Abstract
Most, if not all, cancers are composed of cells in which more than one gene has a cancer-promoting mutation. Although recent evidence has shown the benefits of therapies targeting a single mutant protein, little attention has been given to situations in which experimental tumors are induced by multiple cooperating oncogenes. Using combinations of doxycycline-inducible and constitutive Myc and mutant Kras transgenes expressed in mouse mammary glands, we show that tumors induced by the cooperative actions of two oncogenes remain dependent on the activity of a single oncogene. Deinduction of either oncogene individually, or both oncogenes simultaneously, led to partial or complete tumor regression. Prolonged remission followed deinduction of KrasG12D in the context of continued Myc expression, deinduction of a MYC transgene with continued expression of mutant Kras produced modest effects on life extension, whereas simultaneous deinduction of both MYC and KrasG12D transgenes further improved survival. Disease relapse after deinduction of both oncogenes was associated with reactivation of both oncogenic transgenes in all recurrent tumors, often in conjunction with secondary somatic mutations in the tetracycline transactivator transgene, MMTV-rtTA, rendering gene expression doxycycline-independent. These results demonstrate that tumor viability is maintained by each gene in a combination of oncogenes and that targeted approaches will also benefit from combination therapies.
Keywords: inducible, mammary gland, ras
Recent successes in the treatment of several human cancers using agents directed against the products of single oncogenes (1) and the prospect of many more such agents becoming available (2) raise important questions about long-term outcomes of interfering with certain mutations in cancer cells. Should targeted therapies be directed against all of the genetic damage in a cancer cell? Or do cancer cells display a differential dependence on mutations, and can that differential be used to select the most potent targeted therapies?
Experimental murine models of human cancer that depend on regulated oncogenic transgenes mimic hypothetical therapeutic situations in human cancers, for which highly effective targeted therapies might be used to block the action of one or more oncogenes. These models are indispensable for testing the effects of blocking two common human oncogenes, Myc and Kras, for which no effective therapies currently exist. By using this approach, tumor regression in response to oncogene withdrawal has been observed across multiple tumor types induced by either Myc or mutant Kras transgenes—lymphomas, leukemias, insulinomas, lung, bone, liver, and breast tumors (3, 4). However, inactivation of the same oncogene in different tumor types produced a range of long-term outcomes, from complete cure to invariable relapse.
To reduce the effects of genetic variability on tumor regression and long-term remission after selective oncogene inactivation, we took advantage of the cooperative behavior of Myc and mutant Kras oncogenes in tumorigenesis. Using constitutive and doxycycline-inducible alleles of these oncogenes expressed in the mammary gland (MG) of transgenic mice (5–9), we first confirmed the synergistic effects of Myc and mutant Kras oncogenes on mammary tumorigenesis, leading to rapid autochtonous tumor formation, whether expressed from inducible or constitutive promoters. We then compared the effects of deinducing either one or both oncogenes on cell growth, cell death, tumor regression, and tumor relapse. We observed a hierarchy of responses to deinduction of one or both oncogenes, revealing the existence of oncogene cooperation in tumor maintenance and tumor recurrence, not only in tumor initiation.
Results
Synergistic Pairs of Mammary Oncogenes.
Our goals in this work were to determine whether primary cancer cell growth and viability depend on one or both members of pairs of oncogenes that cooperate during mammary tumorigenesis and whether long-term outcomes may be determined by selective inactivation of either one or both oncogenes. Because we planned to do this by using a combination of doxycycline-inducible and constitutive oncogenic transgenes, it was first important to show that the relevant combinations of inducible and constitutive oncogenic transgenes worked synergistically to cause mammary cancers.
Constitutively expressed transgenes encoding Myc and mutant Hras proteins were shown two decades ago to cooperate during mammary tumorigenesis (10). Our choice of Myc and Ras transgenes for these studies was influenced in part by these early observations, in part by subsequent work implicating spontaneous mutations of Kras genes in the maintenance of tumors induced by regulated Myc (11–13) and in part by the availability of doxycycline-inducible transgenes encoding Myc and mutant Kras (7, 8).
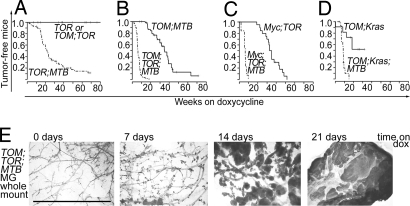
Because the inducible transgene encoding mutant Kras has not been tested for its ability to cause mammary tumors (MT), we first generated bitransgenic mice carrying TetO-KrasG12D (TOR) and MMTV-rtTA (MTB) (5, 11); the latter transgene expresses the reverse tetracycline transactivator (rtTA) in mammary epithelial cells (5). When fed doxycycline after weaning, these mice developed mammary gland hyperplasia as early as 1 week after induction, progressing to hyperplastic alveolar nodules and then palpable oligofocal mammary tumors with a median latency of 22 weeks. No tumors occurred in bitransgenic animals not given the antibiotic (n = 15, data not shown) or in TOR mice lacking the regulator transgene but fed doxycycline (n = 7, see also Fig. 1A). We also confirmed previous studies (11–13) showing that bitransgenic animals with an inducible (human) MYC transgene (TOM;MTB) developed solitary mammary tumors; in our cohort the median latency was 26 weeks (Fig. 1B).
Fig. 1.
Oncogenic Kras synergizes with Myc in mammary tumorigenesis. Tumor incidence in female mice from experimental and control lines from the beginning of doxycycline exposure. (A) TOR; MTB mice (dashed-dot line; n = 80) compared with TOR (n = 7) and TOM;TOR (n = 11) mice (solid line). (B) TOM;TOR;MTB mice (dashed-dot line; n = 60) compared with TOM;MTB mice (solid line; n = 61). (C) Myc;TOR;MTB mice (dashed-dot line; n = 14) compared with Myc;TOR mice (solid line; n = 20). (D) TOM;Kras;MTB mice (dashed-dot line; n = 15) compared with TOM;Kras mice (solid line; n = 11). (E) Diffuse tumor development in mature mouse mammary glands induced with doxycycline to coexpress MYC and mutant Kras transgenes. Whole-mount mammary gland preparations displayed are from tritransgenic TOM;TOR;MTB mice exposed to doxycycline for indicated periods of time. (Scale bar, 5 mm.)
We then determined the potential of the doxycycline-inducible Kras and MYC oncogenes to collaborate with each other and with constitutively expressed transgenes, MMTV-Myc (Myc) and MMTV-KrasG12V (Kras), in tritransgenic virgin females also expressing MTB. As shown in Fig. 1 B–D, expression of two transgenic oncogenes always induced mammary tumors much more efficiently than did a single transgene, regardless of whether either or both of the oncogenes was doxycycline-inducible. Each of the three combinations produced visible tumors in all mammary glands within 10 weeks of induction, with a median latency between 6 and 8 weeks, much shorter than that observed with any of the single inducible or constitutive oncogenic transgenes. Tumors in animals carrying all three combinations of inducible or constitutive Myc and Ras transgenes were phenotypically similar, with keratin 6-, keratin 8-, and smooth muscle actin-positive cell subsets, characteristic of tumors induced by Wnt1, β-catenin, and Myc oncogenes in our earlier study (14), indicating similar differentiation status of the tumors [supporting information (SI) Fig. S1].
The rapid pace of appearance and multifocal character of tumors caused by these combinations of oncogenes suggested that the tumors arose in a polyclonal fashion, implying that dysregulated expression of Myc and mutant Kras is sufficient for mammary transformation. This was further supported by examination of whole mounts of mammary fat pads from TOM;TOR;MTB mice (Fig. 1E). Diffuse mammary hyperplasia was apparent at 7 days after addition of doxycycline, numerous tumor nodules were evident throughout the glandular tree at 14 days, and confluent tumors were present at 21 days. Of the mice with cooperating oncogenes, only the mice with a constitutive KrasG12V gene and inducible MYC showed distinct, multifocal growths. Sinn et al. (10) reported a similar phenotype in mice with two constitutive oncogenes, Myc and MMTV-Hras. It is possible that the result reflects mosaic expression of the Kras transgene or senescence of some mammary epithelial cells in response to early expression of a Ras oncogene, as described in mammary and other cell types (15–17).
Deinduction of Either MYC or Oncogenic Kras Individually, or both Oncogenes Simultaneously, Causes Apoptosis and Reduced Proliferation.
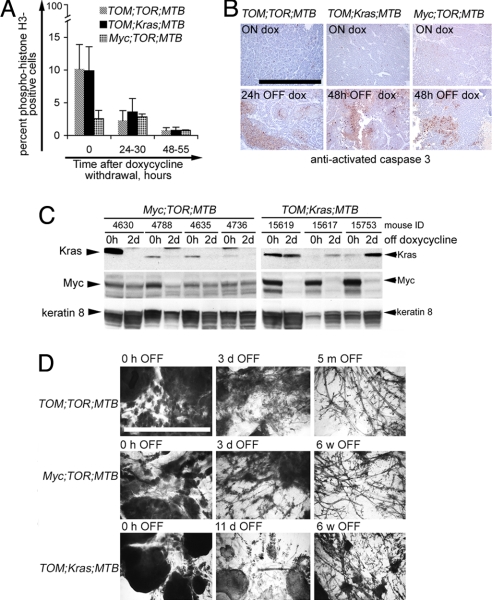
We next asked whether the tumors depended on continued expression of either one or both of these oncogenes for continued growth or survival. As a first step in analyzing the response to the deinduction of oncogenes, we determined mitotic indices (by immunohistological detection of phosphorylated histone H3) and assayed tumor slices for apoptotic cells (by immunostaining for activated caspase-3) at several time points after withdrawal of doxycycline (Fig. 2). Deinduction of TOM and TOR transgenes in all three Myc/Kras combinations was accompanied by a drop in the proportion of mitotic cells (Fig. 2A), although a significantly smaller fraction of cells was initially cycling in tumors from mice with an inducible Kras and a constitutive Myc transgene than in the other two types of tritransgenic mice. Similarly, an increase in the proportion of apoptotic cells was observed in tumors from cohorts of all three tritransgenic mice sampled 1 or 2 days after removal of doxycycline from the diet (Fig. 2B). It was, however, difficult to measure apoptotic indices precisely, because discrete areas with many dying cells were often found surrounded by viable cells, perhaps reflecting the multifocal nature of these rapidly induced tumors.
Fig. 2.
Short-term responses to doxycycline withdrawal in mouse mammary tumors induced by Myc and mutant Kras. (A) Decrease in the number of proliferating cells in mammary tumors after doxycycline withdrawal in TOM;TOR;MTB mice (gray bars), TOM;Kras;MTB mice (black bars), and Myc;TOR;MTB mice (hatched bars). Proliferating cells were detected by immunostaining with anti-phosphorylated (Ser-10) histone H3 on paraffin tissue sections and counted as described in Experimental Procedures. (B) Increase in tumor cell death at 24–48 h after doxycycline withdrawal, assayed by immunodetection of anti-activated caspase-3 (Cell Signaling Technology) on paraffin tissue sections from TOM;TOR;MTB mice (Left), TOM;Kras;MTB mice (Center), and Myc;TOR;MTB mice (Right). The slides were counterstained with hematoxylin. Brown-red cells are positive for activated caspase 3. (Upper) Before removal of doxycycline. (Lower) At 24 or 48 h after removal of doxycycline. (Scale bar, 0.5 mm.) (C) Immunoblotting of total lysates from mammary tumors of tritransgenic Myc;TOR;MTB mice (Left) and TOM;Kras;MTB mice (Right) maintained on doxycycline for 7 weeks (0 h OFF) and contralateral tumors harvested 48–50 h after doxycycline removal (2 d OFF). The blot was probed with anti-Myc, anti-Kras, and anti-keratin-8 antibodies as described in Experimental Procedures. (D) Mammary whole mounts from tritransgenic animals taken off the doxycycline diet for the indicated periods of time. (Scale bar, 5 mm.)
To confirm our prediction that expression levels of the inducible transgenes rapidly decline after doxycycline withdrawal, irrespective of whether the cooperating oncogene is regulated, we measured levels of Myc and Kras proteins by immunoblots of extracts of mammary tumors from tritransgenic mice before and 2 days after removal of doxycycline from the diet (Fig. 2C). After 5–6 weeks on doxycycline, Myc and Kras proteins are detectable in mammary tumors from tritransgenic animals at variable levels (Fig. 2C). The variability may, in part, be due to histological heterogeneity of the tumors. After a 2-day deinduction, protein levels dropped to very low or undetectable levels when expressed from regulated alleles but not when expressed from constitutive alleles (Fig. 2C). These results indicate that we were able to achieve inducible transgene regulation in tumors coexpressing cooperating constitutive oncogenes and that changes observed in tumor proliferation and cell death upon doxycycline withdrawal can be attributed to loss of expression of the respective oncogenic transgenes.
Complete Tumor Regression After Deinduction of Oncogenic Kras, With or Without Simultaneous Deinduction of a Cooperating MYC Oncogene.
The reduced proliferation and appearance of apoptosis upon deinduction of oncogenes in our mouse mammary tumor models suggested that both Myc and mutant Kras play a role in maintaining tumor cell growth and viability. To determine whether deinduction also leads to tumor regression and sustained remission, we observed cohorts of the three types of tritransgenic mice for prolonged periods after removal of doxycycline from the diets of tumor-bearing animals. After doxycycline withdrawal, almost all of the grossly transformed mammary glands that developed in TOM;TOR;MTB mice completely regressed to a nonpalpable state within 2 weeks (Fig. 2D Top); at later time points, mammary glands often displayed normal ductal architecture. All of the mammary tumors that developed through the combined expression of an inducible mutant Kras transgene and a constitutively expressed Myc transgene also completely regressed to a nonpalpable size upon withdrawal of doxycycline, with restoration of a nearly normal architecture in all mammary glands, indicating that expression of mutant Kras is essential for tumor viability even when expression of the cooperating Myc oncogene is maintained (Fig. 2D Middle). However, hyperplastic changes, characteristic of glands expressing the Myc transgene alone, persisted despite deinduction of mutant Kras and the dramatic tumor clearance (data not shown).
Deinduction of MYC in the Presence of Continuous Expression of a Kras Oncogene Results in Widespread but Incomplete Regression and Swift Recurrence of Mammary Tumors.
Loss of expression of a Myc oncogene causes tumor regression and extended survival in several experimental tumor models (7, 18, 19), including TOM;MTB-induced mammary tumors (11–13). Because we showed that deinduction of the MYC transgene in TOM;Kras;MTB mice was associated with a 10-fold reduction in proliferation (Fig. 2A) and increased tumor cell death (Fig. 2B), we expected to observe tumor regression after removal of doxycycline from the diet. In fact, all of the tumors in these animals became smaller within 5–7 days of doxycycline withdrawal (Fig. 2D Bottom). However, none of the palpable tumors regressed completely after 3 weeks of monitoring, at which point some of them began to regrow. Within 3–6 weeks after withdrawal of doxycycline, animals harbored multiple focal tumors. The incomplete regression and relatively rapid recurrence of tumors in these mice suggested that the tumor cells were less dependent for viability on MYC than on mutant Kras.
Tumors Also Recur After Deinduction of a Mutant Kras Oncogene in Tritransgenic Mice.
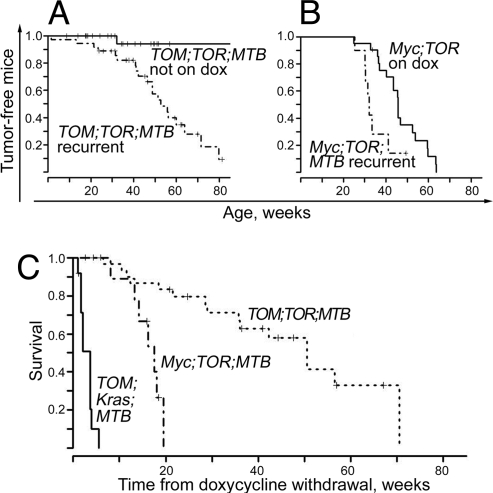
In mice with the two other tritransgenic combinations (TOM;TOR;MTB and Myc;TOR;MTB), apparently complete regression of tumors occurred when mutant Kras or both Kras and MYC were deinduced (Fig. 2 D), and mice entered remission, defined by the absence of palpable tumors. However, tumors ultimately reappeared in most animals. We found that even simultaneous deinduction of MYC and mutant Kras oncogenes did not completely protect from relapse. Solitary mammary tumors developed after withdrawal of doxycycline in 20 of 36 of the TOM;TOR;MTB animals followed up to 80 weeks of age, at a median age of 53 weeks (Fig. 3A). The histological appearance of the recurrent tumors was similar to that of primary tumors, although 3 of the 20 tumors displayed morphologic hallmarks of an epithelial-to-mesenchymal transition (data not shown). Although it is not possible to determine directly whether these tumors arose from surviving cells from the regressed tumors, it is notable that only one mammary tumor was observed in 31 TOM;TOR;MTB mice that never received doxycycline (Fig. 3A).
Fig. 3.
History of mammary tumors is associated with tumor recurrence and reduced survival. (A) Time of appearance of recurrent tumors in a cohort of mature female TOM;TOR;MTB mice exposed to doxycycline from 4 to 20 weeks of age (median, 8 weeks) until tumor burden reached an estimated 10% of body weight and then placed on a doxycycline-free diet (dashed-dot line; n = 36), compared with time of appearance of primary tumors in TOM;TOR;MTB control female mice never exposed to doxycycline (solid line; n = 31). (B) Time of appearance of recurrent tumors in a cohort of mature female Myc;TOR;MTB mice exposed to doxycycline from 4 to 20 weeks of age (median, 9 weeks) until total tumor burden reached an estimated 10% of body weight and then placed on a doxycycline-free diet (dashed-dot line; n = 14) compared with time of appearance of primary tumors in bitransgenic Myc;TOR control female mice on doxycycline (solid line; n = 22). (C) Survival of animals after doxycycline removal in TOM;Kras;MTB mice (solid line; n = 12), Myc;TOR;MTB (dashed-dot line; n = 12), and TOM;TOR;MTB (dotted line, n = 36). The animals were removed from doxycycline when the initial tumor burden reached an estimated 10% of body weight. Mice were euthanized when total recurrent tumor burden reached an estimated 10% of body weight, and these time points were used to calculate survival time after doxycycline withdrawal.
We made similar observations with tritransgenic mice in which tumors that developed on doxycycline regressed fully after deinduction of the TOR transgene, whereas the Myc transgene remained constitutively expressed (Fig. 3B). Again, animals that carried tumors and were apparently successfully treated by doxycycline withdrawal developed recurrent tumors at a much younger age than animals that lacked the regulator transgene developed primary tumors (33 vs. 46 weeks, P = 0.04). Thus, a history of tumor growth and regression predisposes these animals to recurrent mammary tumorigenesis, suggesting that regrowth of residual tumor cells, rather than spontaneous primary tumors, was responsible for the tumors arising late in life.
Deinduction of both MYC and Mutant Kras Improves Survival More than Deinduction of Either Oncogene Alone.
The experimental situations we have created with the three kinds of tritransgenic mice mimic hypothetical therapeutic situations in human cancer, in which highly effective targeted therapies might be used to block the action of one or both of a pair of cooperating oncogenes. Because deinduction of TOR was clearly superior to the deinduction of TOM for tumor regression, we tested whether combined deinduction would produce a more stable remission than that achieved by loss of TOR alone.
We compared the results of deinducing either one or both oncogenes by using a common measure of therapeutic effectiveness, extension of life. We considered the time when the estimated tumor burden becomes large enough (after 7 weeks of doxycycline exposure, on average) to require euthanasia of the animal to be approximately equivalent to the end of life. Because doxycycline was withdrawn when the total size of primary tumors was large enough to require euthanasia, extension of life was measured as the time from doxycycline withdrawal to the time when tumors recurring in the absence of inducer again became large enough to require euthanasia.
In the comparison of the three tritransgenic lines (Fig. 3C), it is apparent that inactivation of the TOM oncogene by doxycycline withdrawal conferred only a few additional weeks of life on “treated” mice. Inactivation of the TOR transgene added ≈14 weeks of life, a significant increase over what is achieved by deinducing TOM. Finally, inactivation of both TOR and TOM oncogenes added approximately another 33 weeks of life beyond what was achieved by inactivation of TOR alone, another significant increase (P value overall <0.001). The analysis thus suggests that, whereas dramatic immediate responses, including reduction in tumor size, are observed with all three “therapies,” targeting Kras is preferable to targeting MYC, and targeting both oncogenes is superior for achieving extended remissions.
Tumor Recurrence in TOM;TOR;MTB and Myc;TOR;MTB, but Not TOM;Kras;MTB Mice, Is Associated with Reactivation of Oncogenic Transgenes and Somatic Mutations in the rtTA Transgene.
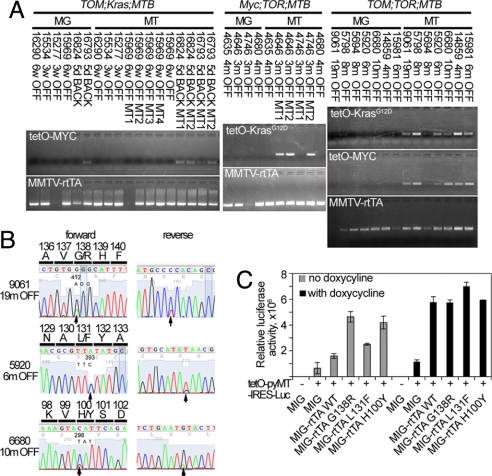
Acquired resistance to targeted therapies in human cancer is often associated with the emergence of secondary mutations in the targeted oncogenes that interfere with drug binding, suggesting continued dependence of the tumor on the initiating oncogenic lesion (20–23). Similarly, doxycycline-independent reexpression of the transgenic oncogene is found in many (for example, refs. 24–26) but not all (refs. 11, 13, and 27) tumors that recur in inducible-oncogene-driven tumor models. To determine whether recurrent tumors in our transgenic lines were associated with doxycycline-independent reexpression of the transgenes, we tested recurrent tumor and tumor-free mammary gland samples from the three models for transgenic transcripts by RT-PCR (Fig. 4A). The MMTV-rtTA transcript was detectable in the majority of the samples, both tumors and tumor-free glands, but no inducible transgene expression was observed in any of the tumor-free mammary gland preparations from aged mice from any of the three lines. Consistent with published data (11, 13), no reexpression of the TOM transgene was found in recurrent tumors from the TOM;Kras;MTB mice, although it could be induced in the recurrent tumors (and mammary glands) when mice were returned to a doxycycline diet (Fig. 4A Upper Left).
Fig. 4.
Loss of doxycycline dependence in tumors from TOM;TOR;MTB and Myc;TOR;MTB mice is associated with somatic mutations in the MMTV-rtTA transgene. (A) Analysis of transgene reactivation in recurrent tumors from TOM;Kras;MTB (Left), Myc;TOR;MTB (Center), and TOM;TOR;MTB (Right) animals. Paired RNA samples from recurrent tumor and non-tumor-bearing mammary gland were collected after removal OFF doxycycline or after placing the animals BACK on doxycycline as indicated and assayed as described in Experimental Procedures. (B) Examples of forward and reverse sequencing traces from tumor samples: 9061, 19 m OFF; 5920, 6 m OFF; 6680 10 m OFF by direct sequencing of rtTA amplification products with nucleotide and predicted amino acid changes indicated in the forward sequencing panel. (C) Luciferase activity of the three mutant rtTA clones is increased compared with wild-type rtTA and to mock-transfected controls with and without doxycycline addition. Wild-type and mutant rtTA was cloned into a modified MigR1 retroviral vector (33) and cotransfected into 293T cells with luciferase reporter pTetO-PyMT-IRES-Luc [Nancy Y.-C. Du (Memorial Sloan–Kettering Cancer Center) and H.E.V., unpublished work]. At 16 h after transfection, doxycycline was added to appropriate samples, and, 24 h later, cells were harvested, and luciferase activity for each sample was compared with the mock-transfected control to calculate the increase in luciferase activity.
Half of the recurrent tumors from the Myc;TOR;MTB mice and all of those from TOM;TOR;MTB mice, however, reexpressed their inducible transgenes. Consistent reexpression of both transgenes in the recurrent tumors from TOM;TOR;MTB mice suggested continued dependence of tumor growth on both oncogenes in this model. To ask whether there might be a common origin of doxycycline independence, we sequenced the 5′ portion (codons 13–210) of the rtTA transgene cDNA; this portion is reported to contain residues that govern binding to tet operators and thus the activation of the promoters (28). Five of seven samples contained nucleotide changes predicted to change amino acids (Fig. 4B), and the nucleotide substitution G412A was found in three independent tumor relapses. This mutation was also found in one of three relapses in Myc;TOR;MTB mice with TOR transgene reexpression. No mutations were found in the rtTA cDNA from tumor-free mammary glands of the same animals (n = 7) or in rtTA cDNA from primary tumors from animals receiving doxycycline (n = 8). Because the amino acid changes (H100Y, L131F, G138R) were distributed close to the region that differs between the rtTA and tTA (amino acids 71–102), they were likely to alter the ability of rtTA to bind and activate the tetO promoter.
To test directly whether mutant rtTA proteins were doxycycline-independent activators, we cloned mutant and wild-type rtTA sequences into a modified MSCV-based vector MigR1 and tested them in a doxycycline-responsive luciferase assay by cotransfection into 293T cells with a reporter plasmid (TetO-PyMT-IRES-Luc, provided by Nancy Y.-C. Du and H.E.V., unpublished work). All three mutants produced 1.5- to 3-fold-higher luciferase activity in the absence of doxycycline than did wild-type rtTA (Fig. 4C). These levels were comparable with 3.6-fold activation observed after addition of doxycycline in the cells transfected with the wild-type rtTA clone. Exposure to doxycycline further increased reporter activity in the mutants. These results are consistent with the observed doxycycline-independent expression of the inducible transgenes in recurrent tumors. Thus, somatic mutations in the MMTV-rtTA transgene appear to have a major role in tumor relapse in TOM;TOR;MTB mice and some of the Myc;TOR;MTB mice.
Because we and others have shown that tumors induced by dysregulated expression of Myc often contain somatic activating mutations in the endogenous Ras genes (11, 29), we screened recurrent tumors from the Myc;TOR;MTB mice with no evident reexpression of TOR for activating mutations in the endogenous Hras, Kras, and Nras genes. One of three tumors had a GGC13GTC mutation in the Hras gene (data not shown), reflecting continued requirement for activated Ras signaling in these tumors.
Discussion
Regression of tumors in response to inactivation of an initiating oncogene in experimental systems and in human tumors suggests that a single oncogene may play a crucial role in the maintenance of genetically complex tumors. However, in the context of a tumor induced by multiple known oncogenes, it has been unclear whether one or a subset of the genetic lesions is essential for tumor maintenance and whether anything can be gained by interfering with cooperating genetic lesions simultaneously.
Using combinations of doxycycline-inducible and constitutively expressed Myc and mutant Kras transgenes in the mouse mammary gland and observing tumor growth, regression, and recurrence, we have established a hierarchy of responses to deinduction of one or both oncogenes. In brief, whereas all three “treatments” produce benefits, deinduction of mutant Kras is significantly more effective than deinduction of Myc, and deinduction of both transgenic oncogenes produces the most durable remissions. However, in all three conditions, tumors ultimately recur in the absence of the inducer, doxycycline, although, in many recurrent tumors, doxycycline regulation was bypassed as a result of secondary somatic mutations in the rtTA transgene.
Why Is Loss of Kras Sufficient for Tumor Regression Despite Continued Expression of Myc?
Our findings with tritransgenic mice indicate that coexpression of MYC and mutant Kras in the mouse mammary gland is sufficient for full and rapid malignant transformation and that loss of mutant Kras expression alone is sufficient for dramatic, but not permanent, regression of the resulting tumors, with arrest of proliferation and increased tumor cell death (Fig. 2).
Why might genetically complex tumors with multiple oncogenic lesions respond to inactivation of a single oncogene? One explanation might be that certain oncogenic events are uniquely important for tumor viability because complementary mutations depend on them. The action of the initiating oncogene violates physiological checkpoints by inducing changes in gene regulation or secondary mutations, which would normally trigger cell death unless neutralized by the effects of another oncogenic mutation. This model predicts that an oncogenic mutation represents an attractive target for therapeutic intervention if it protects tumor cell viability. In fact, down-regulation of antiapoptotic BCL-2 in Myc-induced mouse leukemia resulted in remission of the disease and prolonged survival of the mice (30). Alternatively, the direct participation of one cooperating oncogene, such as Myc, in death signaling could explain why loss of its partner, mutant Kras, alone induces cell death and clinical remission in our dual-oncogene tumor models. In short, the mammary tumor models described here support the view that, although several genetic events may be necessary to confer the traits associated with oncogenic transformation (31), inactivation of a single oncogenic partner may undermine tumor maintenance, leading to tumor regression and temporary disease remission. Continued requirement for activated Ras signaling is reflected by frequent reactivation of the TOR transgene or somatic mutation in the endogenous Hras gene in the recurrent tumors of the Myc;TOR;MTB mice (Fig. 4A and data not shown).
Why Is Loss of MYC Insufficient for the Complete Regression of Tumors?
MYC has been shown to be essential for maintenance of several tumor types (7, 11, 19). We do not know whether the incomplete regression upon deinduction of the MYC transgene in our TOM;Kras;MTB mice is the result of primary resistance, i.e., preexisting tumor cells insensitive to the inactivation of the MYC transgene. Alternatively, recurrence could depend on secondary resistance, with the rapid emergence of recurrent tumors from rare clones that acquired independence through additional oncogenic genetic or epigenetic events. Irrespective of the mechanism, down-regulation of MYC, although itself a poor mediator of tumor regression compared with deinduction of mutant Kras, extended the length of remission almost 3-fold when combined with deinduction of TOR. This provides a strong argument for developing combination therapies against multiple targets to postpone disease recurrence.
Why Does Simultaneous Deinduction of both MYC and Mutant Kras Fail to Prevent Relapse?
Despite the deinduction of even both of two cooperating oncogenes, we found that tumors eventually reappeared in almost all animals maintained on doxycycline-free diets. These tumors likely represent the outgrowth of cells from the original, doxycycline-induced tumors, because tumors were not observed in animals that had not experienced oncogene expression (Fig. 3A). It is expected that tumor cells giving rise to recurrent tumors become independent of the transgenic oncogenes by acquiring genetic lesions or epigenetic changes that substitute for the requirement of MYC and/or mutant Kras, as was demonstrated in other models of doxycycline-independent relapses (7, 11, 25, 27, 34). The high prevalence of doxycycline-bypassing mutations in the MMTV-rtTA transgene that confer doxycycline-independence in the recurrent tumors from TOM;TOR;MTB mice reflects the essential role of oncogene cooperation in tumor relapse, because none of the recurrent tumors displayed reinduction of one oncogene alone. Molecular mechanisms of resistance to doxycycline withdrawal in this model may be analogous to forms of acquired resistance in human tumors treated with targeted therapies.
Experimental Procedures
Animal Husbandry and Genotyping.
All animals were kept in specific pathogen-free housing with abundant food and water under guidelines approved by the Memorial Sloan–Kettering Cancer Center Institutional Animal Care and Use Committee and Research Animal Resource Center. Myc, MTB, TOR, Kras, and TOM transgenes have been described (5–9). Doxycycline was administered by feeding mice with doxycycline-impregnated food pellets (625 ppm; Harlan–Teklad). Mice were placed on doxycycline at various ages. The median age at the beginning of doxycycline exposure was 8 weeks. The median time of tissue sampling before doxycycline withdrawal was 7 weeks. Tail DNA was isolated by using the DNeasy 96 Blood and Tissue kit (Qiagen) according to the manufacturer's protocol. Detection of the transgenes was performed as described (5–9). Animals were inspected for tumors weekly and monitored for tumor size according to institutional guidelines. Regressing tumors that were nonpalpable on at least two consecutive occasions were considered to have fully regressed.
Histology and Immunohistochemistry.
Mammary tissues were obtained by either excision biopsy or postmortem according to institutional guidelines. Mammary glands and tumors were excised; some were flash-frozen in liquid nitrogen for molecular analyses, and some were fixed in 10% buffered formalin for 16–48 h at room temperature, placed in 70% ethanol, and sent for paraffin embedding and sectioning (Histoserv). Whole-mount preparations were done as described (http://mammary.nih.gov/tools/histological/wholemounts/index.html).
Immunohistochemistry was performed with Vectastain ABC and M.O.M. kits (Vector Laboratories) according to the manufacturer's instructions. The following antibodies were used: anti-phospho (Ser-10) histone H3 (1:200 dilution, cat no. 9701; Cell Signaling Technology) and anti-cleaved (Asp-175) caspase-3 (clone 5A1, 1:200 dilution, cat no. 9661; Cell Signaling Technology). To determine proliferation index, phosphohistone H3-positive and -negative cells were counted in three representative fields for at least three tumors at each time point. More than 150 nuclei per field were counted for tumors with the regulated MYC allele, and 700–1,100 nuclei per field were counted for tumors with constitutive Myc and regulated mutant Kras transgenes.
Antibodies and Immunoblotting.
Tissue samples were crushed and lysed in lysis buffer [50 mM Tris·HCl (pH 8.0), 150 mM sodium chloride, 1 mM EDTA, 1% Triton X-100, 0.25% sodium deoxycholate, 1% Nonidet P-40, 40 mM sodium fluoride, 1 mM sodium orthovanadate, and 100 nM okadaic acid] containing protease inhibitors (Roche Diagnostics). After quantitation by Bio-Rad protein assays, 25–50 mg of each sample was separated by gel electrophoresis on 12% Tris-Glycine precast gels (Invitrogen) and transferred to nitrocellulose. Specific proteins were detected by using enhanced chemiluminescence (Amersham Pharmacia) or SuperSignal West Femto Maximum Sensitivity Substrate (Pierce) and the following antibodies: anti-Myc (1:1,000 dilution, cat no. 9402; Cell Signaling Technology), anti-Kras (clone F234, 1:500 dilution, cat. no. sc-30; Santa Cruz Biotechnology); anti-keratin-8 (TROMA, 1:500 dilution; Developmental Studies Hybridoma Bank, Iowa City, IA), HRP-conjugated anti-rabbit Ig (1:5,000 dilution; Amersham Pharmacia), and HRP-conjugated anti-mouse IgG (1:2,000 dilution; Roche Applied Science).
Transgene Reexpression Studies, Mutation Analysis, and Luciferase Assays.
Frozen tissue samples were crushed in liquid nitrogen and total RNA extracted according to standard protocols. After DNase I treatment (Invitrogen), 250 ng of RNA were reverse transcribed with random hexamer primers by using the SuperScript III first-strand synthesis system (Invitrogen), omitting the enzyme for the control (RT) set.
Transgenes were amplified by using the following primers: rtTA.F 5′-tgattaacagcgcattagagc-3′; rtTA.R 5′-ctgtacgcggacccacttt-3′ (the same primers were used for sequencing); tor.F: 5′-aaggacaaggtgtacagttatgtga-3′; tor.R: 5′-ctccgtctgcgacatcttc-3′, and, as reported in ref. 32) full-length wild-type and mutant rtTA sequences were amplified by using primers rtTAclone.F: 5′-gcgcgaattcaccatgtctagattagataaaaag-3′, rtTAclone.R 5′-cgcgctcgagctacccaccgtactcgtcaattc-3′, cloned into a modified MigR1 retroviral vector (33). For luciferase assays, 293T cells were grown to 50% confluence in 12-well plates and cotransfected by using FuGENE 6 (Roche) according to the manufacturer's protocol with MigR1 constructs and a luciferase reporter pTetO-PyMT-IRES-Luc (provided by Nancy Y.-C. Du and H.E.V., unpublished work). At 16 h after transfection, doxycycline or mock treatment was added to samples, and 24 h later, cells were harvested, and luciferase activity was measured by using the luciferase reporter assay system (Promega). Luciferase activity for each sample was compared with the mock-transfected control to calculate the increase in luciferase activity.
Acknowledgments.
We thank Jennifer Demers, Mary Ann Melnick, Anthony Daniyan, and Daisy Chen for expert handling of the mouse colony; Camelia Sima and Adam Olshen for statistical analysis; Lewis Chodosh (University of Pennsylvania School of Medicine, Philadelphia) for MTB transgenic mice; Dean Felsher (Stanford University, Stanford, CA) and Mike Bishop (University of California, San Francisco) for TOM transgenic mice; and Nancy Kohl (Merck Research Laboratories) for Kras transgenic mice. This work was supported in part by National Institutes of Health (NIH) Grant P01 CA94060 and the Martell Foundation (to H.V.), NIH Grant K01 CA118731 (to K. Podsypanina), and American Cancer Society Davidson Sinai Research Fellowship PF-05-078-01-MGO (to K. Politi).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801197105/DCSupplemental.
References
- 1.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 2.Sawyers CL. Making progress through molecular attacks on cancer. Cold Spring Harb Symp Quant Biol. 2005;70:479–482. doi: 10.1101/sqb.2005.70.034. [DOI] [PubMed] [Google Scholar]
- 3.Evan GI. Can't kick that oncogene habit. Cancer Cell. 2006;10:345–347. doi: 10.1016/j.ccr.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Shachaf CM, Felsher DW. Rehabilitation of cancer through oncogene inactivation. Trends Mol Med. 2005;11:316–321. doi: 10.1016/j.molmed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Gunther EJ, et al. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–292. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- 6.Omer CA, et al. Mouse mammary tumor virus-Ki-rasB transgenic mice develop mammary carcinomas that can be growth-inhibited by a farnesyl:protein transferase inhibitor. Cancer Res. 2000;60:2680–2688. [PubMed] [Google Scholar]
- 7.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 8.Fisher GH, et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart TA, Pattengale PK, Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984;38:627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- 10.Sinn E, et al. Coexpression of MMTV/v-Ha-ras and MMTV/c-myc genes in transgenic mice: Synergistic action of oncogenes in vivo. Cell. 2001;49:465–475. doi: 10.1016/0092-8674(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 11.D'Cruz CM, et al. c-MYC induces mammary tumorigenesis by means of a preferred pathway involving spontaneous Kras2 mutations. Nat Med. 2001;7:235–239. doi: 10.1038/84691. [DOI] [PubMed] [Google Scholar]
- 12.Jang JW, Boxer RB, Chodosh LA. Isoform-specific ras activation and oncogene dependence during MYC- and Wnt-induced mammary tumorigenesis. Mol Cell Biol. 2006;26:8109–8121. doi: 10.1128/MCB.00404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boxer RB, et al. Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell. 2004;6:577–586. doi: 10.1016/j.ccr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkisian CJ, et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 16.Collado M, et al. Tumour biology: Senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 17.Braig M, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 18.Pelengaris S, et al. Brief inactivation of c-Myc is not sufficient for sustained regression of c-Myc-induced tumours of pancreatic islets and skin epidermis. BMC Biol. 2004;2:26. doi: 10.1186/1741-7007-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shachaf CM, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 20.Gorre ME, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 21.Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamborini E, et al. A new mutation in the KIT ATP pocket causes acquired resistance to imatinib in a gastrointestinal stromal tumor patient. Gastroenterology. 2004;127:294–299. doi: 10.1053/j.gastro.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Wakai T, et al. Late resistance to imatinib therapy in a metastatic gastrointestinal stromal tumour is associated with a second KIT mutation. Br J Cancer. 2004;90:2059–2061. doi: 10.1038/sj.bjc.6601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beverly LJ, Felsher DW, Capobianco AJ. Suppression of p53 by Notch in lymphomagenesis: Implications for initiation and regression. Cancer Res. 2005;65:7159–7168. doi: 10.1158/0008-5472.CAN-05-1664. [DOI] [PubMed] [Google Scholar]
- 25.Chin L, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- 26.Jain M, et al. Sustained loss of a neoplastic phenotype by brief inactivation of MYC. Science. 2002;297:102–104. doi: 10.1126/science.1071489. [DOI] [PubMed] [Google Scholar]
- 27.Moody SE, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Urlinger S, et al. Exploring the sequence space for tetracycline-dependent transcriptional activators: Novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Podsypanina K, Li Y, Varmus HE. Evolution of somatic mutations in mammary tumors in transgenic mice is influenced by the inherited genotype. BMC Med. 2004;2:24. doi: 10.1186/1741-7015-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Letai A, et al. Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell. 2004;6:241–249. doi: 10.1016/j.ccr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 32.Beer S, et al. Developmental context determines latency of MYC-induced tumorigenesis. PLoS Biol. 2004;2:e332. doi: 10.1371/journal.pbio.0020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pear WS, et al. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 1998;92:3780–3792. [PubMed] [Google Scholar]
- 34.Debies MT, et al. Tumor escape in a Wnt1-dependent mouse breast cancer model is enabled by p19Arf/p53 pathway lesions but not p16 Ink4a loss. J Clin Invest. 2008;118:51–63. doi: 10.1172/JCI33320. [DOI] [PMC free article] [PubMed] [Google Scholar]