Abstract
Background
Aging increases vulnerability to obstructive sleep apnea (OSA), but the underlying mechanisms remain unclear. Recent data in awake healthy volunteers show a decrease in the genioglossus negative pressure reflex and anatomic compromise with increasing age, suggesting an age-related predisposition to pharyngeal collapse. However, aging effects on pharyngeal collapsibility have not been studied extensively during sleep. We tested the hypotheses that upper airway closing pressure (PCLOSE) and the increase in pharyngeal resistance during sleep (primary outcomes) as well as measures of arousal threshold (secondary outcomes) increase with age.
Methods
We studied 21 healthy individuals (8 women [mean (± SD) age, 36 ± 18 years] and 13 men [mean age, 41 ±23 years]) who were between 18 and 75 years of age. During overnight polysomnography, we measured nasal pressure (Pmask) and epiglottic pressure (Pepi) during stage 2 sleep before and after airway occlusion (external valve) until arousal. Pclose was defined as the pressure at which Pmask plateaued despite further decreases in Pepi.
Results
Increasing age was correlated with both pharyngeal collapsibility ([Pclose] r = 0.69; p < 0.01) and an increase in pharyngeal resistance during sleep (r = 0.56; p < 0.01) independent of body mass index (BMI) and gender. There was no evidence for an effect of age on arousal threshold after airway occlusion during stage 2 sleep.
Conclusions
Older age is associated with increased pharyngeal airway collapsibility during sleep independent of gender and BMI. These data may at least partially explain the mechanisms underlying the predisposition for pharyngeal collapse in the elderly.
Keywords: aging, collapsibility, dilator muscles, integrity, sleep-disordered breathing, upper airway
Obstructive sleep apnea (OSA) is a common disorder with important and well-established sequelae such as hypertension1–3; some evidence has also established an increased risk of either stroke or death in older OSA patients,4 yielding considerable interest in the mechanisms underlying OSA in the elderly population.
Most current evidence suggests that OSA patients have an anatomic predisposition to pharyngeal collapse on a biomechanical basis.5,6 Through protective reflex mechanisms that drive the activation of dilator muscles, pharyngeal patency is well-maintained during wakefulness. However, these protective reflexes are diminished during sleep, leading to the collapse of the pharyngeal airway in anatomically predisposed individuals.7
Aging substantially increases the risk of OSA.8,9 The underlying mechanisms are unclear but may include age-related changes in pharyngeal collapsibility, ventilatory control stability, and/or arousal responses. In the elderly, the ventilatory control system is quite stable, suggesting that this is an unlikely mechanism for the increased prevalence of OSA in this population.10,11 However, several lines of evidence10,12–17 suggest that aging could predispose the patient to OSA by increases in pharyngeal collapsibility during sleep, although this has not been demonstrated directly. We have previously observed that pharyngeal airway length (in women)18 and the size of the parapharyngeal fat pads increase with age. Both of these abnormalities may impair pharyngeal mechanics during sleep in the elderly. In men, the decrement in upper airway dilator muscle activity associated with wake-sleep transitions increases with age.15,19 In addition, the genioglossus negative pressure reflex during wakefulness is impaired with aging.18 If this finding were also the case during sleep, the impairment of protective reflexes could be a mechanism underlying the propensity for apnea associated with aging. In general, older adults seem to have a lower arousal threshold as the number of arousals per hour of sleep increases linearly with age.20 However, it is possible that upper airway sensory impairment (as evidenced by an impairment of the genioglossus negative pressure reflex in the elderly)18 could increase the threshold for arousal responses, to upper airway mechanical stimuli in particular.21 Therefore, it is difficult to predict the net effects of age on arousal threshold from the available data. In theory, either a low arousal threshold or a high arousal threshold could increase the propensity for apnea by creating state instability or profound blood gas abnormalities leading to unstable ventilatory control, respectively.
In summary, the available data suggest that deteriorating anatomy, muscle function, and the ability to maintain sleep may be responsible for the increased susceptibility of the elderly to OSA. However, the effects of aging on pharyngeal collapsibility per se and the threshold for arousal to respiratory stimuli have not been studied systematically. During airway occlusion, inspiratory efforts become progressively greater in amplitude until arousal occurs.22 We therefore quantified airway collapsibility and the respiratory arousal threshold23 by hypothesizing that age would be associated with a higher (ie, less negative) upper airway closing pressure (Pclose), a greater increase in pharyngeal resistance during sleep, and a reduced arousal threshold (although changes in either direction would be possible).
Materials And Methods
Subjects
With the approval of institutional review boards, we studied 21 consenting, healthy individuals who were between 18 and 75 years of age. The subjects were recruited using e-mail announcements and posters, and through the Harvard Cooperative on Aging. We studied 8 women (mean age, 36 years [SD, 18 years]; age range, 19 to 72 years) and 13 men (mean age, 41 years [SD, 23 years]; age range, 18 to 75 years). The women who were < 50 years old were premenopausal based on regular menstrual cycles, whereas women > 50 years of age had been postmenopausal for at least 2 years. Subjects took no medications and were free of comorbid conditions, including snoring, based on the findings of a thorough history and physical examination.
Equipment and Techniques
The laboratory procedures, and measurement of ventilation and resistance were conducted as previously described.24 In order to assess the sleep-wake state, subjects were instrumented with two EEG channels, two electrooculography channels, and chin electromyography.
Airway Mechanic
Subjects wore a nasal mask (Respironics, Inc; Murraysville, PA) that was connected to a nonrebreathing valve. Inspiratory and expiratory airflow were determined with a pneumotachometer (model 3700A; Hans Rudolph Inc; Kansas City, MO). In addition, end-tidal carbon dioxide was monitored with an infrared analyzer (Capnograph Monitor; BCI; Waukesha, WI). Mask pressure (Pmask) values were monitored with an open catheter that was attached to a pressure transducer (Validyne Engineering; Northridge, CA), and airway pressures were measured at the level of the epiglottis with a pressure-tipped catheter (MPC-500; Millar; Houston, TX). One nostril was decongested (with oxymetazoline HCl) and anesthetized (with lidocaine HCl), and the pressure catheter (MPC-500; Millar) was passed through the mask and then inserted intranasally to the epiglottis. Prior to insertion, both pressure signals were calibrated simultaneously in a rigid cylinder using a standard water manometer. Any drift in the pressure catheters was corrected on a breath-by-breath basis by an automated computer program that defined end-inspiration and end-expiration by identifying the point of zero flow and correcting any offset in the pressure value. Minute ventilation and resistance measured at a flow rate of 0.2 L/s were calculated on a breath-by-breath basis.
Airway Occlusion
The mask occlusion apparatus is depicted in Figure 1. The nasal mask was sealed with an adhesive elastomer (Gum Adhesive & Remover; Rubie’s Costume Co, Inc; Richmond Hill, NY) and held in place by head straps. The nasal mask was connected directly to a pneumotachograph, which was connected to a Y-piece containing inspiratory and expiratory valves. The inspiratory port was connected to a pneumatic valve (Hans Rudolph) that could rapidly occlude, allowing the inspiration of room air unless the balloon was inflated. Expiration was not occluded such that airway occlusion could be performed at functional residual capacity. Subjects were instructed to breathe exclusively through the nose and were carefully monitored by video camera to ensure that the mouth was completely closed. If mouth opening was detected, the mouth was taped shut. Thus, only data obtained during nasal breathing were used.
Figure 1.
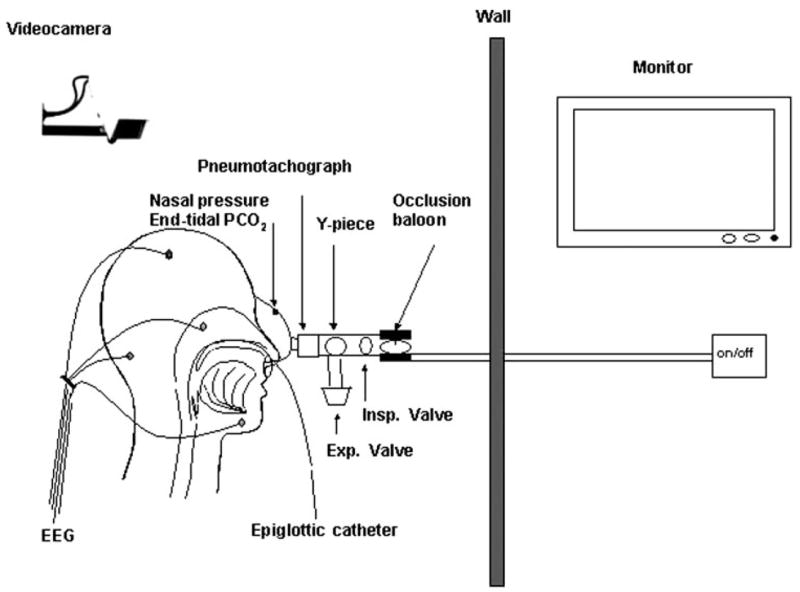
Measurement setup. A face mask covering the nose was sealed with an adhesive elastomer and held in place by head straps. The mask was connected directly to a pneumotachograph, which was connected to a Y-piece containing inspiratory and expiratory valves. The inspiratory port was connected to a pneumatic valve (Hans Rudolph) that could occlude this port while allowing expiration to continue such that airway occlusion could be performed at functional residual capacity.
Protocol
Each subject reported to the laboratory and signed consent forms at approximately 9:00 PM. Sleep-staging electrodes, pressure catheters, and the nasal mask were placed, and subjects assumed the supine posture, which they maintained throughout the study. Subjects subsequently lay with eyes open and were allowed to acclimate to the equipment. After recording for 5 min during wakefulness, subjects were allowed to fall asleep. In order to obtain multiple data points in a standardized sleep stage, subjects were awakened by closing the occlusion valve after they had demonstrated 5 consecutive minutes of stable stage 2 sleep without spontaneous awakenings. Subjects were then allowed to fall asleep again. This procedure was repeated until approximately 4 h of data had been collected.
Data Recording and Analyses
Airway pressure (Pmask and epiglottic pressure [Pepi]), inspiratory flow signals, end-tidal carbon dioxide, and ECG were digitized, and analyzed on a desktop computer (Spike 2 software; Cambridge Electronic Design Ltd; Cambridge, UK), and sleep stage was analyzed by a sleep assessment program (Polysmith, version 4.0, and Polysuite; Nihon Kohden; Foothill Ranch, CA) and scored by a technician.
We recorded tidal breathing for 5 min while the patient was awake to define baseline values of pharyngeal resistance at an inspiration flow of 0.2 L/s. These data were compared with those obtained after 5 min of stable stage 2 sleep (ie, immediately before an airway occlusion was performed). We analyzed at least 15 to 20 breaths during both wake and sleep. Airflow resistance was determined during the first 12 breaths by dropping a vertical line from the point at which flow reached 200 mL/s through both pressure recordings (ie, nasal and Pepi). To limit the noise related to measurement artifacts, the single highest and lowest resistance values were excluded as in prior studies, and the remaining breaths were used in data analysis.19 Pclose was defined as the inflection point of the Pmask trace, as observed during inspiration after external airway occlusion (occlusion valve), while Pepi further decreased (Fig 2, 3), as previously described.23
Figure 2.
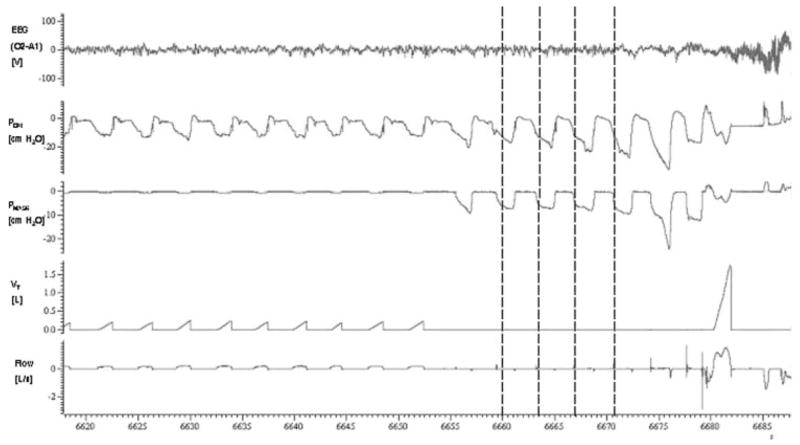
Typical response to airway occlusion (in a 68-year-old subject). During occlusion, each inspiratory effort produced a decrease in Pmask and Pepi. While Pepi progressively decreased to a minimum value, Pmask separated from Pepi during breaths 2 to 5 as the result of pharyngeal airway collapse. The inflection points (Pclose) of the Pmask trace (vertical lines) were defined as the points at which airway collapse occurred. Note that airway collapse (ie, the plateau in Pmask) does not occur on breath 1. This may be due to a decrease in expiratory lung volume between breaths 1 and 2 (subjects were able to exhale through an expiration valve). Minimum Pepi values became more negative with each subsequent respiratory effort until arousal from sleep occurred. The arousal threshold was defined as the nadir Pepi at the respiratory effort preceding arousal.
Figure 3.
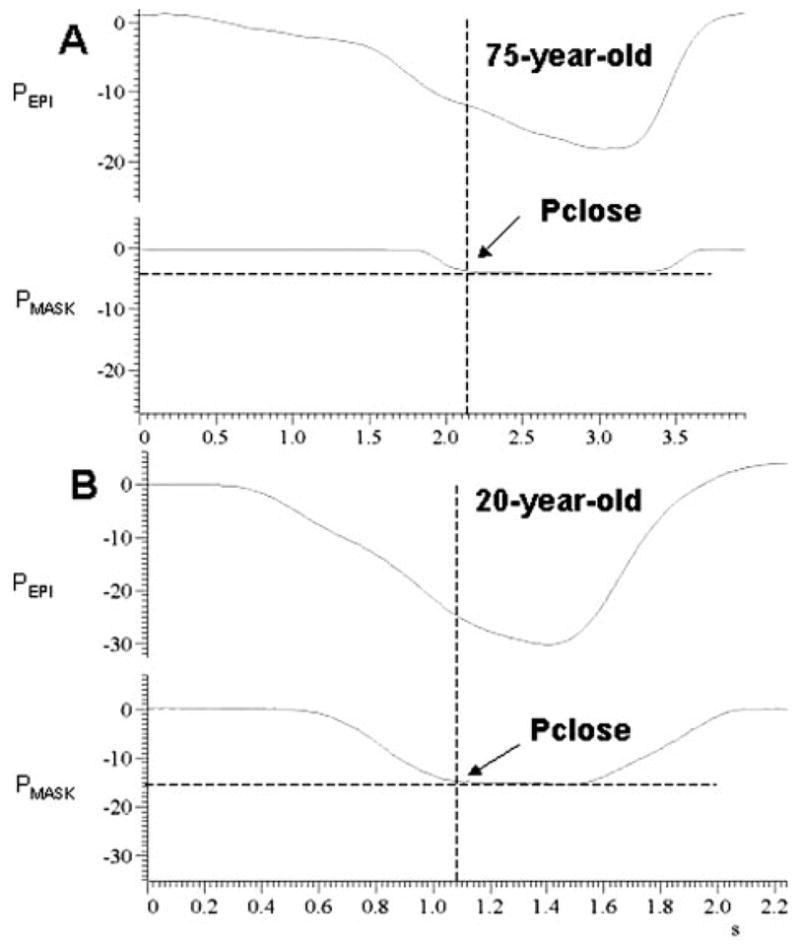
Measurement of Pclose from polygraph tracings of Pepi and Pmask. During occlusion of the valve connected to the subject’s nasal mask, Pepi progressively increased to a maximum value, whereas Pmask plateaued as the result of pharyngeal airway occlusion. The intersections between the two dotted lines represent the Pclose values. Top, A: elderly person 75 years of age. Bottom, B: younger person 20 years of age.
We report the average Pclose values of all occlusive respiratory efforts after inspiratory occlusion but prior to arousal from sleep (as detected by EEG [alpha-wave activity] or video observation [eye opening/movement]). The arousal threshold was defined as the nadir Pepi at the respiratory effort preceding arousal.
Statistical Analysis
Data were analyzed using a statistical software program (SPSS, version 13.0; SPSS Inc; Chicago, IL). Multiple linear regression analysis was used to determine whether age influenced Pclose and pharyngeal resistance (primary outcomes) or nadir Pepi at the respiratory effort preceding arousal (secondary outcome), and the age effects were normalized for “body mass index” (BMI) and “gender” effects. A value of p < 0.05 was used as the threshold for statistical significance. Our sample size estimation was based on a report on the effect of age on upper airway resistance (Rp) in male persons.19 In particular, we assumed a correlation coefficient of 0.65 between the variables age and Pclose (r1) as well as between age and pharyngeal resistance increase (r2). Based on these assumptions, we determined that a sample size of 21 persons would provide a power of > 80% (= Powerr1 × Powerr2) to detect significant age effects regarding both variables of upper airway collapsibility.
Other comparisons were conducted with an exploratory intention only. Seven pairs of younger persons (≤ 32 years of age) and older persons (> 32 years of age) who were closely matched for BMI were selected to test for age effects on variables of upper airway collapsibility. For that purpose, the Wilcoxon test for matched samples was applied.
Results
Two subjects (19 and 75 years old) were unable to sleep well such that the arousal threshold could not be assessed. In another subject (68 years old), pharyngeal resistance could not be measured because of consistent airflow limitation during early inspiration (flow rate, 0.2 L/s). All other subjects completed the entire study, and their mean (± SD) age, weight, height, and BMI were 39 ± 22 years (age range, 18 to 75 years), 66 ± 11 kg (weight range, 48 to 90 kg), 164 ± 8 cm (height range, 147 to 184 cm), and 25.8 ± 5.1 kg/m2 (BMI range, 18 to 35 kg/m2), respectively. In the BMI-matched subgroups, mean age and BMI were 24 ± 5 years, and 26.1 ± 5.5 kilograms/m2, respectively, in the ‘younger’ subjects, compared with 58 ± 14 years 26.0 ± 3.2 kg/ m2, respectively, in the older.
Main Outcome: Upper Airway Collapsibility
Typical collapsibility determinations are shown in Figures 2 and 3. During some of the airway occlusions (first breath in Fig 2), the upper airway did not collapse immediately after occlusion of the inspiration valve, but rather during subsequent breaths. This may be explained by a decrease in expiratory lung volume between consecutive breaths (subjects were able to exhale through an expiration valve).
Age was associated with increased Pclose (r = 0.69; p < 0.01) [Fig 4] and with an increase in Rp during sleep (r = 0.56; p < 0.01 [after correction of α error]) [Fig 5].
Figure 4.
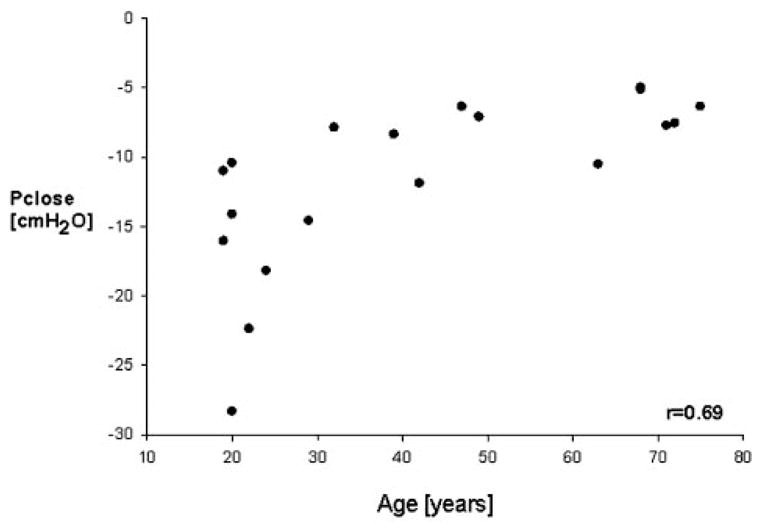
Pclose as a function of age. Mean values from 18 persons. Multiple regression analysis revealed that Pclose became less negative with age (r= 0.75; p = 0.011).
Figure 5.
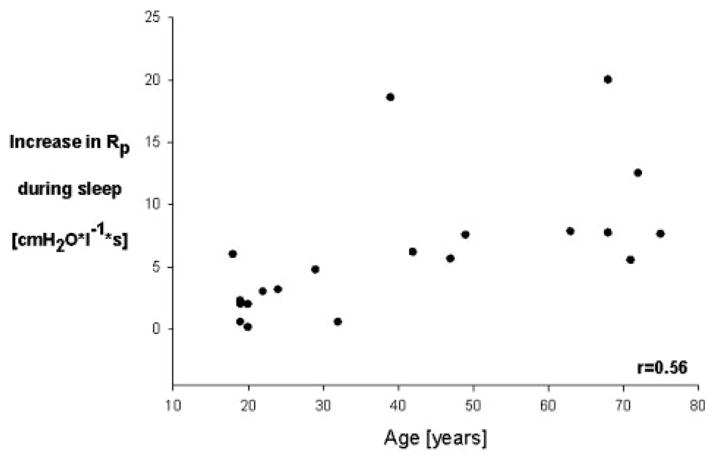
Difference in pharyngeal resistance between wakeful-ness and stable stage 2 sleep as a function of age. Data from 20 persons. Multiple regression analysis revealed that the increase in pharyngeal resistance from wakefulness to stable stage 2 sleep was closely related to age (r = 0.87; p < 0.0001).
From the plot of age vs Pclose, we took curve-linear effects of aging into account. Therefore, our statistical consultant (S.G.) attempted several transformations on age and Pclose. The transformation that yielded the highest correlation (r = 0.69) was “log of age” vs “−1/Pclose.” Since the difference in the correlation of values with and without transformation was only 0.1, we chose to report results in terms of the original variable for simplicity.
BMI did not correlate with Pclose but was significantly higher in the elderly population. Therefore, we compared Pclose in the pairs of volunteers matched for BMI, showing that mean Pclose was significantly higher (less negative) in elderly subjects compared with younger subjects (−8.3 ± 2.3 vs − 16 ± 6.9 cm H2O, respectively; p < 0.01).
During wakefulness, Rp did not significantly differ between younger and older persons. However, the mean increase in Rp during sleep was significantly greater in older subjects (9.91 ± 5.3 H2O/L/s) compared to younger subjects (2.4 ± 1.87 H2O/L/s; p < 0.05).
An exploratory data analysis revealed that subjects with a greater increase in pharyngeal resistance during sleep tended to have significantly smaller (ie, less negative) Pclose values (r = 0.54; p < 0.02). When looking at the distribution of Pclose and Rp with respect to age (Fig 4, 5), it seemed that the values of Pclose and Rp plateaued from the age of 45 years. Post hoc analysis confirmed that Pclose values from subjects aged ≥ 45 years did not significantly contribute to the Pclose age variance. In contrast, the age of subjects who were > 45 years of age significantly contributed to the variance of Rp. When excluding the subjects aged ≥ 45 years, the r2 between age and Rp increase during sleep decreased from 0.30 (whole collective) to 0.15 (subjects aged ≥45 years).
Secondary Outcome: Arousal Threshold
We did not find a significant relationship between age and arousal threshold (ie, the maximum negative Pepi on the final complete inspiratory effort preceding arousal amounted to −20.1 ± 9.4 cm H2O in elderly subjects vs −22.7 ± 10.4 cm H2O in younger subjects [r= 0.031; p = 0.91]). From these data, we calculated that > 8,000 patients would have been required to detect with an 80% power a significant correlation between age and arousal threshold, suggesting that the effect, if present, is weak. Similarly, when pairs (older vs younger) matched for BMI were compared, there were no significant differences in the maximum negative pharyngeal pressure, time to arousal, increase in end-tidal CO2 concentration, pulse oximetric saturation, or mean slope of the maximum pressure deflection.
Discussion
Our study shows that age independently influences both upper airway collapsibility and the increase in pharyngeal resistance during sleep, whereas BMI and gender explained minimal variance of either end point. We did not find any evidence for aging effects on the threshold to arouse from sleep after airway occlusion. In older (nonsnoring) persons, the magnitude of both Pclose 25,26 and the increase in Rp during sleep26 were comparable to those in previous reports25,26 from young persons who snored and did not have without sleep-disordered breathing.
Increased upper airway collapsibility in elderly persons, as observed in our study, might be explained by either anatomic or neuromuscular changes that result from aging.18,19 An age-related decline in the genioglossus negative pressure reflex,18 may contribute to the increased airway collapsibility in the older individuals observed in this study. The negative pressure reflex is a major mechanism whereby animals and humans maintain pharyngeal patency, and the reflex decreases with aging at least as measured during wakefulness.27 Impairment in the negative pressure reflex with aging may reflect a generalized deterioration in reflexes including reduced responses to noxious stimuli, reduced deep tendon reflexes, and poorer control of arterial BP and heart rate during sleep.28,29
The precise events leading to arousal and apnea termination are still not well understood. After airway occlusion, respiratory stimulation from falling PO2 and rising PCO2, and increasing intrathoracic negative pressure appear to work in combination to produce arousal. We used the maximum (negative) Pepi preceding arousal from sleep as our main quantification of arousal threshold, because maximum inspiratory effort in a given subject is similar whether arousal follows induced hypercapnia, hypoxia, or an added resistive inspiratory load.30 It has, however, been suggested31 that the rate of increase of negative airway pressure or measures of effort on the first breath after occlusion also influence the time to arousal. Therefore, we tested for aging effects on the latter variables as well, yielding similar results. Our data show that aging does not have a major impact on arousal threshold following airway occlusion during stage 2 sleep.
Previous data11 suggest that the chemical control system regulating breathing is quite stable in elderly persons, suggesting that the fluctuations in breathing that are characteristic of OSA are not related to a destabilization in the feedback mechanism regulating blood gas chemistry. This, in conjunction with our data in which we found no age-related differences in arousability, would suggest that OSA in the elderly is primarily a result of changes in airway anatomy and physiology.
While our study was not powered to give a final answer on the most important subgroup with respect to aging effects on upper airway collapsibility, our exploratory data analysis revealed that the values of Pclose show a plateau beyond the age of 45 years. This apparent plateau is notable since the prevalence and severity of OSA seems to further increase in subjects > 45 years of age.9 OSA has been considered as a progressively worsening condition32 with the phenotype being influenced by “adaptive” and “maladaptive” pathways. Based on this hypothesis, a decrease in upper airway dilator muscle reflex activity observed in the elderly18 could be temporarily compensated for (eg, by an adaptive increase in muscle mass).32 If, however, the body weight further increases with aging in persons > 45 years of age, adaptive mechanisms might be offset such that the collapsing force of negative intraluminal pressure that is generated by respiratory pump muscles make the airway collapse during sleep. In accordance, some data33 suggest that many patients do progress over time from snoring and mild OSA to more severe levels of disease. Further research is clearly needed to test this speculation.
Our study may suggest effective types of pharmacologic therapies for OSA in elderly patients. The elderly are known to be particularly susceptible to side effects from sedative and hypnotic agents. However, the treatment of OSA patients with sedatives has been a suggested method34–36 for achieving stable breathing by raising the arousal threshold in patients with unstable sleep. Triazolam35 and trazodone36 increase the arousal threshold from sleep, which may increase upper airway patency by stabilizing ventilation,37 particularly in patients with decreased arousal thresholds. Our data suggest that this approach is less promising for the treatment of OSA in older people, because the arousal threshold is not decreased in patients in this age group and thus is unlikely to contribute to the severity of their disorder. Rather, given the predisposition of aging to upper airway collapse, we suggest that drugs that increase upper dilator muscle activity (eg, possibly low doses of barbiturates),38 may be more promising.
Our study has some limitations. First, the collapsibility of the pharyngeal airway39 and the arousal threshold from sleep depend on sleep stage, and older persons tend to experience less slow-wave sleep40 than younger persons. To address this, we standardized the sleep stage by performing airway occlusion only during stage II sleep, which reduced the potential influences of variable sleep depth. However, we cannot rule out the possibility that a decreased arousal threshold during other stages of sleep, or a simple lack of deep sleep in older individuals, contributes to the increased propensity to OSA in this age group. In terms of upper airway collapsibility, our approach of performing measurements only during stage II sleep should have biased the study toward the null hypothesis, but a robust effect of aging was still observed. Second, mask occlusion and naturally occurring upper airway occlusion are not exactly the same, and it might be possible that nasal mechanoreceptors are more stimulated during mask occlusion compared with more physiologic stimuli. Since we applied the same model of airway occlusion throughout the age range, and also measured another variable of upper airway collapsibility (pharyngeal resistance increases during sleep), our study allows us to conclude that increased age is associated with an increase in airway collapsibility during sleep. Third, we may have influenced the outcome of this study by including only healthy older subjects without OSA. The healthy participant effect is a common problem in aging research, since, by excluding OSA and other comorbidities, we may have studied a population of “unusually” healthy older individuals. On the other hand, the study of patients with disease would have not been informative since the increase in pharyngeal collapsibility in patients with OSA is well-known. In addition, airway occlusion during repetitive apnea would not provide meaningful data. Thus, we acknowledge this limitation but point out that studying extremely healthy older subjects would tend to bias toward the null hypothesis and therefore strengthens our findings. This study is cross-sectional in nature. Although our findings are correlative rather than causative, we believe that a more thorough understanding of the pathogenesis of OSA can evolve from these observations. Ultimately, longitudinal studies would be useful to define the natural history of aging on pharyngeal structure and function, although such studies are difficult to complete and likely would be confounded by patients’ weight gain. Despite these limitations, we believe that our new findings importantly add to the existing literature.
In summary, our data show that age independently predicts both Pclose and pharyngeal resistance increase during sleep. We speculate from our data that increasing pharyngeal collapsibility with aging may contribute to the high prevalence of sleep-disordered breathing among older persons.
Acknowledgments
This work was supported by the National Institute of Aging (Beeson Award K23 AG024837–01), grants No. HL 60292 and RO1-HL73146.
Abbreviations
- BMI
body mass index
- OSA
obstructive sleep apnea
- Pclose
upper airway closing pressure
- Pepi
epiglottic pressure
- Pmask
nasal pressure
- Rp
upper airway resistance
Footnotes
The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 3.Faccenda JF, Mackay TW, Boon NA, et al. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Remmers JE, deGroot WJ, Sauerland EK, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 6.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 7.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 9.Dancey DR, Hanly PJ, Soong C, et al. Impact of menopause on the prevalence and severity of sleep apnea. Chest. 2001;120:151–155. doi: 10.1378/chest.120.1.151. [DOI] [PubMed] [Google Scholar]
- 10.Browne HA, Adams L, Simonds AK, et al. Ageing does not influence the sleep-related decrease in the hypercapnic ventilatory response. Eur Respir J. 2003;21:523–529. doi: 10.1183/09031936.03.00039002. [DOI] [PubMed] [Google Scholar]
- 11.Wellman A, Malhotra A, Jordan AS, et al. Chemical control of stability in the elderly. J Physiol. 2007 Feb 22; doi: 10.1113/jphysiol.2006.126409. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J Sleep Res. 2000;9:389–393. doi: 10.1046/j.1365-2869.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 13.Martin SE, Mathur R, Marshall I, et al. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10:2087–2090. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 14.Thurnheer R, Wraith PK, Douglas NJ. Influence of age and gender on upper airway resistance in NREM and REM sleep. J Appl Physiol. 2001;90:981–988. doi: 10.1152/jappl.2001.90.3.981. [DOI] [PubMed] [Google Scholar]
- 15.Worsnop C, Kay A, Kim Y, et al. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88:1831–1839. doi: 10.1152/jappl.2000.88.5.1831. [DOI] [PubMed] [Google Scholar]
- 16.Behan M, Brownfield MS. Age-related changes in serotonin in the hypoglossal nucleus of rat: implications for sleep-disordered breathing. Neurosci Lett. 1999;267:133–136. doi: 10.1016/s0304-3940(99)00337-7. [DOI] [PubMed] [Google Scholar]
- 17.Shochat T, Loredo J, Ancoli-Israel S. Sleep disorders in the elderly. Curr Treat Options Neurol. 2001;3:19–36. doi: 10.1007/s11940-001-0021-x. [DOI] [PubMed] [Google Scholar]
- 18.Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med. 2006;119(72):e79–e14. doi: 10.1016/j.amjmed.2005.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White DP, Lombard RM, Cadieux RJ, et al. Pharyngeal resistance in normal humans: influence of gender, age, and obesity. J Appl Physiol. 1985;58:365–371. doi: 10.1152/jappl.1985.58.2.365. [DOI] [PubMed] [Google Scholar]
- 20.Boselli M, Parrino L, Smerieri A, et al. Effect of age on EEG arousals in normal sleep. Sleep. 1998;21:351–357. [PubMed] [Google Scholar]
- 21.Basner RC, Ringler J, Garpestad E, et al. Upper airway anesthesia delays arousal from airway occlusion induced during human NREM sleep. J Appl Physiol. 1992;73:642–648. doi: 10.1152/jappl.1992.73.2.642. [DOI] [PubMed] [Google Scholar]
- 22.Issa FG, Sullivan CE. Arousal and breathing responses to airway occlusion in healthy sleeping adults. J Appl Physiol. 1983;55:1113–1119. doi: 10.1152/jappl.1983.55.4.1113. [DOI] [PubMed] [Google Scholar]
- 23.Berry RB, McCasland CR, Light RW. The effect of triazolam on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;146:1256–1260. doi: 10.1164/ajrccm/146.5_Pt_1.1256. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra A, Pillar G, Fogel RB, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 25.Gleadhill IC, Schwartz AR, Schubert N, et al. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–1303. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 26.Henke KG, Dempsey JA, Badr MS, et al. Effect of sleep-induced increases in upper airway resistance on respiratory muscle activity. J Appl Physiol. 1991;70:158–168. doi: 10.1152/jappl.1991.70.1.158. [DOI] [PubMed] [Google Scholar]
- 27.Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle responses to upper airway pressure changes: afferent pathways. J Appl Physiol. 1982;52:445–450. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- 28.Erskine RJ, Murphy PJ, Langton JA, et al. Effect of age on the sensitivity of upper airway reflexes. Br J Anaesth. 1993;70:574–575. doi: 10.1093/bja/70.5.574. [DOI] [PubMed] [Google Scholar]
- 29.Sei H, Sano A, Ohno H, et al. Age-related changes in control of blood pressure and heart rate during sleep in the rat. Sleep. 2002;25:279–285. doi: 10.1093/sleep/25.3.279. [DOI] [PubMed] [Google Scholar]
- 30.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142:295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 31.Berry RB, Bonnet MH, Light RW. Effect of ethanol on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;145:445–452. doi: 10.1164/ajrccm/145.2_Pt_1.445. [DOI] [PubMed] [Google Scholar]
- 32.Petrof BJ, Hendricks JC, Pack AI. Does upper airway muscle injury trigger a vicious cycle in obstructive sleep apnea? A hypothesis Sleep. 1996;19:465–471. doi: 10.1093/sleep/19.6.465. [DOI] [PubMed] [Google Scholar]
- 33.Svanborg E, Larsson H. Development of nocturnal respiratory disturbance in untreated patients with obstructive sleep apnea syndrome. Chest. 1993;104:340–343. doi: 10.1378/chest.104.2.340. [DOI] [PubMed] [Google Scholar]
- 34.Berry RB, Koch GL, Hayward LF. Low-dose mirtazapine increases genioglossus activity in the anesthetized rat. Sleep. 2005;28:78–84. doi: 10.1093/sleep/28.1.78. [DOI] [PubMed] [Google Scholar]
- 35.Berry RB, Kouchi K, Bower J, et al. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–454. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 36.Heinzer RC, Malhotra A, Jordan AS, et al. Trazodone increases arousal threshold in response to hyperkapnia in OSA patients [abstract] Proc Am Thorac Soc. 2006;3:A316. [Google Scholar]
- 37.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 38.Younes M, Park E, Horner RL. Pentobarbitol sedation increases genioglossus respiratory activity in sleeping rat. Sleep. 2007 doi: 10.1093/sleep/30.4.478. in press. [DOI] [PubMed] [Google Scholar]
- 39.Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J Appl Physiol. 1984;57:520–527. doi: 10.1152/jappl.1984.57.2.520. [DOI] [PubMed] [Google Scholar]
- 40.Ancoli-Israel S. Sleep and aging: prevalence of disturbed sleep and treatment considerations in older adults. J Clin Psychiatry. 2005;66(suppl):24–30. [PubMed] [Google Scholar]


