Abstract
Recombinant HIV-Tat (Tat) induces extensive apoptosis in peripheral blood mononuclear cells (PBMCs) cultured in typical CO2 incubators, which are equilibrated with air (21% O2). However, as we show here, Tat apoptosis induction fails in PBMCs cultured at physiological oxygen levels (5% O2). Under these conditions, Tat induces PBMCs to divide, efficiently primes them for HIV infection, and supports virus production by the infected cells. Furthermore, Tat takes only 2 h to prime PBMCs under these conditions. In contrast, PHA/IL-2, which is widely used to prime cells for HIV infection, takes 2–3 days. These findings strongly recommend culturing primary cells at physiological oxygen levels. In addition, they suggest HIV-Tat as a key regulator of HIV disease progression.
Keywords: cell culture, HIV infection, HIV-Tat, PBMC
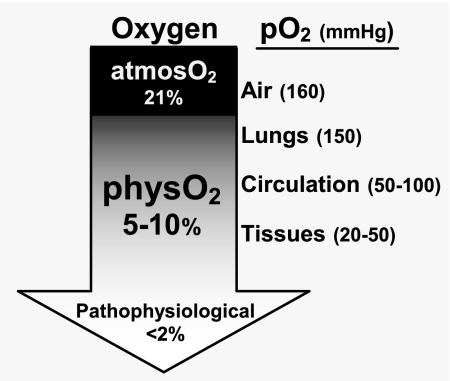
The studies presented here reveal a core problem that potentially poisons interpretations of data from virtually all mammalian cell culture studies. In the overwhelming majority of such studies today, cells are cultured in incubators that are gassed with a mixture of CO2 and air, which maintains the internal oxygen level essentially at atmospheric oxygen (atmosO2) levels (atmosO2, 20–21% O2,). The biomedical community (or at least some portion thereof) has, of course, been aware that these oxygen levels are 2- to 4-fold greater than the oxygen levels that lymphocytes and most other cells encounter in vivo (Fig. 1). However, the implied caution has largely been ignored, keeping the entrenched culture methods in use despite the rapidly growing recognition that all cell types sense and respond to even small shifts in oxygen levels (1–6).
Fig. 1.
Oxygen levels that cells encounter in vivo are lower than oxygen levels in air.
Findings presented here signal an end to this age of innocence. In essence, we demonstrate dramatic differences in the way that human peripheral blood mononuclear cells (PBMCs) respond to a well studied HIV protein depending on whether the cells are cultured at atmosO2 or at physiologically relevant oxygen (physO2) levels that are typical of those lymphocytes encounter in vivo (physO2, 5% O2). The striking qualitative differences in these responses, which shift from the previously demonstrated apoptosis induction at atmosO2 to the stimulation of cell division and support for HIV infection at physO2, suggest that interpretation of other in vitro HIV findings may similarly require evaluation under more physiological culture conditions.
These considerations apply to conclusions from essentially all current culture studies with mammalian cells. However, they may be particularly relevant to those conducted with cell lines (including stem cell lines) because long-term growth at atmosO2 is likely to induce functionally significant mutations, in addition to altering cell functions relative to those the cells perform in vivo.
Important for understanding the dynamics of HIV disease progression, the findings we present here introduce an extracellular role for HIV-Transactivator of transcription (Tat), a multifunctional viral protein named originally for its intracellular role as a transactivator of transcription. Although previously shown to induce T cell apoptosis in vitro (at atmosO2) (7, 8) and proposed to play a similar role in vivo (8–10), we show here that, instead of inducing apoptosis, Tat induces cell division in PBMCs cultured at physO2 as efficiently as the mitogen/cytokine combination (PHA/IL-2) widely used for this purpose. Furthermore, we show that, although PHA/IL-2 stimulation is typically used to prime for and support HIV infection in PBMCs (at atmosO2), Tat at physO2 is substantially more effective. PHA/IL-2 stimulation requires 2–3 days to prime PBMCs for productive HIV infection. In contrast, Tat requires only 2 h to enter and prime significant numbers (perhaps all) of the cultured cells that can host the virus.
At a minimum, these findings introduce recombinant Tat as an effective replacement for the more artificial stimuli commonly used for in vitro HIV-infection studies with primary cells. However, based on findings presented here, Tat also can be envisioned as playing a key role in HIV disease economy. Tat is well known to be released from HIV-infected cells in vitro. The evidence for Tat release in vivo is of necessity indirect because, as we confirm here, Tat is taken up by neighboring cells too rapidly to be reliably detected in vivo. Nevertheless, several studies indicate that Tat is indeed released in vivo (11–15). Our findings support these arguments by suggesting that local Tat release and uptake by neighboring cells may be central to the curious kinetics of HIV disease, which begins with an intense viral storm that only abates after depleting a high percentage of the memory T cells (16) in lymph nodes and other crowded lymphoid sites.
Overall, the studies presented here show that important responses of primary lymphocytes have been masked by studying the behavior of primary lymphocytes at oxygen levels that the cells are highly unlikely to encounter in vivo.
Results
Tat Rapidly Enters Cultured PBMCs.
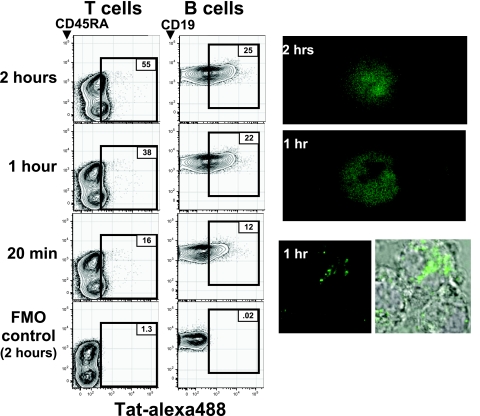
Consistent with a large body of evidence (13, 17–21), FACS and fluorescence microscopy studies with the recombinant Tat used here show that fluorochrome-labeled Tat efficiently enters all cells in PBMC cultures maintained at physO2 (Fig. 2). After 1 h at 37°C, the Tat is detectable in cytoplasm; by 2 h, it is detectable in both the cytoplasm and nuclei of the cells. In contrast, Tat is not detectable in cells cultured at 0°C for this period (data not shown).
Fig. 2.
HIV-Tat rapidly enters PBMCs. Human PBMCs mixed with AlexaFluor488-labeled Tat (Tat-alexa) were incubated at 37°C at physO2 or kept on ice for the indicated times. (Left) Cell-associated fluorescence in the Tat-alexa channel is shown for viable T and B cells in FACS analyses gated to reveal these subsets. Fluorescence-minus-one (FMO) controls show fluorescence detected in the Tat channel when cells were incubated with all stains but Tat. (Right) Intracellular Tat-alexa in PBMCs was revealed by a confocal microscope (LSM 510; Carl Zeiss) in cells incubated with Tat for the indicated times.
Flow-cytometry analyses of PBMCs incubated with Tat coupled to Alexa488 for up to 2 h at physO2 similarly demonstrate that Tat enters cells in all lymphocyte subsets in the PBMC culture (Fig. 2 Left). On average, B cells take up more Tat than T cells, and memory T cells take up more Tat than naïve T cells. However, there is substantial overlap among the populations, and there are many cells in all populations that take up little or no Tat, at least as measured by uptake of the fluorochrome-coupled Tat used here.
Tat Induces Apoptosis in PBMCs Cultured at AtmosO2 levels.
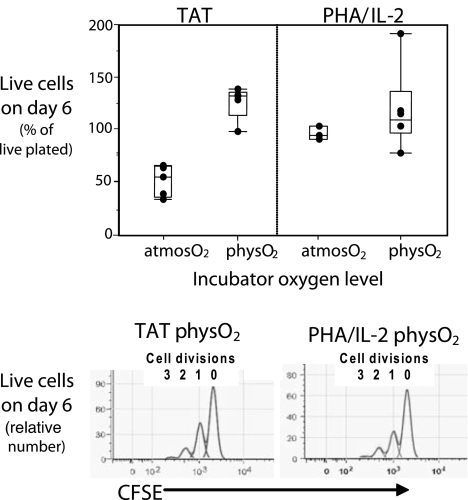
Tat is well known to induce extensive apoptosis in PBMCs cultured in standard CO2 incubators maintained at atmosO2. Data in Fig. 3 confirm these findings by showing that only about half the PBMCs put into culture with recombinant Tat at atmosO2 are detectable 6 days later and that a significant proportion of the remaining cells are apoptotic, as indicated by Annexin-V staining, or are necrotic. In contrast, consistent with previous findings, apoptosis induction is minimal (2–5%) in cultures stimulated at atmosO2 with phytohemagglutinin (PHA) supplemented with IL-2 (PHA/IL-2), a mitogen/cytokine combination used widely to induce cell division and prime PBMCs for HIV infection in in vitro studies. Furthermore, it is minimal in cultures stimulated with Tat at physO2 levels (Fig. 3 Upper).
Fig. 3.
HIV-Tat stimulates proliferation and induces minimal cell death in PBMCs at physO2 levels. PBMCs were cultured with the indicated stimuli (5 μg/ml recombinant HIV-Tat, 2.5 μg/ml PHA, and 50 units/ml IL-2) at physO2 or atmosO2. Viable cell counts were determined at the beginning and end of the culture period by flow cytometry (see Materials and Methods). (Upper) Live cell recoveries at the end of the culture period, expressed as the percentage of live cells plated initially. (Lower) The number of cell divisions occurring during the culture period, computed from the distribution of CFDA-SE (CFSE) staining for live cells recovered from PBMCs that were preincubated with 0.5 μM CFDA for 10 min at 37°C before culturing. Typically, the peak with the highest CFSE staining reflects the frequency of cells that have not divided and the successive peaks with lower fluorescence represent cells that have undergone one, two, or three cell divisions. CFSE data are not shown for PBMCs cultured with Tat at atmosO2 because low cell yields and viability problems prevented accurate data collection and analysis.
Tat Induces Cell Division in PBMCs Cultured at PhysO2 levels.
In contrast to the extensive apoptosis induction and cell loss that occurs when PBMCs are cultured with Tat at atmosO2, the number of cells recovered when PBMCs are cultured with Tat at physO2 for 6 days is 1- to 1.5-fold higher than the number of cells plated (Fig. 3 Upper). Furthermore, very few (2–5%) of the recovered cells are apoptotic.
The increased cell yields in the Tat-stimulated physO2 cultures reflect the induction of cell division at levels and rates equivalent to those obtained in PBMC cultures stimulated with PHA/IL-2 (Fig. 3 Lower). Consistent with previous findings (1, 2), responses to PHA/IL-2 shown in Fig. 3 are equivalent at atmosO2 and physO2. Responses to Tat, however, differ markedly and, in this sense, are more like responses to anti-CD3/CD28 and other physiological stimuli, which we also have shown to differ at physO2 and atmosO2 (1, 2). In any event, at physO2, PBMC responses to Tat approximate PBMC responses to PHA/IL-2. Thus, when cells are maintained at oxygen levels similar to those encountered in vivo, Tat emerges as a potent inducer of cell division and, hence, as a candidate primer for HIV infection.
Tat Primes for HIV Infection and Supports Viral Production in PBMCs Cultured at PhysO2.
PHA and IL-2 are typically used to prime cells for HIV infection and to support viral production by the infected cells. Because Tat mimics PHA/IL-2 in stimulating cell division at physO2, it may efficiently replace this mitogen/cytokine combination in providing priming and support for HIV-infection assays. Evidence presented here, in which Tat is used in place of PHA/IL-2 at various points in the standard three-stage protocol for in vitro HIV-infection protocol, confirms this hypothesis (Figs. 4 and 5).
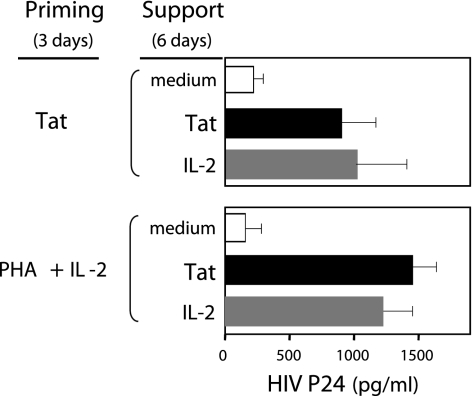
Fig. 4.
Tat efficiently primes PBMCs for HIV infection and supports viral production by the infected cells. PHA/IL-2 and Tat are equivalent for this purpose. In this mix-and-match assay, all cultures were maintained at physO2, and Tat was substituted for PHA/IL-2 during the priming and/or support phases of the standard three-stage in vitro HIV-infection protocol. Thus, PBMCs cultured at physO2 were primed with Tat or PHA/IL-2 as indicated for 3 days, washed to remove free priming agent, incubated for 3 h with HIV (LAI) to allow infection to proceed, washed to remove free virus, and finally cultured for 6 days with Tat or IL-2 as indicated to allow viral production to proceed. Tat and PHA/IL-2 were used at the concentrations indicated in Materials and Methods. HIV-core p24 was measured by ELISA. Viral yields, expressed as pg/ml, were computed from a p24 standard curve. Response values shown in the figure are averages of responses obtained for five subjects. The relative response levels of PBMCs from individual subjects were consistent under the various stimulatory conditions tested. Five to 15 subjects were tested for each stimulatory condition.
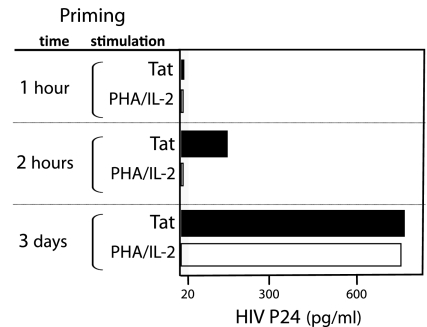
Fig. 5.
Culturing with Tat for 2 h primes PBMCs for HIV infection. PBMCs were primed for the indicated period with Tat or PHA plus IL-2 before infection. After infection, HIV production by the Tat- and PHA/IL-2-primed cells was supported, respectively, with Tat and IL-2. See Fig. 4 legend for additional experiment details. Average responses for two subjects are shown in the figure. Data are representative of two experiments.
For these studies, all cultures were maintained at physO2. PBMCs were primed with Tat or PHA/IL-2 for 3 days, washed to remove free priming agent, incubated for 3 h with HIV (LAI) to allow infection to proceed, washed to remove free virus, and finally cultured for 6 days with Tat or IL-2 as indicated to allow viral production to proceed. HIV p24 in culture supernatants, an index of viral production, was measured by ELISA at the end of the 6-day period (see Materials and Methods for further details).
Replacing PHA/IL-2 with Tat during the priming stage of the protocol demonstrates that Tat and PHA/IL-2 stimulation are equivalent with respect to priming for HIV infection because viral yields obtained from cells primed with Tat match those obtained with the standard PHA/IL-2 protocol (Fig. 4). Viral yields (p24) are maximal when Tat is added at 5 μg/ml and decrease as a function of the amount of Tat added to the culture (data not shown). Tat was not tested at >5 μg/ml because it tends to induce some apoptosis (10–20%) at higher levels, even at physO2.
Replacing IL-2 with Tat during the support phase of the infection protocol shows that Tat provides efficient support for viral production by the primed and infected cells regardless of whether Tat or PHA/IL-2 was used for priming. Substituting Tat for PHA/IL-2 added during the 3-day priming phase of the assay and/or for the IL-2 added during the 6-day support phase results in viral yields comparable to those obtained with the standard PHA/IL-2 protocol in all cases (1,000–1,500 pg/ml of p24) (Fig. 4). Thus, Tat can fully substitute for PHA/IL-2 in the standard HIV infection assay.
Neither the Tat support for HIV production nor the Tat priming for HIV infection is explained by Tat induction of IL-2 production because <0.05 units/ml IL-2 is detectable in supernatants from Tat-primed or Tat-supported cultures assayed with a commercial ELISA kit (Biosource/Invitrogen).
Tat Primes Cells for HIV Infection Much More Rapidly than PHA/IL-2.
Priming PBMCs for just 2 h with Tat (at physO2) is sufficient to enable significant HIV infection and viral production by the primed cells, whereas priming with PHA/IL-2 for 2 h does not result in detectable infection (Fig. 5). In fact, Tat priming for 2 h results in viral yields that are roughly one quarter the yield obtained from cells primed with either Tat or IL-2 for 3 days.
These findings suggest that Tat priming for 2 h may be sufficient to enable infection of all of the PBMCs capable of hosting the virus because such cells are likely to be activatable and, hence, among the cells that Tat (or PHA/IL-2) stimulates to proliferate during the 3-day culture period (Fig. 3). In essence, the 4-fold increase in viral yield that is obtained after 3 days of priming may reflect the ≈4-fold increase in cell number that occurs during this period. In any event, the high viral production obtained after the infection of PBMCs that were primed with Tat for only 2 h demonstrates that HIV infection of PBMCs does not require cell division and requires events that can occur within 2 h.
Only Certain Cysteine (Cys) Residues in Tat Are Required for Tat Priming and Support of HIV Infection.
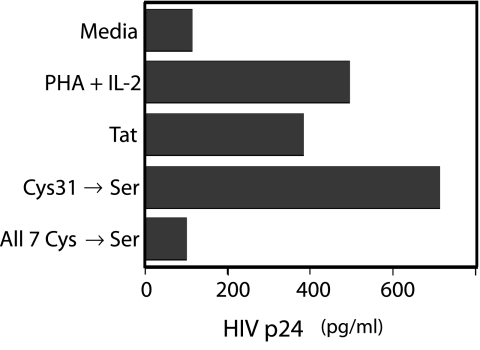
Tat's ability to prime and support HIV infection in PBMCs is abolished when the codons encoding all seven of the Cys residues in the protein are mutated to Serine (Ser) residues (see Fig. 6). However, mutating Cys 31 to Ser does not affect this priming and support capability. Thus, at least one Cys other than Cys 31 is required for priming and support of HIV infection.
Fig. 6.
Mutating the seven Cys residues to Ser in Tat destroys priming for HIV infection. Three Tat proteins were tested for the ability to prime for HIV infection: native Tat and two mutated forms (Cys31Ser and Cys22Ser, Cys25Ser, Cys27Ser, Cys30Ser, Cys31Ser, Cys34Ser, Cys37Ser). IL-2 was used for the support phase in the assay.
In addition to providing initial structure/function insights, these findings sharply decrease the likelihood that a contaminant in the recombinant Tat preparation used here is responsible for the Tat activities we have detected. The recombinant Tat used here was prepared by a method that excludes endotoxin (prepared by J.R. and E.R.) and was shown to have <8 units/ml and is not known to contain other active contaminants. Therefore, the failure to obtain priming and/or support with mutated Tat in which Ser residues replace the 7 Tat Cys residues adds confidence that a functional Tat protein is required for this activity.
Discussion
The findings presented here, which echo and extend results from previous studies (1, 2), raise questions about interpretations of data from virtually all cell culture studies conducted with primary cells or with cell lines developed in atmosO2 incubators. Basically, we show here that key responses to stimulation can differ dramatically even in short-term cultures maintained at physO2 versus atmosO2. Because adaptation to long-term growth perforce requires changes/mutations that alter function, adding the need to adapt to growth at atmosO2 can be expected to have introduced substantial deviation from the in vivo behavior of cells (although creating cell lines in this way may have been the only practical alternative in years gone by). This consideration may be particularly important for the development of stem cell lines, where minimal mutation is clearly a desirable end. In any event, findings presented here suggest that, although results from studies at atmosO2 and physO2 both reveal aspects of the biology of the cells, artifacts potentially introduced by culturing cells at oxygen levels well above those encountered in vivo may cloud the interpretation of the biomedical significance of findings obtained with cells cultured at the higher oxygen level.
There is, of course, room for argument as to whether culturing cells at atmosO2 or physO2 is more useful in different systems. We maintain that it is best to culture cells under conditions that approximate the physiological environment as closely as possible. Other investigators may prefer to continue with current culturing methods because so much work already has been done with cells cultured at atmosO2 and because virtually all cell lines under study today have been developed in atmosO2 incubators. Furthermore, as the PHA/IL-2 data presented here show clearly, some responses are equivalent at the two oxygen levels. Nevertheless, the striking differences we demonstrate between PBMC responses to Tat at atmosO2 versus physO2 strongly recommend a shift toward culturing cells at physO2 whenever findings predictive of in vivo responses are desirable.
Results from the Tat studies described here also show a new perspective on the role(s) that Tat plays in HIV disease. The oxidative stress that develops in HIV-infected subjects as the disease progresses leaves room for Tat to act, as previously proposed (7, 8, 22, 23), to exacerbate oxidative stress and to induce apoptosis in late-stage HIV disease. However, our studies demonstrate that Tat can enter all lymphocytes and can rapidly and efficiently prime cells that can host HIV to render these cells infectable by the virus. Furthermore, our studies demonstrate that Tat can support viral production by the infected cells. Together, these surprising findings open a route through which Tat may influence the course of HIV disease.
Specifically, our findings suggest that Tat released by HIV-infected cells in vivo may act locally to prime neighboring cells and thus to amplify the infection. This Tat release could be particularly important during the early stages of the infection, when HIV-infected cells are seeded into regions that contain a high proportion of uninfected cells capable of hosting the virus. Under these conditions, the Tat that is released would be highly likely to be taken up by HIV-susceptible neighboring cells, which would then be readied within hours for infection by locally released viral particles.
Repeated cycles of Tat priming, HIV infection, and Tat release would drive viral expression up until cells capable of being primed by Tat and infected by the virus become scarce. At this point, the Tat-mediated spread of the infection would be likely to subside because lack of local targets and locally produced Tat would likely be picked up by neighboring cells that cannot be infected by HIV. Thus, Tat may be most important in propagating the infection early in the disease.
This scenario suggests that Tat priming and support for HIV propagation could be central to the propagation of what has been referred to as the viral storm that rages during the acute phase of the infection, when 30–60% of CD4 memory T cells are infected and lost (16, 24, 25). This massive T cell destruction, which heralds subsequent immunodeficiency, occurs to the same extent in many tissues (16, 24–26) and has been recognized in both simian and human HIV disease. Its striking rapidity and short-term voracity argue for a specialized mechanism that can operate locally to increase HIV-infection rates and viral production but will wane as T cells are lost, enabling its replacement with a chronic infection that slowly degrades the immune system, but allow survival of the infected host and thereby optimize the opportunity for passage of the virus to new hosts. Our findings nominate Tat as a key player in this specialized mechanism.
Gallo, Ensoli, and Zagury (11, 27–29), recognizing that anti-Tat antibody production is associated with improved survival in HIV disease, argue that Tat immunization may be effective for preventing or attenuating HIV infection. The unique HIV priming and support capabilities that our studies now demonstrate for Tat also support the idea that stimulating high-titer humoral immunity to Tat may be an important addition to vaccine strategies.
In any event, regardless of whether Tat proves important in the economy of the HIV infection, studies presented here introduce Tat as a potential replacement for PHA/IL-2 for priming PBMCs for HIV infection and supporting viral production by the infected cells. The use of Tat, which requires that the PBMCs be cultured at oxygen levels that approximate those encountered by lymphocytes in vivo, offers the potential for studying HIV infection under conditions that may more closely reflect the in vivo infection conditions and, hence, is likely to provide a more informative environment for mechanism and drug-susceptibility studies.
Materials and Methods
Culture Conditions and Oxygen Level.
Cell stimulation and short-term culture were carried out at two oxygen levels. Cells were maintained at physO2 levels by culturing in a Sanyo “Tri-Gas” MCO-175M O2/CO2 incubator (Sanyo Scientific) in which gas-phase oxygen tension is maintained at 5% oxygen by continuous injection of appropriate amounts of medical-grade N2. Cells maintained at atmosO2 levels (20% O2) by culturing in a standard incubator equilibrated with air (no nitrogen injection). CO2 levels were maintained at 5% in both incubators.
PBMCs.
PBMCs from healthy individuals were isolated from blood drawn immediately before initiation of the experiment (informed consent was obtained before the draw). The blood was drawn into heparin-containing tubes (Vacutainer; Becton Dickinson). PBMCs were isolated by gradient centrifugation on Ficoll-Hypaque (Amersham Pharmacia). RPMI 1640 medium used to isolate and resuspend PBMCs at all stages was equilibrated for >12 h at the oxygen level at which the PBMCs were ultimately cultured.
Recombinant HIV-1 Tat.
Recombinant HIV-1 Tat protein and the Cys to Ser mutant Tat proteins were obtained from J.R. Endotoxin levels were shown to be <8 units/ml.
AlexaFluor-Conjugated Tat Protein.
Tat protein was conjugated to the AlexaFluor-488 carboxylic-acid succinimidyl ester (Molecular Probes/Invitrogen) according to the manufacturer's protocol for conjugation of Alexa dyes to proteins. Slight modifications were made to adjust for the low molecular weight of the protein. The conjugated Tat was separated from the unconjugated dye by centrifugation through a separation filter with a molecular mass cutoff of 2 kDa (Millipore).
p24 ELISA Kit.
HIV-1 p24 levels in the culture supernatant were determined by using a Beckman Coulter kit for quantitative measurement of HIV-1 p24 antigen (EIA assay) according to the manufacturer's instructions. Briefly, the supernatants from cultures were serially diluted in 2-fold steps, as were the negative controls and the positive (purified p24 antigen) provided by the manufacturer. The samples were then added to a 96-well plate precoated with anti-p24, and the plate was sealed and incubated for 60 min at 37°C. After washing, biotin-labeled anti-p24 antibodies were added, followed by enzyme-coupled Streptavidin and the enzyme substrate (with appropriate washing between each step). Absorbance was measured at 450 nm in a Labsystem Multiskan RC (Fisher). A standard curve was constructed from the values obtained for dilutions of the purified p24 antigen and was used to translate supernatant p24 per ml levels from OD values to pg/ml.
High-Dimensional (Hi-D) FACS Staining and Analysis.
Antibodies detecting cell-surface markers (CD3, CD4, CD8, CD45RA, CD45RO, CD62L, and CD11a) that distinguish T cell, B cells, and memory and naïve T cell subsets were produced in our laboratory or obtained from BD PharMingen or Invitrogen. Fluorochrome conjugates were obtained as such from the manufacturer or conjugated according to the protocols available at www.drmr.com/abcon/index.html. Briefly, PBMCs were washed and resuspended in media containing probenecid (Sigma–Aldrich) and FCS (pH 7.4) and then stained with monochlorobimane (MCB; Molecular Probes/Invitrogen) for 20 min at room temperature. Cells were then washed, chilled, and stained with a mixture of fluorophore-conjugated antibodies against cell-subtype markers for 15 min. Propidium iodide (Invitrogen) exclusion was used for dead cell exclusion. Finally, cells were resuspended in staining media with 0.4% formaldehyde and analyzed with the Stanford Shared FACS Facility Flasher-II Hi-D FACS, which uses Becton Dickenson FACS DiVa electronics and software for data collection. Data were analyzed with FlowJo software (Tree Star).
Carboxyfluorescein Diacetate Succinimidyl-Ester (CFDA-SE) Proliferation Assay.
Human PBMCs were stained with CFDA-SE (referred to as CFSE) following a previously described procedure (30) with some modifications. Briefly, PBMCs were suspended at 106 cells per ml in serum-free RPMI medium 1640 and stained with 0.5 μM CFDA-SE for 10 min at 37°C. The reaction was terminated by the addition of a 3-fold excess volume of RPMI medium 1640 with 10% FCS. After two washes, the cells were resuspended at 106 cells per ml in RPMI 1640 with 10% FCS and cultured as indicated. CFSE associated with the cells at the end of the culture period was determined by FACS.
Cell Counts and Identification of Viable Cells.
The numbers of cells in the cultures were determined by FACS using the BD Trucount beads according to the manufacturer's instructions. Cell viability was determined either by MCB stain or Invitrogen live/dead marker.
In Vitro HIV Infection.
PBMCs isolated immediately after blood draw were cultured with PHA/IL-2 (2.5 μg/ml and 50 units/ml IL-2, respectively) or 5 μg/ml Tat protein for the period and at the indicated oxygen levels. For infection, the cultured cells were harvested, washed, resuspended, and cultured with 1,000 IC-50 of the HIV (LAI-1) for 3 h. They were then washed, resuspended, and cultured with Tat or IL-2 as indicated. After 6 days, culture supernatants were obtained, and the accumulated p24 was measured by ELISA with a kit obtained from Beckman Coulter.
Statistical Analysis.
Analyses of FACS data, including calculations of absolute cell numbers, cell division, and cell proliferation indices, were performed by using the FlowJo software (Tree Star). Statistical analyses were performed with the JMP statistical software package (SAS Institute).
Acknowledgments.
We thank Dr. David Katzenstein for making it possible for us to conduct our studies in the Stanford Center for AIDS Research facilities; Dr. David Parks, Dr. James Tung, and the Stanford Shared FACS Facility for expert flow-cytometry support; Ometa Herman and Megan Philips for excellent technical assistance; and Claudia Weber and John Mantovani for administrative support. We are also very fortunate to have had advice from Jon Warren and María García-Moll of the Reagent Resource Support Program for AIDS Vaccine Development (National Institute of Allergy and Infectious Diseases, National Institutes of Health). This work was supported by National Institutes of Health Grant AI 064104.
Footnotes
The authors declare no conflict of interest.
References
- 1.Atkuri KR, Herzenberg LA, Herzenberg LA. Culturing at atmospheric oxygen levels impacts lymphocyte function. Proc Natl Acad Sci USA. 2005;102:3756–3759. doi: 10.1073/pnas.0409910102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkuri KR, Herzenberg LA, Niemi AK, Cowan T, Herzenberg LA. Importance of culturing primary lymphocytes at physiological oxygen levels. Proc Natl Acad Sci USA. 2007;104:4547–4552. doi: 10.1073/pnas.0611732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldwell CC, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 4.Carswell KS, Weiss JW, Papoutsakis ET. Low oxygen tension enhances the stimulation and proliferation of human T lymphocytes in the presence of IL-2. Cytotherapy. 2000;2:25–37. doi: 10.1080/146532400539026. [DOI] [PubMed] [Google Scholar]
- 5.Haddad H, Papoutsakis ET. Low oxygen tension and autologous plasma enhance T-cell proliferation and CD49d expression density in serum-free media. Cytotherapy. 2001;3:435–447. doi: 10.1080/146532401317248045. [DOI] [PubMed] [Google Scholar]
- 6.Haddad H, Windgassen D, Ramsborg CG, Paredes CJ, Papoutsakis ET. Molecular understanding of oxygen-tension and patient-variability effects on ex vivo expanded T cells. Biotechnol Bioeng. 2004;87:437–450. doi: 10.1002/bit.20166. [DOI] [PubMed] [Google Scholar]
- 7.Westendorp MO, et al. HIV-1 Tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulow K, et al. HIV-1 trans-activator of transcription substitutes for oxidative signaling in activation-induced T cell death. J Immunol. 2005;174:5249–5260. doi: 10.4049/jimmunol.174.9.5249. [DOI] [PubMed] [Google Scholar]
- 9.Westendorp MO, et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 10.Krammer PH, Kaminski M, Kiessling M, Gulow K. No life without death. Adv Cancer Res. 2007;97:C111–C138. doi: 10.1016/S0065-230X(06)97005-5. [DOI] [PubMed] [Google Scholar]
- 11.Gallo RC. Tat as one key to HIV-induced immune pathogenesis and Tat (correction of Pat) toxoid as an important component of a vaccine. Proc Natl Acad Sci USA. 1999;96:8324–8326. doi: 10.1073/pnas.96.15.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- 13.Albini A, Barillari G, Benelli R, Gallo RC, Ensoli B. Angiogenic properties of human immunodeficiency virus type 1 Tat protein. Proc Natl Acad Sci USA. 1995;92:4838–4842. doi: 10.1073/pnas.92.11.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ensoli B, et al. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakash O, et al. Human Kaposi's sarcoma cell-mediated tumorigenesis in human immunodeficiency type 1 tat-expressing transgenic mice. J Natl Cancer Inst. 2000;92:721–728. doi: 10.1093/jnci/92.9.721. [DOI] [PubMed] [Google Scholar]
- 16.Mattapallil JJ, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 17.Herce HD, Garcia AE . Molecular dynamics simulations suggest a mechanism for translocation of the HIV-1 TAT peptide across lipid membranes. Proc Natl Acad Sci USA. 2007;104:20805–20810. doi: 10.1073/pnas.0706574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardarelli F, Serresi M, Bizzarri R, Giacca M, Beltram F. In vivo study of HIV-1 Tat arginine-rich motif unveils its transport properties. Mol Ther. 2007;15:1313–1322. doi: 10.1038/sj.mt.6300172. [DOI] [PubMed] [Google Scholar]
- 19.Park J, et al. Mutational analysis of a human immunodeficiency virus type 1 Tat protein transduction domain which is required for delivery of an exogenous protein into mammalian cells. J Gen Virol. 2002;83:1173–1181. doi: 10.1099/0022-1317-83-5-1173. [DOI] [PubMed] [Google Scholar]
- 20.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24:247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- 21.Marchio S, et al. Cell surface-associated Tat modulates HIV-1 infection and spreading through a specific interaction with gp120 viral envelope protein. Blood. 2005;105:2802–2811. doi: 10.1182/blood-2004-06-2212. [DOI] [PubMed] [Google Scholar]
- 22.Katsikis PD, et al. Activation-induced peripheral blood T cell apoptosis is Fas independent in HIV-infected individuals. Int Immunol. 1996;8:1311–1317. doi: 10.1093/intimm/8.8.1311. [DOI] [PubMed] [Google Scholar]
- 23.Ehret A, et al. Resistance of chimpanzee T cells to human immunodeficiency virus type 1 Tat-enhanced oxidative stress and apoptosis. J Virol. 1996;70:6502–6507. doi: 10.1128/jvi.70.9.6502-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattapallil JJ, et al. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J Exp Med. 2006;203:1533–1541. doi: 10.1084/jem.20060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattapallil JJ, Hill B, Douek DC, Roederer M. Systemic vaccination prevents the total destruction of mucosal CD4 T cells during acute SIV challenge. J Med Primatol. 2006;35:217–224. doi: 10.1111/j.1600-0684.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura Y, et al. Resting naïve CD4+ T cells are massively infected and eliminated by X4-tropic simian-human immunodeficiency viruses in macaques. Proc Natl Acad Sci USA. 2005;102:8000–8005. doi: 10.1073/pnas.0503233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezza G, et al. The presence of anti-Tat antibodies is predictive of long-term nonprogression to AIDS or severe immunodeficiency: Findings in a cohort of HIV-1 seroconverters. J Infect Dis. 2005;191:1321–1324. doi: 10.1086/428909. [DOI] [PubMed] [Google Scholar]
- 28.Zagury D, Burny A, Gallo RC. Toward a new generation of vaccines: The anti-cytokine therapeutic vaccines. Proc Natl Acad Sci USA. 2001;98:8024–8029. doi: 10.1073/pnas.141224798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zagury JF, et al. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: A rationale for the use of Tat toxoid as an HIV-1 vaccine. J Hum Virol. 1998;1:282–292. [PubMed] [Google Scholar]
- 30.Mannering SI, et al . A sensitive method for detecting proliferation of rare autoantigen-specific human T cells. J Immunol Methods. 2003;283:173–183. doi: 10.1016/j.jim.2003.09.004. [DOI] [PubMed] [Google Scholar]