Abstract
In a variety of species, development of attachment to a caregiver is crucial for infant survival and partly mediated by the endogenous opioids. Functional mu-opioid receptor gene polymorphisms are present in humans (OPRM1 A118G) and rhesus macaques (OPRM1 C77G). We hypothesized that rhesus infants carrying a gain-of-function OPRM1 77G allele would experience increased reward during maternal contact and would, therefore, display increased measures of attachment. We collected behavioral data from rhesus macaques (n = 97) during early infancy and at 6 months of age, across four cycles of maternal separation (4 days) and reunion (3 days). Animals were genotyped for the OPRM1 C77G polymorphism, and the effects of this allele on attachment-related behaviors were analyzed. Infants carrying the G allele exhibited higher levels of attachment behavior during early infancy. During prolonged periods of maternal separation, although infant macaques homozygous for the C allele exhibited decreases in their levels of distress vocalization with repeated separation, this response persisted in G allele carriers. The OPRM1 77G allele also affected social preference during reunion. C/G infants spent increasing amounts of time in social contact with their mothers as a function of repeated separation and were less likely to interact with other individuals in the social group, a pattern not observed among infants with the C/C genotype. These findings suggest a role for OPRM1 variation in the expression of attachment behavior in human subjects, especially as a function of separation from the caregiver.
Keywords: genetic, rhesus macaque, mother-infant bond, C77G, A118G
Formation and maintenance of the mother–infant attachment bond is essential to infant survival, not only for the provision of nourishment, but also for protection from injury and predation. In a wide variety of animal species, attachment increases the motivation of an infant to use evolutionarily conserved behavioral strategies to maintain proximity to its mother and to gain her attention during periods of separation (1). The separation distress response is an important expression of infant attachment, because cries signal an infant's location, permitting the mother to locate and retrieve it when the two are separated (2). This adaptive response may be especially important during later stages of infancy, when mothers begin to engage in consortships, leaving their infants behind (3). This is also the developmental phase during which infants become more mobile and independent and, therefore, begin to wander from their mothers to play and explore.
The stress-mitigating and rewarding effects that an infant experiences while interacting with its mother are thought to be mediated in part by release of the endogenous opioids (4, 5), and work in rodents, dogs, and primates suggests that the diminution in endogenous opioid system activation during mother–infant separation is important in eliciting a distress vocalization response (6–8). In fact, it has been stated that separation distress may reflect a state of “endorphin withdrawal” (9). Studies performed during mother–infant separation show that nonsedating doses of morphine reduce separation distress vocalization and that mu-opioid receptor blockade with naloxone produces opposite effects (6, 9). During periods of mother–infant reunion, when mothers attend to their infants, groom them, cradle them, and keep them in close physical contact, the endogenous opioid system is said to be reactivated, reinforcing the established attachment bond. Consistent with this, pharmacologic studies demonstrate that the degree to which an infant clings to its mother is decreased with nonsedating doses of morphine and, conversely, potentiated with mu-opioid receptor blockade (10, 11). Effects of mu-opioid receptor activation and blockade on these behavioral responses have been demonstrated in a wide variety of species, including rhesus macaques (6, 8, 11).
Four distinguishing characteristics of attachment have been described for primates: secure base (using the attachment figure as a secure base from which to explore), safe haven (returning to the attachment figure for comfort/safety in the face of danger), proximity maintenance (maintaining physical proximity to the attachment figure), and separation distress (distress in the actual or virtual absence of the attachment source) (2). It is known that both the strength and the quality of the attachment bond differ among human subjects (9). Like humans, rhesus macaques vary in the quality of their attachment relations (3, 12), and social attachment is partially attributable to heritable factors (13). This suggests that genetic factors may contribute to individual variation in attachment behavior during infancy, but no specific loci contributing to this variation have yet been identified. Experimental disruption of the Oprm1 gene has been shown to prevent the emission of separation-induced vocalization in mice (8). However, to date, there have not been any studies examining whether spontaneous variation in the OPRM1 gene influences the quality of attachment in a primate. Such findings could potentially have implications in terms of both vulnerability to psychopathology and natural selection.
In both humans and rhesus macaques, polymorphisms in the OPRM1 gene (OPRM1 A118 G and C77G, respectively) cause amino acid substitutions in the N-terminal arm of the receptor, conferring increased affinity for β-endorphin in vitro (14, 15). Although the exact mechanism remains unclear (16, 17), in vivo data support a gain-of-function role for these variants, as shown, for example, by increased alcohol-induced stimulation, an effect mediated through release of endogenous opioids (18–20). The aim of the present study was to examine whether the rhOPRM1 77G allele would influence attachment behavior in rhesus macaque infants. We predicted that infants carrying this gain-of-function variant would experience increased reward from being in contact with their mothers, resulting in increased measures of attachment and a higher degree of distress during maternal separation. Because of the role of the endogenous opioids in the reinforcement of the attachment bond during reunion, we wanted to test whether this variant influenced attachment behavior as a function of repeated mother–infant separations and reunions. As part of the weaning process, infants were subjected to four cycles of 4-day-long maternal separation, with 3 days of reunion in between. We examined the effect of this variant on the frequency of distress vocalization during periods of maternal separation and on preference for maternal contact during periods of reunion.
Results
The frequency of the G allele was 15%, and genotype frequencies did not deviate from Hardy–Weinberg equilibrium. Consistent with prior studies, homozygosity for the G allele was uncommon (15). Because preliminary analyses demonstrated no difference in outcomes between G/G (n = 1) and C/G animals (n = 26), these were collapsed into a “G allele carrier” group for the all analyses performed.
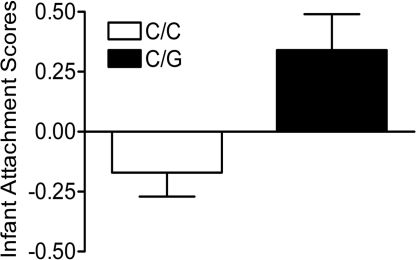
Extraction of Varimax rotated orthogonal factors from behavioral data collected during infancy identified three factors with eigenvalues >1.0, which together explained 58.0% of the variance. Based on factor loadings shown in Table 1(positive loading for social contact-mother and mutual break), one of these was identified as “attachment.” OPRM1 77G carriers scored significantly higher on this factor than 77C homozygous individuals [n = 27 vs. 69; F(1,94) = 5.6, P = 0.017; Fig. 1]. The proportion of variance in attachment behavior accounted for by OPRM1 C77G genotype, measured as partial eta2, was 5.7%. Scores on the other two factors, “gregariousness” and “assertiveness,” were not influenced by genotype (not significant; data not shown).
Table 1.
Behavioral dimensions generated by factor analysis
| Factor | Eigenvalue | Behavior | Factor loading |
|---|---|---|---|
| 1. Gregariousness | 3.684 | Explore environment | 0.553 |
| Locomotion | 0.650 | ||
| Social contact-other | 0.411 | ||
| Mutual ventral | −0.914 | ||
| 2. Assertiveness | 1.714 | Explore environment | 0.520 |
| Vocalization | 0.845 | ||
| Aggression | 0.867 | ||
| 3. Attachment | 1.590 | Social contact-mother | 0.810 |
| Mutual break | 0.834 |
Behavioral dimensions generated by using factor analysis. Behaviors collected over the 18th through the 24th weeks of life were averaged, and extraction of Varimax rotated orthogonal factors identified three factors with eigenvalues >1.0 that together explained 58.0% of the variance. Based on the factor loadings, one of these was identified as infant attachment.
Fig. 1.
Infant attachment as a function of the OPRM1 C77G genotype. Attachment was measured as factor scores (mean ± SEM) extracted from a range of behaviors scored during a critical developmental phase (18–24 months of age) as described in Table 1. Scores were significantly higher in 77G allele carriers [n = 27 vs. 69; F(1,94) = 5.6, P = 0.017]. The proportion of variance in attachment behavior accounted for by the OPRM1 C77G genotype, measured as partial eta2, was 5.7%.
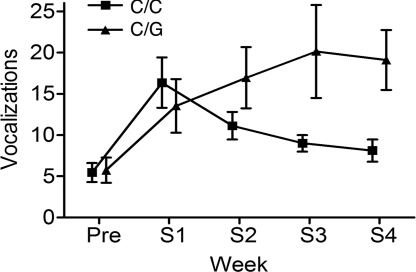
Acute separation distress vocalization [emitted on the first day of separation and known to be a universal response among both free-ranging and captive primates (21–23)] did not show a consistent pattern of differences (not significant; data not shown). In contrast, during protracted periods of separation, there was a main effect of genotype on the level of vocalization [n = 25 vs. 64; F(1,88) = 5.2, P = 0.025] and a differential temporal course of the response over successive separations between genotypes [genotype × time interaction, F(3,246) = 4.2, P = 0.007]. Whereas vocalization among infants homozygous for the major OPRM1 77C allele declined over separation cycles, high levels of distress vocalization persisted with repeated separation among carriers of the 77G allele (Fig. 2). The OPRM1 C77G genotype and its interaction with separation exposure accounted for 9.4% of the observed variance in vocalization.
Fig. 2.
Infant distress vocalization as a function of the OPRM1 C77G genotype (C/C, ■, or C/G, ▴). Values are mean frequencies of vocalizations (± SEM) during the chronic phase of four consecutive (S1–S4) cycles of mother–infant separation. 77G carriers showed overall higher vocalization [main effect of genotype: n = 25 vs. 64; F(1,88) = 5.2, P = 0.025], and had a differential temporal course of the response over successive separations [genotype × time interaction, F(3,246) = 4.2, P = 0.007]. The OPRM1 C77G genotype and its interaction with separation exposure accounted for 9.4% of the observed variance in vocalization.
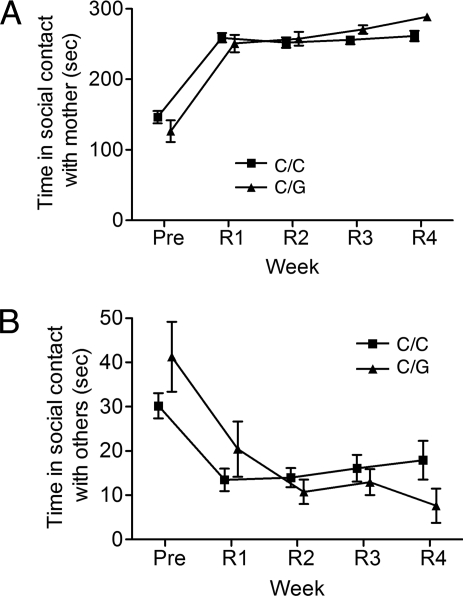
The OPRM1 77G allele also affected social preference during reunion (Fig. 3). In general, the time that an infant spent with its mother during reunion was much higher than that observed before the first mother–infant separation. When data collected during mother–infant reunion were considered over repeat separation cycles and as a function of genotype, G allele carriers exhibited a different course of behavior than did 77C homozygotes [n = 26 vs. 64; OPRM1 genotype × time interaction, F(3,252) = 3.2, P = 0.02]. Whereas C/G infants exhibited progressive increases in the amount of time spent in contact with their mothers with repeated separation, those with the C/C genotype did not (Fig. 3A). The interaction of the OPRM1 genotype with time accounted for 5.6% of the variance. Among G allele carriers, there was also a decrease in the amount of time spent with other group members over repeated separation–reunion cycles [Fig. 3B; F(3,243) = 3, P = 0.03], demonstrating that the increased time spent in maternal contact was not reflective of a nonspecific increase in sociability and, therefore, was suggestive of increased preference for maternal contact.
Fig. 3.
Social preference mother-infant reunion as a function of the OPRM1 C77G genotype (C/C, ■, or C/G, ▴). Values are given as the average duration of time that an infant spent in social contact with its mother or with other members of the social group (sec ± SEM) in a 300-sec scoring session during each of four reunion cycles (R1–R4). (A) Social Contact-Mother: 77G allele carriers had a different course of behavior over separation cycles than did 77C homozygotes [n = 26 vs. 64; OPRM1 genotype x time interaction, F(3,252) = 3.2, P = 0.02]. The interaction of OPRM1 genotype with time accounted for 5.6% of the variance in this behavior. (B) Social Contact-Other: Among carriers of the G allele, there was a decrease in the amount of time spent with other group members over repeated separation–reunion cycles [F(3,243) = 3, P = 0.03].
Discussion
A child's fear of being left alone is among those referred to as the “irrational fears of childhood.” Bowlby (2) used an ethological approach to argue that such fears could be highly adaptive, stating that “all accounts agree on the intensity of protest exhibited whenever a baby primate loses its mother figure and the intensity of distress that follows when she cannot be found. All agree, too, on the intensity of clinging that occurs after the two are reunited.” We have shown that a functional genetic variant in the mu-opioid receptor gene (OPRM1 C77G) is associated with increases in such behaviors in infant macaques.
We found that the OPRM1 77G allele was associated with the attachment behavioral dimension, assessed at a developmental time point at which rhesus infants are weaned from their mothers and, as such, begin to wander, play, and explore. We also demonstrated that these infants exhibit more of a protest response when separated from their mothers. Although most infants decreased their levels of vocalization during protracted periods of maternal separation, carriers of the OPRM1 77G allele were more likely to persist in this response, even after repeated separation exposures. The fact that rhesus macaque infants with the C/G genotype persist, despite the absence of maternal responding, represents a behavior that could be highly adaptive, especially during periods of stress or when food resources are limited. Under these conditions, mothers may need to frequently leave infants behind for protracted periods of time to forage for food or to engage in consortships or aggressive encounters with conspecifics (3). Proximity maintenance after mother–infant reunion would also be predicted to confer selective advantage, because, during times of stress or social conflict, when mother–infant separation may become more common, OPRM1 77G allele carriers would be expected to remain in close proximity to their mothers, making them less vulnerable to injury, predation, or aggression from conspecifics.
We report a consistent behavioral pattern indicative of increased attachment in rhesus macaques carrying a variant in the OPRM1 gene that appears to confer increased affinity for β-endorphin. The effect size contributed by this variant is in the range expected for complex, polygenic behavioral traits, and the combined use of spontaneous genetic variation and an experimental challenge seems to be advantageous for detecting this type of effect. The OPRM1 variation accounted for ≈5–10% of the variance in the different attachment behaviors observed. Notably, frequency-based association studies have a limited power to detect effect sizes at this level, and therefore require considerably larger sample sizes (24). Our study adds to a line of experimental studies in humans and nonhuman primates (25–28) that illustrate the gain in power that comes from measuring quantitative traits under experimental conditions and applying general linear models to test an a priori hypothesis.
Several studies have demonstrated that cases exist in which genetic variants that are functionally similar to those that exist in humans are present in macaques (15, 18, 27, 29). This affords us the opportunity to examine how genetic variation may influence complex behavioral traits in subjects living in a controlled environment (30). Similar to the rhOPRM1 C77G, a polymorphism encoding an amino acid substitution in the N-terminal arm of OPRM1 has independently arisen in humans (OPRM1 A118G) (14). Although its consequences at the molecular level remain to be fully elucidated (14, 16, 17, 31, 32), recent in vivo data support the notion that the OPRM1 118G is indeed a gain-of-function variant, because it is associated with increased pain threshold, increased sensitivity to euphorogenic effects of addictive drugs, increased cortisol response after challenge with the opioid antagonist naltrexone, and higher rates of therapeutic response to the opioid antagonist naltrexone in alcoholism (19, 20, 33–35). Findings in rhesus macaques suggest that rhOPRM1 77G has a gain-of-function role as well (15, 18), indicating the human and rhesus OPRM1 variants are functionally similar. Based on the present data, we cannot exclude the possibility that another variant in LD with OPRM1 77G contributes to the functional effects observed here. However, given the functional parallels between the human and the rhesus variants, the most parsimonious explanation for their similar functional role in a range of behavioral and endocrine phenotypes is a direct functional effect of these markers. Because these two variants confer similar functional effects and, further, both are observed at relatively high frequencies, it might be hypothesized that they have evolved as result of similar selective pressures in the two species. Data to directly address this hypothesis are not presently available.
Infants carrying the OPRM1 77G allele exhibited stronger attachment to their mothers in baseline conditions. Unlike other infants, who spent time playing, exploring, or interacting with other members of the social group, these infants remained in close contact with their mothers. The more intense distress responses to separation and greater contact time with their mothers during reunion, which emerged among G allele carriers after repeated mother–infant separation, shares some characteristics with what has been proposed as an anxious/ambivalent form of insecure attachment in human children (9). Because of the involvement of the reward systems in the development of attachment, it has been proposed that social attachment could be considered an addictive disorder (36), and “insecure early attachment” is believed to be a risk factor for substance abuse and addiction (37, 38). Of interest, we have shown there to be increased levels of alcohol preference in rhesus monkeys carrying the OPRM1 77G allele (15, 18), and OPRM1 variation has been linked to various addictive disorders in human populations (25). Our findings may, therefore, lend support to ethological arguments in favor of attachment theory and its association with psychopathology, not necessarily in terms of causality, but by pointing to common underlying genetic or neurobiological substrates. They also highlight how traits that could have theoretically conferred selective advantage at some point in the evolutionary history of humans can increase risk for addictive disorders in modern society.
Bowlby (2) proposed that “a child's safety lay in proximity to its mother and that the behaviors of both child and mother must have adapted to maintain it.” He also stated that, despite the fact that such behaviors have no obvious role in improving infant survivability in contemporary society, they persist. The results reported here are in agreement with what would be predicted by the established role for opioid transmission in mediation of attachment behavior (4–8, 39, 40), together with the more recent suggestion of rhOPRM1 77G as a gain-of-function variant (15, 18). It may be that the OPRM1 A118G variant influences the development of attachment in human subjects and raises the question of whether, by influencing attachment behavior, the OPRM1 C77G and A118G polymorphisms could have conferred selective advantage at some point in the evolutionary histories of both species.
Materials and Methods
Early Rearing and Mother–Infant Separation.
All procedures were approved by the National Institutes of Health Animal Care and Use Committee. Infant rhesus macaques (total n = 97; number of subjects available for the respective analyses is given together with each result) were reared in indoor–outdoor enclosures with their mothers in mixed-sex social groups containing two adult males and six to eight adult females with their infant offspring (National Institutes of Health Animal Center, Poolesville MD). The sample included infants from seven birth-year cohorts housed at the facility between 1995 and 2001.
At ≈6 months of age, infants were separated from their mothers for 4 days, with 3 days of reunion between each of four repetitions of the separation procedure. To avoid potential confounds of stress resulting from exposures to a novel environment or social isolation, infants remained in the home cage with the social group (consisting of adult females, males, and infants) for the duration of study, and their mothers were removed from the enclosure. No maternal siblings or older juveniles were present in any infant's social group. Separation was initiated between 12 and 1 p.m. on Monday of each week. The first day (Monday) of each separation week was designated as the “acute” phase of mother–infant separation. The second through the fourth days (Tuesday through Thursday) were designated as the “chronic” phase of mother–infant separation (see below). After each separation week, infants were reunited with their mothers for 3 days (Friday through Sunday).
Behavioral Observations.
Focal behavioral scoring was performed in 5-min sessions. Infants were scored two times a week in the social group for the first 24 weeks of life and then once daily for the 2 weeks preceding the first mother–infant separation (baseline). Three observations were made on the first day of mother–infant separation: two observations were made immediately after separation, and another during the second hour of separation (averaged as acute separation). Two observations were made each day on the second, third, and fourth days of mother–infant separation (averaged as chronic separation). Infants were then scored during mother–infant reunion, twice on the first day of reunion (Friday, averaged as reunion).
Definitions for behaviors that were recorded are listed in supporting information (SI) Table S1 (environmental exploration, locomotion, mutual ventral, mutual break, passive, self-direct, social contact-mother, social contact-other, play, vocalization, and aggression). All behaviors were scored in duration, with the exception of vocalizations, which were scored in frequency. Behavioral observations were made by multiple observers, who achieved interobserver reliabilities of at least 85%. In rare instances, behaviors during either separation or reunion were not scored because of a necessity to remove an animal from the group for medical observation or because observers could not reliably visualize the infant to score it among the other members of the social group.
Genotyping.
By using standard extraction methods, DNA was isolated from whole blood, collected from the femoral vein under ketamine anesthesia (15 mg/kg, i.m.). Genotyping was performed by using the procedure modified from Miller et al. (15). A portion of OPRM1 exon 1 was amplified from 25 ng of genomic DNA with flanking oligonucleotide primers museekf1 (5′-TCA GTA CCA TGG ACA GCA GCG CTG TCC CCA CGA A-3′) and museekr1 (5′-GTC GGA CAG GTT GCC ATC TAA GTG-3′) in 15 μl reactions by using AmpliTaq Gold and 2.5 mM MgCl2 according to the manufacturer's instructions (Invitrogen). Amplifications were performed on a PerkinElmer thermocycler (9700) with one cycle at 96°C followed by 30 cycles at 94°C/15 sec, 56°C/15 sec, 72°C/30 sec, and a final 3-min extension at 72°C. Restriction digest by Fnu4HI (New England Biolabs) was then performed with 0.5 μl of PCR product in a total volume of 20 μl for 2 h at 37°C. Samples were separated by electrophoresis on 10% polyacrylamide gels, and the C and G alleles were identified by direct visualization after ethidium bromide staining.
Data Analysis.
Because scores of attachment-related behaviors during baseline were intercorrelated, potentially invalidating results of multiple independent analyses, we performed factor analysis to reduce the dimensionality of the data. By using this approach, we generated behavioral dimensions from behaviors averaged over the 18th through the 24th weeks of life, a developmental stage at which both captive and free-ranging infants are being weaned by their mothers and begin to spend much of their time interacting with other members of the social group, playing, and exploring. We used a principal component extraction to construct independent, orthogonal behavioral dimensions from the data, followed by standard Varimax normalized rotation aimed at minimizing loading of individual items on multiple factors. Behavioral factor scores were then used as dependent variables in ANOVA to test the hypothesis that infants with different genotypes would exhibit differences in one or more behavioral dimensions related to social interactions.
We also wanted to determine whether there were associations of the OPRM1 77G allele with various behavioral responses to repeat cycles of maternal separation and reunion. Four objective behavioral measures (vocalization, clinging, variation in social preference, and proximity seeking for the attachment source) have been established for distinguishing quality and strength of attachment in rhesus monkey infants (41). Because vocalization during separation is an opioid-dependent response known to signify attachment across species, we selected this measure a priori as the dependent variable for our analysis of separation response. We used repeated measures ANOVA to examine the effect of OPRM1 C77G genotype on distress vocalization during both the acute (agitation) and chronic (adaptation) phases of four repeated maternal separation cycles. First, we performed factor analysis to determine the underlying data structure. The objective was to examine whether acute (i.e., emitted on day 1 of each separation), and chronic (i.e., emitted during days 2–4 of each separation cycle) vocalizations reflect a common, or separate behavioral dimensions. By using all measures of vocalization available, a principal component extraction, followed by Varimax rotation, identified two factors with eigenvalues >1.0. Together, these explained 60.0% of the variance. All acute vocalizations loaded >0.7 onto the first factor, which accounted for 36.6% of the variance. Chronic vocalizations loaded 0.4–0.8 on the second factor, which accounted for 23.0% of the variance. Because of this, acute and chronic vocalizations were analyzed separately.
For behaviors collected during mother–infant reunion, we wanted to examine the effects of genotype on behaviors relating to proximity seeking and social preference. We used repeated measures ANOVA to determine whether the 77G allele contributed to the time infants spent in social contact with their mothers. Because we were specifically interested in associations of this variant with the strength of the mother–infant bond (i.e., preference for the mother), we also tested whether the amount of time infants spent interacting socially with other members of the social group varied as a function of the G allele.
The frequency of the G allele was 15%, and genotype frequencies did not deviate from Hardy–Weinberg equilibrium. Because preliminary analyses demonstrated no difference in outcomes between G/G (n = 1) and C/G animals (n = 26), these were collapsed into a “G allele carrier” group for all of the analyses performed. Sex and preseparation baseline behaviors were evaluated as covariates and kept in the model if they reduced residual variance. In instances in which there was nonhomogeneity of variance, analyses were repeated on rank-transformed data, but yielded very similar results on both approaches. A priori power calculation determined that with the available sample size, the repeated measures analyses would have a power of >0.70 to detect a small effect size (Cohen d = 0.2) at α = 0.05. Analyses were performed by using Statistica Statistical software (Statsoft).
The average identity by descent (IBD) for the animals included in the study, based on the known pedigrees within the colony, has previously been determined to be <1.45%. This indicates that two randomly selected macaques would share only 1.45% of their genes by descent (approximately equivalent to a degree of relationship that is observed between second cousins once removed and third cousins). This demonstrates that most pairs of individuals have a low degree of relationship, approximating that observed in some human populations of study (27). To corroborate this for the present study, and exclude a possible confound of relatedness, we analyzed eight additional, functionally unrelated markers, six of which had a comparable number of animals genotyped. None of these markers displayed a pattern that bore any resemblance to that shown by OPRM1 77G, i.e., a consistent difference in the various categories of attachment-related behaviors. This remained true after controlling for sex differences in this response. For these markers, among all uncorrected P values generated for individual behaviors and time points, all but one exceeded 0.35, a distribution indistinguishable from that predicted to result from random variation. Because the IBD was sufficiently low, standard statistical procedures were applied for testing the association of OPRM1C77G with attachment behavior.
Acknowledgments.
We thank Karen Smith for assistance in the preparation of this manuscript and the research and animal care staff at the National Institutes of Health Animal Center for their assistance in data collection. This work was supported by the National Institute of Child Health and Human Development and National Institute on Alcohol Abuse and Alcoholism Intramural Research Programs.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710225105/DCSupplemental.
References
- 1.Hinde RA. In: Annals of Child Development. Vasta R, editor. Greenwich, CT: JAI Press; 1989. pp. 251–285. [Google Scholar]
- 2.Bowlby J. Attachment and Loss. New York: Basic Books; 1969. [Google Scholar]
- 3.Berman CM, Rasmussen KLR, Suomi SJ. Responses of free-ranging rhesus-monkeys to a natural form of social separation. 1. Parallels with mother-infant separation in captivity. Child Dev. 1994;65:1028–1041. [PubMed] [Google Scholar]
- 4.Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105:e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- 5.Weller A, Feldman R. Emotion regulation and touch in infants: The role of cholecystokinin and opioids. Peptides. 2003;24:779–788. doi: 10.1016/s0196-9781(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 6.Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- 7.Kehoe P, Blass EM. Opioid-mediation of separation distress in 10-day-old rats: Reversal of stress with maternal stimuli. Dev Psychobiol. 1986;19:385–398. doi: 10.1002/dev.420190410. [DOI] [PubMed] [Google Scholar]
- 8.Moles A, Kieffer BL, D'Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 9.Ainsworth MDS. Patterns of Attachment A Psychological Study of the Strange Situation. Hillsdale, NJ: Lawrence Erlbaum Associates; 1978. [Google Scholar]
- 10.Kalin NH, Shelton SE, Lynn DE. Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology. 1995;20:735–742. doi: 10.1016/0306-4530(95)00023-2. [DOI] [PubMed] [Google Scholar]
- 11.Schino G, Troisi A. Opiate receptor blockade in juvenile macaques: Effect on affiliative interactions with their mothers and group companions. Brain Res. 1992;576:125–130. doi: 10.1016/0006-8993(92)90617-i. [DOI] [PubMed] [Google Scholar]
- 12.Maestripieri D. Similarities in affiliation and aggression between cross-fostered rhesus macaque females and their biological mothers. Dev Psychobiol. 2003;43:321–327. doi: 10.1002/dev.10143. [DOI] [PubMed] [Google Scholar]
- 13.Segal NL, MacDonald KB. Behavioral genetics and evolutionary psychology: Unified perspective on personality research. Hum Biol. 1998;70:159–184. [PubMed] [Google Scholar]
- 14.Bond C, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proc Natl Acad Sci USA. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller GM, et al. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: Association with stress response and aggression. Mol Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- 16.Kroslak T. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Wang DX, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 18.Barr CS, et al. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- 19.Ray LA. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: A double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- 20.Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- 21.Jensen GD, Tolman CW. Mother-infant relationship in monkey, macaca-nemestring—Effect of brief separation and mother-infant specificity. J Comp Physiol Psychol. 1962;55:131–136. doi: 10.1037/h0048498. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman IC, Rosenblu LA. Depression in infant monkeys separated from their mothers. Science. 1967;155:1030–1031. doi: 10.1126/science.155.3765.1030. [DOI] [PubMed] [Google Scholar]
- 23.van Lawick-Goodall J. The behavior of free-living chimpanzees in the Gombe Stream Reserve. Anim Behav Monogr. 1968;1:161–311. [Google Scholar]
- 24.Goldman D, Oroszi G, Ducci F. The genetics of addictions: Uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 25.Zubieta JK, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 26.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 27.Barr CS, et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci USA. 2004;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barr CS, et al. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- 29.Newman TK, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Barr CS, Goldman D. Non-human primate models of inheritance vulnerability to alcohol use disorders. Addict Biol. 2006;11:374–385. doi: 10.1111/j.1369-1600.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 31.Befort K, et al. A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling. J Biol Chem. 2001;276:3130–3137. doi: 10.1074/jbc.M006352200. [DOI] [PubMed] [Google Scholar]
- 32.Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89:553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- 33.Fillingim RB, et al. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. J Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Oslin DW, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 35.Wand GS, et al. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 36.Insel TR. Is social attachment an addictive disorder? Physiol Behav. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- 37.McNally AM, Palfai TP, Levine RV, Moore BM. Attachment dimensions and drinking-related problems among young adults—The mediational role of coping motives. Addict Behav. 2003;28:1115–1127. doi: 10.1016/s0306-4603(02)00224-1. [DOI] [PubMed] [Google Scholar]
- 38.Vungkhanching M, Sher KJ, Jackson KA, Parra GR. Relation of attachment style to family history of alcoholism and alcohol use disorders in early adulthood. Drug Alcohol Depend. 2004;75:47–53. doi: 10.1016/j.drugalcdep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: Evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–220. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- 40.Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: Opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- 41.Mason WA, Capitanio JP. Formation and expression of filial attachment in rhesus monkeys raised with living and inanimate mother substitutes. Dev Psychobiol. 1988;21:401–430. doi: 10.1002/dev.420210502. [DOI] [PubMed] [Google Scholar]