Abstract
OBJECTIVE
Obstructive sleep apnea has a strong male predominance in adults but not in children. The collapsible portion of the upper airway is longer in adult men than in women (a property that may increase vulnerability to collapse during sleep). We sought to test the hypothesis that in prepubertal children, pharyngeal airway length is equal between genders, but after puberty boys have a longer upper airway than girls, thus potentially contributing to this change in apnea propensity.
METHODS
Sixty-nine healthy boys and girls who had undergone computed tomography scans of their neck for other reasons were selected from the computed tomography archives of Rambam and Carmel hospitals. The airway length was measured in the midsagittal plane and defined as the length between the lower part of the posterior hard palate and the upper limit of the hyoid bone. Airway length and normalized airway length/body height were compared between the genders in prepubertal (4- to 10-year-old) and postpubertal (14- to 19-year-old) children.
RESULTS
In prepubertal children, airway length was similar between boys and girls (43.2 ± 5.9 vs 46.8 ± 7.7 mm, respectively). When normalized to body height, airway length/body height was significantly shorter in prepubertal boys than in girls (0.35 ± 0.03 vs 0.38 ± 0.04 mm/cm). In contrast, postpubertal boys had longer upper airways (66.5 ± 9.2 vs 52.2 ± 7.0 mm) and normalized airway length/body height (0.38 ± 0.05 vs 0.33 ± 0.05 mm/cm) than girls.
CONCLUSIONS
Although boys have equal or shorter airway length compared with girls among prepubertal children, after puberty, airway length and airway length normalized for body height are significantly greater in boys than in girls. These data suggest that important anatomic changes at puberty occur in a gender-specific manner, which may be important in explaining the male predisposition to pharyngeal collapse in adults.
Keywords: upper airway, pharynx, children, sleep apnea
Obstructive sleep apnea (OSA) is a common syndrome with important complications in both children and adults. Although the exact mechanism is still being elucidated, the pathogenesis probably consists of a combination of anatomic predisposition along with dysfunction of the muscles responsible for airway patency.1,2 The prevalence of OSA ranges between 2% to 4% in adults, and roughly 2% to 3% in children.3,4 One of the interesting findings in this regard is the age-related difference in gender predominance. Whereas in adults, OSA is 2 to 8 times more prevalent in men compared with women,5–7 in children, OSA is equally prevalent in boys and girls.3,8,9 Furthermore, even in healthy adults it was shown that as sleep deepens, pharyngeal resistance increases more in men than women.10
Several potential factors may result in the strong male predominance to OSA in adults. First, after puberty boys begin to secrete testosterone, which has been shown to increase upper-airway collapsibility and respiratory disturbance index.11,12 In parallel, girls secrete estrogen and progesterone, both thought to be protective against upper-airway collapse, for unclear reasons.13–18 In addition, weight gain, which commonly occurs with age, differs in character between genders. Whereas in men obesity is commonly central, which predisposes them to upper-airway collapse, it is usually peripheral and less compromising to airway mechanics in girls.19–21 However, we recently studied the gender-related differences in upper-airway collapsibility in normal, healthy, non-obese young adults and found that men had increased upper-airway collapsibility in response to loading, despite similar central respiratory drive (response to load) and similar upper-airway protective muscle activation (genioglossus and tensor palatini).22 Therefore, we concluded that there was a fundamental gender-related difference in upper-airway anatomy and/or tissue characteristics. Because cross-sectional upper-airway area is generally higher in men than women, intrinsic pharyngeal size is an unlikely explanation. However, in a subsequent study, we did find that men have longer upper airways than women, even after normalizing for body height.23 By using finite element modeling, we further demonstrated a major impact of pharyngeal airway length (AL) on upper-airway mechanics. In fact, the magnitude of the difference in pharyngeal mechanics between genders was largely attributable to discordant pharyngeal airway lengths. On the basis of the rules of buckling of cylindrical shells,24 and because as nonrigid tubes become longer they are increasingly prone to collapse, we concluded that upper-airway length (UAL) is a possible additional mechanism responsible for the increased upper-airway collapsibility seen in adult men compared with adult women.25 Obviously, this statement assumes that the airway is held at its 2 edges to a rigid noncollapsible stable structure, and it depends on the tissues’ Young’s modulus.26 In these conditions, according to Bernoulli’s law, a fluid (much like air stream) flows through a tube and is susceptible to an increase in velocity and a decrease in pressure in a narrow segment.
In prepubertal children, the prevalence of OSA is similar between genders. This could result from similar hypertrophy of tonsils and adenoids, which are the leading causes of OSA in children. However, the correlation between these tissue sizes and OSA is poor, and furthermore it is relatively common to see residual OSA even after adenotonsilectomy.27,28 Thus, additional mechanisms must exist. MRI assessment of soft tissue in children aged 4 to 5 years revealed OSA-related differences in neck soft tissues size, but UAL and differences between genders have not been evaluated to our knowledge.29 Although some literature refers to testosterone-mediated “laryngeal descent” in peripubertal boys, we are unable to determine, on the basis of the literature, what this actually means from the standpoint of upper-airway structure and mechanics. Thus, in the current study we aimed to examine potential gender-related differences in UAL of prepubertal and postpubertal children. We hypothesized that excessive lengthening of the pharyngeal airway during development in boys compared with girls would explain the change in gender-related OSA predisposition with puberty.
METHODS
Patient Selection
This was a retrospective study. Because we did not feel it would have been ethical to perform computed tomography (CT) scans in healthy children, the subjects for this study were selected from Rambam and Carmel medical centers’ CT scan archives. A query was performed on the archives to search for children who underwent neck CT scan for several reasons that we thought (a priori) would not affect the length of their upper airways. Thus, children with head concussion or minimal head injury, with a normal CT of the head, were retrieved from the hospital archives. In addition to their CT, the hospital charts were assessed, and children with a history or physical examination suggestive of OSA were excluded (ie, snoring, mouth breathing, hyperextention of the head during sleep, substantial tonsillar or adenoid hypertrophy, or a known diagnosis of OSA). In addition, patients with craniofacial diseases, previous trauma, or head and neck operations; patients with cancer on chronic medications or having other chronic diseases; and patients below the third percentile or above the 97th percentile of normal height and weight for their age were also excluded. Patients who had their CT scan performed while having a nasogastric tube or an endotracheal tube were also excluded from the study. Finally, CT scans that were not performed in the neutral anatomic position (Frankfort plane) were also excluded.
Data Collection
Demographic data and patients’ height and weight were collected from the hospital charts, and when missing were completed by a telephone call to the patients. The children were divided into a prepubertal group, aged 4 to 10 years old, and a postpubertal group, aged 14 to 19 years old. UAL was determined from the upper margin of the hyoid bone (corresponds to the valleculla and epiglottic base) up to the lower margin of the hard palate, as measured in midsagittal CT scan (Fig 1). The measurements were performed by a single scorer, who was blinded to the rest of the data at the time of evaluation (ie, gender or age). UAL was, thereafter, divided by body height to calculate the normalized ratio of airway length/patient’s height (UAL/height). For reproducibility purposes, 20 CT scans were reassessed, and the findings were highly reproducible (±2 mm = less than ±4.6%).
FIGURE 1.
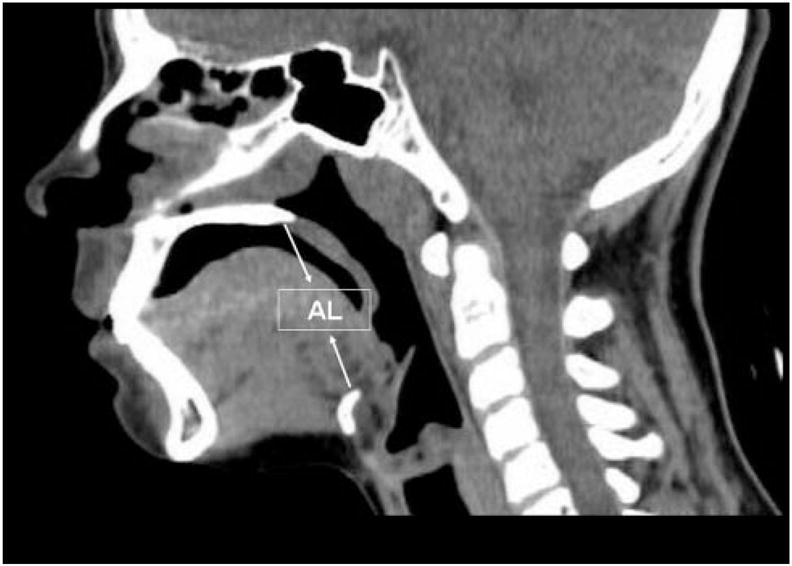
An example of a midsagittal CT scan showing UAL between the lower margin of the hard palate and the upper margin of the hyoid bone.
After completion of data collection, the participants were classified into 4 groups according to their gender and pubertal status. Comparison between groups was performed by using Student’s t tests. In addition, a multiple regression analysis and a forward stepwise regression model to predict UAL were performed, both for the whole group and separately for boys and girls. All data are expressed as averages ±SD, and a P value of <.05 was considered significant.
RESULTS
The research included 69 patients. Thirty-two were pre-pubertal children (aged 4–10 years old), 16 boys and 16 girls. Thirty-seven CT scans were of postpubertal children (aged 14–19 years old): 22 boys and 15 girls. The average age of prepubertal children was 7.3 ± 1.7 years (both boys and girls), and during puberty was 15.1 ± 2.1 year for girls and 15.7 ± 1.3 years for boys. Height and weight are presented in Table 1. Height and weight of postpubertal boys were significantly greater than post-pubertal girls. UAL and corrected UAL per height values are presented in Table 2. An individual representative example of 1 CT scan from each group is presented in Fig 2. The measured length (in millimeters) is shown on the CT, and the normalized number for body height (UAL/height) on top of each CT. In this example, the longest UAL and AL per height is that of the postpubertal boy. For the group results, UAL (measured in millimeters) was significantly longer in postpubertal boys than in girls (Fig 3). Interestingly, corrected UAL per height was significantly lower in prepubertal boys than girls, but significantly longer in postpubertal boys than girls (Fig 4).
TABLE 1.
Height and Weight
| Group | Age, Mean ± SD, y | Height, Mean ± SD, cm | Weight, Mean ± SD, kg | BMI, Mean ± SD, kg/m2 | BMI, Mean ± SD, Percentile |
|---|---|---|---|---|---|
| Prepubertal girls | 7.3 ± 1.8 | 122.6 ± 11.6 | 24.4 ± 5.7 | 16.0 ± 0.8 | 53.1 ± 8.9 |
| Prepubertal boys | 7.3 ± 1.7 | 124.6 ± 10.0 | 25.4 ± 5.0 | 16.1 ± 1.0 | 56.1 ± 14.9 |
| Postpubertal girls | 15.1 ± 2.1 | 160.1 ± 5.1 | 52.3 ± 9.7 | 20.3 ± 3.3 | 50.4 ± 17.5 |
| Postpubertal boys | 15.7 ± 1.3 | 170.6 ± 4.1a | 59.2 ± 7.4a | 21.0 ± 2.0 | 52.8 ± 14.5 |
P < .05.
TABLE 2.
UALs and Normalized UAL
| Group | AL, Mean ± SD, mm | UAL/Height, Mean ± SD, mm/cm | UAL/Weight, Mean ± SD, mm/kg |
|---|---|---|---|
| Prepubertal girls | 46.8 ± 7.7 | 0.38 ± 0.04a | 2.0 ± 0.3a |
| Prepubertal boys | 43.2 ± 5.9 | 0.35 ± 0.03 | 1.7 ± 0.3 |
| Postpubertal girls | 52.2 ± 7.0 | 0.33 ± 0.05 | 1.03 ± 0.2 |
| Postpubertal boys | 66.5 ± 9.2a | 0.38 ± 0.05a | 1.13 ± 0.1 |
P < .05.
FIGURE 2.
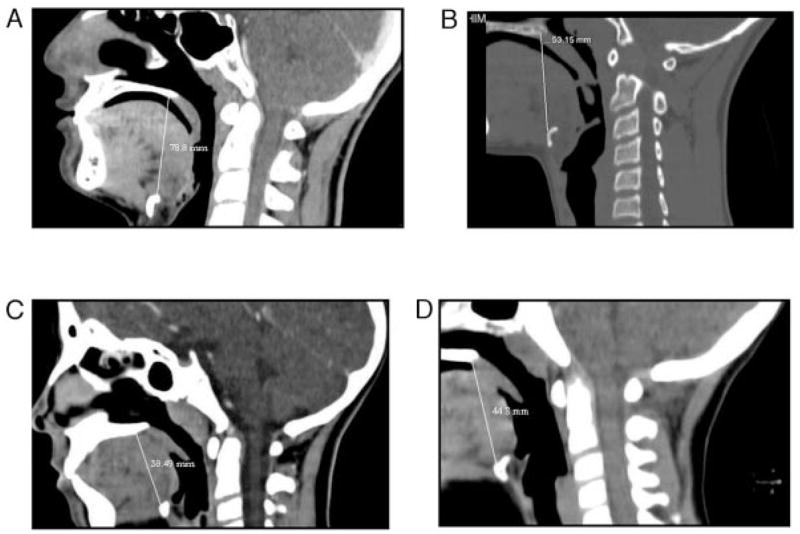
An example of CT and UAL measurement from 1 child from each group. The measured length is shown on the CT. In this example, the longest AL and AL/height ratio is that of the postpubertal boy. A, Postpubertal boy: UAL/height = 0.45 mm/cm; B, postpubertal girl: UAL/height = 0.39 mm/cm; C, postpubertal boy: UAL/h = 0.32 mm/cm; D, postpubertal girl: UAL/height = 0.35 mm/cm.
FIGURE 3.
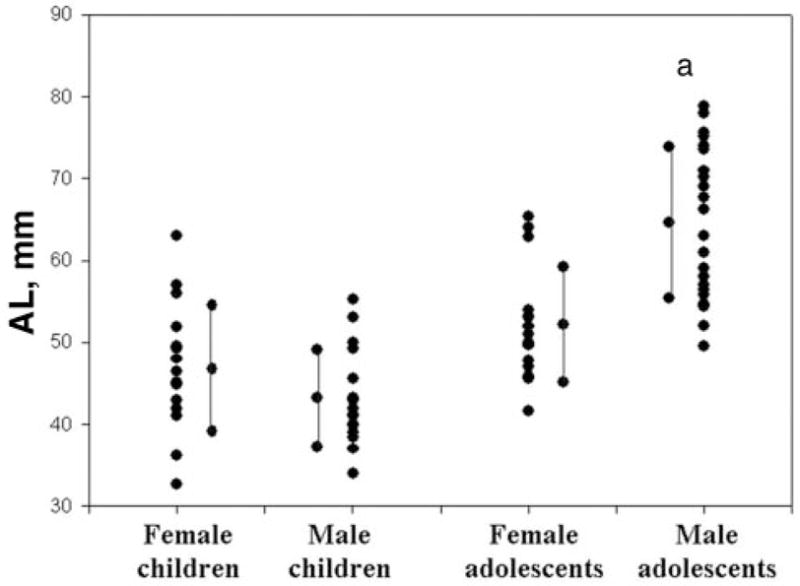
Plot of individual UAL of subjects in each group along with the average and SD for each group (children = prepubertal; adolescents = postpubertal). a P < .05.
FIGURE 4.
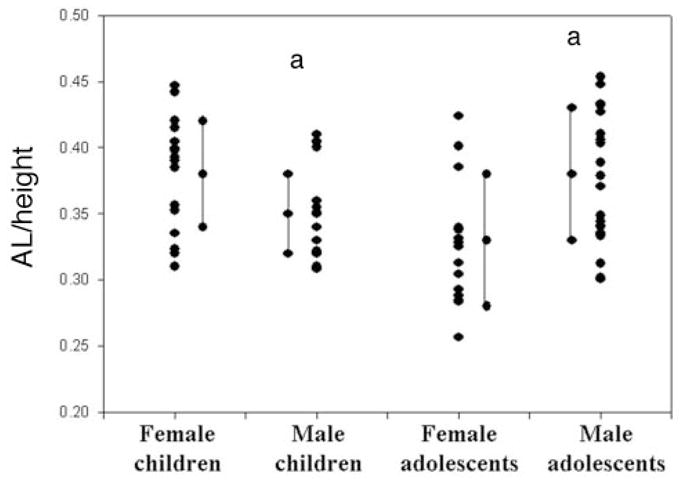
Plot of individual UAL/height ratio of subjects in each group along with the average and SD for each group (children = prepubertal; adolescents = postpubertal). a P < .05 (between girls and boys in each pubertal group).
Multiple regression analysis model for the whole group indicated that age (t = 3.3; P =.002) and gender (male = 1; female = 2; t = −2.1; P =.046) are the 2 most important factors with predictive value for UAL, whereas other factors such as weight, BMI, or percentile of BMI did not significantly contribute to UAL. Forward stepwise regression revealed that age and gender enter the model with r = 0.71 and 0.73, respectively, and the remaining variables did not enter the model. Stepwise regression in an attempt to predict UAL per height failed because all variables were removed from the model. When performing these statistics on girls and boys separately, it seemed that the effect of age in predicting UAL was significantly greater in boys than in girls (r = 0.85 vs 0.45), with other factors not contributing significantly in both genders.
DISCUSSION
The main and most important finding of our research is that there is a marked age- and gender-dependent difference in UAL. Whereas the UAL of prepubertal boys and girls is similar, during puberty the pharyngeal airway becomes significantly longer in boys. Furthermore, when normalizing it to body height, whereas UAL per height of prepubertal boys is shorter than that of girls, postpubertal boys have a larger UAL/height ratio than girls. As we have previously shown, a major impact of pharyngeal AL on airway mechanics, we speculate that our new findings may have important implications in the pathophysiology of OSA, partially explaining the similar gender prevalence in early childhood but strong male predominance in adulthood.
The most recognized risk factor for OSA is compromised anatomy of the upper airway, with smaller cross sectional airway area in patients with OSA than controls.30,31 However, when gender effects have been examined, pharyngeal area and volume were actually larger in men than women.32,33 Thus, from an anatomic point of view, it was not clear why men are at increased risk of OSA. Recently, we reported that men have longer upper airways than women, even when normalized to patients’ height.23 During wakefulness, even long airways do not collapse because protective reflexes activate dilator muscles via negative pressure receptors.34 However, during sleep these reflexes are ineffective,35 and the nonrigid tube tends to collapse in direct relation to its length (assuming similar longitudinal tethering). The finding of the current study, that this increase in AL occurs during puberty, may explain the equal prevalence of OSA in prepubertal children versus male predominance in adults with OSA. Furthermore, residual OSA long-term (12 years) after adenotonsillectomy may be present in men somewhat more than in women,36 and it was shown recently that adenotonsillectomy completely resolves OSA in only 25% of cases.37 In light of our results, we speculate it may result at least in part from the longer airway of boys after puberty.
Our results are in contrast to results reported from studies in experimental animals.38–40 These showed that increasing the length of the upper airway by pulling caudally on the trachea stiffens the pharynx, making it more resistant to collapse. The key difference between these 2 observations is that in experimental animals, when pulling the trachea (generating traction or longitudinal tethering) it changes the tissue characteristics, stiffens them, and indeed reduces their collapsibility. However, with fixed tissue characteristics (ie, intrinsically long versus stretched shorter airway), the longer the tube is, the more easily it can bend and collapse with negative pressure within the lumen.
The clinical relevance of our finding that normal healthy prepubertal girls have a larger UAL/height ratio (0.38 mm/cm) than boys (0.35 mm/cm) is unclear. It is possible that some of the girls have already started their natural growth, being in early puberty, although there were not statistically significant differences in weight and height within that age group. This prepubertal finding, however, only makes it more robust that boys outgrow that difference during puberty. As expected, during puberty the boys had weight and height that are significantly greater than girls, although their BMIs were similar. The upper airways were significantly longer in postpubertal boys even when it was normalized to their height. In fact, it is arguable whether the UAL should or should not be normalized to body height. Theoretically (and on the basis of our finite element modeling), the longer the tube the more it is collapsible, regardless of other body characteristics. Thus, we believe that the long airway seen in boys during puberty (Fig 3) is much more important than the corrected UAL/height ratio, which although significantly greater in postpubertal boys than in girls, is similar to prepubertal girls (Fig 4). In our previous studies in adults, we observed no important correlation between body height and AL, which led us to further question the logic of this normalization strategy. However, to avoid the potential criticism that we are simply measuring surrogates for overall growth, we include the normalized and nonnormalized results. Interestingly, Campbell et al41 reported recently the absence of OSA in children with short stature. In this study, UAL was not reported. We postulate it was shorter than in average stature children. If in short children UAL as divided by their (short) height, the ratio of UAL per height would be expected to be relatively high. Thus, we believe it supports the thought that the absolute UAL rather than the ratio of UAL per height is more important as a risk for OSA. As for BMI, in our cohort most participants were around 50th percentile, and we did not find an effect of BMI on UAL. However, theoretically, shorter airway may better withstand the effects of obesity than a longer airway, in which case increased AL may have an additive procollapsibility effect in addition to obesity.
During the process of puberty, anatomic differences develop between the genders that might have influences on the male tendency toward OSA. One of these changes is the laryngeal descent that happens in both genders as they grow up, but could be more pronounced in boys after a certain age. It has been shown that the human hyoid and larynx descent is the largest in the first years after birth, when it is similar between genders, whereas several aspects of this (eg, hyoid-palatal plane and genion-hyoid body) becomes greater in boys starting at an age of ~8 years.42 Thus, laryngeal descent in boys seems to be associated with increased UAL, which is likely to make the upper airway more collapsible. This may explain our finding that in boys, age has a significant positive predictive value for AL, whereas in girls, it is only a borderline contribution.
Our study had several limitations. First, we did not select patients from the general population but rather looked retrospectively at children and adolescents who underwent head and neck CT, usually after minimal head injury. It could be argued that these do not represent the general healthy population, but rather a selection bias of those who underwent minor trauma (although had normal CT without signs of hematoma or other injury). We have shown previously that minimal head injury may be associated with some sleep disturbances but not usually OSA.43,44 Nevertheless, our results should be taken cautiously from standpoint of generalizability. Second, we did not have accurate Tanner pubertal scale data in our patients’ charts. For this reason, we decided a priori to take ages up to 10 as prepubertal, and >14 as postpubertal, leaving the “gray” zone of 10 to 14 of the study. Because girls tend to mature earlier than boys, it is possible that some of the prepubertal girls had actually started puberty and some of the postpubertal boys were still peripubertal. We believe that this is unlikely but even if so, this misclassification should bias toward the null hypothesis, making the findings of the present study more robust. Third, CT scans in children are usually performed under mild sedation. Sedation can affect not only ventilation, but also upper-airway muscle tone. However, we doubt this had major influences on our results because the effects should be similar in all groups. Fourth, we assessed UAL as a straight line (from the upper margin of the hyoid bone up to the lower margin of the hard palate), and we cannot be sure it accurately reflects the length of a curved airway. In addition, we have no data regarding whether the curvature of the upper-airway changes by age or gender, but we believe that the maintenance of the Frankfort plane in all subjects should help to minimize the impact of airway curvature. Our previous findings in adults used similar techniques (straight line rather than curvature); therefore, we believe that the findings of the present study may help to explain the differences in AL in adults. Finally, it should be emphasized that this study was performed in children without known OSA. To prove that these findings have clinical implications, such a study should include children with OSA as well. Thus, we believe that additional studies are needed to investigate the importance of UAL in the pathophysiology of OSA.
CONCLUSIONS
Despite these limitations, we believe our study supports a substantially greater relative elongation of the upper airway during puberty in boys versus girls, which may have importance in the male predominance in adults versus prepubertal children with OSA.
Acknowledgments
Dr Malhotra is funded by NIH grants AG024837, HL 73146, and P50 HL060292.
Abbreviations
- OSA
obstructive sleep apnea
- AL
airway length
- UAL
upper-airway length
- CT
computed tomography
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.White DP, Mezzanotte WS. Neuromuscular compensation in the human upper airway. Sleep. 1993;16:S90–S91. doi: 10.1093/sleep/16.suppl_8.s90. [DOI] [PubMed] [Google Scholar]
- 2.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–1326. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med. 2001;164:16–30. doi: 10.1164/ajrccm.164.1.2008171. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin JL, Babar SI, Kaemingk KL, et al. Symptoms related to sleep-disordered breathing in white and Hispanic children: the Tucson Children’s Assessment of Sleep Apnea Study. Chest. 2003;124:196–203. doi: 10.1378/chest.124.1.196. [DOI] [PubMed] [Google Scholar]
- 5.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149:722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;32:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 7.Guilleminault C, Quera-Salva MA, Partinen M, Jamieson A. Women and the obstructive sleep apnea syndrome. Chest. 1988;93:104–109. doi: 10.1378/chest.93.1.104. [DOI] [PubMed] [Google Scholar]
- 8.Guilleminault C, Pelayo R. Sleep-disordered breathing in children. Ann Med. 1998;30:350–356. doi: 10.3109/07853899809029934. [DOI] [PubMed] [Google Scholar]
- 9.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100:31–40. doi: 10.1016/s0022-3476(82)80231-x. [DOI] [PubMed] [Google Scholar]
- 10.Trinder J, Kay A, Kleiman J, Dunai J. Gender differences in airway resistance during sleep. J Appl Physiol. 1997;83:1986–1997. doi: 10.1152/jappl.1997.83.6.1986. [DOI] [PubMed] [Google Scholar]
- 11.Cistulli PA, Grunstein RR, Sullivan CE. Effect of testosterone administration on upper airway collapsibility during sleep. Am J Respir Crit Care Med. 1994;149:530–532. doi: 10.1164/ajrccm.149.2.8306057. [DOI] [PubMed] [Google Scholar]
- 12.Carden K, Malhotra A. The debate about gender differences in obstructive sleep apnea. Sleep Med. 2003;4:485–487. doi: 10.1016/s1389-9457(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 13.Wilhoit SC, Suratt PM. Obstructive sleep apnea in premenopausal women. A comparison with men and with postmenopausal women. Chest. 1987;91:654–658. doi: 10.1378/chest.91.5.654. [DOI] [PubMed] [Google Scholar]
- 14.Cistulli PA, Barnes DJ, Grunstein RR, Sullivan CE. Effect of short-term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax. 1994;49:699–702. doi: 10.1136/thx.49.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collop NA. Medroxyprogesterone acetate and ethanol-induced exacerbation of obstructive sleep apnea. Chest. 1994;106:792–799. doi: 10.1378/chest.106.3.792. [DOI] [PubMed] [Google Scholar]
- 16.D’Ambrosio C, Stachenfeld NS, Pisani M, Mohsenin V. Sleep, breathing, and menopause: the effect of fluctuating estrogen and progesterone on sleep and breathing in women. Gend Med. 2005;2:238–245. doi: 10.1016/s1550-8579(05)80053-1. [DOI] [PubMed] [Google Scholar]
- 17.Popovic RM, White DP. Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol. 1998;84:1055–1062. doi: 10.1152/jappl.1998.84.3.1055. [DOI] [PubMed] [Google Scholar]
- 18.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 19.Pillar G, Peled N, Katz N, Lavie P. Predictive value of specific risk factors, symptoms and signs, in diagnosing obstructive sleep apnoea and its severity. J Sleep Res. 1994;3:241–244. doi: 10.1111/j.1365-2869.1994.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 20.Brown LK. A waist is a terrible thing to mind: central obesity, the metabolic syndrome, and sleep apnea hypopnea syndrome. Chest. 2002;122:774–778. doi: 10.1378/chest.122.3.774. [DOI] [PubMed] [Google Scholar]
- 21.Schafer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–839. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 22.Pillar G, Malhotra A, Fogel RB, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Crit Care Med. 2000;162:1627–1633. doi: 10.1164/ajrccm.162.5.2003131. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra A, Huang Y, Fogel RB, et al. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 24.Ekstrom RE. Buckling of cylindrical shells under combined torsion and hydrostatic pressure. Exp Mech. 1963;3:192–197. [Google Scholar]
- 25.Dawson SV, Elliott EA. Wave-speed limitation on expiratory flow: a unifying concept. J Appl Physiol. 1977;43:498–515. doi: 10.1152/jappl.1977.43.3.498. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, White DP, Malhotra A. The impact of anatomic manipulations on pharyngeal collapse: results from a computational model of the normal human upper airway. Chest. 2005;128:1324–1330. doi: 10.1378/chest.128.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laurikainen E, Erkinjuntti M, Alihanka J, Rikalainen H. Suonpaa, Radiological parameters of the bony nasopharynx and the adenotonsillar size compared with sleep apnea episodes in children. Int J Pediatr Otorhinolaryngol. 1987;12:303–310. doi: 10.1016/s0165-5876(87)80006-x. [DOI] [PubMed] [Google Scholar]
- 28.Marcus CL, Ward SL, Mallory GB, et al. Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr. 1995;127:88–94. doi: 10.1016/s0022-3476(95)70262-8. [DOI] [PubMed] [Google Scholar]
- 29.Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea. Am J Repir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 30.Schwab RJ, Gefter WB, Hoffman EA, Gupta KB, Pack AI. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148:1385–1400. doi: 10.1164/ajrccm/148.5.1385. [DOI] [PubMed] [Google Scholar]
- 31.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 32.Lee JJ, Ramirez SG, Will MJ. Gender and racial variations in cephalometric analysis. Otolaryngol Head Neck Surg. 1997;117:326–329. doi: 10.1016/S0194-5998(97)70121-9. [DOI] [PubMed] [Google Scholar]
- 33.Mohsenin V. Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest. 2001;120:1442–1447. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 34.Malhotra A, Pillar G, Fogel RB, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–77. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- 35.Wheatley J, Mezzanotte W, Tangel D, White D. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- 36.Tasker C, Crosby JH, Stradling JR. Evidence for persistence of UAW narrowing during sleep, 12 years after adenotonsillectomy. Arch Dis Child. 2002;86:34–37. doi: 10.1136/adc.86.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149:803–808. doi: 10.1016/j.jpeds.2006.08.067. [DOI] [PubMed] [Google Scholar]
- 38.Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol. 1996;80:2171–2178. doi: 10.1152/jappl.1996.80.6.2171. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz AR, Rowley JA, Thut DC, Permutt S, Smith PL. Structural basis for alterations in upper airway collapsibility. Sleep. 1996;19:S184–S188. doi: 10.1093/sleep/19.suppl_10.184. [DOI] [PubMed] [Google Scholar]
- 40.Thut DC, Schwartz AR, Roach D, Wisew RA, Permutt S, Smith PL. Tracheal and neck position influence upper airway airflow dynamics by altering airway length. J Appl Physiol. 1993;75:2084–2090. doi: 10.1152/jappl.1993.75.5.2084. [DOI] [PubMed] [Google Scholar]
- 41.Campbell TA, Papadopoulosverge DJ, Verge CF, Williamson BD, Teng A. Incidence of sleep disorders in children with presumed normal variant short stature. J Paediatr Child Health. 2005;41:358–360. doi: 10.1111/j.1440-1754.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman DE, McCarthy RC, Hiiemac KM, Palmer JB. Ontogeny of postnatal hyoid and larynx descent in humans. Arch Oral Biol. 2001;46:117–128. doi: 10.1016/s0003-9969(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 43.Kaufman Y, Tzichinski O, Epstein R, Etzioni A, Lavie P, Pillar G. Sleep disorders in children in the long-term after minimal head injury. Pediatr Neurol. 2001;24:129–134. doi: 10.1016/s0887-8994(00)00254-x. [DOI] [PubMed] [Google Scholar]
- 44.Pillar G, Averbooch E, Katz N, Peled N, Kaufman Y, Shahar E. Prevalence and risk factors of long-term sleep disturbances in adolescents after minor head injury. Pediatr Neurol. 2003;29:131–135. doi: 10.1016/s0887-8994(03)00149-8. [DOI] [PubMed] [Google Scholar]


