Abstract
Androgen receptor (AR) mediates transcriptional activation of diverse target genes through interactions with various coactivators that may alter its function and help mediate the switch between prostate cell proliferation and differentiation. We recently identified p44/MEP50 as an AR coactivator and further showed that it is expressed primarily in the nucleus and cytoplasm of benign prostate epithelial and prostate cancer cells, respectively. We also showed that haploinsufficiency in p44+/− mice causes prostate epithelial cell proliferation. To establish direct cause-and-effect relationships, we have used p44 fusion proteins that are selectively expressed in the nucleus or cytoplasm of prostate cancer cells (LNCaP), along with RNAi analyses, to examine effects of p44 both in vitro and in vivo (in tumor xenografts). We show that preferential expression of p44 in the nucleus inhibits proliferation of LNCaP cells in an AR-dependent manner, whereas preferential expression of p44 in the cytoplasm enhances cell proliferation. These effects appear to be mediated, at least in part, through the regulation of distinct cell-cycle regulatory genes that include p21 (up-regulated by nuclear p44) and cyclin D2 and CDK6 (up-regulated by cytoplasmic p44). Importantly, we also demonstrate that altered p44 expression is associated with androgen-independent prostate cancer. Our results indicate that nuclear p44 and cytoplasmic p44 have distinct and opposing functions in the regulation of prostate cancer cell proliferation.
Keywords: Mep50, prostate cancer growth
Hormone refractory or androgen-independent prostate cancer remains the primary cause of cancer-related death. Androgens and androgen receptors (ARs) play crucial roles in prostate cancer oncogenesis and progression, including progression into androgen-independent cancer (1–3). AR, a member of the steroid hormone receptor family (4), is a transcription factor with an important role in the regulation of normal prostate growth and differentiation. On the one hand, AR can increase prostate cell proliferation; on the other hand, AR can also inhibit proliferation of prostate cells (5, 6). The mechanism underlying the AR-mediated switch between proliferation and growth suppression remains to be elucidated. In association with its coactivators, AR binds to androgen-responsive elements and activates transcription of AR target genes (4). AR coactivators affect the transcriptional activity of AR by altering its association with either the general transcriptional machinery (7) or chromatin-modifying complexes such as those with histone acetylation or methylation activity (8, 9).
Increasing numbers of AR coactivators have been described recently. We and others have observed diverse expression patterns of some cofactors involved in human prostate cancer. Increased expression of some cofactors (ARA24, PIAS1, cyclinD1, SRC1, FHL2, and Par4) and decreased expression of others (ARA70 and ART-27) are found in prostate cancer tissue when compared with adjacent benign tissue (10–15). Thus alterations in the cellular concentrations of AR cofactors may influence selective expression of AR target genes and thereby govern the switch between proliferation and growth inhibition.
We recently identified p44/Mep50 as an AR coactivator (16) and further showed that while p44 is expressed as a nuclear protein in benign prostate, the level of nuclear p44 is decreased and the level of cytoplasmic p44 is increased in prostate cancer (17). In addition, transgenic mice lacking one allele for p44 (p44+/−) exhibited excessive epithelial cell proliferation that resulted in a precancerous lesion termed high-grade prostatic intraepithelial neoplasia (17). These results suggest that the cellular localization of p44 regulates prostate cancer cell growth suppression and proliferation.
The p44 C-terminal region contains numerous WD40 repeats. Thus, p44 may mediate interactions between AR and multiple protein complexes through WD40-mediated protein–protein interactions. Indeed, p44 also exists in a methylsome complex with protein arginine methyl transferase 5 (PRMT5) (16, 18), a member of the protein arginine methyltransferase family of coactivators (8). Further linking p44 function to transcription regulation, p44 has been reported to bind to polycomb group protein SUZ12 (19), histone H2A (19), and a phosphatase (FCP1) that dephosphorylates the C-terminal domain of RNA polymerase II (20). Intriguingly, p44 is also a subunit of the survival of motor neuron (SMN) complex, which is active in the cytoplasm in small nuclear ribonucleoprotein (snRNP) complex assembly. SMN is not active in the nucleus, whereas it is active in the cytoplasmic compartment as a result of phosphorylation (21). These findings support the argument that p44 has a dual role: as a transcriptional factor in the nucleus and as a splicing-associated factor in the cytoplasm.
The purpose of this study was to determine the function of nuclear and cytoplasmic forms of p44 in human prostate cancer cell proliferation and growth inhibition. We reason that decreased nuclear p44 and/or the level of cytoplasmic p44 in prostate cells may play a key role in mediating the switch from a nonproliferative to a proliferative state. Indeed, we show in both in vitro assays and in vivo tumor xenograft experiments that nuclear p44 inhibits prostate cancer growth, whereas cytoplasmic p44 promotes prostate cancer growth. We also show that nuclear exclusion of p44 is associated with androgen-independent prostate cancer.
Results
Establishment of Cell Lines Expressing Nuclear and Cytoplasmic Forms of p44.
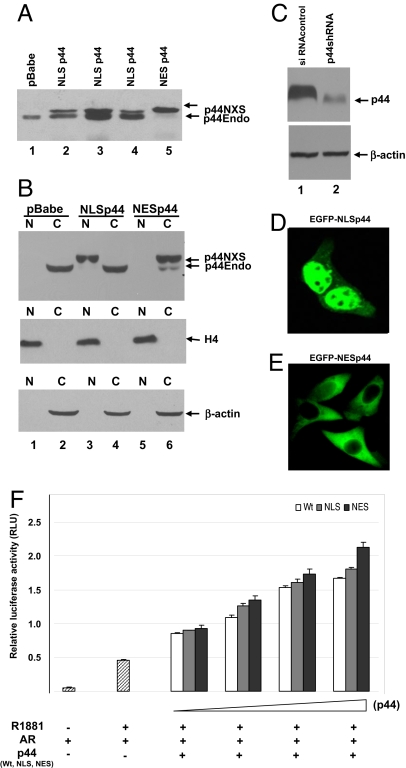
To dissect the nuclear and cytoplasmic functions of p44, we fused either a nuclear import signal (NLS, nuclear localization signal) or sequence (PKKKRKV) or a nuclear export signal (NES) sequence (MLQKKLEELE) to the N terminus of p44, resulting in NLSp44 or NESp44, respectively. LNCaP cell lines that stably express NLSp44 or NESp44 were established and termed LNCaP-NLSp44 and LNCaP-NESp44, respectively. An immunoblot showed that NLSp44 (Fig. 1A, lanes 2–4; three clonal lines) and NESp44 (Fig. 1A, lane 5) were both expressed as full-length proteins. NLSp44 and NESp44 migrated more slowly than did endogenous p44 because of the flag-NLS and flag-NES tags (Fig. 1A). We also used shRNA interference technology to establish an LNCaP cell line with reduced p44 expression (Fig. 1C Upper, lane 2 vs. lane 1). Similarly, PC3 cells that stably overexpress NLSp44 (PC3-NLSp44) and NESp44 (PC3-NESp44) were also established.
Fig. 1.
Subcellular locations and functional analyses of endogenous p44, NLSp44, and NESp44. (A) Immunoblots of whole-cell extracts from LNCaP cells stably transfected with pBabe (lane 1), pBabe-NLSp44 (lanes 2–4; three stable clonal lines), and pBabe-NESp44 (lane 5) retroviral vectors. This analysis used a rabbit polyclonal antibody that detects endogenous (p44Endo) and ectopic (p44NXS) p44. (B) Immunoblot of nuclear (N) and cytoplasmic (C) fractions of LNCaP cells stably transfected with pBabe, pBabe-NLSp44, or pBabe-NESp44 vectors. The analysis used antibodies to p44 (Top), histone H4 as internal control for the nuclear fraction (Middle), and β-actin as an internal control for the cytoplasmic fraction (Bottom). (C) Immunoblots of whole-cell extracts from LNCaP cells that stably express a control siRNA (lane 1) or a p44 shRNA (lane 2). Antibodies to p44 (Upper) and β-actin as a loading control (Lower) were used. (D) Fluorescence microscopy of LNCaP cells expressing EGFP-tagged p44 (NLSp44). (E) Fluoresence microscopy of LNCaP cells expressing EGFP-tagged p44 (NESp44). (F) Luciferase reporter assays for effects of NLSp44, NESp44, and wild-type p44 on AR-dependent transcription. PC3 cells were transfected with the luciferase reporter gene, an AR expression vector, and increasing amounts (100, 200, 400, and 600 ng) of p44, NLSp4, and NESp44 expression vectors in the presence of 10 nM R1881 as indicated. (Magnification: D and E, ×400.)
The subcellular localization of NLSp44 and NESp44 was demonstrated by immunoblots of nuclear and cytoplasmic fractions from LNCaP cells that stably express the fusion proteins. An immunoblot indicated that NLSp44 is localized exclusively in the nuclear fraction (Fig. 1B, lanes 3 and 4) and NESp44 exclusively in the cytoplasmic fraction (Fig. 1B, lanes 5 and 6). Endogenous p44 was identified primarily in the cytoplasmic fraction of LNCaP cells (Fig. 1B, lanes 2 and 4 and lower band of lane 6). Similarly, cellular fluorescence assays indicated localization of EGFP-NLSp44 (Fig. 1D) and EGFP-NESp44 (Fig. 1E) to the nuclear and cytoplasmic compartments, respectively, of intact cells. These experiments confirmed the nuclear localization of NLSp44 and the cytoplasmic localization of NESp44. In a dual luciferase assay, flag-NLSp44, flag-NESp44, and wild-type p44 showed comparable effects on AR-dependent transcription (Fig. 1F). This result suggests that fusion of a flag-NLS or flag-NES tag to the N terminus of p44 does not affect its activity.
NLSp44 Mediates AR-Dependent Growth Inhibition.
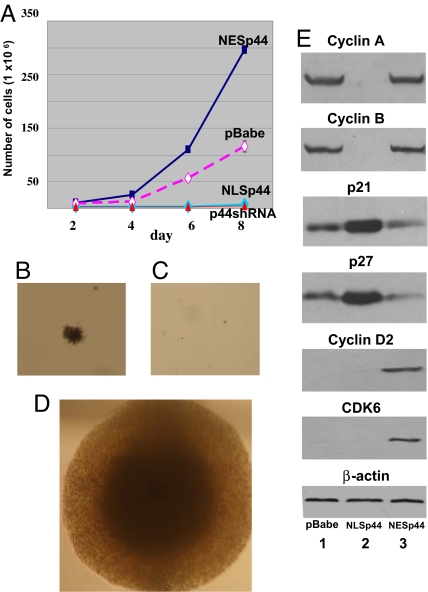
Cell proliferation was examined after p44 overexpression in the nucleus in LNCaP-NLSp44 cells in both the presence and absence of androgen. Consistent with the results of our previous study (17), NLSp44 inhibited growth of the LNCaP prostate cancer cell line in both androgen-containing (Fig. 2A) and androgen-free media (data not shown). To investigate the level of cellular growth inhibition in suspension media, we examined the transforming ability of NLSp44 by using anchorage-independent assays. Similarly, whereas a moderate number of small colonies grew in cells containing the pBabe control vector (Fig. 2B and Table 1), LNCaP cells expressing NLSp44 were unable to grow in soft agar (Fig. 2C; P < 0.0001).
Fig. 2.
Regulation of cell proliferation, anchorage-independent growth, and cell cycle regulatory genes by NLSp44 and NESp44 in vitro. (A) Cell growth kinetics of LNCaP stable cell lines stably transfected with pBabe (purple line), pBabe-NLSp44 (light blue line), pBabe-NESp44 (dark blue line), or pBabe-p44shRNA (red line). Cells were seeded in androgen-containing media (10 nM R1881) on day 0, and the total number of viable cells was determined every other day by trypan blue exclusion. (B) Anchorage-independent growth of control LNCaP cells. (C) Anchorage-independent growth of LNCaP cells stably transfected with pBabe-NLSp44. (D) Anchorage-independent growth LNCaP cells stably transfected with pBabe-NLSp44. Quantitation of the results indicating the number of colonies with >50 cells in B, the absence of colonies in C, and the number of large colonies in D are presented in Table 1. (E) Immunoblots of whole-cell extracts from LNCaP cells stably transfected with pBabe (lanes 1), pBabe-NLSp44 (lanes 2), or pBabe-NESp44 (lanes 3) retroviral vectors. The antibodies used are indicated. β-Actin was included as a loading control. (Magnification: B–D, ×200.)
Table 1.
Colony formation in anchorage-independent assays by NLSp44 and NESp44 in LNCaP and PC3 cells
| Cell line | Construct | Hormone-free medium | Androgen medium |
|---|---|---|---|
| LNCaP | pBabe | 12 ± 1.5 | 12.6 ± 0.6 |
| NLSp44 | 0 | 0 | |
| NESp44 | 25 ± 1 | 32 ± 2 | |
| PC3 | pBabe | 8.3 ± 0.58 | 8.7 ± 5.7 |
| NLSp44 | 8 ± 1 | 8.3 ± 5.7 | |
| NESp44 | 9 ± 1 | 9.3 ± 5.7 |
Flow cytometry analyses showed that NLSp44 caused a 2.4-fold reduction in the number of cells in S phase (from 17.67% to 7.38%) and a corresponding increase in the number of cells in G0/G1 phase. Immunoblot analysis revealed that, compared with cells expressing the pBabe control vector, cells expressing NLSp44 showed decreased levels of cyclin A and cyclin B (Fig. 2E). Conversely, the levels of cyclin inhibitors p21 and p27 (Fig. 2E) increased up to 5- and 4-fold, respectively. We monitored cell proliferation after p21 knockdown by small interfering RNA (siRNA) technology in LNCaP-NLSp44 cells (Fig. 3A Top, lane 2 vs. lane 1). As shown in Fig. 3B, the p21 knockdown resulted in a faster growth rate of LNCaP-NLSp44 cells (Fig. 3B), indicating that the growth-inhibitory effects of nuclear p44 are effected, at least partially, through CDK inhibitor p21.
Fig. 3.
AR-dependent, p21-regulated growth inhibition by NLSp44. (A) Immunoblots of whole-cell extracts from LNCaP-NLSp44 cells transiently transfected either with control or p21 siRNA (Top), control (Middle), or AR siRNA (Bottom). Immunoblots used antibodies against p21, AR and, as a loading control, β-actin. (B) Cell growth kinetics of LNCaP-NLSp44 cells transiently transfected with p21 siRNA or control siRNA. (C) Cell growth kinetics of LNCaP-NLSp44 cells transiently transfected with AR siRNA or control siRNA. (D) Cell growth kinetics of LNCaP-pBabe cells transiently transfected with AR siRNA or control siRNA. In all cases, cells were transfected with 10 mM siRNAs.
We also performed cell proliferation and anchorage-independent assays with PC3-NLSp44 and PC3-pBabe cells. We did not observe a growth difference upon expression of NLSp44 in PC3 cells in comparison with control PC3-pBabe cells (data not shown). In addition, the results of anchorage-independent assays did not reveal an decrease in colony formation for PC3-NLSp44 cells in comparison with PC3-pBabe cells (Table 1).
Because growth inhibition by nuclear p44 occurs only in LNCaP and not PC3 cells, the growth-inhibitory effects of NLSp44 may be AR-dependent. We used siRNA to knock down AR in androgen-treated LNCaP-NLSp44 and control LNCaP-pBabe cells (Fig. 3A Middle, lane 2 vs. lane 1). Cell proliferation assays revealed a statistically significant increase in cell growth after AR knockdown in LNCaP-NLSp44 cells (Fig. 3C; P < 0.0001), but not after AR knockdown in LNCaP-pBabe control cells (Fig. 3D; P = 0.019). Subsequently, the results of flow cytometry analyses showed that the percentage of S-phase LNCaP-NLSp44 cells increased from 6% to 14% after AR knockdown in the presence of androgen.
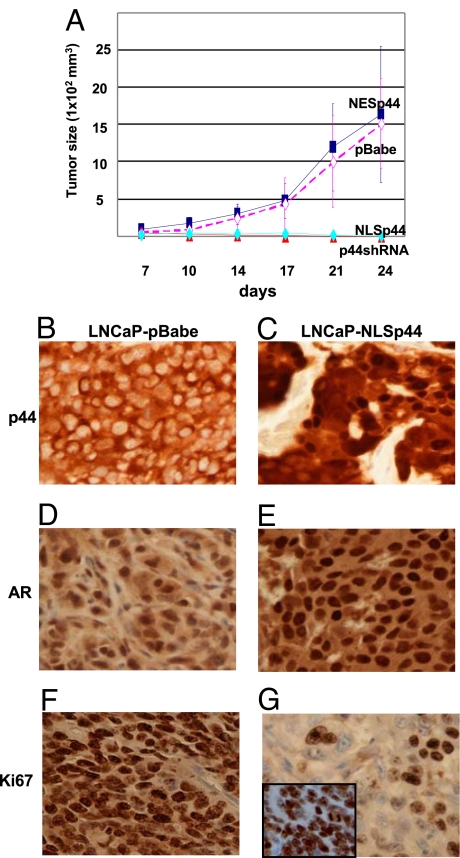
To confirm the growth inhibitory effects of nuclear p44 in vivo, we examined tumor growth in nude mice xenografts overexpressing nuclear p44. A total of 7 × 106 LNCaP or PC3 cells stably overexpressing NLSp44 or pBabe vector were injected s.c. into the flank region of 4- to 5-week-old male nude mice. As shown in Fig. 4A, NLSp44 suppressed tumor growth compared with the pBabe vector control. Tumor growth in mice xenografts with LNCaP cells overexpressing NLSp44 was not observed until 45 days postinjection.
Fig. 4.
Tumor growth kinetics of LNCaP-NLSp44 and NESp44 cells in tumor xenografts. (A) Tumor growth kinetics of nude mice xenografts from LNCaP-pBabe, LNCaP-NLSp44, LNCaP-NESp44, and LNCaP-p44shRNA cells. (B) IHC of tumor xenograft from LNCaP cells stably transfected with pBabe control vector showing cytoplasmic p44 expression. (C) IHC of tumor xenograft from LNCaP cells stably transfected with pBabe-NLSp44 showing nuclear (from NLSp44) and cytoplasmic (endogenous) p44 expression. (D and E) IHC of tumor xenograft from LNCaP cells with pBabe control vector (D) or tumor xenograft from LNCaP cells stably transfected with pBabe-NLSp44 retroviral vector (E) showing AR expression. (F and G) IHC of Ki67 expression in tumor xenograft from LNCaP cells with pBabe control vector (F) or in tumor xenograft from LNCaP cells stably transfected with pBabe-NLSp44 (G) and pBabe-NESp44 (G Inset) retroviral vectors. (Magnification: ×400.)
We used immunohistochemistry (IHC) to examine expression of AR, p44, Ki67, and cleaved Caspase3 in tumors from nude mice xenografts. IHC confirmed that p44 was localized to the nucleus in LNCaP-NLSp44 cells (Fig. 4C) and the cytoplasm in LNCaP-pBabe control cells, consistent with the cell fractionation data. AR was expressed in both NLSp44 and pBabe control cells (Fig. 4 D and E). We observed decreased Ki67 expression in LNCaP-NLSp44 tumor cells (Fig. 4G). The percentage of Ki67-positive cells decreased from 90% with the control pBabe vector to 45% (P < 0.0001) in tumors with NLSp44 overexpression. IHC analyses of cleaved Caspase3 in LNCaP-NLSp44 and LNCaP-pBabe cells did not show a statistically significant difference [supporting information (SI) Fig. 6]. Thus, the observed tumor growth inhibition is caused by reduced cell proliferation rather than increased apoptosis.
Cytoplasmic NESp44 Promotes Growth Through Cyclin D2 and CDK6 Activation.
To assess possible growth-regulatory effects of cytoplasmic p44 on prostate cancer cells in vitro, cell proliferation assays were performed on LNCaP cell lines that overexpress cytoplasmic p44 (NESp44) and LNCaP cell lines with the control pBabe vector. As shown in Fig. 2A, the expression of p44 as a cytoplasmic protein (NESp44) promoted the growth of LNCaP prostate cancer cells when compared with LNCaP cells transfected with a control vector. In addition, p44 knockdown by shRNA interference (Fig. 1C Upper, lane 2 vs. 1) completely inhibited LNCaP cell proliferation (Fig. 2A). This result is consistent with the concept that cytoplasmic p44 stimulates cell growth because p44 is expressed mainly in the cytoplasm in LNCaP cells. In an anchorage-independent assay, we observed that NESp44 expression increased the number of colonies compared with the pBabe control vector (Table 1) in LNCaP cells. Further, the colony size observed with LNCaP-NESp44 cells was markedly increased relative to that observed with control LNCaP-pBabe cells (Fig. 2 D vs. B).
Flow cytometric analyses showed that NESp44 expression resulted in an expansion of LNCaP cells in S phase from 17.67% to 40.21% and an expansion of cells in G2/M phase from 7.64% to 12.15% (Table 2), consistent with the results in our growth kinetic studies. We performed immunoblot analyses to examine a panel of cell cycle regulatory proteins affected by cytoplasmic p44 (NESp44). This experiment revealed that cytoplasmic p44 expression decreased slightly (up to 2-fold) the expression of cyclin inhibitors p21 and p27 (Fig. 2E), but not the levels of cyclin A (Fig. 2E) and cyclin B (Fig. 2E) proteins. Strikingly, an Affymetrix oligonucleotide microarray analysis revealed drastic increases in the levels of cyclin D2 (128-fold) and CDK6 (256-fold) RNA expression in LNCaP-NESp44 cells compared with control LNCaP-pBabe cells (Y.P and P.L., unpublished data). The increased levels of cyclin D2 and CDK6 (Fig. 2E) were confirmed by immmunoblot. Because it has been reported that cyclin D2 and CDK6 can form the preferred complex for promoting cell proliferation (22), we used corresponding siRNAs to knock down cyclin D2 (SI Fig. 7A Top) and CDK6 (SI Fig. 7A Middle) to examine whether the enhanced proliferation of cells overexpressing NESp44 is cyclin D2- and CDK6-dependent. The results indeed indicate slowed growth after knockdown of cyclin D2 (Fig. 5B) or CDK6 (Fig. 7B) in LNCaP-NESp44 cells. There was no dramatic change in the levels of CDK2 and CDK4 expression in LNCaP-NESp44 cells compared with control LNCaP-pBabe cells (data not shown). These changes in the levels of cell cycle regulatory proteins are consistent with the growth kinetics that cytoplasmic p44 increases cell proliferation.
Table 2.
Flow cytometric analysis of cell cycle regulation by NLSp44 and NESp44 overexpression in LNCaP cells
| Cell type | G1/G0, % | S, % | G2/M, % |
|---|---|---|---|
| pBabe | 74.68 ± 0.13 | 17.67 ± 0.52 | 7.64 ± 0.39 |
| NLSp44 | 89.22 ± 0.60 | 7.38 ± 0.58 | 3.40 ± 0.25 |
| NESp44 | 47.63 ± 0.51 | 40.21 ± 0.51 | 12.15 ± 0.31 |
Data are mean ± SEM.
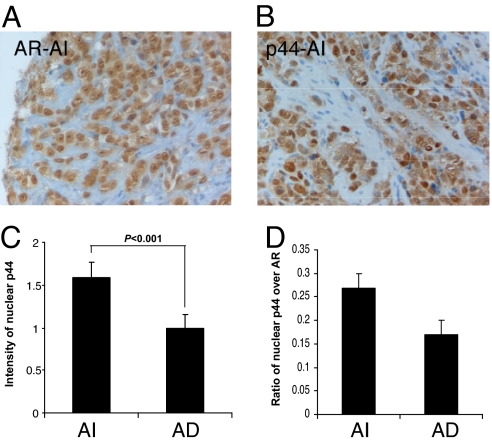
Fig. 5.
Expression pattern of nuclear p44 in relation to AR in androgen-dependent (AD) and androgen-independent (AI) prostate cancer. (A) IHC of a serial section of a TMA AI prostate cancer case showing nuclear AR expression. (B) IHC of serial section of a TMA AI prostate cancer case showing nuclear p44 expression. (C) Statistical analysis of the difference between the levels of nuclear p44 expression in AD and AI prostate cancer cases. (D) Ratio of p44 over AR in AI and AD prostate cancer cases. The levels of nuclear p44 and AR expression were averaged from four cores for each case. (Magnification: A and B, ×200.)
To confirm the growth-promoting effects of cytoplasmic p44 in vivo, we examined tumor growth in nude mice xenografts with LNCaP cells that express cytoplasmic p44 (LNCaP-NESp44) or LNCaP cells in which p44 is knocked down (LNCaP-shRNAp44). Somewhat to our surprise, the tumor generated with LNCaP-NESp44 cells did not show an increased rate of growth relative to that of the tumor generated with the LNCaP-pBabe cells (Fig. 4A). Moreover, Ki67 labeling was comparable between LNCaP-NESp44 and LNCaP-pBabe tumors (Fig. 4G Inset). However, there was suppression of tumor growth when endogenous p44 in LNCaP cells was knocked down by shRNAp44 (Fig. 4A). Thus, the results of this study indicate that whereas an increase in the level of cytoplasmic p44 through expression of NESp44 did not result in faster tumor growth, cytoplasmic p44 does have a function in promoting growth of LNCaP cells.
High Levels of Nuclear and Cytoplasmic p44 Are Associated with Androgen-Independent Prostate Cancer.
We used IHC to determine whether nuclear p44 or cytoplasmic p44 is associated with prostate cancer progression, especially to androgen-independent disease by using prostate cancer tissue microarrays (TMAs). The TMAs incorporated a total of 133 cases, including 36 nonrecurrent and 36 recurrent prostatectomy cases and 17 androgen-dependent (hormone naive) and 18 androgen-independent (hormone refractory) transurethral resection (TURP) cases and 26 cases of benign prostate tissue. We scored p44 nuclear and cytoplasmic expression semiquantitatively (3+ as strong, 2+ as moderate, 1+ as weak, and 0 as negative expression) (SI Fig. 8).
In confirmation of our previous report, there was a statistically significant (P < 0.001) reduction in nuclear p44 staining and a statistically significant (P < 0.001) increase in cytoplasmic p44 staining in prostate cancer (SI Fig. 8 A vs. C) compared with benign glands. We first examined associations of cytoplasmic p44 expression with androgen-dependent and androgen-independent cancer. When the data from the 16 scorable androgen naïve cases were dichotomized into zero/weak and strong staining, 10 cases (63%) showed no or weak (1+) cytoplasmic p44 staining and 6 cases (37%) showed strong (2+ and 3+) cytoplasmic p44 staining. Among 18 androgen-independent cases, there was an increase in cytoplasmic p44 staining with 3 cases (17%) showing no or weak staining and 15 cases (83%) with strong staining (2+ and 3+). These data indicate an association between high levels of cytoplasmic p44 expression and androgen-independent cancer (Table 3).
Table 3.
Nuclear and cytoplasmic expression in androgen-dependent (AD) and -independent (AI) cases
| p44 localization | p44 intensity | AI, % | AD, % |
|---|---|---|---|
| Nuclear p44 | Weak(0 and 1+) | 44 [67] | 71 [94] |
| Strong(2+ and 3+) | 56 [33] | 29 [6] | |
| Cytoplasmic p44 | Weak(0 and 1+) | 17 | 63 |
| Strong(2+ and 3+) | 83 | 37 |
The percentage number in brackets is when the data are dichotomized as no or weak expression for 0–2+ and strong for 3+.
Next, we examined the levels of nuclear p44 expression between androgen-dependent and androgen-independent cases. There was a statistically significant increase (P < 0.001) in the intensity of nuclear p44 in androgen-independent cases compared with androgen-dependent cases (Fig. 5C; P < 0.001). We next examined the frequencies of high-level nuclear p44 expression in androgen-dependent and -independent cases (Table 3). Of the 17 androgen naïve cases, 12 cases (71%) revealed no or weak (1+) staining and 5 cases (29%) showed strong (2+ and 3+) nuclear p44 staining. Surprisingly, in 18 androgen-independent cases, there was also an increase in the number of cases with strong nuclear staining: 8 cases (44%) with no or weak 1+ staining and 10 cases (56%) with strong (2+ and 3+) nuclear p44 staining. The association between nuclear p44 and the androgen-independent group was even stronger when the levels of p44 were dichotomized as no and weak for 1+ and 2+ staining versus 3+ as strong staining. Of the 17 androgen-naïve cases, 16 cases (94%) showed no or weak (1+ and 2+) staining and 1 case (6%) showed strong (3+) staining. In the 18 androgen-independent cases, there was an increase in the number of cases with strong nuclear staining: 12 cases (67%) with no or weak (1+ and 2+) staining and 6 cases (33%) with strong 3+ staining. Because the function of nuclear p44 depends on AR, we further compared AR (Fig. 5A) and p44 (Fig. 5B) expression levels in serial sections. We obtained nuclear p44-to-AR ratios that were correlated with androgen-dependent and -independent cancer. This analysis revealed an increased nuclear p44/AR ratio in androgen-independent cancer compared with androgen-dependent cancer (Fig. 5D). These results indicate that high levels of nuclear p44 are associated with AR-independent cancer regardless of AR expression. When comparing recurrent and nonrecurrent prostate cancer, there was no statistically significant difference for the levels and frequencies of the expression of either nuclear or cytoplasmic p44. These results indicate that high levels of both nuclear and cytoplasmic p44 are associated with androgen-independent prostate cancer.
Discussion
It is well known that androgens and AR play roles in both cell proliferation and differentiation in the prostate. However, the mechanism responsible for the switch between cell proliferation and differentiation remains to be elucidated. It has been suggested that that AR cofactors may direct the specificity of AR target genes, therefore determining cell proliferation versus differentiation. A number of AR coactivators, including ART27 (11) and ARA70 (10), have been reported to inhibit prostate cancer growth. In this study we show a dual function of the AR coactivator p44/Mep50 in regulating prostate cancer cell proliferation and growth suppression.
Beyond its demonstrated function as an AR coactivator (16), p44/Mep50 has been identified as a component of both a PRMT complex and the SMN complex. p44 appears to be an AR coactivator with unique properties in benign and cancerous prostate because nuclear p44 is decreased in prostate cancer compared with adjacent benign prostate epithelium. Our cell proliferation, anchorage-independent assays, and nude mice xenograft experiments showed that LNCaP cell proliferation and tumor growth are inhibited when p44 is expressed in the nucleus, suggesting a tumor suppressor function of nuclear p44. Two lines of evidence strongly suggest that growth inhibition by nuclear p44 is AR-dependent. First, nuclear p44 inhibits the growth of LNCaP cells but does not inhibit the growth of PC3 cells. Second, knockdown of AR in LNCaP cells that express nuclear p44 (LNCaP-NLSp44) reverses the growth inhibition.
Importantly, our immunocytological analyses clearly showed increased cytoplasmic p44 in human prostate cancer. As cytoplasmic p44 could be an epiphenomenon (bystander effect) of prostate cancer, we sought to determine whether cytoplasmic p44 has an active role in promoting LNCaP cell growth. Several lines of evidence strongly suggest that cytoplasmic p44/Mep50 stimulates cell proliferation. First, we showed that overexpression of cytoplasmic p44 (NESp44) induced cell proliferation in in vitro cell proliferation and soft agar anchorage-independent assays. The cell growth effect could be a result of the relaxation of growth inhibition by decreased nuclear p44. However, a knockdown of cytoplasmic p44 by shRNA resulted in retarded LNCaP cell growth both in vitro and in vivo. These results strongly support the view that cytoplasmic p44 promotes cell proliferation. Somewhat surprisingly, in nude mice xenografts, LNCaP cells overexpressing cytoplasmic p44 (NESp44) showed no increase in tumor growth rate compared with control LNCaP cells. The possibilities for the discrepancy between the in vitro and in vivo experiments might relate to the regulation of tumor growth by stromal cells and angiogenesis involved in xenografts.
Our flow cytometry data suggest that the growth suppression effects associated with ectopic nuclear p44 (NLSp44) expression, and the growth promotion effects associated with ectopic cytoplasmic p44 (NESp44) expression, are largely regulated through S-phase cell cycle regulatory genes. We show that nuclear NLSp44 completely inhibits the expression of cyclin A and cyclin B and, conversely, increases the expression of cyclin inhibitors p21 and p27. These results are consistent with our previous reports that nuclear p44 inhibits p21 as an AR target gene. On the other hand, ectopic cytoplasmic p44 (NESp44) promotes cell growth through an increase in cyclin D2 and CDK6. These data suggest that the growth inhibitory effect of nuclear p44 and the enhanced cell proliferation effect of cytoplasmic p44 are regulated by different cell cycle gene pathways.
We examined the expression of p44 to determine whether p44 dysregulation is associated with either aggressive prostate cancer that recurs after prostatectomy or androgen-independent prostate cancer. We showed that high levels of cytoplasmic p44 are associated with androgen-independent prostate cancer. To our surprise, there were also a considerably higher number of androgen-independent cases showing high levels of nuclear p44 expression. These data suggest that nuclear p44, which inhibits prostate cancer growth in earlier-stage disease, fails to exert growth inhibition effects in late-stage advanced disease. In contrast to cohorts of androgen-dependent and -independent TURP cases, we did not find a statistically significant difference in nuclear and cytoplasmic p44 in prostate cancer that recurs after radical prostatectomy. This group constitutes androgen-dependent cancer.
In summary, we have shown that nuclear p44 inhibits, and cytoplasmic p44 promotes, prostate cancer cell proliferation through the regulation of S-phase cell cycle genes. Further, growth inhibition by nuclear p44 is an AR-dependent process. These results are of considerable significance with respect to an understanding of the mechanisms by which AR regulates prostate cancer cell proliferation and growth suppression. Equally important, high levels of both cytoplasmic and nuclear p44 are associated with androgen-independent prostate cancer.
Materials and Methods
Construction of Retroviral pBabe Vectors Expressing NLSp44, NESp44, and shRNAs.
We created a p44 fusion protein in which the strong NLS PKKKRKV (23) or the strong NES MLQKKLEELE was fused to the N terminus of p44. A retroviral-based mammalian expression vector, pBabe, was used to stably express NLSp44 and NESp44 proteins in LNCaP cells. shRNAp44 was constructed by using the pBabe/U6 plasmid with sequences: 5′-TCGAGGGAGAGAGGTATTCTAGTGTTCAAGAGACACTAGAATACCTCTCTCCCCTTTTTT G GAAG-3′ and 5′-AATTCTTCCAAAAAAGGGGAGAGAGGTATTCTAGTGTCTCTTG A A CACTAGAATACC T C TCTCCC-3′.
Cell Culture, Luciferase, Transient Transfection, and Retroviral Infection Assays.
LNCaP and PC3 cells were maintained in complete medium (RPMI medium 1640 plus 10% FBS), androgen-free medium (phenol-free medium with charcoal-treated FBS), or androgen media (androgen-free medium with 10 nM R1881). Luciferase assays were performed with Lipofectamine (Invitrogen) with 4XARE reporter, 20 ng of AR, 100 ng of reporter, 2.5 ng of pR-LUC internal control plasmids, and varying amounts of p44 or NLSp44 expression vector as described (16). siRNAs for AR, p21, p44, cyclin D2 and CDK6, and control siRNA were purchased from Ambion and transfected with HyperFect Transfection Reagents (Qiagen) 24 h before immunoblot analysis of whole-cell extract or cell kinetic studies. For retroviral infection (17), Phoenix A amphotropic packaging cells (American Type Culture Collection) were transfected with NLSp44, NESp44, shRNAp44, and pBabe retroviral constructs to produce virus. The virus-containing supernatant was collected by centrifugation and filtered before retroviral infection.
Florescence Microscopy.
EGFP and NLSp44 or NESp44 fusion proteins was constructed by subcloning the NLSp44 or NESp44 fragment of pBabe-NLSp44 and pBabe-NESp44 into pCDNA-EGFP vector, respectively, by using the NdeI and BamHI sites. The resulting EGFP-NLSp44 and EGFP-NESp44 were transfected into LNCaP cells. Images were captured 48 h later with a Nikon Digital Sight DS-U1 camera under inverted Nikon Eclipse TS100 microscope with appropriate filter.
Cell Proliferation Assays by Cell Counting and Anchorage-Independent Assays.
For cell proliferation assay, cells (1 × 104) were plated into six-well plates and counted with a hemocytometer every other day. Anchorage-independent cell growth in soft agar was performed in triplicate with cells (1 × 104) suspended in 2 ml of medium containing 0.35% agar (Becton Dickinson) spread on top of 5 ml of 0.7% solidified agar. Colony volume was calculated from the average radius of representative colonies. Numbers of colonies were also calculated.
Nude Mice Xenografts.
Male nude mice (5 weeks old) were purchased from NCI and maintained in accordance with the Institutional Animal Care and Use Committee-approved protocol. Seven million cells were used to perform s.c. injection into the flank region of the mice with Matrigel ECM (reconstituted basement membrane) to support tumor growth (24). Each experimental group contained 10 mice. Tumors were measured with calipers every 3 days.
Immunoblot Analysis.
Whole-cell extracts were prepared from LNCaP and PC3 cells containing NLSp44, NESp44, or pBabe (16). Nuclear and cytoplasmic extracts were prepared with the Active-Motif nuclear extract kit. Portions of both fractions were subjected to electrophoresis on SDS/PAGE and then transferred to nitrocellulose membranes for immunoblot analysis as described (16).
TMA Construction and IHC.
The study protocol was approved by the New York University institution review board. Nonrecurrent and recurrent prostate cancer cases were androgen-dependent cases from prostatectomy. Hormone-resistant samples were derived from patients who underwent TURP at least 6 months after surgical orchiectomy. Hormone naïve specimens were derived from patients who were diagnosed with prostate cancer by TURP, having high grade (Gleason 8 or higher) and volume of disease.
Prostate cancer TMA was constructed by using a semiautomated tissue TMA1 puncher/array (Beecher Instruments). Because of the heterogeneous nature of prostate cancer, each case was represented by four 0.6-mm cores. Immunohistochemical staining of AR, p44, Ki67, and Caspase3 was performed by using single-label IHC on a NexES automated immunostainer as described (17).
Statistical Analysis of the Results.
Statistical analyses of the above results were performed by pairwise Student's t test. Differences are considered statistically significant if P < 0.05.
Supplementary Material
Acknowledgments.
This study was supported by grants from the Veterans Affairs Seed and Merit Review and Department of Defense (to P.L.) and National Institutes of Health Grants DK065156 01 (to Z.X.W.), DK058024 (to M.J.G.), and DK071900 (to R.G.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712262105/DC1.
References
- 1.Buchanan G, Irvine RA, Coetzee GA, Tilley WD. Contribution of the androgen receptor to prostate cancer predisposition and progression. Cancer Metastasis Rev. 2001;20:207–223. doi: 10.1023/a:1015531326689. [DOI] [PubMed] [Google Scholar]
- 2.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–344. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, Tindall DJ. The role of the androgen receptor in prostate cancer. Crit Rev Eukaryotic Gene Expression. 2002;12:193–207. doi: 10.1615/critreveukaryotgeneexpr.v12.i3.30. [DOI] [PubMed] [Google Scholar]
- 4.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitacre DC, et al. Androgen induction of in vitro prostate cell differentiation. Cell Growth Differ. 2002;13:1–11. [PubMed] [Google Scholar]
- 6.Yuan S, et al. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304–1311. [PubMed] [Google Scholar]
- 7.Xu L, Glass CK, Rosenfeld MG. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 8.Stallcup MR, et al. Cooperation between protein-acetylating and protein-methylating coactivators in transcriptional activation. Biochem Soc Trans. 2000;28:415–418. [PubMed] [Google Scholar]
- 9.Chen H, Lin RJ, Xie W, Hoshino A. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell. 1999;98:675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 10.Li P, et al. Heterogeneous expression and functions of androgen receptor cofactors in primary prostate cancer. Am J Pathol. 2002;161:1467–1474. doi: 10.1016/S0002-9440(10)64422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taneja SS, et al. ART-27, an androgen receptor coactivator regulated in prostate development and cancer. J Biol Chem. 2004;279:13944–13952. doi: 10.1074/jbc.M306576200. [DOI] [PubMed] [Google Scholar]
- 12.Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem. 2002;277:7076–7085. doi: 10.1074/jbc.M108255200. [DOI] [PubMed] [Google Scholar]
- 13.Muller JM, et al. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J. 2002;21:736–748. doi: 10.1093/emboj/21.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drobnjak M, Osman I, Scher HI, Fazzari M, Cordon-Cardo C. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin Cancer Res. 2000;6:1891–1895. [PubMed] [Google Scholar]
- 15.Gao S, et al. Androgen receptor and prostate apoptosis response factor-4 target the c-FLIP gene to determine survival and apoptosis in the prostate gland. J Mol Endocrinol. 2006;36:463–483. doi: 10.1677/jme.1.01991. [DOI] [PubMed] [Google Scholar]
- 16.Hosohata K, et al. Purification and identification of a novel complex which is involved in androgen receptor-dependent transcription. Mol Cell Biol. 2003;23:7019–7029. doi: 10.1128/MCB.23.19.7019-7029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, Wu H, Lee P, Wang Z. Roles of the androgen receptor cofactor p44 in the growth of prostate epithelial cells. J Mol Endocrinol. 2006;37:283–300. doi: 10.1677/jme.1.02062. [DOI] [PubMed] [Google Scholar]
- 18.Friesen WJ, et al. A novel WD repeat protein component of the methylosome binds Sm proteins. J Biol Chem. 2002;277:8243–8247. doi: 10.1074/jbc.M109984200. [DOI] [PubMed] [Google Scholar]
- 19.Furuno K, Masatsugu T, Sonoda M, Sasazuki T, Yamamoto K. Association of Polycomb group SUZ12 with WD-repeat protein MEP50 that binds to histone H2A selectively in vitro. Biochem Biophys Res Commun. 2006;345:1051–1058. doi: 10.1016/j.bbrc.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Licciardo P, et al. The FCP1 phosphatase interacts with RNA polymerase II, with MEP50 a component of the methylosome complex involved in the assembly of snRNP. Nucleic Acids Res. 2003;31:999–1005. doi: 10.1093/nar/gkg197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimmler M, et al. Phosphorylation regulates the activity of the SMN complex during assembly of spliceosomal U snRNPs. EMBO Rep. 2005;6:70–76. doi: 10.1038/sj.embor.7400301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ely S, et al. Mutually exclusive cyclin-dependent kinase 4/cyclin D1 and cyclin-dependent kinase 6/cyclin D2 pairing inactivates retinoblastoma protein and promotes cell cycle dysregulation in multiple myeloma. Cancer Res. 2005;65:11345–11353. doi: 10.1158/0008-5472.CAN-05-2159. [DOI] [PubMed] [Google Scholar]
- 23.Barry DM, Wente SR. Nuclear transport: Never-ending cycles of signals and receptors. Essays Biochem. 2000;36:89–103. doi: 10.1042/bse0360089. [DOI] [PubMed] [Google Scholar]
- 24.Lim DJ, et al. Growth of an androgen-sensitive human prostate cancer cell line, LNCaP, in nude mice. Prostate. 1993;22:109–118. doi: 10.1002/pros.2990220203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.