | Normal/Uninjured | |
|---|---|---|---|---|---|---|
| L3 DRG (n) | 72.04% ± 0.02% †(4) | 1.84% ± 0.02% * (4) | 0.43% ± 0% (4) | 0.84% ± 0% (4) | 0% ± 0% (4) | - |
| L4 DRG (n) | 23.50% ± 0.09% † (4) | 0.65% ± 0% (4) | 0.39% ± 0% (4) | 0.45% ± 0% (4) | 0.21% ± 0% (4) | 0.26% ± 0%(3) |
| L5 DRG(n) | 0.61% ± 0% (3) | 0.33% ± 0% (3) | 0.41% ± 0% (3) | 0.13% ± 0% (3) | 0.25% ± 0% (3) | 0.41% ± 0% (2) |
All values are mean ± SEM and were rounded up to two decimal places as required; n, number of animals used listed as appropriate, 4 sections examined per animal in all cases. NB: ATF3 expression in L3 uninjured DRG was not measured.
p < 0.05, one-tailed Mann-Whitney test, significant increase compared to sham ipsilateral and to SMIR contralateral L3 DRG
p < 0.05, one-tailed Mann-Whitney test, significant increase compared to SMIR ipsilateral L3/L4 DRG
4. Discussion
This study reports the development of a new rat model of persistent postoperative pain evoked by skin/muscle incision and retraction (SMIR), akin to a clinical procedure. SMIR-operated rats displayed at least three weeks of hypersensitivity to mechanical stimulation of the plantar ipsilateral paw. SMIR surgery did not evoke mechanical hypersensitivity in the contralateral paw. Mechanical hypersensitivity was also not evoked following sham surgery i.e. incision of thigh skin and muscle without retraction. The SMIR model is reproducible, with a high proportion (over 80%) of animals operated over a 10-month period developing SMIR-evoked pain. The data presented here also demonstrate that SMIR surgery does not induce significant peripheral neuronal damage suggesting that nerve damage is not a causal factor in persistent postoperative pain evoked by SMIR.
Secondary hyperalgesia (an enhanced response to stimulation at dermatomes outside the injury site) is a common phenomenon in the postoperative patient that is driven by central sensitization, (cf. Stubhaug et al. 1997). Central sensitization is also the likely explanation of enhanced mechanical hypersensitivity shown here in SMIR-operated rats because the hindpaw dermatomes tested are innervated in normal rats predominantly by the tibial branch of the sciatic nerve, not the saphenous nerve (Bajrovic and Sketelj 1998). In addition, mechanical hypersensitivity was not found in the plantar hindpaw region of SMIR-operated rats that is reportedly innervated by the saphenous nerve alone (Bajrovic and Sketelj 1998). Whether SMIR affects innervation territories in the hind paw or evokes expansion of peripheral receptive fields as observed in other pain models e.g. spinal nerve ligation (Suzuki et al. 2000), remains to be determined.
Neither heat hyperalgesia nor cold allodynia were observed in the plantar hindpaw following SMIR surgery. However, these findings are not necessarily detrimental to the usability of the SMIR model as an animal model of postoperative pain considering the clinical symptoms. Heat hyperalgesia and cold allodynia are not commonly reported symptoms in patients following surgeries that involve prolonged tissue retraction. The most widely reported pain symptoms in these post surgical patients are spontaneous/ongoing pain and pain exacerbated by mechanical stimulation e.g. coughing following a thoracotomy or climbing stairs following inguinal herniorrhaphy (Bay-Nielsen et al. 2001). The absence of heat hyperalgesia has been previously reported with animal models of incisional pain (Zahn and Brennan 1999; Pogatzki et al. 2002). Heat hyperalgesia was only found in the paw incisional model at the plantar hindpaw incisional site, not at a plantar hindpaw location distant to the incision (Zahn and Brennan 1999). As discussed in the results, in the SMIR model it was not possible to gain access and directly test the surgical site due to its location on the medial thigh. In addition, incision in the skin and muscle of the gastrocnemius region of the hindpaw did not produce heat hyperalgesia in the plantar hindpaw (Pogatzki et al. 2002).
There was no enhanced response to pinprick stimulation in the plantar hindpaw of SMIR-operated rats at postoperative time points when pronounced sensitivity to von Frey stimulation was present. This suggests that the SMIR-evoked changes in mechanical sensitivity occur at the lower end of the force stimulation spectrum (von Frey range 4g – 15g) and that at the higher end (e.g. pinprick) there is no SMIR-evoked change. It could also suggest that there is insufficient SMIR-evoked nerve damage to affect responses to pinprick stimulation, as observed in a model of direct saphenous nerve trauma (partial ligation, Walczak et al. 2005). The lack of nerve damage following SMIR (discussed below) may also account for the absence of cold allodynia, as this symptom was also present following partial saphenous nerve ligation (Walczak et al. 2005).
During SMIR surgery the saphenous nerve is displaced and potentially stretched along with the retraction of the superficial layer of thigh muscle. It is important to note however that this nerve is not compressed against any hard surface e.g. bone, due to its superficial location just under the medial skin of the thigh. The ipsilateral saphenous nerve and DRG of SMIR-operated rats were examined for evidence of SMIR-evoked nerve damage at the maximum SMIR-evoked pain. There was no evidence of degeneration or oedema in the saphenous nerve proximal, adjacent or distal to the SMIR surgical site as the number/density of myelinated axons and the nerve cross-sectional area were unaffected in all regions. ATF3 expression in L3-L5 DRG was largely unaltered in SMIR-operated animals at the maximum SMIR-evoked pain. However, there was a very small, but significant (1%) increase of ATF3 expression in ipsilateral L3 DRG of SMIR-operated animals compared to sham-operated animals. In comparison, a complete saphenous nerve transection resulted in almost three-quarters and one-quarter of cells expressing ATF3 in L3 and L4 DRG, respectively. This illustrates that although a significant increase in ATF3 expression is observed in L3 DRG following SMIR, a potential saphenous nerve stretch; it is not remotely within the same magnitude as that evoked by direct nerve injury. In summary, the findings from the light microscopy and immunohistochemistry studies performed here, demonstrate that the damage to the saphenous nerve trunk and its afferents ascending to the spinal cord is negligible at the maximum SMIR-evoked pain. Therefore neither neural oedema nor neurodegeneration are likely to be contributory factors in the SMIR-evoked postoperative pain syndrome.
There are three rat models of pain that are most relevant for comparison to the SMIR model of postoperative pain; the paw incision model (Brennan et al. 1996), the gastrocnemius incision model (Pogatzki et al. 2002) and the thoracotomy model (Buvanendran et al. 2004). The paw incision model (Brennan et al. 1996) displays up to 4 days of mechanical hypersensitivity, whereas the SMIR model displays over 3 weeks of mechanical hypersensitivity. Thus the SMIR model is advantageous for the persistence of the evoked postoperative pain syndrome. In addition, the majority of the surgeries that evoke the clinical problem of persistence postoperative pain involve both skin incision and the retraction of tissue, as the SMIR model aims to replicate. The gastrocnemius incision model (Pogatzki et al. 2002) is more similar to the SMIR model, as it involves an incision of both skin and muscle at a site remote to the area of evoked mechanical hypersensitivity on the plantar hindpaw. The gastrocnemius incision model evokes up to 8 days of mechanical hypersensitivity, twice the length of paw incision evoked changes. This provides further evidence that the skin/muscle retraction, per se, in the SMIR model is instrumental in the persistence of postoperative pain.
The thoracotomy model (Buvanendran et al. 2004) is the only other animal model where persistent pain is evoked through retraction, in this case, the ribs and covering muscles are retracted for one hour to expose the intercostal muscle. In this model, mechanical and cold allodynia are observed by postoperative day 10 and are present up to 40 days following surgery. Hence thoracotomy-evoked mechanical hypersensitivity is longer lasting than SMIR-evoked mechanical hypersensitivity. Importantly, however, thoracotomy-evoked pain was only present in 50% of operated animals whereas SMIR-evoked pain is present in over 80% of operated animals. Moreover, on examination of nerve morphology it was found that thoracotomy-evoked pain was only present in animals with severe degeneration (> 90% loss of myelinated axons) of the 4th intercostal nerve at postoperative day 14, whereas this nerve was largely unaltered in operated animals without pain. Degeneration was caused by the retractor compressing the nerve against the rib which is often unavoidable due to the location of this nerve. Therefore, in contrast to the thoracotomy model where tissue retraction causes neurodegeneration resulting in persistent pain, the SMIR model employs tissue retraction that does not cause neurodegeneration, but still evokes persistent pain. The mechanisms underlying SMIR-evoked pain remain to be determined but could involve a combination of nociceptive and inflammatory processes. The potential stretch of the saphenous nerve during SMIR surgery does not appear to cause neurodegeneration, however this does not eliminate the possibility of neuronal hyperexcitability in saphenous afferents. This could be examined using the well-established skin-saphenous nerve preparation (Reeh 1986) to study how peripheral sensory mechanisms are altered during persistent postoperative pain.
5. Conclusion
This study reveals a new rat model of persistent postoperative pain evoked through prolonged tissue retraction, akin to clinical surgical procedures e.g. inguinal hernia repair. Mechanical hypersensitivity was present by postoperative day 3 and persisted for over 3 weeks. Studies on peripheral neuronal tissue did not indicate that neurodegeneration or neuritis was responsible for the SMIR-evoked persistent pain syndrome. The SMIR model provides a new tool to explore mechanisms and treatments for postoperative pain and demonstrates a persistent pain syndrome that is not driven by neuronal damage.
Acknowledgments
SJLF is supported by an intramural grant from the Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital. This work was supported in part by NIH grant CA080153. Thanks to Prof. Gary R. Strichartz for his useful comments and discussion on this work and manuscript. Thanks to Howard L. Mulhern, Technical EM Manager, Dept. Pathology, Children’s Hospital, Harvard Medical School, for his technical expertise in preparing the saphenous nerve sections. Thanks to Dr. Ru-Rong Ji for the use of the cryostat and fluorescence microscope in his laboratory and Dr. Yasuhiko Kawasaki for advice on immunohistochemistry techniques.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bajrovic F, Sketelj J. Extent of nociceptive dermatomes in adult rats is not primarily maintained by axonal competition. Exp Neurol. 1998;150(1):115–121. doi: 10.1006/exnr.1997.6734. [DOI] [PubMed] [Google Scholar]
- Bay-Nielsen M, Perkins FM, Kehlet H. Pain and functional impairment 1 year after inguinal herniorrhaphy: a nationwide questionnaire study. Ann Surg. 2001;233(1):1–7. doi: 10.1097/00000658-200101000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64(3):493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Buvanendran A, Kroin JS, Kerns JM, Nagalla SN, Tuman KJ. Characterization of a new animal model for evaluation of persistent postthoracotomy pain. Anesth Analg. 2004;99(5):1453–1460. doi: 10.1213/01.ANE.0000134806.61887.0D. table of contents. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Temple WJ, Mitchell P, Nixon JA, Preshaw RM, Hagen NA. Cooperative hernia study. Pain in the postrepair patient. Ann Surg. 1996;224(5):598–602. doi: 10.1097/00000658-199611000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte AM, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz GR. Reduction of postincisional allodynia by subcutaneous bupivacaine: findings with a new model in the hairy skin of the rat. Anesthesiology. 2005;103(1):113–125. doi: 10.1097/00000542-200507000-00018. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109(1–2):150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Strichartz GR. Characterization of a new model of post-operative pain evoked by skin/muscle incision and retraction (SMIR) Society of Neurosci Abstracts. 2006:738.13. [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93(4):1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- Pogatzki EM, Niemeier JS, Brennan TJ. Persistent secondary hyperalgesia after gastrocnemius incision in the rat. Eur J Pain. 2002;6(4):295–305. doi: 10.1053/eujp.2002.0339. [DOI] [PubMed] [Google Scholar]
- Poobalan AS, Bruce J, Smith WC, King PM, Krukowski ZH, Chambers WA. A review of chronic pain after inguinal herniorrhaphy. Clin J Pain. 2003;19(1):48–54. doi: 10.1097/00002508-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neuroscience Letters. 1986;66(2):141–146. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Stubhaug A, Breivik H, Eide PK, Kreunen M, Foss A. Mapping of punctuate hyperalgesia around a surgical incision demonstrates that ketamine is a powerful suppressor of central sensitization to pain following surgery. Acta Anaesthesiologica Scandinavica. 1997;41(9):1124–1132. doi: 10.1111/j.1399-6576.1997.tb04854.x. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Kontinen V, Matthews E, Williams E, Dickenson A. Enlargement of receptive field size to low intensity mechanical stimulation in the rat spinal nerve ligation model of neuropathy. Experimental neurology. 2000:163. doi: 10.1006/exnr.2000.7371. [DOI] [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57(3):375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15(2):170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- Walczak JS, Pichette V, Leblond F, Desbiens K, Beaulieu P. Behavioral, pharmacological and molecular characterization of the saphenous nerve partial ligation: a new model of neuropathic pain. Neuroscience. 2005;132(4):1093–1102. doi: 10.1016/j.neuroscience.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90(3):863–872. doi: 10.1097/00000542-199903000-00030. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals [editorial] Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]