Abstract
A current paradigm in immunology is that the strength of T cell responses is governed by antigen dose, localization, and costimulatory signals. This study investigates the influence of antigen kinetics on CD8 T cell responses in mice. A fixed cumulative antigen dose was administered by different schedules to produce distinct dose-kinetics. Antigenic stimulation increasing exponentially over days was a stronger stimulus for CD8 T cells and antiviral immunity than a single dose or multiple dosing with daily equal doses. The same was observed for dendritic cell vaccination, with regard to T cell and anti-tumor responses, and for T cells stimulated in vitro. In conclusion, stimulation kinetics per se was shown to be a separate parameter of immunogenicity. These findings warrant a revision of current immunization models and have implications for vaccine development and immunotherapy.
Keywords: antigen presentation, antiviral immunity, CD8 T cell responses, tumor vaccine
The immune system has evolved to respond to pathogens (1–3). Therefore, pathogens' characteristics have been adopted to enhance vaccine efficacy. For example, vaccines are often delivered as emulsions or particles with comparable dimensions to pathogens (4), and pathogen-associated molecular patterns (PAMPs) stimulating the immune system via Toll-like receptors (TLR) are used as adjuvants to activate antigen-presenting cells (4, 5). Another hallmark of pathogens is that replication exposes the immune system to increasing amounts of antigen and PAMPs.
The present study investigates whether the dose-kinetics of antigen is a separate parameter of immunogenicity. Mice were immunized with a fixed cumulative dose of antigenic peptides and CpG ODN or with antigen-pulsed dendritic cells (DCs), but following different kinetics. MHC class-I binding peptides were chosen, because their short in vivo half-life allows the production of sharp kinetics (6, 7). When administered in a dose-escalating fashion, the vaccines stimulated much stronger CD8 T cell responses than when administered as a single shot or at uniform daily doses. Thus, the immune system seems to interpret exponentially increasing antigenic stimulation per se as a signal related to pathogen replication, and consequently enhances T cell activity.
Results
Exponentially Increasing Antigenic Stimulation Enhances CD8 T Cell Responses.
Transgenic TCR318 T cells (106 cells) were transferred into C57BL/6 mice, which were immunized with 125 μg of gp33 peptide and 12.5 nmol of CpG using different protocols [supporting information (SI) Table 1 and Fig. 1C]. Mice infected with 250 pfu of lymphocytic choriomeningitis virus (LCMV) served as a positive control. On day 6 (Fig. 1A), CD8 T cell responses were quantified by intracellular IFN-γ staining in blood. Exponentially increasing immunizations produced a stronger response than uniform daily doses of gp33 and CpG (P = 0.0001). If either one of the vaccine components was given as a single dose, the efficacy was weak but still significant compared with the naive control (2.23 ± 0.84% vs. 0.19 ± 0.12% P = 0.02); day 6 represents the peak of the immune, and the response retracted after day 12 (data not shown). Also in C57BL/6 WT mice (Fig. 1B), dose escalation enhanced induction of IFN-γ-producing CD8 T cells (2.1 ± 0.4%) compared with other vaccination protocols (P < 0.008), which barely induced detectable frequencies. Similar observations were made in HLA A2.1 transgenic mice immunized with the influenza matrix peptide (data not shown).
Fig. 1.
Exponentially increasing doses of both gp33 peptide and CpG enhance CD8 T cell response. C57BL/6 mice injected with 1 × 106 TCR CD8 T cells (A) or C57BL/6 WT mice (B) were s.c. immunized with identical cumulative vaccine doses, but using the immunization schedules s1–s6 illustrated in C. s1, single dose of gp33 peptide (125 μg) and CpG (12.5 nmol); s2, equivalent doses of gp33 peptide and CpG; s3, exponentially decreasing doses of gp33 peptide and CpG; s4, exponentially increasing doses of gp33 peptide and CpG; s5, exponentially increasing doses of gp33 peptide and an initial single dose of CpG; s6, initial single dose of gp33 peptide and exponentially increasing doses of CpG; naive, untreated mice; LCMV, mice immunized with 250 pfu of LCMV i.v. on day 0. CD8 T cells were analyzed for IFN-γ production after in vitro restimulation of blood lymphocytes with gp33 peptide on day 6 (A), or day 8 (B). Values represent the means and SEM of four mice per group. The experiment was repeated twice.
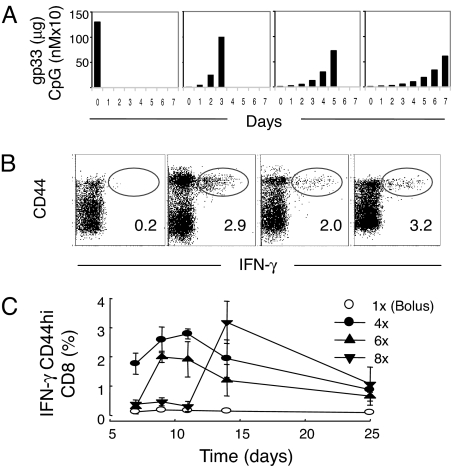
Compared with single bolus injection, exponential vaccination over 4–8 days (Fig. 2A), significantly enhanced the immune response at its peak, which was 4–7 days after the last injection (Fig. 2B). Four weeks later, there was no significant difference in the number of CD44-positive resting memory cells between the different groups (Fig. 2C). T helper (Th) epitopes can be crucial for functional CD8 T cell immunity (8–10), but exponential vaccination with a mixture of the class-I gp33 and the class-II binding gp61 peptides did not affect the outcome (data not shown), suggesting that the above findings were Th independent.
Fig. 2.
Four days of antigenic stimulation is necessary for optimal for CD8-T cell induction. C57BL/6 mice were immunized with fixed cumulative dose of 125 μg of gp33 peptide and 12.5 nmol of CpG according to different schedules (A) with peaks at day 0 (bolus), day 3, day 5, or day 7. At different time points thereafter, mice were bled, and the CD44 expression and the IFN-γ secretion of CD8 T cells after restimulation in vitro with gp33 peptide were analyzed (B and C). The FACS density blots illustrate the frequencies of CD44hi and IFN-γ-producing CD8-positive lymphocytes as measured by FACS at the peak of the immune response, and the numbers show the mean percentage of IFN-γ-producing CD44hi CD8+ T cells. The mean percentage of IFN-γ-producing CD44hi CD8+ T cells is also illustrated as a function of time (C). One of two similar experiments is shown (n = 3–4).
Exponentially Increasing Stimulation Prolongs T Cell Proliferation.
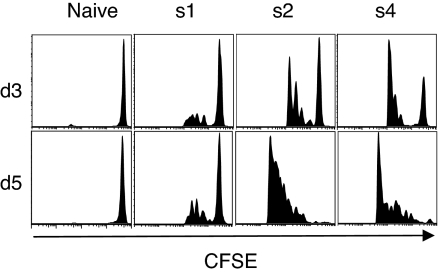
To test how the kinetics of immunization affected the proliferation of carboxyfluorescein diacetate-succinimidyl ester (CFSE)-labeled TCR318 CD8 T cells, mice were injected with a single dose [schedule 1 (s1)], uniform daily doses (s2), or exponentially increasing doses (s4) of gp33 peptide and CpG. A bolus injection of gp33 peptide and CpG triggered the cells to divide after 3 days (Fig. 3). On day 5, precursor cells still entered division although to a lower extent than at day 3. By day 7, the CFSE-labeled cells had ceased to enter new divisions (data not shown). In contrast, four equally sized or four dose-escalating vaccine doses produced much stronger stimuli for proliferation, even at day 3 after priming, despite not yet having received the whole immunization regime. Moreover, the division index, i.e., the average number of division undergone, was significantly higher (P < 0.05 by Mann–Whitney) for s4 than for s2.
Fig. 3.
Exponential immunization favors prolonged T cell proliferation. C57BL/6 mice received by i.v. adoptive transfer 1.5 × 106 CFSE-labeled and magnetic cell separation (MACS) selected CD8 cells from TCR318 spleens and LNs and 1 day later were immunized s.c. with fixed cumulative vaccine doses of gp33 peptide and CpG according to the immunization protocol s1, s2, s4 or left untreated as described above. Lymphocytes were isolated by tail bleeding and analyzed for CD8 expression and CFSE staining by flow cytometry.
Exponentially Increasing Immunization Enhances Antiviral Immunity.
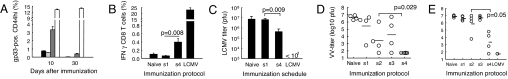
To evaluate the functional relevance of the above observations, immunized C57BL/6 mice were challenged with LCMV or a LCMV glycoprotein-expressing vaccinia virus (vacc-gp) (11); protection against both viruses depends on CD8 T cells (12). Exponentially increasing doses of gp33 and CpG induced significantly higher frequencies of gp33-tetramer-positive memory (CD44hi) cells (Fig. 4A) and of IFN-γ-producing effector and memory cells (Fig. 4B) than did a bolus vaccination. On day 30, all mice were challenged with 250 pfu of LCMV. Whereas bolus-vaccinated mice were not significantly protected against viral replication (Fig. 4C), exponential vaccination induced strong protection with LCMV titers 10- to 20-fold higher than naive or bolus-vaccinated mice (P < 0.009). Similarly, a challenge with 1.5 × 106 pfu of vacc-gp 8 (Fig. 4D) or 24 (Fig. 4E) days after priming revealed significantly inhibited viral replication in mice immunized with the dose-escalating protocol as compared with s1 and s2.
Fig. 4.
Exponentially increasing doses of both gp33 peptide and CpG enhance antiviral CD8 T cell responses. C57BL/6 mice were s.c. immunized with fixed cumulative doses of gp33 peptide and CpG using the different immunization schedules (s1–s4; see also SI Table 1), left untreated (Naive), or immunized by i.p. injection of 250 pfu of LCMV on day 0 (n = 4). The mice were bled on day 10 and day 30 for analysis of gp33-specific effector or memory CTLs using gp33-MHC-tetramers and flow cytometry (A) or on day 30 for analysis of IFN-γ-producing CD8 T cells after restimulation in vitro with gp33 (B) (A describes on day 10 and day 30, from left to right, Naive, s1, s4, and LCMV). On day 30 (C), the mice were challenged i.p. with 250 pfu of LCMV (C). Alternatively, the mice were challenged on day 8 (D) or 24 (E) with 1.5 × 106 pfu of vacc-gp. Four or five days later, spleens or ovaries were harvested for determination of LCMV or vaccinia titers, respectively.
Exponential Immunization Delays Maximal Activation of DCs.
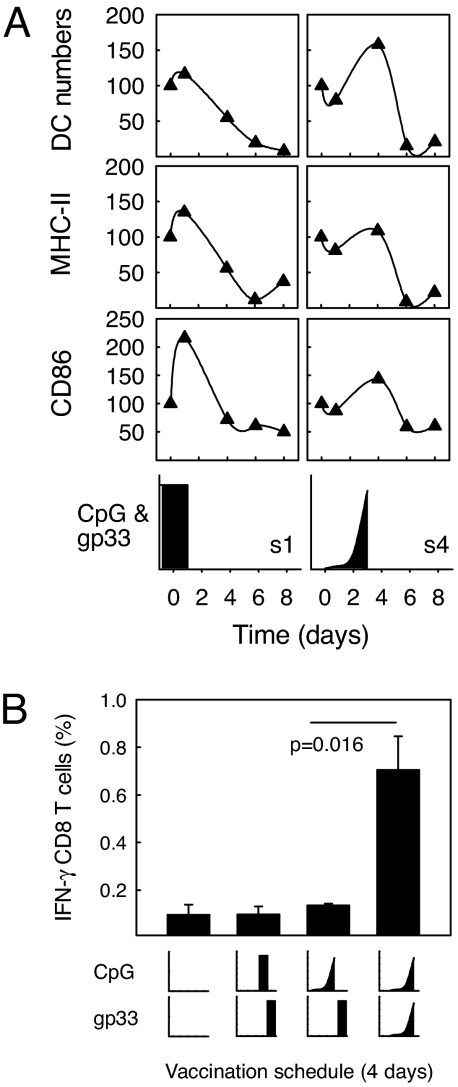
To test whether the immunization kinetics affected the numbers and activation status of DCs, mice were immunized with exponentially increasing doses (s4) or by bolus injection (s1). The kinetics type did not crucially affect the numbers of DCs in the draining lymph nodes, or their activation status as monitored by MHC-class II (I-Ab) and CD86 expression (Fig. 5A), but the peaks of DC numbers and activation were separated by 2–3 days.
Fig. 5.
Exponential immunization delays DC recruitment. C57BL/6 mice were immunized with gp33 peptide and CpG according to the immunization protocol s1 (bolus injection) and s4 (exponentially increasing doses) as described in Fig. 1. The vaccines were administered s.c. in the inguinal region. After 1, 4, 6, and 8 days, the iLN were removed, and single cell suspensions thereof were analyzed by flow cytometry for the expression of the DC marker CD11c, as well as CD86 and the MHC class II marker I-Ab (A). The results in the Top are illustrated as the relative frequency of cells expressing both CD11c and I-Ab. The Middle illustrates the relative MFI of CD86 expression and the Bottom the relative MFI of I-Ab expression on DCs. In all cases, the results are normalized to that of naive controls (day 0) for which reason the starting point is always 100. Mice were also immunized with gp33 peptide and CpG according to modified protocols as illustrated (B). One group received a CpG bolus on day 3 and a gp33 peptide bolus on day 4. One group received exponentially increasing CpG doses on days 0–3 followed by a gp33 peptide bolus on day 4. The last group received exponentially increasing doses of gp33 peptide and CpG on days 1–4 as described above (s4). The frequency of IFN-γ-producing CD8 T cells was measured in peripheral blood on day 10. The results show means and SEM of one of two comparable experiments (n = 3).
Because DC activation reached its maximum 1 day after the maximal CpG dose, independent of the type of immunization kinetics, we tested whether exponentially vaccination was beneficial for CD8 T cell induction, simply because the peptide is delivered when DCs are mostly activated. If so, administration of a high peptide dose 1 day after a bolus CpG injection or after the last dose of exponentially given CpG should result in a T cell response comparable with giving both peptide and CpG in a dose-escalating fashion. However, prestimulation with CpG resulted in immune responses that were significantly lower than those produced by concomitant dose-escalating gp33 and CpG administration (Fig. 5B; P = 0.016).
Exponentially Increasing Numbers of Peptide-Pulsed DCs Enhances Tumor Protection.
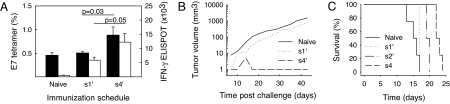
To further investigate the contribution of APCs, C57BL/6 mice were immunized with 1.11 × 105 peptide-pulsed DCs by different kinetics (SI Materials and Methods). Bone-marrow derived DCs were loaded with the HPV E7 peptide, and then injected as a bolus on day 1 (s1′), or in an increasing (s4′) pattern on days 1 (103 cells), 3 (104 cells), and 6 (105 cells). The vaccines were administered directly into a s.c. lymph node (LN) to ensure that all DCs were available for T cell priming. Again, DC dose-escalation (s4′) induced higher numbers of antigen-specific CD8 T cells than a bolus injection of DCs (s1′) as shown for both tetramer and IFN-γ enzyme-linked immunospot assays (ELISPOTs) on days 17 and 22, respectively (Fig. 6A). Importantly, mice vaccinated with the dose-escalating protocol, but not the bolus, rejected a challenge with the HPV-transformed tumor cell line C3.43 (Fig. 6B). By the same token, mice immunized with the s4′-protocol of DCs loaded with the VSV np52 peptide showed improved survival after a challenge with mouse lymphoma cells EL-4 transfected to express the VSV nucleoprotein (Fig. 6C). The survival of s4′ immunized was also significant better than mice immunized according to the s2′ protocol with DCs given in uniform numbers on all 3 days (P = 0.0084).
Fig. 6.
Exponential immunization with peptide-loaded DCs induces strong T cell and anti-tumor responses. Groups of 10 C57BL/6 mice were immunized by intralymphatic injection of bone-marrow-derived DCs loaded with HPV17 E7 peptide (A and B). The DCs were given as a bolus on day 1 (s1′) or equally (s2′) or exponentially increasingly distributed on day 1, day 3, and day 6 (s4′). Naive mice were used as negative controls. (A) On day 17 and on day 22, the frequency of E7-tetramer positive CD8 T cells in peripheral blood was analyzed by flow cytometry (means and SEM; n = 10), and IFN-γ ELISPOTs were analyzed from spleens (means and SEM; n = 7). (B) On day 21, three vaccinated mice and 10 naive mice were challenged with the HPV-transformed tumor cell line C3.43. Tumor progression was monitored by caliber measurements (mm) from which tumor volumes were calculated. (C) C57BL/6 mice were immunized by s.c. injection of DCs loaded with the VSV np53 peptide (n = 4). DCs (1.11 × 105) were given as a bolus on day 1 (s1′) or as equal (s2′) or dose-escalating doses (s4′) on days 1, 3, and 6. Naive mice were used as controls. On day 14, all mice were challenged with 106 EL-4 N.1 cells i.p (50). Log-rank tests of Kaplan Meier curves: s4′ ≠ s2′, P = 0.0084; s2′≠ s1′: P = 0.0082; s1′ ≠ Naive, P = 0.401.
Exponentially Increasing Antigenic Stimulation Enhanced IL-2 Production of T Cells: The Impact of Antigen Kinetics Occurs at the Level of T Cell Clones.
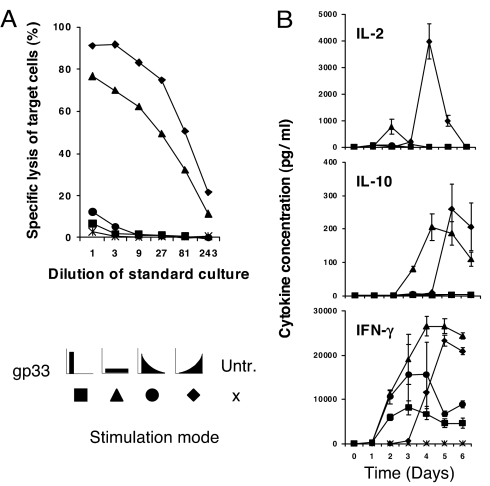
We next investigated whether the above observations could be explained at the level of a T cell clone, or whether they were the result of in vivo T cell selection processes, involving T cell clonotypes of different affinity, avidity and functionality. Splenocytes from TCR318 mice were stimulated in vitro with 10−9 M gp33, either as a bolus on day 0, with exponentially increasing or decreasing doses over four days, or with a uniform dose every day; the splenocytes also contain macrophages, DCs and B cells. IL-2, IL-10, and IFN-γ were determined daily in supernatants, and on day 6, cytotoxic T lymphocyte (CTL) activity was determined in a 51Cr release assay (Fig. 7). Exponentially increasing antigen doses induced the strongest CTL response, followed by daily administrations of a uniform dose, whereas antigen given as a bolus or in a decreasing dose profile generated weaker responses. The difference was even greater, when cells were stimulated with one tenth of the peptide doses (data not shown). The cytotoxicity correlated with the production of IL-2. Uniform daily stimulation induced high amounts of IL-10 with an earlier onset as compared with exponentially increasing stimulation. IFN-γ was transiently produced at an early stage in cells stimulated with an antigen bolus or decreasing amounts of antigen. In contrast, daily stimulation by uniform or exponentially increasing doses induced higher amounts of IFN-γ.
Fig. 7.
Exponential in vitro stimulation of CD8 T cells enhances IL-2 production and cytotoxicity. TCR318 T cells (1 × 105) were cocultured with 2 × 106 irradiated syngeneic splenocytes serving as feeder cells. Cultures were stimulated with the same total doses of gp33, but following different kinetics: ■, a single dose of 10−9 M at day 0; ▲, four equal doses of 0.25 × 10−9 M during 4 days; ●, four exponentially decreasing doses of 10−9, 10−10, 10−11, and 10−12 M at days 0, 1, 2, and 3, respectively; and ♦, four exponentially increasing doses of 10−12, 10−11, 10−10, and 10−9 M at days 0, 1, 2, and 3, respectively. ×, control culture without gp33 peptide. (A) After 6 days, CTL activity was measured by using gp33-pulsed EL-4 target cells in a 5-h 51Cr-release assay. Values represent means of duplicate cultures. One representative of two similar experiments is shown. (B) Supernatants were analyzed for IL-2, IL-10, and IFN-γ. Values represent means of triplicate culture wells, and one representative of two experiments is shown.
Discussion
This study demonstrates that the kinetics of antigenic stimulation is a key parameter of immunogenicity. Exponentially increasing antigen doses stimulated stronger immune responses than constant stimulation with uniform doses or immunization with a bolus. Such dose escalation reflects the increasing amounts of antigen associated with highly virulent pathogens and calls for strong immune response. In contrast, nonreplicating pathogens represent less danger, require less T cell activity and are well controlled by innate immunity. Likewise, maximal T cell responses were induced by antigenic stimulation over 4 days, whereas rapid exponential growth of antigenic stimulation over 1–3 days induced weaker T cell responses. This observation reflects the fact that proliferation and differentiation of T cells takes several days, and it is impossible for the immune system to race a pathogen that overwhelmingly infects the host within shorter time.
Likely candidates for mediating this perceptive faculty are the DCs. Whereas the different vaccination protocols did not induce different numbers and activation levels of DCs in the lymph node, they differed in the time required to produce the peak of DC activation and numbers, which by dose-escalating vaccination was delayed by 3 days as compared with bolus vaccination. Because both vaccination regimes induced peak DC activation 1 day after the maximal vaccine dose was administered, it could imply that an optimal vaccination schedule would be a single injection of antigen 1 day after the adjuvant injection. However, our data demonstrates that the latter was inferior to doses of adjuvant and antigen that escalated exponentially in parallel. This result illustrates the importance for antigen and adjuvant to be administered together and not separated in time (1, 3, 13).
That the observed effect was not exclusively mediated by the number of antigen presenting DCs nor by their activation status, was demonstrated by immunizing mice with DCs that were pulsed with MHC class-I binding peptides ex vivo. Also here, exponentially increasing vaccination was superior to other schedules. In these experiments, DC activation was kept at the same level throughout immunization, and the total number of DCs was the same, demonstrating that the strength of the immune response is most probably enhanced by the synchronization of antigen-presenting cell numbers and the frequency of available T cell precursors. Whereas the low frequency of specific T cells during the early response can be efficiently stimulated with a low number of antigen pulsed DCs, it seems important to restimulate the high frequency of specific T cells during the later primary response with a high number of DCs. Moreover, because proliferation is an exponential process, it is likely that exponential vaccination may match this process by allowing quantitative synapses generation on available progenitor CD8 T cells (14). Indeed, whereas dividing cells were observed for only 2–3 days after bolus immunization, exponentially increasing stimulation triggered precursor cells to enter division over a period of at least one week.
Furthermore, antigen persistency has been accepted to promote efficient immune responses (15). Also, when TCR signaling persists for hours, it has a cumulative effect that is necessary for the maintenance of the immunological synapse, for T cell proliferation and for IL-2 production (14). To what extent it applies for exponential vaccination schedules is currently not known. However, our work does not necessarily imply that effector T cells require daylong stimulation in vivo. Because vaccination with increasing antigen kinetics also enhances clonal expansion of specific T cells, stimulation of the single T cell must not be prolonged. Hence, our work is also compatible with the temporal summation model that offers an explanation for how signals originating from serially triggered TCRs are accumulated and integrated over the period required for T cell activation (16–18).
The production of IL-2 is a hallmark of T cell activation and plays a key role in regulating several stages of the T cell response. Engagement of the TCR (signal 1) and costimulatory molecules (signal 2) induces only limited clonal expansion of T cells. Extensive amplification of T cells as well as differentiation into effector cells requires signaling via the IL-2R (signal 3) (19), and autocrine IL-2 production by CD8 T cells subsequently triggers in vivo CD8 T cell expansion (19, 20). However, IL-10 is a main inhibitor of T cell proliferation partly via modulation of DCs (21). Consistent with these findings, we observed that exponentially increasing antigen doses stimulated IL-2 production in vitro more efficiently than other regimens and that production of immunosuppressive IL-10 occurred at a later time point compared with constant antigenic stimulation. These phenomena have furthermore shown to be accompanied by higher T cell avidity, which again is crucial for efficient interaction between T cells and DCs (22). Thus, these in vitro observations may explain our in vivo findings and also suggest that, at a clonal level, T cells are capable of decoding the kinetics of antigen exposure.
The more an antigen resembles a virulent pathogen, the more immunogenic it is likely to be. High antigen doses (23–25) and the presence of antigen in lymphoid organs (26, 27), both mimicking widespread replication of a virulent pathogen, induce strong immune responses. Particulate antigens that resemble the structure of viruses or bacteria induce stronger immune responses than soluble antigens (4, 28, 29). Finally, presentation of an antigen together with pathogen components such as bacterial DNA, lipopolysaccharide or viral RNA strongly enhances the immune response. Based on this study, it seems that exponentially increasing antigenic stimulation (a further hallmark of virulent pathogens) is also recognized by the immune system as a pattern associated with pathogens, driving strong immune responses.
The presented findings offer an additional explanation for the observation that live attenuated vaccines usually induce strong and long lasting immune responses after only one injection, such that many viral vaccines of this type have efficacies >90% (30). In contrast, vaccines consisting of killed microorganisms, toxins, subunit vaccines including peptide vaccines, or naked DNA vaccines, are of considerably lower efficacy and boosting immunizations are essential. Whereas live vaccines produce increasing antigen doses that call for strong immune responses, nonreplicating vaccines produce a decreasing antigen profile, which we demonstrate here to be a weak stimulus of T cells. Because the trend in vaccine development goes toward subunit vaccines that are safer than live vaccines, more consideration must be given to the dose-kinetics of antigenic stimulation.
Current strategies to improve the efficiency of vaccination aim at increasing the duration of antigen presentation (31–40). As also shown in this study, constant daily antigenic stimulation enhanced the CD8 T cell response when compared with single shot administration. However, our findings show that optimal T cell induction requires an exponentially increasing immunization regimen. Therefore, a vaccine should not be administered as one single bolus (a “shot”) or in a depot formulation, but rather in a dose escalating fashion over several consecutive days. This strategy could be used for immunotherapeutic approaches to enhance T cell responses against chronic infectious diseases or cancer.
Materials and Methods
Mice.
Female C57BL/6 mice were purchased from Harlan or The Jackson Laboratory at 6–12 weeks of age. TCR318 transgenic mice expressing a T cell receptor specific for the lymphocytic choriomeningitis virus (LCMV) glycoprotein H-2Db epitope aa33-41 (gp33) on a C57BL/6 background were obtained from M. F. Bachmann and R. M. Zinkernagel (41). HHD transgenic mice expressing HLA A2.1 were originally obtained from the MannKind Corporation (42) and were bred and kept in a SPF facility at the University Hospital Zurich according to Swiss guidelines.
Viruses, Peptides, and Oligodeoxynucleotides.
LCMV isolate WE titers were determined by using a focus-forming assay on MC57 fibroblasts (43). Recombinant vaccinia virus expressing the LCMV glycoprotein (vacc-gp) (44) was grown and plaqued on BSC40 cells (45). LCMV peptides gp33 (KAVYNFATM) and gp61 (GLNGPDIYKGVYQFKSVEFD) and VSV peptide np52 (SDLRGYVYQGLKSG) were purchased from EMC Microcollections (Tübingen, Germany). Influenza matrix peptide (GILGFVFTL) was obtained from Neosystems (Strasbourg, France). The HPV16 E7 (aa49-57; RAHYNIVTF) peptide used was synthesized at MannKind Corporation (Valencia, CA) to >99% purity. Phosphorothioate-modified CG-rich oligodeoxynucleotide CpG 1668 (5′-TCC ATG ACG TTC CTG AAT AAT-3′) was synthesized by Microsynth (Balgach, Switzerland). CpG ODN was chosen as adjuvant because it strongly enhances CD8 T cell responses (46, 47) and is cleared from plasma with a half-life of 30–60 min (48). In tissue, CpG ODN is relatively stable with a half-life of 48 h (49).
Peptide-CpG Immunization Schedules.
The different immunization schedules s1 to s6 were designed to deliver the same cumulative dose of 125 μg of peptide and 12.5 nmol of CpG over a time frame of 1–4 days (SI Table 1). Schedules 3 (s3) and 4 (s4) follow an exponentially decreasing or increasing pattern at 5-fold dilution steps. In some experiments, C57BL/6 mice received 106 sex-matched TCR318 cells to increase precursor T cell frequencies and facilitate assessment of the immune response. One day later, the recipients were s.c. vaccinated in the neck region with peptide and CpG at the indicated doses.
Assessment of Antiviral Immunity in Vivo.
Vaccinated mice were infected with 1.5 × 106 pfu of vacc-gp. Five days later, ovaries were isolated and the vaccinia titers were determined on BSC 40 cells (45). Alternatively, the mice were infected with 250 pfu of LCMV-WE, and viral titers in spleens were determined on MC57 cells (43).
Cytotoxicity Assay and Cytokine Secretion Analysis.
TCR318 transgenic T cells (105) were cultured with syngeneic irradiated feeder cells (2 × 106 cells per well; 2,000 rad) for 6 days in 24-well plates and pulsed with the indicated amounts of gp33 peptide. Effector cells were then resuspended in 300 μl of fresh medium and 3-fold dilutions were made. EL-4 cells were pulsed with 10−6 M gp33 peptide and used as target cells in a 5-h 51Chromium release assay (44). Radioactivity in cell culture supernatants was measured with a Cobra II Counter (Canberra Packard, Downers Growe, IL). Nonradioactive culture supernatants were assessed daily for IFN-γ, IL-2, and IL-10 concentrations using bead-multiplex-assays and flow cytometry.
Statistical Analysis.
Nonparametric or nonnormally distributed data were analyzed by using the Mann–Whitney U test or by Kurskal-Wallis ANOVA. The comparison of Kaplan Meier survival curves was performed by using log rank test.
Supplementary Material
Acknowledgments.
We thank R. M. Zinkernagel and M. F. Bachmann for comments and for providing virus, gp33 tetramers, and TCR318 mice. The HPV transformed tumor cell line C3.43 was provided by W. Martin Kast (Loyola University, Chicago, IL). We also thank Adrian Urwyler and Anna Flace for technical assistance and Nicole Graf for help with statistics. This project was supported by Swiss National Science Foundation Grant SNF 3200B0-100622/1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706296105/DC1.
References
- 1.Janeway CA., Jr Cold Spring Harb Symp Quant Biol. 1989;54:1–13. [PubMed] [Google Scholar]
- 2.Zinkernagel RM. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 3.Germain RN. Nat Med. 2004;10:1307–1320. doi: 10.1038/nm1159. [DOI] [PubMed] [Google Scholar]
- 4.O'Hagan DT, Valiante NM. Nat Rev Drug Discov. 2003;2:727–735. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen P, Senti G, Martinez Gomez JM, Storni T, von Beust BR, Wuthrich B, Bot A, Kundig TM. Clin Exp Allergy. 2005;35:1591–1598. doi: 10.1111/j.1365-2222.2005.02384.x. [DOI] [PubMed] [Google Scholar]
- 6.Falo LD, Jr, Colarusso LJ, Benacerraf B, Rock KL. Proc Natl Acad Sci USA. 1992;89:8347–8350. doi: 10.1073/pnas.89.17.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widmann C, Maryanski JL, Romero P, Corradin G. J Immunol. 1991;147:3745–3751. [PubMed] [Google Scholar]
- 8.Johansen P, Stamou P, Tascon RE, Lowrie DB, Stockinger B. Eur J Immunol. 2004;34:91–97. doi: 10.1002/eji.200324231. [DOI] [PubMed] [Google Scholar]
- 9.Shedlock DJ, Shen H. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 10.Sun JC, Bevan MJ. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaech SM, Wherry EJ, Ahmed R. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 12.Binder D, Kundig TM. J Immunol. 1991;146:4301–4307. [PubMed] [Google Scholar]
- 13.Schwartz RH. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 14.Huppa JB, Gleimer M, Sumen C, Davis MM. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 15.Gray D. Nat Rev Immunol. 2002;2:60–65. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- 16.Iezzi G, Karjalainen K, Lanzavecchia A. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 17.Rachmilewitz J, Lanzavecchia A. Trends Immunol. 2002;23:592–595. doi: 10.1016/s1471-4906(02)02342-6. [DOI] [PubMed] [Google Scholar]
- 18.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 19.Malek TR, Bayer AL. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 20.D'Souza WN, Schluns KS, Masopust D, Lefrancois L. J Immunol. 2002;168:5566–5572. doi: 10.4049/jimmunol.168.11.5566. [DOI] [PubMed] [Google Scholar]
- 21.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 22.Bousso P, Robey E. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 23.Mitchison NA. Proc R Soc London B Biol Sci. 1964;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- 24.Weigle WO. Adv Immunol. 1973;16:61–122. doi: 10.1016/s0065-2776(08)60296-5. [DOI] [PubMed] [Google Scholar]
- 25.Nossal GJ. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- 26.Zinkernagel RM. Semin Immunol. 2000;12:163–171. doi: 10.1006/smim.2000.0253. [DOI] [PubMed] [Google Scholar]
- 27.Zinkernagel RM, Hengartner H. Science. 2001;293:251–253. doi: 10.1126/science.1063005. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 29.Storni T, Kundig TM, Senti G, Johansen P. Adv Drug Deliv Rev. 2005;57:333–355. doi: 10.1016/j.addr.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Nossal G. In: Fundamental Immunology. Paul WE, editor. Philadelphia: Lippincot-Raven; 1999. pp. 1387–1425. [Google Scholar]
- 31.Lofthouse S. Adv Drug Deliv Rev. 2002;54:863–870. doi: 10.1016/s0169-409x(02)00073-x. [DOI] [PubMed] [Google Scholar]
- 32.Ehrenhofer C, Opdebeeck JP. Vet Parasitol. 1995;59:263–273. doi: 10.1016/0304-4017(94)00747-z. [DOI] [PubMed] [Google Scholar]
- 33.Guery JC, Galbiati F, Smiroldo S, Adorini L. J Exp Med. 1996;183:485–497. doi: 10.1084/jem.183.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu G, Mallery SR, Schwendeman SP. Nat Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 35.Borbulevych OY, Baxter TK, Yu Z, Restifo NP, Baker BM. J Immunol. 2005;174:4812–4820. doi: 10.4049/jimmunol.174.8.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitsky V, Zhang QJ, Levitskaya J, Masucci MG. J Exp Med. 1996;183:915–926. doi: 10.1084/jem.183.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen JL, Stewart-Jones G, Bossi G, Lissin NM, Wooldridge L, Choi EM, Held G, Dunbar PR, Esnouf RM, Sami M, et al. J Exp Med. 2005;201:1243–1255. doi: 10.1084/jem.20042323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivoltini L, Squarcina P, Loftus DJ, Castelli C, Tarsini P, Mazzocchi A, Rini F, Viggiano V, Belli F, Parmiani G. Cancer Res. 1999;59:301–306. [PubMed] [Google Scholar]
- 39.Brinckerhoff LH, Kalashnikov VV, Thompson LW, Yamshchikov GV, Pierce RA, Galavotti HS, Engelhard VH, Slingluff CL., Jr Int J Cancer. 1999;83:326–334. doi: 10.1002/(sici)1097-0215(19991029)83:3<326::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Ayyoub M, Mazarguil H, Monsarrat B, Van den Eynde B, Gairin JE. J Biol Chem. 1999;274:10227–10234. doi: 10.1074/jbc.274.15.10227. [DOI] [PubMed] [Google Scholar]
- 41.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 42.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. J Exp Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel RM. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 44.Bachmann MF, Kundig TM, Freer G, Li Y, Kang CY, Bishop DH, Hengartner H, Zinkernagel RM. Eur J Immunol. 1994;24:2228–2236. doi: 10.1002/eji.1830240944. [DOI] [PubMed] [Google Scholar]
- 45.Kundig TM, Castelmur I, Bachmann MF, Abraham D, Binder D, Hengartner H, Zinkernagel RM. J Virol. 1993;67:3680–3683. doi: 10.1128/jvi.67.6.3680-3683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarz K, Storni T, Manolova V, Didierlaurent A, Sirard JC, Rothlisberger P, Bachmann MF. Eur J Immunol. 2003;33:1465–1470. doi: 10.1002/eji.200323919. [DOI] [PubMed] [Google Scholar]
- 47.Krieg AM. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 48.Farman CA, Kornbrust DJ. Toxicol Pathol. 2003;31(Suppl):119–122. doi: 10.1080/01926230390174995. [DOI] [PubMed] [Google Scholar]
- 49.Mutwiri GK, Nichani AK, Babiuk S, Babiuk LA. J Control Release. 2004;97:1–17. doi: 10.1016/j.jconrel.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 50.Kundig TM, Bachmann MF, Lefrancois L, Puddington L, Hengartner H, Zinkernagel RM. J Immunol. 1993;150:4450–4456. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.