Bile acids are potent detergents and are essential for efficient digestion and absorption of dietary fat. Bile acids are synthesized by the liver from cholesterol, excreted into bile, and stored in the gallbladder. After ingestion of a fat-containing diet, the gallbladder contracts, forcing bile to enter the lumen of the proximal duodenum. Lipids in bile acid emulsions are absorbed in the proximal jejunum, whereas the majority of bile acids are absorbed in distal ileum, whereupon they are transported back to the liver through the portal blood. Each cycle of the bile acid enterohepatic circulation pathway is associated with a small (≈5%) loss, which is replaced through de novo hepatic synthesis (1).
The same amphipathic properties that allow bile acids to emulsify lipids also make them membrane-disruptive agents (2). Thus, limiting the accumulation of bile acids within cells making up the enterohepatic circulation helps to prevent cytotoxicity. Because bile acids have vastly different structures and abilities to emulsify lipids (2), the composition of the bile acid pool can markedly influence their ability to facilitate lipid digestion and absorption.
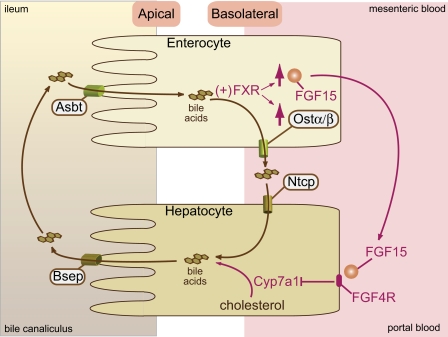
The itinerary of bile acids involves transport across apical and basolateral surfaces of cells in the liver (hepatocytes) and intestine (enterocytes) (Fig. 1). Identification of the apical ileal sodium-dependent bile acid transporter (Asbt) explained how bile acids are efficiently reabsorbed by the ileal epithelial cell (3). This discovery stimulated the search for a transporter that would efficiently transport bile acids across the basolateral surface membrane of the ileal enterocyte.
Fig. 1.
Regulation of bile acid synthesis by an ileal bile acid sensing system. Bile acids are synthesized from cholesterol in the liver. The rate-limiting enzyme in the pathway is cholesterol-7α-hydroxylase (Cyp7a1). Bile acids are secreted across the apical (canalicular) membrane into the bile canaliculus via the Bsep transporter). Bile is then released into the duodenum and flows through the intestinal lumen, where it emulsifies lipids. Lipids are primarily absorbed by enterocytes in the jejunum. The bile acids are transported through the apical Asbt transporter across into ileal enterocytes. Bile acids activate the farnesoid X receptor/retinoid X receptor (FXR/RXR), leading the induction of Fgf15 and Ostαβ. The bile acids are then released into the portal circulation via basolateral Ostα/β and reabsorbed by the hepatic basolateral transporter, Ntcp. FGF15 binds to the FGF4 receptor, leading to the repression of Cyp7a1 expression and reduced bile acid synthesis.
In a recent issue of PNAS, Rao et al. (4) identify Ostα/Ostβ as the heterodimeric bile acid transporter responsible for the basolateral transport of bile acids out of the ileal epithelial cell. The new insights provided by these studies provide an integrated pathway through which the expression of multiple distinct high-affinity bile acid transporters efficiently retrieves bile acids from the distal ileum and delivers them to the liver.
The farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily and, as a heterodimer with RXR, is activated by bile acids. Because FXR induces the expression of Ostα and Ostβ, it seemed plausible that the Ostα/β heterodimer could be the ileal basolateral bile acid transporter (5). Whereas cell culture studies showed that coexpression of Ostα and Ostβ could enhance the apical-to-basolateral transport of taurocholate, it was not clear that this is the major transporter responsible for basolateral ileal bile acid transport in vivo (6). The studies of Rao et al. (4) clearly show that the expression of Ostα is necessary for the hetero dimeric complex Ostα/Ostβ to be expressed at the basolateral surface and for the bile acids to be efficiently absorbed in mice. Moreover, using everted gut sacs, Rao et al. show that the trans-ileal transport of taurocholate is reduced by >80% in Ostα−/− mice vs. sacs from wild-type mice. These data clearly establish the Ostα/β heterodimer as the major transporter responsible for the ileal basolateral transport of bile acids.
A parallel story involves the mechanism by which bile acid levels in the enterocytes synchronize bile acid production in hepatocytes. This story emerges from the paradoxical observation that although feeding animals diets enriched in bile acids suppresses their own production by decreasing CYP7A1 expression, adding bile acids to the medium of isolated hepatocytes has no effect (7). FXR is essential for bile acid feedback regulation through the suppression of Cyp7a1 expression, but quite surprisingly, it is intestinal FXR, not hepatic FXR, that mediates the tuning of this pathway (8). The abilities of individual bile acids to activate FXR (9, 10) correlate with their detergent properties (2). Thus, nonamphipathic bile acids (ursodeoxycholic acid) neither activate FXR nor are helpful in digestion and absorption of lipids. Uptake of bile acids that activate FXR by the ileal enterocyte induces the expression of fibroblast growth factor is (FGF15) (11–13). FGF15 is released into the portal circulation and then binds to the hepatocyte basolateral receptor FGFR4, leading to the repression of Cyp7A1 expression (8, 12–15). In short, FGF15 is the link between FXR sensing of bile acids in enterocytes and expression of Cyp7A1 in hepatocytes.
Rao et al. (4) show that the expression of Asbt, Ostα/β, and FGF15 is confined mainly to enterocytes located in a fairly narrow portion located in the distal ileum. Thus, a distinct population of intestinal epithelial cells is responsible for the FGF15-mediated communication between the intestine and the liver.
Bile acids have vastly different structures and abilities to emulsify lipids.
Because ileal FXR acts as a bile acid sensor, this pathway synchronizes hepatic bile acid production to the enterocyte bile acid pool size. Accordingly, reduction of the enterocyte bile acid pool size by deletion of the apical bile acid transporter, Asbt, leads to an induction of hepatic Cyp7A1 (16). Conversely, Rao et al. (4) show that deletion of Ostα increases the enterocyte bile acid pool size, but reduces the overall bile acid pool size, because of the repression of Cyp7A1. Furthermore, the inability of nonamphipathic bile acids to activate FXR in the distal ileal epithelial cell would prevent FXR-mediated induction of FGF15 and repression of CYP7A1. As a result, enhanced expression of CYP7A1 would increase the production of primary amphipathic bile acids (e.g., cholic and chenodeoxycholic acids), thus maintaining the composition of the bile acid pool in a manner that ensures efficient digestion and absorption of lipids.
These findings expose the complex circuitry through which the amount and composition of bile acids recovered by the distal ileal epithelial cell adjusts de novo hepatic bile acid synthesis, thus promoting efficient digestion and absorption of essential lipid nutrients by maintaining both the size and the composition of the bile acid pool.
Footnotes
The authors declare no conflict of interest.
See companion article on page 3891 in issue 10 of volume 105.
References
- 1.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 2.Heuman DM. Bile salt-membrane interactions and the physico-chemical mechanisms of bile salt toxicity. Ital J Gastroenterol. 1995;27:372–375. [PubMed] [Google Scholar]
- 3.Wong MH, Oelkers P, Craddock AL, Dawson PA. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994;269:1340–1347. [PubMed] [Google Scholar]
- 4.Rao A, et al. The organic solute transporter α-β, Ostα-Ostβ, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci USA. 2008;105:3891–3896. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankenberg T, et al. Regulation of the mouse organic solute transporter α-β, Ostα-Ostβ, by bile acids. Am J Physiol. 2006;290:G912–G922. doi: 10.1152/ajpgi.00479.2005. [DOI] [PubMed] [Google Scholar]
- 6.Dawson PA, et al. The heteromeric organic solute transporter α-β, Ostα-Ostβ, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trawick JD, et al. Rat hepatoma L35 cells, a liver-differentiated cell line, display resistance to bile acid repression of cholesterol 7α-hydroxylase. J Lipid Res. 1996;37:588–598. [PubMed] [Google Scholar]
- 8.Kim I, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Parks DJ, et al. Bile acids: Natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Pircher PC, Schulman IG, Westin SK. Regulation of complement C3 expression by the bile acid receptor FXR. J Biol Chem. 2005;280:7427–7434. doi: 10.1074/jbc.M411473200. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez A, et al. Bile acids decrease hepatic paraoxonase 1 expression and plasma high-density lipoprotein levels via FXR-mediated signaling of FGFR4. Arterioscler Thromb Vasc Biol. 2005;26:301–306. doi: 10.1161/01.ATV.0000195793.73118.b4. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki T, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Yu C, Wang F, Jin C, Huang X, McKeehan WL. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. J Biol Chem. 2005;280:17707–17714. doi: 10.1074/jbc.M411771200. [DOI] [PubMed] [Google Scholar]
- 15.Shih DM, et al. A role for FXR and human FGF-19 in the repression of paraoxonase-1 gene expression by bile acids. J Lipid Res. 2006;47:384–392. doi: 10.1194/jlr.M500378-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Dawson PA, et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–33927. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]