Abstract
Purpose
To assess the association of cardiovascular risk factors, ocular perfusion pressure with early and advanced age-related macular degeneration (AMD) in Latinos.
Design
Population-based, cross-sectional study.
Methods
Data were collected from a population-based sample of self-identified adult Latinos using standardized protocols for assessing blood pressure and intraocular pressure (IOP) measurement and stereoscopic macular photography. Hypertension was defined as either a history of hypertension or systolic blood pressure (SBP) >140mmHg +/− diastolic blood pressure (DBP) ≥85mmHg. Ocular perfusion pressure (OPP) was defined as the difference between mean arterial blood pressure and IOP. AMD was diagnosed from photographic grading by masked trained graders. Logistic regression was used to assess associations.
Results
Gradable retinal photographs were available in 5875 participants. After adjusting for age, sex, and cigarette smoking, higher DBP and uncontrolled diastolic hypertension were associated with exudative AMD (Odds ratio [OR], 1.8; 95% confidence interval [CI], 1.1−2.8; and OR, 3.3; CI, 1.2−9.3, respectively). Higher OPP was associated with a decreased risk of GA (OR, 0.4 per 10mmHg; CI, 0.3−0.5). Low pulse pressure was associated with a lower risk of exudative AMD (OR, 0.2; CI, 0.1−0.6). Obesity was associated with increased retinal pigment (OR, 1.6; CI, 1.0−2.3).
Conclusion
These data suggest that in Latinos cardiovascular risk factors may play a role in advanced AMD. Given that Latinos have a high prevalence of cardiovascular risk factors, an intervention aimed at reducing these risk factors may also have a beneficial impact on the risk of having early and advanced AMD.
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible visual impairment and blindness in individuals aged 60 years and older in the US. (1-3) Despite the magnitude of this problem, the pathogenesis of AMD remains poorly understood. The prevalence of both AMD and cardiovascular disease are strongly age-dependent, but there are conflicting reports about the independent associations of cardiovascular disease, its risk factors and AMD. Factors such as hypertension, (4-10) previous vascular events, (9, 11-15) obesity, (4, 11, 16-19) and diabetes (11, 20, 21) have been significantly associated with early and advanced AMD in several studies. Hypertension and atherosclerosis have been hypothesized by Friedman (22) to increase the risk of AMD through the reduction of blood flow through the choroidal vasculature and lipid deposition in Bruch's membrane with a reduction of permeability leading to the upregulation of vascular endothelial derived growth factor. Inflammation has been postulated to be important in the pathogenesis of atherosclerosis and may be also be important in AMD. C-reactive protein has been associated with AMD (23, 24) and also cardiovascular disease. (25) Genes for complement factor H which is involved with inflammation has also been associated with AMD. (26)
Most data on the association between various cardiovascular risk factors and AMD have been derived from studies among non-Hispanic Whites. (4-8, 11, 13, 15-21) To date, there have been few published risk factor analyses of AMD and cardiovascular disease and cardiovascular risk factors among Latinos. (9, 10, 12, 14) The Latino population is the largest minority group in the United States (US) and the fastest growing segment of the US population. (27)
This paper aims to explore the relationship of self-reported cardiovascular disease (including angina, congestive cardiac failure, acute myocardial infarction, stroke), various cardiovascular disease risk factors (including history of hypertension, measured systolic and diastolic blood pressures, diabetes), and both early and advanced AMD.
Methods
The Los Angeles Latino Eye Study (LALES) is a population-based prevalence study of eye disease. Data was collected from 2000−2003. The study cohort consisted of self-identified Latinos, aged 40 years and older, living in six census tracts in the city of La Puente, California. The survey design and methods have been reported in detail elsewhere. (28, 29) The study protocol was approved by the Institutional Review Board (IRB)/Ethics Committee at the University of Southern California and all study procedures adhered to the recommendations of the Declaration of Helsinki. Written consent was obtained from all participants.
Interview and Examination Procedures
Eligible residents were informed of the study and invited to participate in an at-home interviewer-administered questionnair and an in-clinic examination. Demographic factors, various risk factors, ocular and medical histories, and access to medical and ocular care was assessed by the questionnaire. Trained ophthalmologists and technicians used standardized protocols in performing a comprehensive ocular examination, which included 30° stereoscopic color retinal photographs of Diabetic Retinopathy Study fields (30) one, two and a modified field three on all participants.
Cardiovascular Risk Factors
History of cardiovascular events, hypertension and diabetes was obtained during the interview. Blood pressure was measured twice during the clinic examination by trained technicians in accordance with standardized study protocol using the random zero sphygmomanometer. Cuff of the appropriate size for the participant's arm was chosen by ensuring that the rubber bladder encircled at least two-thirds of the arm and the inflatable inner bladder centered over the brachial artery. Participants were seated and were allowed to rest for at least 5 minutes prior to the first measurement, and at least 2 minutes in between the first and second measurements. Quality control was performed through periodic repeated measurements taken by a second technician. All technicians received standard training for BP measurement at the start of the study and were retrained annually. The average systolic (SBP) and diastolic blood pressures (DBP) were used in the analyses. Hypertension was defined as either a history of hypertension, use of ant-hypertensive medications, or SBP >140mmHg +/− DBP ≥85mmHg.
Body mass index (BMI), computed as weight (kg) divided by height squared (meters2), was used to assess level of obesity. Low/normal weight was defined as BMI <25 kg/m2, overweight BMI 25−30 kg/m2, and obese BMI ≥30 kg/m2.
Presence of diabetes was defined by self-reported history of diabetes, and/or Hemoglobin A1c (HbA1c) greater than 7.0% and/or random glucose greater than 200mg%. HbA1c and random blood glucose was measured using the DCA 2000+, the Bayer glucometer and the Hemocue B-Glucose analyzer from a finger-prick blood sample.
Ocular perfusion pressure (OPP) was defined as two thirds of the mean arterial blood pressure (MAP) minus the intraocular pressure (IOP), that is OPP = 2/3 MAP-IOP. IOP was measured by Goldmann tonometry and the median of 3 measurements was used in the analyses. The MAP was calculated by DBP plus one third of the pulse pressure, that is DBP+1/3 pulse pressure. Pulse pressure was defined as SBP-DBP.
Age-related Macular Degeneration (AMD) Grading
A modification of the Wisconsin Age-related Maculopathy Grading System (30) was used to grade individual AMD lesions by masked graders at the Wisconsin Ocular Epidemiology Grading Center. A more detailed description about all grading procedures and definitions has been presented elsewhere. (28, 29) Early AMD was defined as the absence of signs of advanced AMD and the presence of 1) soft indistinct or reticular drusen or 2) hard distinct or soft distinct drusen with pigmentary abnormalities (retinal pigment epithelial (RPE) depigmentation or increased retinal pigment). Advanced AMD was defined as the presence of 1) geographic atrophy or 2) exudative AMD. Signs of exudative AMD were RPE detachment or serous detachment of the sensory retina, subretinal or sub-RPE hemorrhages, and subretinal fibrous scars. Geographic atrophy was defined as a circular discrete area (of at least 350 μm in diameter) of retinal depigmentation with visible choroidal vessels, in the absence of exudative AMD.
Data and Statistical Analyses
Data were entered into an automated database using Microsoft Access-98, with internal automated quality control checks. The Statistical Analysis System (version 8; SAS Institute Inc, Cary, NC) was used for tabulations and statistical analyses. All odds ratios were adjusted for age, sex and smoking, and all confidence interval presented were 95%. Inter-grader and intra-grader agreement was assessed using the quadratic weighted kappa statistic on a random subset of 30 eyes. There was moderate to excellent inter- and intra-observer agreement (κ=0.8−1.0 for early AMD, exudative AMD and geographic atrophy and κ=0.4−0.9 for individual early AMD lesions). Logistic regression was performed using each AMD lesion as a dependent variable.
Results
Of the 7789 eligible self-identified Latinos, 6870 individuals (88%) completed an in-home interview and 6357 (82%) participated in a clinical examination. Comparison of the demographic, socioeconomic and clinical characteristics of participants included and excluded from LALES has been previously described. (33, 34) In brief, the mean age of participants was 54.6 years, 58% were female, and 95% had Mexican-American ancestry. A total of 5875 participants with complete clinical examination, gradable retinal photographs and medical history were included in these analyses (Figure 1).
Figure 1.
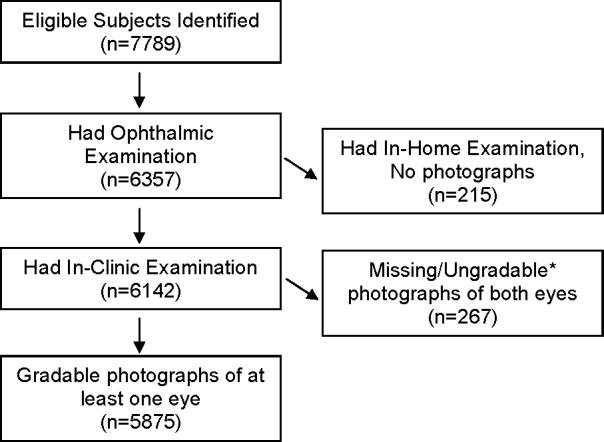
Participation Flowchart for the Assessment of Various Cardiovascular Factors with Age-related Macular Degeneration (AMD) in the Los Angeles Latino Eye Study. *Photographs were ungradable due to media opacities, poor camera focus, or maculopathies believed to be secondary to other conditions.
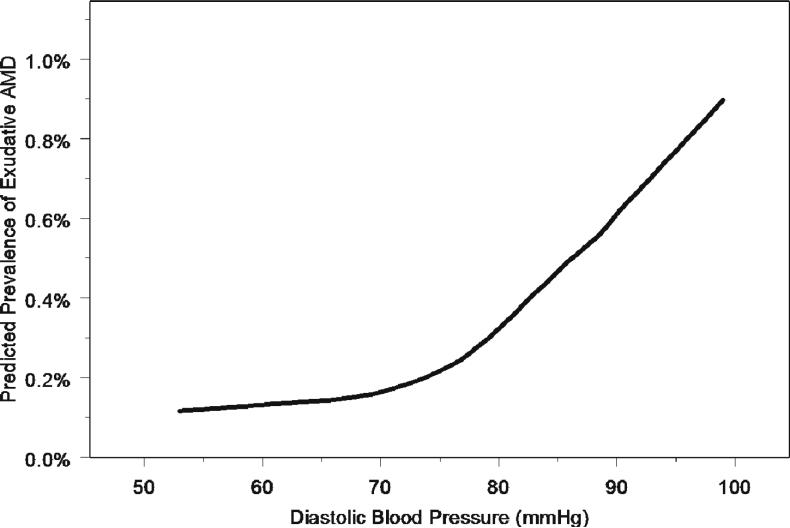
Hypertension was present in 43.2% of participants. Men and women were equally likely to be hypertensive (43.9% vs. 42.6%, respectively; p=0.32). Those with advanced AMD were more likely to have a history of hypertension (52%) compared to those with no AMD (29%) (p=0.004) (Table 1). After adjustment for age, sex and smoking, there was no association of history of hypertension with any AMD lesions (Table 2). However, individuals with diastolic hypertension were significantly more likely to have exudative AMD (OR, 3.3; p=0.02). The association remained if age and age squared were included in the model. Further, this association was maintained when analyzing DBP per 10mmHg increase (OR, 1.8; p=0.01). The relationship between diastolic blood pressure and the risk of having exudative age-related macular degeneration (AMD) is nonlinear and increases sharply with diastolic blood pressures that range from 75mmHg and higher (Figure 2). Diastolic hypertension was also independently associated with soft indistinct drusen (OR, 1.4; p=0.02). There was a trend towards a significant association between diastolic hypertension and RPE depigmentation (OR, 1.5; p=0.05). There was no significant association between systolic hypertension and any AMD lesions. Individuals with pulse pressure higher than 90 mmHg had over a three-fold risk for RPE depigmentation (OR, 3.37), while individuals with lower pulse pressures (40−90 mmHg) had a reduced risk for exudative AMD (OR, 0.2).
Table 1.
Frequency Distribution of Hypertension, Diabetes, Body Mass Index and History of Cardiovascular Disease in Latinos by Presence of Age-related Macular Degeneration (AMD) and Specific AMD Lesions in the Los Angeles Latino Eye Study
| Cardiovascular Risk Factor | No AMD (N=5299) | Early AMD (N=551) | Soft Indistinct Drusen (N=421) | IRP (N=328) | RPE depigmentation (N=133) | Advanced AMD (N=25) | GA*(N=9) | Exudative AMD*(N=17) |
|---|---|---|---|---|---|---|---|---|
| History of hypertension | 1536 (29.0) | 186 (33.8) | 161 (38.2) | 106 (32.3) | 47 (35.3) | 13 (52.0) | 5 (55.6) | 9 (52.9) |
| SBP ≥ 140 mmHg | 930 (17.6) | 141 (25.6) | 118 (28.0) | 80 (24.4) | 30 (22.6) | 5 (20.0) | 1 (11.1) | 4 (23.5) |
| DBP ≥ 85 mmHg | 993 (18.7) | 121 (22.0) | 100 (23.8) | 73 (22.3) | 35 (26.3) | 7 (28.0) | 0 (0.0) | 7 (41.2) |
| Pulse pressure > 90 mmHg | 56 (1.1) | 14 (2.5) | 15 (3.6) | 9 (2.7) | 6 (4.5) | 2 (8.0) | 0 (0.0) | 2 (11.8) |
| History of diabetes | 853 (16.1) | 91 (16.5) | 70 (16.6) | 55 (16.8) | 22 (16.5) | 5 (20.0) | 2 (22.2) | 3 (17.7) |
| Definite diabetes | 1033 (19.5) | 115 (20.9) | 87 (20.7) | 68 (20.7) | 26 (19.5) | 4 (16.0) | 2 (22.2) | 2 (11.8) |
| HbA1c ≥ 7.0% | 786 (14.8) | 90 (16.3) | 66 (15.7) | 54 (16.5) | 21 (15.8) | 4 (16.0) | 2 (22.2) | 2 (11.8) |
| Random glucose ≥ 200mg% | 451 (8.5) | 50 (9.1) | 36 (8.6) | 29 (8.8) | 14 (10.5) | 1 (4.0) | 0 (0.0) | 1 (5.9) |
| BMI <25 (low/normal) | 595 (11.2) | 53 (9.6) | 43 (10.2) | 27 (8.2) | 13 (9.8) | 4 (16.0) | 3 (33.3) | 2 (11.8) |
| BMI 25−30 (overweight) | 2005 (37.8) | 233 (42.3) | 173 (41.1) | 147 (44.8) | 62 (46.6) | 10 (40.0) | 2 (22.2) | 8 (47.1) |
| BMI ≥ 30 (obese) | 2645 (49.9) | 256 (46.5) | 196 (46.6) | 148 (45.1) | 54 (40.6) | 10 (40.0) | 4 (44.4) | 6 (35.3) |
| History of acute myocardial infarction | 158 (3.0) | 21 (3.8) | 19 (4.5) | 13 (4.0) | 7 (5.3) | 3 (12.0) | 2 (22.2) | 1 (5.9) |
| History of angina | 184 (3.5) | 21 (3.8) | 20 (4.8) | 13 (4.0) | 6 (4.5) | 4 (16.0) | 1 (11.1) | 3 (17.7) |
| History of stroke / transient ischemic attack | 152 (2.9) | 18 (3.3) | 19 (4.5) | 16 (4.9) | 8 (6.0) | 4 (16.0) | 3 (33.3) | 1 (5.9) |
AMD = age-related macular degeneration; IRP = increased retinal pigment; RPE = retinal pigment epithelium; GA = geographic atrophy.
One participant had geographic atrophy in the left eye and exudative AMD in the right eye.
Table 2.
Hypertension, Ocular Perfusion Pressure and the Risk of Age-related Macular Degeneration (AMD) in the Los Angeles Latino Eye Study, Odds Ratio (95% Confidence Interval)
| Risk Factor | Early AMD (N=551) | Soft Indistinct Drusen (N=421) | IRP (N=328) | RPE depigmentation (N=133) | Advanced AMD (N=25) | GA*(N=9) | Exudative AMD*(N=17) |
|---|---|---|---|---|---|---|---|
| History of hypertension | |||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.96 (0.79−1.17) | 1.08 (0.87−1.34) | 0.93 (0.72−1.20) | 1.10 (0.75−1.60) | 1.30 (0.58−2.94) | 1.50 (0.39−5.76) | 1.35 (0.51−3.59) |
| Systolic blood pressure (SBP) (mmHg) | |||||||
| <140 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥140 | 1.18 (0.95−1.46) | 1.19 (0.93−1.51) | 1.14 (0.86−1.50) | 1.03 (0.66−1.59) | 0.4 (0.14−1.12) | 0.19 (0.02−1.58) | 0.52 (0.16−1.69) |
| Per 10mmHg higher SBP | 1.01 (0.97−1.06) | 1.02 (0.97−1.08) | 1.00 (0.94−1.07) | 1.04 (0.94−1.14) | 0.95 (0.77−1.17) | 0.74 (0.51−1.08) | 1.04 (0.82−1.33) |
| Diastolic blood pressure (DBP) (mmHg) | |||||||
| <85 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥85 | 1.19 (0.96−1.48) | 1.35 (1.06−1.72) | 1.19 (0.90−1.56) | 1.48 (0.995−2.20) | 1.72 (0.69−4.32) | Insufficient data | 3.33 (1.19−9.26) |
| Per 10mmHg higher DBP | 1.01 (0.93−1.10) | 1.03 (0.94−1.13) | 1.03 (0.93−1.14) | 1.12 (0.96−1.31) | 1.16 (0.81−1.67) | 0.58 (0.32−1.06) | 1.79 (1.14−2.82) |
| Pulse pressure (mmHg) | |||||||
| <40 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 40−90 | 1.02 (0.83−1.25) | 0.96 (0.76−1.23) | 0.86 (0.67−1.12) | 1.06 (0.71−1.60) | 0.41 (0.15−1.14) | 1.38 (0.16−11.71) | 0.19 (0.06−0.61) |
| >90 | 1.56 (0.82−2.94) | 1.79 (0.95−3.39) | 1.58 (0.73−3.40) | 3.37 (1.23−8.82) | 1.19 (0.21−6.67) | Insufficient data | 1.11 (0.19−6.33) |
| Per 10mmHg higher OPP | 0.99 (0.89−1.12) | 0.94 (0.86−1.02) | 1.10 (0.90−1.22) | 1.22 (0.90−1.63) | 0.82 (0.48−1.48) | 0.35 (0.28−0.48) | 1.48 (0.82−2.84) |
Odds ratios are adjusted for age, sex and smoking. Significant values are indicated in bold.
AMD = age-related macular degeneration; IRP = increased retinal pigment; RPE = retinal pigment epithelium; GA = geographic atrophy, OPP = ocular perfusion pressure
One participant had geographic atrophy in the left eye and exudative AMD in the right eye.
Figure 2.
Estimated Prevalence of Exudative Age-Related Macular Degeneration (AMD) by Diastolic Blood Pressure with Locally Weighted Regression Line in the Los Angeles Latino Eye Study
Ocular perfusion pressure per 10mmHg increase was found to be protective for geographic atrophy (OR, 0.4; p<0.0001). No significant association was demonstrated between OPP and exudative AMD, nor with any early AMD lesions.
The presence of diabetes among LALES participants by maculopathy status is presented in Table 1. Definite diabetes was present in 19.5% of the overall LALES population. Table 3 shows the odds ratios of AMD lesions by presence/absence of diabetes. There was no significant association between diabetes or hyperglycemia and any AMD lesions after adjustment for age, sex and smoking status. No significant relationship was found between AMD and BMI (Table 4); however being either overweight or obese was independently associated with an increased risk of increased retinal pigment (OR, 1.6; p=0.03).
Table 3.
Diabetes mellitus and the Risk of Age-related Macular Degeneration (AMD) in the Los Angeles Latino Eye Study, Odds Ratios (95% Confidence Interval)
| Risk Factor | Early AMD (N=551) | Soft Indistinct Drusen (N=421) | IRP (N=328) | RPE depigmentation (N=133) | Advanced AMD (N=25) | GA*(N=9) | Exudative AMD*(N=17) |
|---|---|---|---|---|---|---|---|
| History of diabetes mellitus | |||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.83 (0.65, 1.06) | 0.79 (0.60, 1.04) | 0.84 (0.62, 1.13) | 0.82 (0.51, 1.31) | 0.88 (0.32, 2.40) | 1.00 (0.20, 4.89) | 0.77 (0.22, 2.75) |
| Presence of Diabetes mellitus on examination | |||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.90 (0.72, 1.13) | 0.85 (0.66, 1.09) | 0.90 (0.68, 1.19) | 0.83 (0.53, 1.28) | 0.59 (0.20, 1.75) | 0.85 (0.17, 4.14) | 0.42 (0.09, 1.85) |
| Glycosylated Hemoglobin (%) | |||||||
| <7.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥7.0 | 1.01 (0.80, 1.29) | 0.94 (0.71, 1.24) | 1.04 (0.77, 1.41) | 0.99 (0.61, 1.59) | 1.21 (0.40, 3.66) | 1.54 (0.31, 7.57) | 0.89 (0.20, 4.06) |
| Random blood glucose (mg%) | |||||||
| <200 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ≥200 | 1.04 (0.76, 1.41) | 0.98 (0.68, 1.40) | 1.00 (0.67, 1.49) | 1.22 (0.69, 2.14) | 0.63 (0.08, 4.82) | Insufficient data | 1.04 (0.13, 8.23) |
Odds ratios are adjusted for age, sex and smoking.
AMD = age-related macular degeneration; IRP = increased retinal pigment; RPE = retinal pigment epithelium; GA = geographic atrophy;
One participant had geographic atrophy in the left eye and exudative AMD in the right eye.
Table 4.
Body Mass Index (BMI) and the Risk of Age-related Macular Degeneration (AMD) in the Los Angeles Latino Eye Study, Odds Ratios (95% Confidence Interval)
| Risk Factor | Early AMD N=551 | Soft Indistinct Drusen N=421 | IRP N=328 | RPE depigmentation N=133 | Advanced AMD N=25 | GA*N=9 | Exudative AMD*N=17 |
|---|---|---|---|---|---|---|---|
| Body Mass Index (kg/m2) | |||||||
| <25 (low/normal) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| 25−30 (overweight) | 1.42 (1.03, 1.96) | 1.36 (0.95, 1.95) | 1.71 (1.12, 2.61) | 1.50 (0.81, 2.76) | 1.22 (0.36, 4.08) | 0.34 (0.05, 2.12) | 1.91 (0.39, 9.42) |
| ≥ 30 (obese) | 1.29 (0.94, 1.76) | 1.30 (0.91, 1.85) | 1.43 (0.94, 2.20) | 1.11 (0.60, 2.06) | 1.28 (0.38, 4.40) | 0.68 (0.14, 3.32) | 1.51 (0.29, 8.03) |
| Overweight/Obese | |||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.34 (0.99, 1.82) | 1.32 (0.94, 1.86) | 1.55 (1.03, 2.34) | 1.29 (0.72, 2.31) | 1.24 (0.40, 3.85) | 0.50 (0.12, 2.13) | 1.73 (0.37, 8.01) |
Odds ratios are adjusted for age, sex and smoking. Significant value is indicated in bold.
AMD = age-related macular degeneration; IRP = increased retinal pigment; RPE = retinal pigment epithelium; GA = geographic atrophy.
One participant had geographic atrophy in the left eye and exudative AMD in the right eye.
History of cardiovascular diseases by AMD is shown in Table 1. After adjustment for age, sex, and smoking in the multivariate model, only history of stroke or transient ischemic attacks (TIAs) have significant association with geographic atrophy (OR, 6.4; p=0.01) (Table 5).
Table 5.
History of Cardiovascular Disease and the Risk of Age-related Macular Degeneration (AMD) in the Los Angeles Latino Eye Study, Odds Ratios (95% Confidence Interval)
| Risk Factor | Early AMD N=551 | Soft Indistinct Drusen N=421 | IRP N=328 | RPE depigmentation N=133 | Advanced AMD N=25 | GA*N=9 | Exudative AMD*N=17 |
|---|---|---|---|---|---|---|---|
| History of Acute Myocardial Infarction | |||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.85 (0.53, 1.37) | 0.91 (0.55, 1.50) | 0.88 (0.49, 1.59) | 1.19 (0.54, 2.63) | 1.88 (0.53, 6.61) | 3.83 (0.76, 19.39) | 0.87 (0.11, 6.84) |
| History of Angina | |||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.87 (0.54, 1.39) | 1.01 (0.62, 1.64) | 0.91 (0.51, 1.63) | 1.04 (0.45, 2.42) | 2.72 (0.89, 8.32) | 1.69 (0.20, 14.01) | 3.04 (0.83, 11.06) |
| History of Stroke or Transient Ischemic Attacks | |||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.83 (0.50, 1.38) | 1.04 (0.63, 1.73) | 1.30 (0.76, 2.24) | 1.63 (0.77, 3.44) | 2.33 (0.71, 7.61) | 6.42 (1.44, 28.61) | 0.63 (0.08, 5.31) |
| History of Acute Myocardial Infarction, Angina, Stroke or Transient Ischemic Attacks | |||||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.87 (0.63, 1.20) | 0.97 (0.69, 1.35) | 0.96 (0.65, 1.42) | 1.14 (0.65, 2.00) | 1.80 (0.72, 4.55) | 2.23 (0.53, 9,49) | 1.39 (0.43, 4.49) |
Odds ratios are adjusted for age, sex and smoking. Significant value is indicated in bold.
AMD = age-related macular degeneration; IRP = increased retinal pigment; RPE = retinal pigment epithelium; GA = geographic atrophy;
One participant had geographic atrophy in the left eye and exudative AMD in the right eye.
Discussion
The LALES, a cross-sectional population-based survey of prevalence and risk factors for eye disease in Latinos, provided a unique opportunity to assess the relationship of cardiovascular disease and AMD in what is the largest minority group and fastest growing segment of the US population. (27) Strengths include use of standardized protocols for data collection including interviews, blood pressure, height and weight measurements, and grading of fundus photographs (28), as well as having a large number of participants with gradable stereoscopic macular photographs. The procedure for the grading of AMD lesions was similar to that used in the Beaver Dam Eye Study (31), the Blue Mountains Eye Study (32) and the Rotterdam Study (33). The limitations of our study include the small number of cases of advanced AMD which only allows us to identify strong relationships and limits our ability to study moderate or weaker relationships. Furthermore, our study is a prevalence study and thus we are unable to discuss any temporal relationships between these risk factors and the development of AMD. Such an assessment would be possible with incident data which is currently being collected.
In previous LALES publications, we found that older age, male sex, having ever smoked, and heavy consumption of alcohol were independently associated with AMD. (38, 39) However, there were too few cases of heavy alcohol consumption associated with cardiovascular risk factors in our population. Thus, only age, sex and smoking were included in the multivariate model for the current analysis.
Hypertension
It has been proposed that exudative AMD is caused by the deposition of lipid in the sclera and Bruch's membrane, which leads to increased choriocapillary pressure, decreased choroidal blood flow, and calcification and fragmentation of Bruch's membrane. (22) This deposition of lipid is enhanced by high choroidal arterial and venous hydrostatic pressures (22) thus contributing to the development of exudative AMD.
Diastolic hypertension was associated with a three-fold increased risk of exudative AMD (p=0.02), after controlling for age, sex and smoking. Moreover, each 10mmHg increment of DBP imparted an almost two-fold increased risk of exudative AMD (p=0.01). This finding was further strengthened by the significant association of diastolic hypertension with soft indistinct drusen (OR, 1.3; p=0.02), a well-recognized risk factor for the development of exudative AMD. (22, 34)
The association between blood pressure and AMD has been well documented in various population-based studies. (6-10, 12) In the Framingham Heart and Eye Studies systemic hypertension was significantly associated with AMD. (7) In the Beaver Dam Eye Study (BDES), individuals with uncontrolled hypertension had over a three-fold risk of developing exudative AMD. (8) Higher SBP and pulse pressure at baseline were associated with higher risk of exudative AMD (8) and RPE depigmentation (6, 8), while higher pulse pressure was also associated with increased retinal pigment. (8) BDES did not find any significant associations between hypertension and exudative AMD at baseline. (36) However, the prevalence of hypertension at baseline in the BDES population was lower than that of the LALES population (36.7% and 43.2%, respectively) while the prevalence of exudative AMD was much higher in BDES compared to LALES (1.2% and 0.3%, respectively). Therefore despite the small number of cases of exudative AMD, the significant relationship that we found in our study between hypertension and exudative AMD further emphasizes the role that hypertension may play in exudative AMD.
Data from case-control studies have suggested that different underlying pathogenesis for neovascular and non-neovascular AMD may be at work. (5) A large case-control study by Hyman et al found that neovascular AMD cases were more likely to have elevated DBP than controls, and the strength of association increased with more severe hypertension. Similarly, they too found no association between hypertension and geographic atrophy. (5) Similarly, the Age Related Eye Disease Study (AREDS) reported a significant association between hypertension and exudative AMD as well as large drusen, but not geographic atrophy. (4) In our study we did not find any association between hypertension and geographic atrophy, although our small sample size limits our ability to examine these relationships.
Ocular perfusion pressure
It has been hypothesized that decreased OPP is associated with an accumulation of lipofucsin in the RPE cells. (37) Increase in RPE lipofucsin levels preceded the development of new GA lesions and the enlargement of existing lesions. (37) Higher OPP was found to be protective for geographic atrophy (OR, 0.4; p<0.0001) in our study. No association was demonstrated between OPP and exudative AMD, nor with any early AMD lesions. To the best of our knowledge, no other population-based studies have found a relationship between OPP and geographic atrophy. Thus, our finding supports the hypothesis that greater ocular perfusion may be protective and hence reduced choroidal blood flow may be particularly relevant in the development of geographic atrophy (GA).
Body mass index and markers of obesity
In our data, only increased retinal pigment was noted to have a significant association with overweight/obese persons compared to those with low/normal BMI. Early AMD was associated with overweight/obese BMI, but this relationship was only of borderline significance. Although there was an increased risk of all early AMD lesions in individuals outside the normal BMI range, these results were not significant.
The relationship between BMI and AMD has been previously studied (4, 11, 16-19). The Beaver Dam Eye Study (BDES) showed a significant relationship between higher BMI, higher waist-to-hip ratio and early AMD in women, (16) while the Blue Mountains Eye Study (BMES) reported an increased risk of early AMD associated with BMI outside the normal range. (17) No significant associations for BMI were seen with advanced AMD in both BDES and BMES. Nevertheless, both studies did not examine the relation of BMI to individual AMD lesions, and thus the significant association between higher BMI and early AMD may in part be due an underlying association between BMI and increased retinal pigment, which in part defines early AMD. Meanwhile, the Pathologies Oculaires Liees a l'Age (POLA) study in France demonstrated an increased risk of advanced AMD and pigmentary abnormalities in obese participants. (18) In the AREDS study significant associations between greater BMI, neovascular AMD and geographic atrophy were observed. (4, 19)
In sum these findings contribute to the body of evidence that overweight/obesity and markers of obesity are associated with AMD, possibly through an increase in oxidative damage and inflammation in overweight/obese persons (11). Obesity is also associated with an increase in C-reactive protein, a marker for systemic inflammation which in turn is also significantly associated with AMD. (23)
Diabetes mellitus
Our study was unable to detect any association between diabetes mellitus or hyperglycemia and any AMD lesion. Other population-based studies that have examined the relationship of diabetes to AMD did not detect any association. (10, 12, 18, 35) Only few studies showed any association. (11, 19-21)
While the lack of a positive finding in our study could be due to numerous reasons including a survivor effect and systematic grading errors, it is also possible that diabetes mellitus may have a different impact on AMD in different populations. Our lack of an association is particularly important among Latinos who have a significantly high prevalence and incidence of diabetes mellitus.
History of cardiovascular disease
In our study population we found that only history of stroke or transient ischemic attacks (TIA) showed a significant association with AMD; individuals who reported ever having an episode of stroke or TIA were at an increased risk for geographic atrophy. No other association between cardiovascular disease (CVD) and AMD type or lesion was seen. Although statistically significant, the association between stroke or TIA and geographic atrophy may be due to chance finding, particularly with the low number of individuals with both conditions in this baseline cohort. Conversely, the lack of association seen between other CVD factors and AMD may be due to limited statistical power.
Many studies that examined the relationship between CVD and AMD did not find significant or consistent associations. (5, 6, 8, 17, 35, 36) In the Blue Mountains Eye Study (BMES), association between CVD and AMD was only observed after 10 years of follow-up and not at baseline or 5-year examinations. (15) The Age-Related Eye Disease Study (AREDS) found at baseline that individuals with intermediate drusen, extensive small drusen, or pigment abnormalities were less likely to have history of angina (4), but that this association was not present in the follow-up study. (19)
The first National Health and Nutrition Examination Survey (NHANES-I) showed an association between history of cerebrovascular disease and higher prevalence of AMD. (9) In the subsequent national survey (NHANES-III), the authors found an association between history of angina and increased retinal pigment, and demonstrated that this finding holds true only for Mexican-Americans. (12) Interestingly we did not find this association in our population of Latinos where the majority was also of Mexican-American ancestry. This disparity in findings may have been due to different sample size (only 1,925 Mexican-Americans had gradable fundus photographs in NHANES-III) and different examination protocol (NHANES-III captured non-mydriatic photographs and only one eye per participant was graded for AMD).
Unlike most association studies which examined CVD as risk factor for AMD, investigators of the Atherosclerosis Risk in Communities (ARIC) Study postulated that AMD could in turn be a risk factor for stroke, and found that presence of early AMD at baseline predicted the 10-year cumulative incidence of stroke. (40) The authors proposed 3 possible explanations for this association, which included the shared mechanism for atherosclerosis, inflammation and other pathogenic pathways such as Alzheimer disease in both AMD and stroke. (40) Due to differences in study design and analytical strategy it is difficult to draw conclusions by direct comparisons of the results from ARIC and LALES. Nonetheless, the findings from ARIC demonstrated the relationship between AMD and CVD events, and thus highlighted the importance of conducting longitudinal follow-up for LALES for a more robust assessment of risk factor associations.
While many have examined the association of AMD with classic clinical manifestations of CVD (such as myocardial infarction and stroke), more studies are beginning to assess its association with subclinical CVD and direct measures of atherosclerosis instead. In the Rotterdam Study, presence of plaques in the carotid artery and carotid bifurcation increased the risk of AMD. (13) More recently, Klein et al presented findings from the Multiethnic Study of Atherosclerosis (MESA) which showed that certain signs of subclinical CVD were associated with early AMD, and that these associations varied by race/ethnicity. (14) The reason for these ethnicity-specific associations remains unclear, but may suggest that different pathogenic pathways for AMD could exist for different ethnic groups. Further investigations are therefore needed in order to understand the pathogenesis of AMD and its association with cardiovascular factors specific to populations of similar genetic admixtures. This may in turn impact the clinical management, treatment and prevention of AMD.
In summary, our findings suggest that advanced AMD lesions may share some pathogenic mechanisms associated with cardiovascular disease in the Latino population. Any intervention to reduce the prevalence of these risk factors may reduce the burden of AMD in this population. However, longitudinal data to confirm these findings would be useful to further determine the potential value of any intervention to reduce these cardiovascular risk factors.
Acknowledgements
A. Support: National Institutes of Health Grants: NEI U10-EY-11753 and EY-03040 and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Rohit Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
B. Financial Disclosure: The authors have no proprietary or commercial interest in any materials discussed in the manuscript.
C. Statement about Conformity: The study protocol was approved by the Institutional Review Board (IRB)/Ethics Committee at the University of Southern California and all study procedures adhered to the recommendations of the Declaration of Helsinki. Written consent was obtained from all participants.
D. Contributions: design and conduct of the study (RV, SA); collection, management, analysis, and interpretation of the data (SFB, JW, RK, SA, CH, AWPF, RV); and preparation, review, or approval of the manuscript (SFB, JW, RK, SA, CH, AWPF, RV).
E. Acknowledgements: The Los Angeles Latino Eye Study Group, University of Southern California, Los Angeles, CA.-Rohit Varma, MD, MPH; Sylvia H. Paz, MS; Stanley P. Azen, PhD; Lupe Cisneros, COA; Elizabeth Corona; Carolina Cuestas, OD; Denise R. Globe, PhD; Sora Hahn, MD; Mei-Ying Lai, MS; George Martinez; Susan Preston-Martin, PhD; Ronald E. Smith, MD; LaVina Tetrow, Mina Torres, MS; Natalia Uribe, OD; Jennifer Wong, MPH; Joanne Wu, MPH; Myrna Zuniga.
Battelle Survey Research Center, St. Louis, MO- Sonia Chico, BS; Lisa John, MSW; Michael Preciado, BA; Karen Tucker, MA.
Ocular Epidemiology Grading Center, University of Wisconsin, Madison, WI Ronald Klein, MD, MPH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Advisory Eye Council (U.S.) Vision research: a national plan, 1994−1998. Vol. 1993. U.S. Department of Health and Human Services, National Institutes of Health; Washington, D.C.: pp. 1–396. [Google Scholar]
- 2.Klein R, Wang Q, Klein BE, et al. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182–191. [PubMed] [Google Scholar]
- 3.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Age-Related Eye Disease Study Research Group Risk factors associated with age-related macular degeneration. A case-control study in the Age-Related Eye Disease Study: Age-Related Eye Disease Study report number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyman L, Schachat AP, He Q, Leske C. Age-Related Macular Degeneration Risk Factors Study Group. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch Ophthalmol. 2000;118:351–8. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BEK, Jensen SC. The relation of cardiovascular disease and its risk factors to the 5-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:1804–12. doi: 10.1016/s0161-6420(97)30023-2. [DOI] [PubMed] [Google Scholar]
- 7.Sperduto RD, Hiller R. Systemic hypertension and age-related maculopathy in the Framingham Study. Arch Ophthalmol. 1986;104:216–19. doi: 10.1001/archopht.1986.01050140070022. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Klein BE, Tomany SC, et al. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110:1273–80. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg J, Flowerdew G, Smith E, Brody JA, Tso MO. Factors associated with age-related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol. 1988;128(4):700–10. doi: 10.1093/oxfordjournals.aje.a115023. [DOI] [PubMed] [Google Scholar]
- 10.Cruickshanks K, Hamman RF, Klein R, Nondahl DM, Shetterly SM. The prevalence of age-related maculopathy by geographic region and ethnicity: the Colorado-Wisconsin Study of Age-Related Maculopathy. Arch Ophthalmol. 1997;115(2):242–250. doi: 10.1001/archopht.1997.01100150244015. [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Deng Y, Klein BEK, Hyman L, Seddon J, Frank RN, Wallace RB, Hendrix SL, Kuppermann BD, Langer RD, Kuller L, Brunner R, Johnson KC, Thomas AM, Haan M. Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: Women's Health Initiative Sight Exam Ancillary Study. Am J Ophthalmol. 2007;143:473–483. doi: 10.1016/j.ajo.2006.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein R, Klein BEK, Jensen SC, Mares-Perlman JA, Cruickshanks KJ, Palta M. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–65. doi: 10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 13.Vingerling JR, Dielemans I, Bots ML, Hofman A, Grobbee DE, de Jong PTVM. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am J Epidemiol. 1995;142:404–9. doi: 10.1093/oxfordjournals.aje.a117648. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BEK, Knudtson M, Cotch MF, Wong TY, Liu Kiang, Burke GL, Saad MF, Jacobs DR, Sharrett AR. Subclinical atherosclerotic cardiovascular disease and early age-related macular degeneration in a multiracial cohort: the Multiethnic Study of Atherosclerosis. Arch Ophthalmol. 2007;125:534–543. doi: 10.1001/archopht.125.4.534. [DOI] [PubMed] [Google Scholar]
- 15.Tan JSL, Mitchell P, Smith W, Wang JJ. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2007;114:1143–50. doi: 10.1016/j.ophtha.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Klein BE, Klein R, Lee KE, et al. Measures of obesity and age-related eye diseases. Ophthalmic Epidemiol. 2001;8:251–62. doi: 10.1076/opep.8.4.251.1612. [DOI] [PubMed] [Google Scholar]
- 17.Smith W, Mitchell P, Leeder SR, Wang JJ. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 1998;116:583–587. doi: 10.1001/archopht.116.5.583. [DOI] [PubMed] [Google Scholar]
- 18.Delcourt C, Michel F, Corvez A, Lacroux A, Delage M, Vernet MH, POLA Study Group Associations of cardiovascular disease and its risk factors with age-related macular degeneration: the POLA study. Ophthalmic Epidemiol. 2001;8:237–49. doi: 10.1076/opep.8.4.237.1613. [DOI] [PubMed] [Google Scholar]
- 19.Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL., 3rd Age-Related Eye Disease Study Research Group. Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112:533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein R, Klein BE, Moss SE. Diabetes, hyperglycemia, and age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:1527–34. doi: 10.1016/s0161-6420(92)31770-1. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell P, Wang JJ. Diabetes, fasting blood glucose and age-related maculopathy: the Blue Mountains Eye Study. Aust N Z J Ophthalmol. 1999;27:197–9. doi: 10.1046/j.1440-1606.1999.00211.x. [DOI] [PubMed] [Google Scholar]
- 22.Friedman E. The role of the atherosclerotic process in the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2000;130:658–6.3. doi: 10.1016/s0002-9394(00)00643-7. [DOI] [PubMed] [Google Scholar]
- 23.Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291:704–10. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 24.Vine AK, Stader J, Branham K, Musch DC, Swaroop A. Biomarkers of cardiovascular disease as risk factors for age-related macular degeneration. Ophthalmology. 2005;112:2076–2080. doi: 10.1016/j.ophtha.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. Am J Epidemiol. 1996;144:537–47. doi: 10.1093/oxfordjournals.aje.a008963. [DOI] [PubMed] [Google Scholar]
- 26.Despriet DD, Klaver CC, Witteman JC, Bergen AA, Kardys I, de Maat MP, Boekhoorn SS, Vingerling JR, Hofman A, Oostra BA, Uitterlinden AG, Stijnen T, van Duijn CM, de Jong PT. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA. 2006;296:301–9. doi: 10.1001/jama.296.3.301. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Census Bureau [October 4, 2007];Projections of the resident population by race, Hispanic origin, and nativity: middle series, 2050 to 2070. Available at http://www.census.gov/population/projections/nation/summary/np-t5-g.txt.
- 28.Varma R, Paz SH, Azen SP, Klein R, Globe D, Torres M, Shufelt C, Preston-Martin S. Los Angeles Latino Eye Study Group. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–31. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Varma R, Fraser-Bell S, Tan S, Klein R, Azen SP. Los Angeles Latino Eye Study Group. Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1288–97. doi: 10.1016/j.ophtha.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia: the Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–60. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 33.Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CFL, de Jong PTVM. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102:205–10. doi: 10.1016/s0161-6420(95)31034-2. [DOI] [PubMed] [Google Scholar]
- 34.Sigelman J. Foveal drusen resorption one year after perifoveal laser photocoagulation. Ophthalmology. 1991;98(9):1379–1383. doi: 10.1016/s0161-6420(91)32122-5. [DOI] [PubMed] [Google Scholar]
- 35.Klein R, Klein BEK, Marino EK, Kuller LH, Furberg C, Burke GL, Hubbard LD. Early age-related maculopathy in the Cardiovascular Health Study. Ophthalmology. 2003;110:25–33. doi: 10.1016/s0161-6420(02)01565-8. [DOI] [PubMed] [Google Scholar]
- 36.Klein R, Klein BEK, Franke T. The relationship of cardiovascular disease and its risk factors to age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1993;100:406–414. doi: 10.1016/s0161-6420(93)31634-9. [DOI] [PubMed] [Google Scholar]
- 37.Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J, Scholl HPN, Schmitz-Valckenberg S, the FAM-Study Group Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–472. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 38.Fraser-Bell S, Donofrio J, Wu J, Klein R, Azen SP, Varma R, the Los Angeles Latino Eye Study Group Sociodemographic factors and age-related macular degeneration in Latinos: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2005;139:30–38. doi: 10.1016/j.ajo.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 39.Fraser-Bell S, Wu J, Klein R, Azen SP, Varma R, the Los Angeles Latino Eye Study Group Smoking, alcohol intake, estrogen use, and age-related macular degeneration in Latinos: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2006;141:79–87. doi: 10.1016/j.ajo.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 40.Wong TY, Klein R, Sun C, Mitchell P, Couper DJ, Lai H, Hubbard LD, Sharrett AR, the Atherosclerosis Risk in Communities Study Age-related macular degeneration and risk for stroke. Ann Intern Med. 2006;145:98–106. doi: 10.7326/0003-4819-145-2-200607180-00007. [DOI] [PubMed] [Google Scholar]