Abstract
Ullrich congenital muscular dystrophy and Bethlem myopathy are skeletal muscle diseases that are due to mutations in the genes encoding collagen VI, an extracellular matrix protein forming a microfibrillar network that is particularly prominent in the endomysium of skeletal muscle. Myoblasts from patients affected by Ullrich congenital muscular dystrophy display functional and ultrastructural mitochondrial alterations and increased apoptosis due to inappropriate opening of the permeability transition pore, a mitochondrial inner membrane channel. These alterations could be normalized by treatment with cyclosporin A, a widely used immunosuppressant that desensitizes the permeability transition pore independently of calcineurin inhibition. Here, we report the results of an open pilot trial with cyclosporin A in five patients with collagen VI myopathies. Before treatment, all patients displayed mitochondrial dysfunction and increased frequency of apoptosis, as determined in muscle biopsies. Both of these pathologic signs were largely normalized after 1 month of oral cyclosporin A administration, which also increased muscle regeneration. These findings demonstrate that collagen VI myopathies can be effectively treated with drugs acting on the pathogenic mechanism downstream of the genetic lesion, and they represent an important proof of principle for the potential therapy of genetic diseases.
Keywords: mitochondria, muscular dystrophy
Considerable progress in understanding the pathogenesis of collagen VI diseases has been made in mice with targeted disruption of the Col6a1 gene, which display an early-onset, mild myopathic syndrome due to lack of collagen VI (1). Mitochondria in flexor digitorum brevis fibers from Col6a1−/− mice depolarize in response to oligomycin (2). Our interpretation of this abnormal event is that flickering of the permeability transition pore (PTP), an inner-membrane, high-conductance channel, is increased and causes depletion of pyridine nucleotides (3, 4), progressive impairment of respiration (3), and the switch of F1FO ATP synthase into an ATP hydrolase that maintains the membrane potential at the expense of glycolytic ATP (5, 6). Because ADP inhibits the PTP (7), ATP hydrolysis may lead to pore closure and to at least partial restoration of respiration. The maximal attainable respiratory rate would be lower than normal, however, and determined by the residual matrix levels of pyridine nucleotides. As long as the energy demand can be matched, the fibers would behave normally, but dysfunction would be precipitated by increased workload and/or by increased PTP flickering, events that will eventually lead to individual muscle fiber death observed in vivo (2). This sequence of events is consistent with the occurrence of transient PTP openings in isolated mitochondria (8, 9) and intact cells (10, 11) and with the therapeutic effect of short-term treatment of Col6a1−/− mice with cyclosporin A (CsA) at 10 mg·kg−1·day−1 (2), which desensitizes the PTP in vivo (12). Myoblasts from patients affected by Ullrich congenital muscular dystrophy (UCMD; Mendelian Inheritance in Man no. 254090) have a similar mitochondrial defect that could be normalized with CsA in vitro (13), suggesting that PTP desensitization might have a therapeutic potential in humans as well. Several issues have to be considered, however, to address this key question.
The genotype–phenotype correlation of human collagen VI myopathies is extremely complex. UCMD is a rare, severe wasting dystrophy normally present at birth. Muscle biopsies usually show marked decrease or complete absence of collagen VI (14), and the disease is classically regarded as autosomal recessive (15, 16), yet UCMD cases with de novo dominant mutations have been described (13, 14). Homozygous or compound heterozygous mutations of COL6A1–A3 genes typically lead to a severe UCMD phenotype (17, 18), although they may occasionally present a Bethlem myopathy-like disease (19). Bethlem myopathy (Mendelian Inheritance in Man no. 158810) is a milder disease with autosomal dominant inheritance, and mutations can affect any of the three COL6 genes. Expression of collagen VI is normal or mildly reduced in cultured fibroblasts of most Bethlem myopathy patients (14). The differences in the clinical phenotype, the unpredictability of the spontaneous course of the disease, the limited number of patients, and the potentially harmful side effects of long-term immunosuppression with CsA (in particular the occurrence of pulmonary infections) induced us to design a short-term open trial whose endpoint was not based on clinical assessment but, rather, on measurements of mitochondrial function and apoptosis in biopsies taken before and after treatment with CsA. Here, we report the results of this open pilot trial with CsA in five patients with collagen VI myopathies.
Results
An essential preliminary question that we had to address was how long the mitochondrial effects of CsA persist after a biopsy is taken. Indeed, our experimental design is based on ex vivo measurements of mitochondrial function before and after treatment of the patients with CsA, and it can succeed only if the minimum time required to perform the measurement of mitochondrial function is comparable to or shorter than the lifetime of the protective effect of CsA. To address this issue, experiments were performed in Col6a1−/− mice treated for 4 days with CsA at 5 mg/kg twice daily, a regimen that leads to normalization of mitochondrial ultrastructure and incidence of apoptosis (2). Animals were killed, and flexor digitorum brevis fibers were prepared 4 h after the last administration of CsA, a time point at which the desensitizing effects on the PTP are maximal in liver and measurable in muscle mitochondria (L. Nicolosi, E. Palma, A.A., M. E. Soriano, A. Rasola, F. Chiara, F. Finetti, G. Vuagniaux, J.-M. Dumont, C. T. Baldari, and P. Bernardi, unpublished results). Mitochondrial function was assessed 3 h later; that is, after the time required for the preparation of muscle fibers (≈2 h) and their attachment to the coverslips.
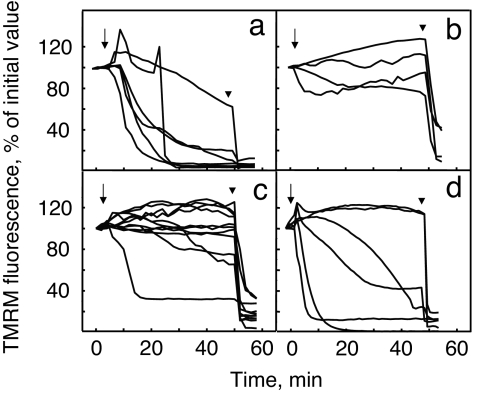
At variance from the case of untreated animals (2), mitochondria in fibers assayed 7 h after the last in vivo treatment with CsA did not depolarize (compare Fig. 1 a and b). We then increased the time elapsing between treatment with CsA and preparation of fibers. It can be seen that when the time elapsed was 17 h, addition of oligomycin still did not depolarize mitochondria in the majority of fibers (Fig. 1c). The protective effect of CsA was instead almost completely lost after 27 h, a time point at which most fibers depolarized upon addition of oligomycin (Fig. 1d).
Fig. 1.
Changes of mitochondrial TMRM fluorescence induced by oligomycin in muscle fibers isolated from Col6a1−/− mice. Flexor digitorum brevis muscle fibers were isolated from Col6a1−/− mice treated with vehicle (a) or with 10 mg/kg CsA in two divided doses per day for 4 days and assayed 7 h (b), 17 h (c), or 27 h (d) after the last treatment with CsA. Fibers from two individuals per time point were loaded with TMRM and studied as described in Experimental Procedures, and the results for each time point were pooled. Where indicated, 6 μM oligomycin (arrows) or 4 μM carbonylcyanide-p-trifluoromethoxyphenyl hydrazone (arrowheads) was added. Each line represents one individual fiber.
The next step was to assess the optimal conditions for obtaining viable, nonreplicating muscle cells amenable to measurements of mitochondrial function. To this end, a series of preliminary experiments was carried out on cells prepared as detailed in Experimental Procedures from biopsies of healthy human volunteers (data not shown). Cell adhesion and spreading onto the substrate occurred within 4–9 h of plating, as revealed by phosphorylated focal adhesion kinase immunostaining. Only after 24 h did cells start to proliferate, as indicated by KI-67 staining. The fraction of myoblasts relative to muscle fibroblasts was 25–35% of the total, as assessed by labeling with desmin and vimentin antibodies. A reliable measurement of mitochondrial function with tetramethyl rhodamine methyl ester (TMRM) could be performed after ≈9 h of plating.
Having established that the approach was feasible, five patients representing the clinical and molecular variety of collagen VI diseases were recruited in the study (13, 16, 19, 20). Four were affected by UCMD and one by Bethlem myopathy; three were of pediatric age and two were adults; collagen VI ranged from almost normal to marked depletion; and the pathogenic mutation involved each of the three COL6 genes (Table 1). All patients were treated with 5 mg/kg CsA per day, a dose that lies in the lower end of the range used for immunosuppression according to established protocols.
Table 1.
Features of the patients included in the study
| P | Phenotype | Age, yr | Collagen VI | Mutation(s) | Refs. |
|---|---|---|---|---|---|
| 1 | UCMD, NW | 9 | Mild reduction in muscle fibers and fibroblasts | COL6A1 de novo heterozygous Gly284Arg | 13 |
| 2 | Mild UCMD, W | 23 | Mild reduction in muscle fibers and fibroblasts | COL6A3 homozygous Arg465 stop | 19 |
| 3 | UCMD, NW | 6 | Marked reduction in muscle fibers | COL6A2 compound heterozygous | 16 |
| Gly487-Ala495delAspfsX48 and Glu591- | |||||
| Cys605delThrfsX148 | |||||
| 4 | UCMD, NW | 8 | Marked reduction in muscle fibers | COL6A1 de novo heterozygous del275–280/insGlu275 | 13 |
| 5 | Bethlem myopathy, W | 57 | Normal in muscle fibers, decreased in fibroblasts | COL6A1 heterozygous Tyr122-Gly143del | 20 |
The features of the patients (P) are summarized. Collagen VI levels are based on immunohistochemistry as described in the original references, which also contain further details about the gene mutations and their mode of inheritance. NW, nonwalker, W, walker.
The vast majority of mitochondria in cells prepared from muscle biopsies of two healthy individuals with the above protocol did not depolarize upon the addition of oligomycin, indicating that mitochondrial function had not been perturbed by the isolation procedures (Fig. 2 a and b). On the other hand, most mitochondria within the cells isolated from patients depolarized after the addition of oligomycin (Fig. 2 c–g), confirming the presence of the latent dysfunction we had previously identified in muscle fibers from Col6a1−/− mice (2) and in cultures from UCMD patients (13). Similar effects of oligomycin on mitochondrial TMRM fluorescence were observed in myoblasts from two patients incubated in KCl- rather than NaCl-based medium (results not shown), indicating that TMRM is reporting a mitochondrial event rather than undergoing a change of distribution secondary to plasma membrane depolarization (21). One month after oral administration of CsA at a dose of 5 mg·kg−1·day−1 in two divided doses, a new biopsy was taken at the controlateral site of the first biopsy, and the experiment was repeated. Strikingly, the mitochondrial membrane potential response to oligomycin was largely normalized in the muscle cells from all patients (Fig. 2 c′–g′). If a threshold is arbitrarily set at 90% fluorescence, the number of cells with depolarizing mitochondria shifted from 89%, 92%, 91%, 95%, and 84% to 45%, 41%, 38%, 41%, and 19% before and after treatment with CsA for patients 1, 2, 3, 4, and 5, respectively (on average from 90% to 37%). These findings are in keeping with the results obtained in cultures from UCMD patients (13) and in fibers from Col6a1−/− mice (2), and they indicate that, at the dose used in this study, CsA reaches pharmacologically active concentrations in muscle.
Fig. 2.
Changes of mitochondrial TMRM fluorescence induced by oligomycin in muscle cells isolated from healthy donors and from UCMD and Bethlem myopathy patients before and after treatment with CsA. Muscle cells obtained from biopsies of two healthy donors (a and b), four patients with UCMD (c–f; patients 1–4, respectively), and one with Bethlem myopathy (g; patient 5) were seeded onto glass coverslips precoated with laminin/poly-l-lysine, loaded with TMRM and studied as described in Experimental Procedures. The procedure was repeated after 1 month of oral treatment with 5 mg/kg CsA per day (c′–g′; patients 1–5, respectively). Where indicated, 6 μM oligomycin (arrows) or 4 μM carbonylcyanide-p-trifluoromethoxyphenyl hydrazone (arrowheads) was added. Each line represents one individual cell.
The incidence of apoptosis was assessed in the same bioptic materials used for the measurements of mitochondrial function. Unlike samples from healthy donors, where apoptosis was undetectable (results not shown), all samples from patients had a sizeable number of apoptotic nuclei, which was particularly prominent in patients 1 and 3 (Fig. 3a, open bars), and in all patients treatment with CsA considerably decreased the occurrence of apoptosis (Fig. 3a, filled bars), indicating a cause–effect relationship between normalization of mitochondrial function and decreased cell death. We also tested the expression of developmental myosin heavy chain (Fig. 3b, green fluorescence) and desmin (Fig. 3b, red fluorescence) in the muscle biopsies. After treatment with CsA, we found an increased number of myofibers positive for developmental myosin heavy chain (Fig. 3b Lower) also displaying diffuse staining for desmin (Fig. 3b Right), which are characteristic signs of regeneration. The effect was particularly prominent in patients 1, 3, and 4; that is, the younger patients included in this study (Fig. 3c). No regenerating fibers could be detected in samples from healthy donors (results not shown).
Fig. 3.
Apoptosis and regeneration in muscle biopsies from UCMD and Bethlem myopathy patients before and after treatment with CsA. (A) (Bottom) Biopsies from four patients with UCMD (P1–P4) and one patient with Bethlem myopathy (P5) were scored for the presence of apoptotic nuclei with the TUNEL reaction as described in Experimental Procedures before (open bars) and after 1 month of oral treatment with 5 mg/kg CsA per day (filled bars). Data are expressed as mean ± SEM of three independent experiments. *, P < 0.05. The incidence of apoptotic nuclei in healthy donor (control) biopsies was 0.39 ± 0.3 per square millimeter. (Top and Middle) Representative fields of sections derived from the biopsy of one healthy donor (control) and of patient 1 (P1) before and after CsA treatment, stained with TUNEL and Hoechst. Arrows indicate TUNEL-positive nuclei. (Scale bar: 50 μm.) (B) Representative cross-sections of muscle biopsies of UCMD patient 3 before (basal) and after (CsA) 1 month of oral treatment with 5 mg/kg CsA per day. The sections were stained with DAPI to identify nuclei (blue) and with antibodies against developmental myosin heavy chain (green) and desmin (red). (Left) Overlay between DAPI and developmental myosin heavy chain. (Right) Overlay between developmental myosin heavy chains and desmin. (C) Occurrence of regenerating fibers before (open bars) and after treatment with CsA (filled bars) identified as illustrated for B. Data represent mean ± SD. *, P < 0.05.
Discussion
A remarkable result of the present manuscript is the demonstration that the PTP-desensitizing effects of CsA persist up to 17 h in flexor digitorum brevis fibers prepared from CsA-treated Col6a1−/− mice and at least 18 h in primary cultures prepared from CsA-treated patients. It should be recalled that the binding of CsA to cyclophilins, which also mediates the desensitizing effects on the mitochondrial PTP (22), is noncovalent (23); yet, the off constant for CsA binding is extremely low, a property that we successfully exploited to demonstrate the desensitizing effects of the drug on the PTP after isolation of mitochondria from CsA-treated animals (12) and of cells from patients (present work). This finding is of practical value because it allows an objective readout of the efficacy of CsA in vivo, which is expected to be more predictive of the potential clinical outcome than the plasma levels of the drug for future randomized clinical studies. We would like to point out that, in the present study, the clinical response to CsA was not assessed because no reliable measurements can be performed in the limited time frame imposed by ethical considerations. Immunosuppression caused by CsA could expose the patients to pulmonary infections, and this forced us to perform a short-term trial that, in turn, leaves little potential for clinical improvement in a disease where fibrosis is so prominent.
It is quite encouraging that CsA decreased apoptosis in all treated patients within a month, because this finding suggests that assessing the effects of CsA on the clinical course of the disease may be feasible in future clinical trials. Some reason for hope also comes from the observation that treatment with CsA increased muscle regeneration. At first sight, this finding may appear paradoxical, because regeneration should match the rate of muscle fiber death, which is lower when CsA is present. An appealing explanation is that in collagen VI diseases, differentiating muscle cells are undergoing early apoptosis together with mature fibers, a situation that would be similar to the ineffective erythropoiesis seen in many hemoglobinopathies. By decreasing ineffective myogenesis, CsA could increase the overall efficiency of muscle regeneration, in keeping with the response of all patients. This is another promising indication that long-term PTP desensitization may do more than just stop the progression of the disease. Additional studies and a larger sample size will be required before firm conclusions can be made about this issue and about the correlation between regeneration and patient age observed here.
Our results prove that treatment with moderate doses of CsA favorably affects mitochondrial function in collagen VI myopathies in vivo and dramatically decreases the frequency of muscle cell death in the patients. Whether this treatment will eventually stop progression of the disease and/or translate into a better muscle performance depends on multiple factors, such as muscle loss and extent of fibrosis at the time of treatment, and potential for muscle regeneration in individual patients. Furthermore, the potential benefit must be carefully weighed against increased occurrence of infections that may follow immunosuppression during treatment with CsA. It should be mentioned, however, that other cyclosporins, like MeAla3EtVal4-cyclosporin (Debio 025), may represent an alternative to CsA. We have shown that Debio 025, a nonimmunosuppressive cyclosporin that retains the PTP-desensitizing properties of CsA (24), is as effective as CsA at protecting mitochondrial function and preventing apoptosis in muscle cultures from UCMD patients (13), a finding that holds great promise for the treatment of collagen VI disorders.
The results obtained in this study indicate that mitochondrial dysfunction plays a critical role in human muscle diseases in vivo, as was first hypothesized 30 years ago (25). Moreover, they represent an important proof of principle that hereditary muscle diseases can be cured with proper drugs downstream of the genetic lesion if the pathogenetic mechanisms are understood (26). This is a useful example of how translational medicine can rapidly move from animal models to treatment of human diseases and how mitochondrial medicine may be useful beyond the cure of primary mitochondrial diseases (27).
Experimental Procedures
Mouse Treatment and Preparation of Muscle Fibers.
All animal manipulations conformed to the rules of the Ethics Committee of the University of Padova. We treated Col6a1−/− mice (1) i.p. with 10 mg·kg−1·day−1 CsA (Novartis) in two divided doses for 4 days or with vehicle (olive oil). Fibers from flexor digitorum brevis muscles of Col6a1−/− mice were isolated as described (2), plated onto 24-mm round glass coverslips coated with 3 μg/cm2 Engelbreth–Holm–Swarm sarcoma mouse laminin (Roche) and cultured for 1 h in DMEM (Sigma) containing 10% FCS (Sigma) before starting the experiments.
Participants.
UCMD and Bethlem myopathy were diagnosed according to the criteria of the European NeuroMuscular Center (28). All probands were examined, and they all underwent a muscle biopsy. Genetic data for all of the patients have been published previously (13, 16, 19, 20). All participants provided written informed consent, and approval was obtained from the Ethics Committee of the University of Ferrara. The basic features of the patients analyzed in this study are summarized in Table 1. CsA was administered as an oral formulation (Sandimmun Neoral; Novartis).
Biopsies and Primary Cell Cultures.
We obtained muscle biopsies from healthy donors and patients after informed consent and approval of the Ethics Committee of the University of Ferrara. Biopsies of tibialis anterior were obtained from one Bethlem myopathy and four UCMD patients. Part of the biopsy was frozen in isopentane prechilled in liquid nitrogen and used for TUNEL assay and immunohistochemistry. Ten muscle biopsies were obtained from healthy donors of ages ranging between 3 and 65 years during orthopedic surgical treatment. Muscle samples selected for culture preparation were transferred in DMEM supplemented with penicillin, streptomycin, and amphotericin B (complete medium). To remove erythrocytes, muscle fragments were soaked in a 0.25% trypsin solution, which was discarded after 3–5 minutes. The muscle preparations were then mixed with 200 units/ml collagenase type I (Sigma) in DMEM and incubated at 37°C for 20 min. The medium (containing detached cells) was collected, an equal volume of complete medium supplemented with 20% FCS was added, and the mixture was set aside. Collagenase I was added back to the residual muscle fragments, and the above procedure was repeated four times. Cell suspensions were pooled, centrifuged for 10 min at 800 × g, resuspended in complete medium plus 20% FCS, and finally plated on laminin/poly-l-lysine-coated coverslips (BD Laboratories). To define suitable conditions for measuring the mitochondrial membrane potential, muscle cultures from healthy donors were checked at different times for phosphorylated focal adhesion kinase, KI-67, desmin, and vimentin. At 4, 9, 12, and 24 h of plating, muscle cell cultures were fixed with cold methanol, washed with PBS, and incubated with the following polyclonal or monoclonal antibodies, properly diluted in PBS with 2% BSA: phosphofocal adhesion kinase (1:10; Santa Cruz Biotechnology), KI-67 (1:50; Santa Cruz Biotechnology), desmin (1:10; Abcam), and vimentin (1:10; Sigma). The immunoreactions were revealed with FITC-conjugated swine anti-rabbit or TRITC-conjugated rabbit anti-mouse antibodies (DAKO), mounted with Pro-long anti-fade reagent (Molecular Probes) and examined with a Nikon epifluorescence microscope at ×100 magnification.
Immunofluorescence on Muscle Sections.
Unfixed frozen sections of muscle biopsies from patients were double labeled with a rabbit polyclonal anti-desmin antibody (undiluted; Abcam), followed by a TRITC-conjugated swine anti-rabbit antibody (DAKO). Then, samples were incubated with a monoclonal anti-developmental myosin heavy chain antibody (1:10; Novocastra), washed extensively with PBS, and treated with a secondary FITC-conjugated rabbit anti-mouse antibody (DAKO). Samples were mounted with Pro-long anti-fade reagent (Molecular Probes) and examined with a Nikon epifluorescence microscope at a ×40 magnification. For statistical analysis, 1,000 fibers from three different regions of the biopsy for each sample were considered. Data were analyzed with the unpaired Student t test, and values with P < 0.05 were considered as significant.
Detection of Apoptosis.
We measured the rate of apoptosis in muscle biopsies by using the TUNEL method. Seven-micrometer-thick frozen sections were prepared from muscle biopsies, fixed in 50% acetone/50% methanol and processed for TUNEL analysis by using the Apoptag peroxidase in situ apoptosis detection kit (Chemicon). Visualization of all nuclei was performed by staining with Hoechst 33258 (Sigma). The number of TUNEL-positive nuclei was determined in randomly selected fields with a Zeiss Axioplan microscope (×40 magnification) equipped with a digital camera. For each biopsy, 30–85 fields containing muscle fibers and covering a total area of ≈1–7 mm2 were observed. Data were analyzed with the unpaired Student t test and values with P < 0.05 were considered as significant.
Mitochondrial Membrane Potential.
This was measured based on the accumulation of TMRM (Molecular Probes). Flexor digitorum brevis myofibers plated onto 24-mm round glass coverslips coated with 3 μg/cm2 Engelbreth–Holm–Swarm sarcoma mouse laminin (Roche) were incubated with 20 nM TMRM for 15 min in 1 ml of glucose-free Tyrode buffer (2). Imaging was performed with a Zeiss Axiovert 100 TV inverted microscope equipped with a mercury light source (100 W) for epifluorescence illumination and with a 12-bit digital cooled CCD camera (Micromax; Princeton Instruments). We analyzed data with the MetaFluor Imaging software. Primary cell cultures obtained as described above from healthy donor or patient biopsies were plated within 4 h of the surgical biopsy in complete medium plus 20% FCS on laminin/poly-l-lysine–coated coverslips (BD Laboratories) and allowed to attach and spread for 9–14 h. The medium was then replaced with serum-free DMEM supplemented with 10 nM TMRM for 30 min, and cellular fluorescence images were acquired with an Olympus IX71/IX51 inverted microscope equipped with a xenon light source (75 W) for epifluorescence illumination, and with a 12-bit digital cooled CCD camera (Micromax). Data were acquired and analyzed by using Cell R software (Olympus). For detection of fluorescence, 568 ± 25-nm bandpass excitation and 585-nm longpass emission filter settings were used. Images were collected with an exposure time of 100 msec by using a ×20 objective (Nikon) for the mouse muscle fibers and a ×40, 1.3 N.A. oil immersion objective (Nikon) for the patients' cells. The extent of cell and, hence, mitochondrial loading with potentiometric probes are affected by the activity of the plasma membrane multidrug resistance pump, which is inhibited by CsA. Treatment with this drug may, therefore, cause an increased mitochondrial fluorescence that can be erroneously interpreted as an increase of the mitochondrial membrane potential (29). To prevent this artifact and to normalize the loading conditions, the medium in all experiments with TMRM was supplemented with 1.6 μM CsH, which inhibits the multidrug resistance pump but not the PTP (22). At the end of each experiment, mitochondria were fully depolarized by the addition of 4 μM protonophore carbonylcyanide-p-trifluoromethoxyphenyl hydrazone. Clusters of several mitochondria (n = 10–30) were identified as regions of interest, and fields not containing cells were taken as the background. Sequential digital images were acquired every 1 min for the fibers and every 2 min for the cells, and the average fluorescence intensity of all relevant regions was recorded and stored for subsequent analysis.
Acknowledgments.
We thank all individuals who participated in this study and their families, Andrea Franchella and Claudio Vella (University of Ferrara) for performing the muscle biopsies, and Enrico Bertini (Ospedale Bambino Gesù, Rome) for referring one of the patients. This study was supported by Telethon-Italy Grant GGP04113, the Fondazione Carisbo, Association Française contre les Myopathies Grant 9398, and the Italian Ministry for the University.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bonaldo P, et al. Collagen VI deficiency induces early onset myopathy in the mouse: An animal model for Bethlem myopathy. Hum Mol Genet. 1998;7:2135–2140. doi: 10.1093/hmg/7.13.2135. [DOI] [PubMed] [Google Scholar]
- 2.Irwin WA, et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:267–271. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 3.Vinogradov A, Scarpa A, Chance B. Calcium and pyridine nucleotide interaction in mitochondrial membranes. Arch Biochem Biophys. 1972;152:646–654. doi: 10.1016/0003-9861(72)90261-5. [DOI] [PubMed] [Google Scholar]
- 4.Di Lisa F, Menabò R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 5.Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition: Implications for tumor necrosis factor-a apoptotic signaling. J Biol Chem. 2001;276:12035–12040. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- 6.Nicholls DG, Ward MW. Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci. 2000;23:166–174. doi: 10.1016/s0166-2236(99)01534-9. [DOI] [PubMed] [Google Scholar]
- 7.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 8.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 9.Hüser J, Rechenmacher CE, Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophys J. 1998;74:2129–2137. doi: 10.1016/S0006-3495(98)77920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petronilli V. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hüser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999;343:311–317. [PMC free article] [PubMed] [Google Scholar]
- 12.Soriano ME, Nicolosi L, Bernardi P. Desensitization of the permeability transition pore by cyclosporin A prevents activation of the mitochondrial apoptotic pathway and liver damage by tumor necrosis factor-α. J Biol Chem. 2004;279:36803–36808. doi: 10.1074/jbc.M405297200. [DOI] [PubMed] [Google Scholar]
- 13.Angelin A, et al. Mitochondrial dysfunction in the pathogenesis of Ullrich congenital muscular dystrophy and prospective therapy with cyclosporins. Proc Natl Acad Sci USA. 2007;104:991–996. doi: 10.1073/pnas.0610270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampe AK, Bushby KM. Collagen VI related muscle disorders. J Med Genet. 2005;42:673–685. doi: 10.1136/jmg.2002.002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ullrich O. Congenital, atonic-sclerotic muscular dystrophy, a further type of hereditary degenerative disease of the neuromuscular system (translated from German) Z Ges Neurol Psychiatr. 1930;126:171–201. [Google Scholar]
- 16.Camacho Vanegas O, et al. Ullrich scleroatonic muscular dystrophy is caused by recessive mutations in collagen type VI. Proc Natl Acad Sci USA. 2001;98:7516–7521. doi: 10.1073/pnas.121027598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan TC, et al. New molecular mechanism for Ullrich congenital muscular dystrophy: A heterozygous in-frame deletion in the COL6A1 gene causes a severe phenotype. Am J Hum Genet. 2003;73:355–369. doi: 10.1086/377107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker NL, et al. Dominant collagen VI mutations are a common cause of Ullrich congenital muscular dystrophy. Hum Mol Genet. 2005;14:279–293. doi: 10.1093/hmg/ddi025. [DOI] [PubMed] [Google Scholar]
- 19.Demir E, et al. Mutations in COL6A3 cause severe and mild phenotypes of Ullrich congenital muscular dystrophy. Am J Hum Genet. 2002;70:1446–1458. doi: 10.1086/340608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camacho Vanegas O, et al. Novel COL6A1 splicing mutation in a family affected by mild Bethlem myopathy. Muscle Nerve. 2002;25:513–519. doi: 10.1002/mus.10100. [DOI] [PubMed] [Google Scholar]
- 21.Rottenberg H, Wu S. Quantitative assay by flow cytometry of the mitochondrial membrane potential in intact cells. Biochim Biophys Acta. 1998;1404:393–404. doi: 10.1016/s0167-4889(98)00088-3. [DOI] [PubMed] [Google Scholar]
- 22.Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, a cyclosporin A-sensitive channel. J Biol Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 23.Pflugl G, et al. X-ray structure of a decameric cyclophilin-cyclosporin crystal complex. Nature. 1993;361:91–94. doi: 10.1038/361091a0. [DOI] [PubMed] [Google Scholar]
- 24.Hansson MJ, et al. The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J Bioenerg Biomembr. 2004;36:407–413. doi: 10.1023/B:JOBB.0000041776.31885.45. [DOI] [PubMed] [Google Scholar]
- 25.Wrogemann K, Pena SD. Mitochondrial calcium overload: A general mechanism for cell-necrosis in muscle diseases. Lancet. 1976;1:672–674. doi: 10.1016/s0140-6736(76)92781-1. [DOI] [PubMed] [Google Scholar]
- 26.Burton EA, Davies KE. Muscular dystrophy—Reason for optimism? Cell. 2002;108:5–8. doi: 10.1016/s0092-8674(01)00626-2. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepe G, et al. Surgical treatment of neck hyperextension in myopathies. Neuromuscul Disord. 2002;12:984–993. [Google Scholar]
- 29.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]