Abstract
IL-10-secreting regulatory T cell lines specific to glatiramer acetate [poly(Y,E,A,K)n] or poly(Y,F,A,K)n have been established from the enlarged spleen and lymph nodes that result from copolymer treatment of SJL mice in which experimental autoimmune encephalomyelitis was induced by PLP139-151. These CD4+CD25+T cell lines secrete high levels of IL-10 and IL-13 but only small amounts of IL-4 and virtually no TGF-β, IL-17, IL-6, IFN-γ, or TNF-α. Their phenotypes are particularly characterized by the absence of Foxp3 and the presence of two TNFR family members, CD30 and GITR. The lines proliferated specifically to the immunizing copolymers but were autoantigen-nonspecific, in that the same T cell line could suppress autoimmunity induced by three different autoantigens in SJL mice, i.e., PLP139-151(EAE), MBP85-99 (EAE), and bovine peripheral nerve myelin (experimental autoimmune neuritis), indicating they function by bystander suppression.
Keywords: CD30, dendritic cells, glatiramer acetate, IL-13, multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease with a frequency of ≈1/1,000 in Western populations and is thought to be due to an expansion of T cells autoreactive to a myelin protein, e.g., myelin basic protein (MBP), phospholipid protein (PLP), or myelin oligodendrocyte protein. Its animal model, experimental autoimmune encephalomyelitis (EAE), can be induced in appropriate strains of mice or rats by immunization with these proteins or peptides derived from them. The human disease MS and some forms of EAE in mice are relapsing-remitting diseases that can continue for decades with incremental permanent CNS damage after each relapse. Several decades ago, two new therapies were introduced for MS, β-IFN and glatiramer acetate [(GA) Copolymer 1, Cop1, Copaxone, poly(Y,E,A,K)n, YEAK], each of which can reduce the relapse frequency by ≈30%. Although these therapies are valuable, more beneficial therapies are clearly desirable.
GA (earlier called Copolymer 1) is an amino acid copolymer synthesized by random polymerization of the acid anhydrides of its 4-aa constituents. It was originally designed to be a synthetic substitution for MBP as an immunogen but was found instead to ameliorate the disease (1, 2). Extensive laboratory study of its effects on EAE and clinical studies of its effects on MS have led to some understanding of the mechanism(s) by which it suppresses disease (reviewed in ref. 2). MS is genetically linked to HLA-DR2 (DRA/DRB1*1501), and more recently, additional copolymers poly(Y,F,A,K)n and poly(V,W,A,K)n were designed based on the binding motif for peptides to HLA-DR2 (3). These new copolymers were shown to form complexes with HLA-DR2 (DRA/DRB1*1501), to effectively compete with MBP85-99 for binding and for stimulation of T cells, and to have enhanced activity in amelioration of EAE in two different mouse models (PLP139-151-induced EAE in the SJL mouse and MBP85-99-induced EAE in a humanized double-transgenic mouse) using three different administration protocols, termed vaccination, prevention, and treatment (4–7). In the course of these studies, a variety of mechanisms of immunosuppression were described, including competition for binding of autoantigen to MHC proteins (blocking), induction of T cell unresponsiveness (anergy), and stimulation of copolymer-specific splenocytes that produce immunosuppressive cytokines, particularly IL-10 (Th1/Th2 deviation).
Here, we describe copolymer-specific T cell lines (TCL) that can mediate suppression of several different autoimmune diseases and their characterization. These copolymer-specific T cells are a set of IL-10-secreting Tr1-like regulatory T cells that differ from those that have been described (8–11), and that function independently of the immunizing autoantigen. Additional facets of the immunosuppression have been described (12–15) and will be discussed.
Results
Establishment and Properties of PLP139-151 and Copolymer-Specific TCL from SJL Mice.
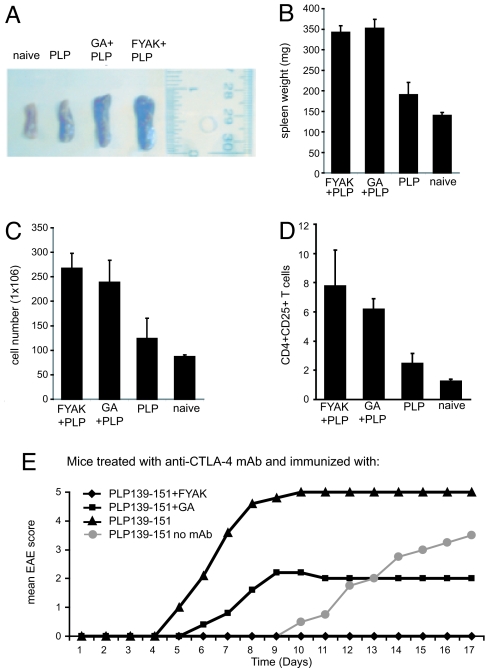
Coimmunization of SJL mice with PLP139-151 and the amino acid copolymers GA (YEAK), FYAK, or VWAK induced a robust immune response, manifest by an increase in spleen size and cell number (including an increase of CD4+CD25+T cells) not seen after immunization with PLP139-151 alone (Fig. 1 A–D). Coimmunization using either complete Freund's adjuvant (CFA) as used in Fig. 1, incomplete Freund's adjuvant (IFA), or mannitol as the vehicle has been shown to ameliorate EAE induced in SJL mice by PLP139-151 or in double-transgenic humanized mice by MPB85-99 (4–7). In addition, these copolymers ameliorated a much more severe disease induced by PLP139-151 in SJL mice pretreated with a mAb to CTLA-4 that normally induces an inhibitory signal (16). In this rapidly progressive model in which disease appeared at day 5 and nearly all mice were dead by day 8 (compared with appearance at days 10–11 and severe illness by day 17 in mice that did not receive mAb), FYAK was again more effective than GA (Fig. 1E).
Fig. 1.
Amelioration of PLP-139–151-induced EAE with amino acid copolymers. (A–D) Effect of amino acid copolymers on spleen size and cellularity. (A) SJL mice were immunized with 75 μg of PLP139-151 with or without amino acid copolymers, 500 μg of GA or FYAK in CFA, followed by i.v. pertussis toxin (200 ng) 1 day later, as described (4–7). Mice were killed on day 20, after which spleens were removed and (A) measured, (B) weighed, and (C) single-cell suspensions prepared and total cell number enumerated. In each of these analyses, P < 0.01 for GA vs. FYAK vs. naïve or PLP139–151. (D) Finally, CD4+CD25+ T cells were enumerated by using FACS analysis. Data are shown as means of triplicates. Bars show SD values. (E) Effects of anti-CTLA-4 mAb on disease course and copolymer therapy. SJL mice were injected i.p. with 200 μg of anti-CTLA-4 mAb in saline and then on the next day, immunized with PLP139-151 alone or with PLP139-151 plus either FYAK or GA in CFA followed by pertussis toxin as in A. Anti-CTLA-4 mAb was again injected i.p. on days 3 and 5. Mean EAE scores for five mice in each group are shown. All mice in the group immunized with PLP139-151 that had received anti-CTLA-4 mAb were dead by day 9 (score 5). P < 0.001 for FYAK or P < 0.01 for GA vs. PLP139-151 at day 7; GA vs. PLP139-151 not significant P = 0.07 after day 9; P < 0.001 for PLP139-151 no mAb vs. PLP139–151 with mAb.
TCL were readily established from splenocytes after immunization of SJL mice with PLP139-151 or with each of the three copolymers, GA(YEAK), FYAK, or VWAK alone. Cell lines were obtained by restimulation of splenocytes in vitro, using 10 μg/ml for each copolymer and for PLP139-151. The GA- and FYAK-induced cell lines were restimulated three to four times at 2-week intervals in vitro and continued to proliferate. Aliquots of cells could be kept frozen after the third restimulation, and restimulation was repeated after thawing many months later. However, VWAK TCL became anergic after two to three restimulations. For this reason, more studies have been carried out with the GA- and FYAK-specific TCL and, for comparison, PLP139-151-specific TCL.
Properties of TCL.
(i) Proliferation.
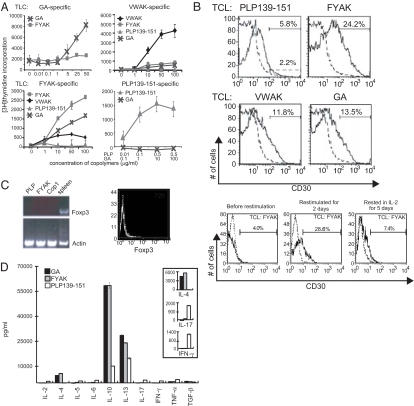
These cell lines proliferated specifically to PLP139-151 or to the copolymer used to establish them, although some cross-reaction to related copolymers was present in the FYAK cell line (Fig. 2A).
Fig. 2.
Properties of TCL. (A) Proliferation of TCL obtained after immunization with PLP139-151 (75 μg), GA, FYAK, or VWAK (250 μg each) alone. Each well contained 5 × 105 cells in 200 μl; 3H-thymidine incorporation was measured after 3 days of incubation. (B) CD30 expression on TCL (Upper). Also shown is CD30 expression on FYAK-specific TCL before restimulation, restimulated for 2 days, and then after resting in IL-2 for 5 additional days (Lower). Similar results were obtained for a VWAK-specific TCL. (C) Foxp3 expression on established TCL determined by RT-PCR. Foxp3 was measured on established TCL by RT-PCR by using ≈1 μg of total RNA (Left). The primers used were described in ref. 34. Thirty-five cycles were used. The products were separated on an agarose gel and detected by ethidium bromide staining. Foxp3 was also measured by intracellular cytokine staining of CD4+CD25+ T cells (Right). The FYAK-specific T cells were restimulated for 72 h. CD4+CD25+ T cells were gated by FACS and then analyzed for intracellular expression of the transcription factor Foxp3 as described by the mAb manufacturer (eBioscience). (D) Cytokine production by TCL. ELISA was used to measure cytokines in the supernatants of 1 × 106/ml TCL. Inset shows amplification of the scales for IL-4, IL-17, and IFN-γ. Supernatants were assayed 3 days after the last restimulation. Data are shown as means of triplicates. Bars show SD values. P < 0.01 for IL-10, P < 0.01 for IL-13, P < 0.001 for IL-4, P = 0.01 for IL-17, and P < 0.01 for IFN-γ for either FYAK or GA vs. PLP139–151.
(ii) Phenotype.
The copolymer-specific TCL were phenotypically similar to PLP139-151-specific TCL, i.e., they were CD4+, CD25+, CD69+, CD45+, CD30+, GITR+, CTLA4+, CD71low, and CD62L− (data not shown). Two members of the TNFR superfamily, GITR and CD30, were present, and CD30 was up-regulated (Fig. 2B). CD30 expression was found 2–3 days after stimulation with relevant copolymer but disappeared after 3–5 days in IL-2 (Fig. 2B).
(iii) FoxP3 expression.
Notably, these CD4+CD25+T cells either did not express FoxP3 or expressed only very low levels (Fig. 2C), distinguishing them from FoxP3+ regulatory T cells that have been studied extensively in the recent past (17, 18).
(iv) Cytokine secretion.
Cytokine profiles were determined by ELISA by using TCL derived from SJL mice immunized either with PLP139-151 or copolymers and restimulated in vitro with the respective antigen. Splenocytes from these PLP139-151- and copolymer-immunized mice were restimulated three times semiweekly to establish lines. The PLP139-151-specific TCL secreted high amounts of IL-17 and IFN-γ. Similar TCL established after the third stimulation with copolymers produced very large amounts of IL-10 and IL-13, relatively small amounts of IL-4, but virtually no TGFβ, IL-2, IL-5, IL-6, IL-17, IFN-γ, or TNF-α (Fig. 2D).
(v) TCR Vβ and Vα usage.
By using a panel of Vβ-specific monoclonal antibodies, all of the lines were found to be heterogeneous with respect to usage of TCR Vβ segments, although in each case, two or three Vβ segments predominated. The usage of Vβ4 (24–30%) and Vβ14 (25–30%) by FYAK-specific TCL after three restimulations was particularly striking. Similarly, in the GA-specific TCL, the most highly expressed segments were Vβ2 (24%) and Vβ6 (22%) and in PLP139-151-specific TCL Vβ17a (22%) and Vβ14 (18%). Analysis of the TCR Vα chain expression was carried out by RT-PCR, because only a few Vα-specific antibodies are available. The usage of the TCR Vα segments was even more heterogeneous in all three TCL. The heterogeneity of both TCR Vβ and Vα segments is not surprising in view of the fact that random copolymers are being presented to generate these T cells, and several different peptide sequences of each random copolymer may be presented.
Suppression of Proliferation of PLP139-151 TCL Mediated by Supernatants of Copolymer-Specific TCL.
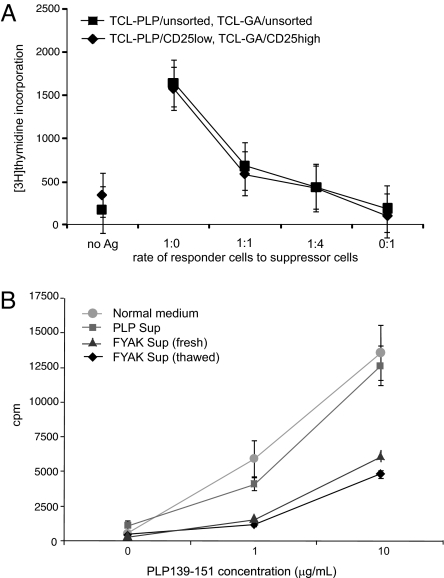
CD4+CD25+FoxP3+ natural regulatory T cells (17, 18) require cell–cell contact for mediating inhibition of proliferation. Similarly, the proliferation of PLP139-151 TCL studied here was inhibited by admixture of the GA-specific TCL (Fig. 3A). However, inhibition was also obtained by addition of supernatants of GA- or FYAK-specific TCL (Fig. 3B). Thus, materials in the supernatants (presumably cytokines) mediated inhibition of proliferation. It is not possible in these experiments to ascertain whether an additional inhibitory effect is provided by cell–cell contact.
Fig. 3.
Suppression of proliferation in vitro. (A) Suppression of proliferation of PLP139-151-specific TCL by the GA-specific TCL. Activated GA- and PLP139-151-specific TCL were each purified with the murine T cell enrichment kit (SpinSep, StemCell Technologies) and sorted into two populations of CD4+CD25low and CD4+CD25high. Sorted 5 × 104 CD4+CD25low or unsorted PLP139-151-specific cells as responders were mixed with CD4+CD25high or unsorted GA-specific cells as suppressors at the ratios shown and cocultured with 1 × 105 irradiated splenocytes as APC, antigens (Ag) 0.5 μg/ml PLP139-151, and 5 μg/ml of GA per well in triplicate for 96 h; cultures were pulsed with 3H-thymidine during the last 24 h of incubation. Bars show SD values. P values at the 1:4 ratio compared with 1:0 were <0.01 for unsorted and <0.05 for sorted cells. (B) Suppression of proliferation by culture supernatant. PLP139-151-specific T cells were resuspended in normal medium (DMEM-10) or supernatant collected from restimulated PLP139-151- or FYAK-specific TCL. T cells (5 × 104) were mixed with an equal number of irradiated APC and the antigen PLP139-151 (0, 1, or 10 μg/ml) and were then plated into a well of a round-bottom 96-well plate. Data are shown as means of triplicates. Bars show SD values. The supernatant from FYAK-specific T cells inhibited the proliferation of PLP139-151-specific T cells by 60%. P < 0.001 for comparison of FYAK supernatant with either control at 10 μg/ml PLP139-151.
Adoptive Transfer (ATx) of Copolymer-Specific Regulatory T Cells Inhibited Two Additional Autoimmune Diseases in SJL Mice.
Previously, a TCL generated by immunization of naive SJL mice with copolymers was shown on ATx to ameliorate the subsequent induction of EAE induced by PLP-139-151 (4). ATx has been used in two additional models of autoimmune diseases in SJL mice.
(i) MBP peptide (MBP85-99)-induced EAE.
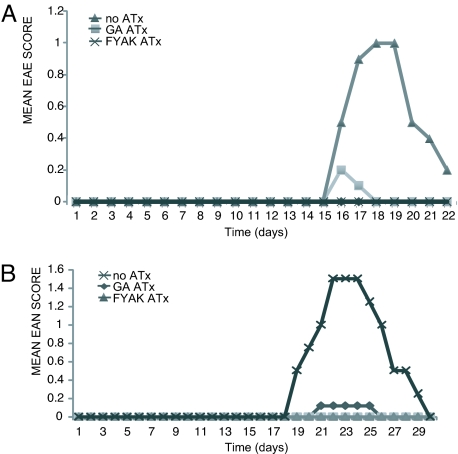
MBP85-99-induced EAE in SJL mice is a mild disease with a maximal score of ≈1–2 followed by remission. After immunization with MPB85-99, all mice developed disease between days 15 and 17 with a mean score of 1.0 (Fig. 4A). After ATx of 5 × 106 T cells from either a GA or a FYAK cell line at day 0 and immunization with MPB85-99 the next day, essentially no disease was evident.
Fig. 4.
ATx of GA- or FYAK-specific TCL prevents induction of MBP-induced EAE and BPNM-induced EAN. Cells (1 × 106) of GA- or FYAK-specific TCL were injected i.v. into SJL mice followed by immunization at day 1 with 250 μg of MBP85-99 in CFA (A) or with 2.5 mg of BPNM in CFA (B). Controls received no ATx. Mean disease scores for each group of five mice are shown. (A) At days 18–19, P < 0.01 for FYAK or GA ATx vs. control. (B) At days 22–24, P = 0.01 for FYAK or GA ATx vs. control.
(ii) Bovine peripheral nerve myelin (BPNM)-induced EAN.
BPNM-induced EAN (19) is also a mild disease with a maximal score of ≈1.5–2. After immunization with BPNM alone, all mice in the group developed signs of EAN between days 17 and 18 with a mean score of 1.7 (Fig. 4B). However, little or no disease was observed after transfer of 5 × 106 cells of either the GA- or FYAK-specific TCL.
Discussion
T cells that expand after treatment with amino acid copolymers belong to the group of regulatory T cells that secrete immunosuppressive cytokines and mediate bystander immunosuppression. They are similar in these properties to Tr-1 cells that have also been called IL-10-secreting regulatory T cells (9–11). These cells are generated in the periphery and thus belong to the mechanisms that contribute to peripheral rather than central tolerance. The IL-10-secreting T cells described here differ from those previously studied, in that they secrete large amounts of IL-10 and IL-13, small amounts of IL-4, but no TGF-β, whereas the originally described Tr-1 cells secrete IL-10 and TGF-β but no IL-4. The secretion of IL-13 by these cells has not been reported. In addition, an earlier-described family of cells referred to as Th3 cells (8) also belongs in this group. These cells were generated after oral feeding of antigen and were reported to secrete TGF-β. However, cells that secrete both TGF-β and IL-10 were also observed. A variety of regulatory T cells of this type may be induced in the periphery under various circumstances and possibly at different locations, e.g., in intestinal Peyer's patches as distinct from spleen and lymph nodes. The particular immunosuppressive cytokines they secrete may depend on particular locations and stimuli that have not been defined.
The expression of CD30 (which plays a major role in the protective responses to parasites) on these regulatory T cells may have special significance. CD30 expression has been reported to be expressed on activated T cells and to have a role in the regulation of cytokine secretion (20). Particularly interesting in the present context is the reported induction of IL-13 secretion after ligation of CD30 in the absence of TCR engagement (21). Correspondingly, CD30−/− mice were reported to have augmented T cell autoreactivity (22). This molecule was also reported to be important in the prevention of complications during allogeneic bone marrow transplantation in mice (22), an effect ascribed to regulatory T cells. Its role in the secretion of cytokines induced by amino acid copolymers requires further study.
Moreover, multiple mechanisms and additional cell types may also be involved in the tolerance induced by amino acid copolymers. Both macrophages and dendritic cells that can differentiate into regulatory cells (23, 24) have been reported to be important in the mechanism (12–15). In the case of macrophages, these have been referred to as Type II or alternatively activated macrophages. Clearly, CNS microglia that can differentiate under different influences into either DC or macrophages (25) play an important role in immunity within the CNS. Down-regulation of expression of MHC Class II proteins on microglia induced by amino acid copolymers (7) could be a critical feature of protection against EAE and, by extension, against MS. Inhibition of transcription of some genes and down-regulation of MHC Class II surface expression are among the mechanisms proposed for the action of IL-10 (26). This cytokine could indirectly or directly affect surface expression of MHC Class II genes in microglia. Neurotrophic factors have been reported to be induced by copolymers (27, 28), and the direct effects of copolymers on nerve cells have been suggested to be important (29). Neurodegeneration is the hallmark of pathologies in these CNS diseases (30).
Finally, much recent work has focused on Th17 cells and the cytokine IL-17 they secrete in the pathogenesis of neurodegeneration, in addition to IFN-γ secreted by Th1 cells. Still another potentially important mechanism of neuroprotection afforded by amino acid copolymers may be the inhibition of the generation of Th17 cells (31–33).
The enlarged spleen and lymph nodes that occur after immunization with copolymers (in CFA, IFA, or mannitol to different extents) is notable. The increase in spleen weight is reflected in a large increase in the number of cells, but CD4+CD25+ T cells to which the regulatory T cells described here belong represent only a fraction of the expansion (Fig. 1). In addition, an expansion of CD11b+CD11c− (macrophages), CD11b+CD11c+ (DC), and CD11b−CD11c+ (DC) cells is also apparent (data not shown). Whether and how these different cell types contribute to tolerance remain for further investigation. It is notable, however, that IL-13 produced by the IL-10-secreting T cells described here is a prominent factor in inducing activation and expansion of regulatory Type II macrophages, of which several kinds have been described (23, 24). Both regulatory macrophages and regulatory DC have been reported to secrete IL-10. Thus, these different cell types may all participate in the protective mechanism induced by amino acid copolymers and indeed may secrete cytokines that serve to amplify the protection in either autocrine or paracrine fashion. Recent data suggest that IL-27 secreted by one of these cell types may be a primary event that initiates many of the observed phenomena (34–37).
Materials and Methods
Copolymers and Peptides.
Copolymers FYAK and VWAK were synthesized as described (4–6). FYAK (PI-2301) was a gift of Peptimmune, Inc.. GA (Copaxone) was the commercial product (Teva). Peptides synthesized were PLP 139-151 and MBP 85-99. BPNM was a gift of Ralf Gold (Department of Neurology, Ruhr University, Bochum, Germany) (19).
Generation of Copolymer-Specific TCL.
SJL/J mice were immunized with PLP 139-151, FYAK, VWAK, or GA (200 μg per mouse) emulsified in CFA (Difco). Ten days later, single-cell suspensions were prepared from spleens and lymph nodes and were stimulated with corresponding peptides or copolymers, at a concentration of 10 μg/ml in the presence of antigen-presenting cells (APC) (irradiated splenocytes) at 37°C (4). After two rounds of restimulation with peptide or copolymers alone for 1 week followed by addition of IL-2 (20 units/ml) and incubation for a second week, viable lymphocytes were separated by Ficoll–Hypaque density gradient centrifugation, and 5 × 105 cells in 200 μl were used to test their specificity at a dose range of 0–50 μg/ml.
FACS Analysis of Copolymer-Specific TCL and Control PLP139-151 TCL.
TCL were stained with specific antibodies for CTLA-4, CD30, CD25, CD4, CD3, CD45, CD71, and GITR (BD Biosciences). Vβ analysis of TCL was performed by using the Vβ screening kit (BD Biosciences). All samples were detected by using FACSCalibur (BD Biosciences) and analyzed by using CellQuest software (BD Biosciences). Cell sorting was performed by using MoFlo (DAKO).
RT-PCR Analysis of Foxp3 Expression in TCL.
Total RNA was extracted from copolymer-specific TCL or PLP139-151-specific TCL by using an RNA STAT-30 kit according to the manufacturer's protocol (Tel-Test). Foxp3 primers were described in ref. 38. γ-Actin fragment was used as a control (39).
Cytokine Measurement by ELISA.
Lymphocytes from SJL mice immunized with PLP 139-151 or copolymers (FYAK, VWAK, and GA) were restimulated with corresponding peptides at a concentration of 10 μg per ml in the presence of APC in 24-well plates for 2 days. Cytokine mAb (BD Biosciences) for IL-2, IL-4, IL-10, IL-13, TGF-β, IL-6, and IFN-γ were coated on 96-well plates at a concentration of 1 μg/ml overnight. Plates were washed and treated with blocking solution (Kierkegaard & Perry), followed by incubation of cytokine standards and culture supernatants overnight at 4°C. After washing, the plates were incubated with their corresponding biotinylated α-cytokine-detecting mAb (1 μg/ml) for 2 h. They were developed after adding avidin/peroxidase and its substrate.
Effect of Anti-CTLA-4 mAb on the Induction of EAE.
SJL/J mice were injected with 100 μg of anti-CTLA-4 mAb i.p. 1 day before administration of PLP139-151 (75 μg) in CFA and then again on days 3 and 5 of the experiment. Pertussis toxin, 200 ng (List Biological Laboratories), was given i.v. on the day after immunization. Mice were monitored for the appearance of clinical signs of EAE as described (4, 5).
ATx of Copolymer-Specific TCL.
To establish lines, SJL/J mice were immunized with either GA or FYAK, and lines were propagated as described. The next day, the mice (five per group) were immunized with either 100 μg of MBP85-99 peptide or 2.5 mg of BPNM in CFA. Pertussis toxin, 200 ng (List Biological Laboratories), was given i.v. on the day of immunization and on day 2 postimmunization. T cells (5 × 106) from these lines were injected i.v. into naïve 6- to 8-week-old SJL/J mice. Mice were scored daily for 22–30 days, as described (4, 5).
Acknowledgments.
We thank J. Reddy, Z. Illes, J. Passanese, and C. Li for helpful discussions and M. Mapa and M.L. Nicotra for assistance with figures. This work was supported by research grants from the National Institutes of Health (Grant 5RO1 AI049524) and the National Multiple Sclerosis Society (Award no. RG3796A3).
Footnotes
Conflict of interest statement: J.L.S. declares a conflict of interest. He is a member of the Scientific Advisory Board of Peptimmune, Inc., who are developing FYAK for clinical trial. All other authors declare no conflict of interest.
References
- 1.Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur J Immunol. 1971;1:242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- 2.Farina C, Weber MS, Meinl E, Wekerle H, Hohlfeld R. Glatiramer acetate in multiple sclerosis: update on potential mechanisms of action. Lancet Neurol. 2005;4:567–575. doi: 10.1016/S1474-4422(05)70167-8. [DOI] [PubMed] [Google Scholar]
- 3.Fridkis-Hareli M, et al. Novel synthetic amino acid copolymers that inhibit autoantigen-specific T cell responses and suppress experimental autoimmune encephalomyelitis. J Clin Invest. 2002;109:1635–1643. doi: 10.1172/JCI15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern JN, et al. Amelioration of proteolipid protein 139–151-induced encephalomyelitis in SJL mice by modified amino acid copolymers and their mechanisms. Proc Natl Acad Sci USA. 2004;101:11743–11748. doi: 10.1073/pnas.0403832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Illes Z, et al. Modified amino acid copolymers suppress myelin basic protein 85–99-induced encephalomyelitis in humanized mice through different effects on T cells. Proc Natl Acad Sci USA. 2004;101:11749–11754. doi: 10.1073/pnas.0403833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern JN, et al. Peptide 15-mers of defined sequence that substitute for random amino acid copolymers in amelioration of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2005;102:1620–1625. doi: 10.1073/pnas.0409022102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Illes Z, et al. Copolymer effects on microglia and T cells in the central nervous system of humanized mice. Eur J Immunol. 2005;35:3683–3693. doi: 10.1002/eji.200526121. [DOI] [PubMed] [Google Scholar]
- 8.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 9.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 11.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 12.Hussien Y, Sanna A, Soderstrom M, Link H, Huang YM. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J Neuroimmunol. 2001;121:102–110. doi: 10.1016/s0165-5728(01)00432-5. [DOI] [PubMed] [Google Scholar]
- 13.Vieira PL, Heystek HC, Wormmeester J, Wierenga EA, Kapsenberg ML. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J Immunol. 2003;170:4483–4488. doi: 10.4049/jimmunol.170.9.4483. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi R, Anderson DE, Weiner HL. Cutting Edge: Immature human dendritic cells express latency-associated peptide and inhibit T cell activation in a TGF-beta-dependent manner. J Immunol. 2007;178:4017–4021. doi: 10.4049/jimmunol.178.7.4017. [DOI] [PubMed] [Google Scholar]
- 15.Weber MS, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 16.Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakaguchi S, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 18.Shevach EM, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 19.Stienekemeier M, et al. Vaccination, prevention, and treatment of experimental autoimmune neuritis (EAN) by an oligomerized T cell epitope. Proc Natl Acad Sci USA. 2001;98:13872–13877. doi: 10.1073/pnas.241504598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowen MA, Lee RK, Miragliotta G, Nam SY, Podack ER. Structure and expression of murine CD30 and its role in cytokine production. J Immunol. 1996;156:442–449. [PubMed] [Google Scholar]
- 21.Harlin H, Podack E, Boothby M, Alegre ML. TCR-independent CD30 signaling selectively induces IL-13 production via a TNF receptor-associated factor/p38 mitogen-activated protein kinase-dependent mechanism. J Immunol. 2002;169:2451–2459. doi: 10.4049/jimmunol.169.5.2451. [DOI] [PubMed] [Google Scholar]
- 22.Zeiser R, et al. Early CD30 signaling is critical for adoptively transferred CD4+CD25+ regulatory T cells in prevention of acute graft-versus-host disease. Blood. 2007;109:2225–2233. doi: 10.1182/blood-2006-07-038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Santambrogio L, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 27.Aharoni R, et al. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci USA. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorantla S, et al. Copolymer-1 induces adaptive immune anti-inflammatory glial and neuroprotective responses in a murine model of HIV-1 encephalitis. J Immunol. 2007;179:4345–4356. doi: 10.4049/jimmunol.179.7.4345. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, et al. T cell independent mechanism for copolymer-1-induced neuroprotection. Eur J Immunol. 2007;37:3143–3154. doi: 10.1002/eji.200737398. [DOI] [PubMed] [Google Scholar]
- 30.Friese MA, et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 31.Ehirchiou D, et al. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J Exp Med. 2007;204:1519–1524. doi: 10.1084/jem.20062292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batten M, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 33.Stumhofer JS, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 34.Jankovic D, Trinchieri G. IL-10 or not IL-10: that is the question. Nat Immunol. 2007;8:1281–1283. doi: 10.1038/ni1207-1281. [DOI] [PubMed] [Google Scholar]
- 35.Awasthi A, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald DC, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 37.Stumhofer JS, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 38.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 39.Keskin DB, Marshall B, Munn D, Mellor AL, Gearhart DA. Decreased protein nitration in macrophages that overexpress indoleamine 2, 3-dioxygenase. Cell Mol Biol Lett. 2007;12:82–102. doi: 10.2478/s11658-006-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]