Abstract
Elevated levels of atmospheric carbon dioxide (CO2), a consequence of anthropogenic global change, can profoundly affect the interactions between crop plants and insect pests and may promote yet another form of global change: the rapid establishment of invasive species. Elevated CO2 increased the susceptibility of soybean plants grown under field conditions to the invasive Japanese beetle (Popillia japonica) and to a variant of western corn rootworm (Diabrotica virgifera virgifera) resistant to crop rotation by down-regulating gene expression related to defense signaling [lipoxygenase 7 (lox7), lipoxygenase 8 (lox8), and 1-aminocyclopropane-1-carboxylate synthase (acc-s)]. The down-regulation of these genes, in turn, reduced the production of cysteine proteinase inhibitors (CystPIs), which are specific deterrents to coleopteran herbivores. Beetle herbivory increased CystPI activity to a greater degree in plants grown under ambient than under elevated CO2. Gut cysteine proteinase activity was higher in beetles consuming foliage of soybeans grown under elevated CO2 than in beetles consuming soybeans grown in ambient CO2, consistent with enhanced growth and development of these beetles on plants grown in elevated CO2. These findings suggest that predicted increases in soybean productivity under projected elevated CO2 levels may be reduced by increased susceptibility to invasive crop pests.
Keywords: Diabrotica virgifera, global change, Glycine max, plant–insect interactions, Popillia japonica
At present rates of anthropogenic emissions, the atmospheric carbon dioxide (CO2) concentration of 381 μmol mol−1 is predicted to rise to 550 μmol mol−1 by 2050 (1). The CO2 increase is projected to increase the productivity of agroecosystems by enhancing photosynthesis and water use efficiency, particularly in C3 crops (2), although precise estimates of the magnitude of the enhancement vary with experimental approach (3). Such projections, however, generally do not take into account the potential for interactions between plants and herbivorous insects to modify the fertilization effect of elevated CO2 on plant production (4). Impacts of herbivory on plant responses to elevated CO2, however, can be readily measured in Free Air gas Concentration Enrichment (FACE) experiments, where the movement of insects into field plots is unrestricted.
Typically, elevated CO2 diminishes plant host quality by increasing leaf C:N ratio, specific leaf mass and thickness, the proportion of nonstructural carbohydrates, and allocation to phenolic compounds (5–7). However, other compounds, such as proteinase inhibitors (PIs), can play a role in plant defense, affecting the digestibility of proteins and decreasing the availability of free amino acids required by insects for growth, development, and reproduction (8). Cysteine proteinases are common in the slightly acidic midgut (pH 5–7) of many coleopterans (9, 10), and cysteine PIs (CystPIs) in plant tissues decrease growth and development by inhibiting these proteinases (10, 11). Induction of the jasmonate signaling pathway in plants by herbivore damage leads to increased synthesis of CystPIs (12).
Soybean (Glycine max), the world's most widely grown seed legume, has one constitutive CystPI gene (L1) and two inducible CystPI genes (N2 and R1) (13, 14). Soybean CystPIs, as well as the synthetic CystPI, transepoxysucciny-l-leucyl-amido (4-guanidino) butane (E-64), inhibit gut cysteine (cathepsin L-like) activity, growth, and survival of larval and adult western corn rootworm (WCR; Diabrotica virgifera virgifera LeConte: Coleoptera) (10, 13, 15, 16). Although WCR normally causes economic injury by damaging roots of corn (Zea mays), a variant of WCR feeds on soybean foliage and lays eggs in soybean fields (17). The Japanese beetle (JB; Popillia japonica Newman: Coleoptera), a broadly polyphagous species introduced into the United States in 1916 and now expanding its range throughout the Midwest, feeds on ≈300 species of wild and cultivated plants in 79 families; soybeans are among its many host plants (18). Elevated CO2 increased herbivory and oviposition by both JB and WCR in soybean grown in FACE experiments (19–21). Although sugars can stimulate feeding in JB (18), higher carbohydrate levels in leaves did not account for changes in preference or fecundity of this species (21), leaving open the possibility that plant chemical defenses, such as CystPIs, may mediate these changes.
To examine the role of cysteine proteinases in altered resistance to coleopteran herbivores, soybeans were grown at the SoyFACE facility established at the University of Illinois at Urbana–Champaign. The SoyFACE facility, which elevates CO2 under fully open-air field conditions without any barriers, allowed us to investigate the mechanism whereby elevated CO2 increases susceptibility of soybean to naturally occurring herbivores. Specifically, we examined whether growth in elevated CO2 down-regulates the expression of genes associated with signaling hormones that regulate CystPI and other defense genes, whether CystPI activity in soybean foliage is reduced under elevated CO2, and whether JB and WCR display higher levels of digestive cysteine proteinase activity in their guts when consuming soybean leaves grown under elevated CO2. A reduction in the ability to mobilize defenses in response to herbivory is a potential mechanism to account for the enhanced performance of beetles consuming foliage of soybean grown under elevated CO2 and for the increased amount of damage sustained by soybean plants under elevated CO2 under field conditions.
Results
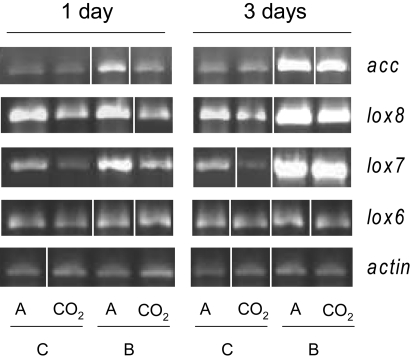
To determine the effect of elevated CO2 on the expression of soybean defense genes, five genes from fully expanded leaves grown either under ambient or elevated CO2 were analyzed by semiquantitative RT-PCR. We analyzed the expression of 1-aminocyclopropane-1-carboxylate synthase (acc), which is the key regulatory point in the biosynthesis of the signaling hormone ethylene, two genes related to the signaling hormone jasmonic acid (JA), lipoxygenase 7 (lox7) and 8 (lox8), and the gene encoding vegetative lipoxygenase 6 (lox6), which is not related to plant signaling and served as an internal control (22–24).
There was no main effect of elevated CO2 on the expression of acc (P = 0.92). However, the significant interaction between elevated CO2 and herbivory by JB (P ≤ 0.01) indicates that the induction of acc after herbivory was reduced in leaves grown under elevated CO2, compared with ambient CO2 (Fig. 1). One day after damage by JB, acc was induced by 86% in leaves grown in ambient CO2, but this induction was only 54% in leaves grown in elevated CO2. The induction of acc increased to 222% in ambient leaves 3 days after attack, but only to 155% after 3 days in leaves grown in elevated CO2. Similar results were found in response to damage inflicted by WCRs (data not shown).
Fig. 1.
Expression analysis of genes related with JA and ethylene biosynthesis. Quantitative RT- PCR of five genes from fully expanded leaves of soybean grown either under elevated [CO2] (CO2) or ambient [CO2] (A): 1-aminocyclopropane-1-carboxylate synthase (acc), lipoxygenase 7 (lox7), 8 (lox8) and 6 (lox6). RNA was extracted from four replicates (one replicate per plot) of either unattacked (C) or attacked leaves by Japanese beetles (B) for 1 or 3 days and reverse-transcribed to cDNA. PCR reactions were replicated from four independent cDNA samples for all primers. Prior to statistical analysis, the spot intensity values generated by image analysis software for each gene was normalized to the intensity of actin to correct for differences in amplification of cDNA. The figure is a composite of multiple experiments and contains images spliced into place.
Elevated CO2 inhibited constitutive levels of lox7 and lox8 by 30% and 28%, respectively (P ≤ 0.01). As with acc, the magnitude of induction after beetle attack was lower for plants grown under elevated than ambient CO2 (Fig. 1). Averaged across both time points, the expression of lox7 after herbivory increased under ambient CO2 by 98%, but only by 77% under elevated CO2. Similarly, the expression of lox8 after herbivory increased by 49% under ambient CO2, but only by 5% under elevated CO2. The expression level of both genes after induction by herbivory increased with time (P ≤ 0.01). As expected, there were no changes in the expression of lox6 by either elevated CO2 or beetle damage (P = 0.5) (Fig. 1).
Our results indicate that elevated CO2 not only decreased the expression of genes related to the signaling hormones JA and ethylene, but also decreased their induction after beetle damage. The positive interaction between JA and ethylene after wounding synergistically induces PI genes, such as CystPI genes in soybean (14). However, changes in the synthesis of JA by elevated CO2 can affect constitutive and inducible CystPI activity levels.
Jasmonic acid (JA), a ubiquitous wound hormone known to increase the synthesis of diverse defense-related metabolites, is strongly implicated in activating CystPI synthesis (12). Endogenous levels of JA increase (5–500 ng per plant), in proportion to the amount of damage, within 90 min (25, 26). The addition of JA and its methyl ester, methyl jasmonate (MeJA), to leaves mimics leaf damage by increasing the synthesis of CystPIs (12).
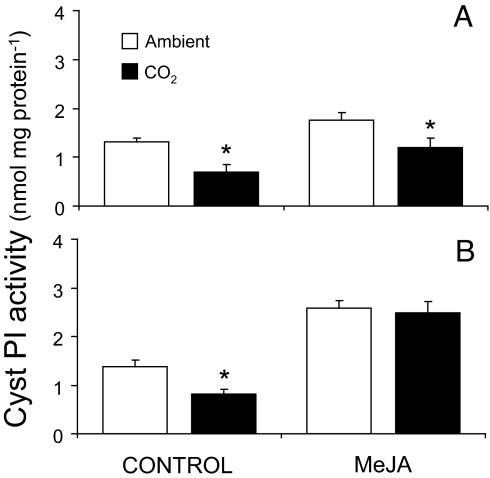
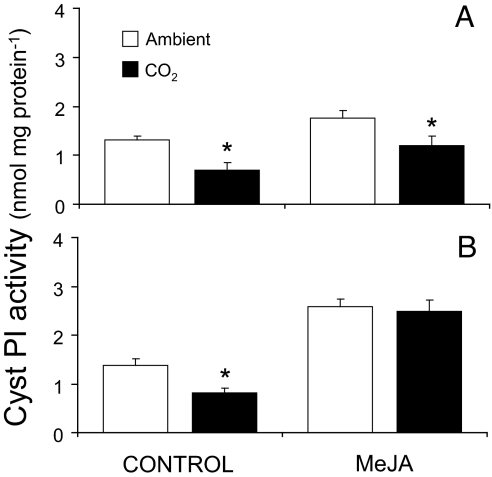
Growth of soybeans under elevated CO2 resulted in a 47% reduction in constitutive CystPI activity in leaves (P = 0.006), and treatment of plants grown under elevated CO2 with MeJA restored CystPI activity to levels comparable to plants grown under ambient CO2 (Fig. 2). These findings are consistent with the down-regulation of JA signaling by elevated CO2. After 1 day of MeJA elicitation, CystPI activity levels increased to a greater extent in plants grown under elevated CO2 (42%) than under ambient CO2 (25%; P = 0.003) (Fig. 2). Similar results were found after 3 days of MeJA elicitation. CystPI activity levels increased to a greater extent in plants grown under elevated CO2 (71%) than in those grown under ambient CO2 (49%; P < 0.0001), resulting in similar CystPI activity levels in both environments (P = 0.7) (Fig. 2). Although elevated CO2 decreased constitutive and inducible CystPI activity, inducible CystPI activity in plants grown under elevated CO2 was restored by the application of MeJA.
Fig. 2.
MeJA restored CystPI activity in elevated CO2 treatments. (A and B) CystPI activity (mean ± SEM) from fully expanded soybean leaves grown under ambient CO2 (open bars) or elevated CO2 (filled bars) 1 (A) or 3 (B) days after the application of 150 μg of MeJA in lanolin paste or pure lanolin (control). Asterisks indicate the level of significant differences between ambient and elevated CO2 treatments (*, P < 0.05; **, P < 0.001; ***, P < 0.0001).
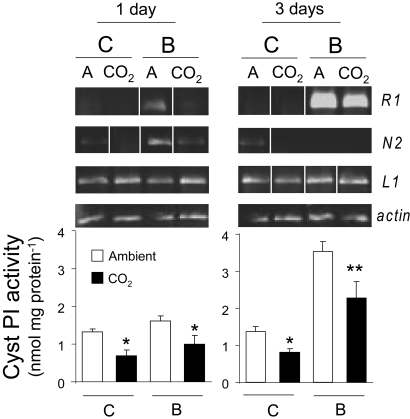
The down-regulation of JA and ethylene by elevated CO2 decreased both expression levels of CystPI-inducible genes R1 and N2 and constitutive and inducible CystPI activity levels in leaves (P < 0.0001) (Fig. 3). Damage by JB induced CystPI genes R1 and N2 under ambient CO2, but not under elevated CO2 (Fig. 3). Three days after insect damage, R1 gene expression was up-regulated in both ambient and elevated CO2, but the magnitude of up-regulation was greater for plants grown in ambient atmosphere (P = 0.0003) (Fig. 3). As expected, the treatments did not change the expression of the L1 gene (P = 0.4) (Fig. 3).
Fig. 3.
Expression analysis of CystPI genes and activity of the inhibitor. Quantitative RT-PCR analysis and cysteine proteinase inhibitor (CystPI) activity (mean ± SEM) from fully expanded leaves of soybean grown either underelevated [CO2] (CO2) or ambient [CO2] (A): R1, N2 and L1 (14). RNA and protein for CystPI determination were extracted from four replicates (one replicate per plot) of either unattacked (C) or attacked leaves by Japanese beetles (B) for 1 or 3 days and reverse-transcribed to cDNA. Actin was used as an internal standard to control for differences in amplification of cDNA. The figure is a composite of multiple experiments and contains images spliced into place. Asterisks indicate the level of significant differences between ambient and elevated [CO2] treatments (*, P < 0.05; **, P < 0.001; ***, P < 0.0001).
Elevated CO2 not only decreased CystPI gene expression, but also affected CystPI activity levels. One day after beetle attack, CystPI activity levels were higher in plants grown under ambient CO2 than in those grown under elevated CO2 (P = 0.006) (Fig. 3). Three days after beetle attack, CystPI elicitation was similar for both treatments (62% and 64%), resulting in higher activity levels in plants grown under ambient CO2 (3.5 nmol mg protein−1) than in those grown under elevated CO2 (2.2 nmol mg protein−1; P < 0.0001) (Fig. 3). The potent inhibitory capacity and the effectiveness of these soybean CystPIs (N2 and R1) against many coleopteran species have been demonstrated by feeding experiments with plants or with diets containing recombinant inhibitors (13–15).
CystPIs can affect the behavior and performance of adult coleopterans. CystPI E-64 in the diet of adult female WCR reduced the number of eggs laid as a result of a combination of the direct inhibition of protein digestion and a postingestive negative feedback, which reduced food intake and insect mass (10). To determine the consequences of changes in soybean CystPI activity in naturally occurring herbivores, we estimated total and cysteine proteinase activity in the guts of JB and WCR that fed on plants grown under ambient or elevated CO2. Cysteine proteinase activity increased 50% (P = 0.03) in JB and 37% (P = 0.01) in WCR, and total proteinase activity increased 41% (P = 0.006) in JB and 41% (P = 0.01) in WCR that fed on leaves developed under elevated compared with ambient CO2 (Fig. 4). Our results suggest that changes of CystPI activity produced in plants grown under elevated CO2 increased the digestive proteinase activity in the guts of herbivores by improving foliage digestibility for adult JB and WCR and enhancing their performance (19–21).
Fig. 4.
Total proteinase and cysteine proteinase activities in the gut of beetles. (A and B) JB (A) or WCR (B) fed for 3 days on soybean leaves grown under ambient or elevated CO2. Values represent the average of four independent samples (one replicate per FACE plot). Azocaseinolytic and cysteine proteinase activities are represented in different units. Asterisks indicate the level of significant differences between ambient and elevated CO2 treatments (*, P < 0.05; **, P < 0.001; ***, P < 0.0001).
Although digestive enzymes in larval WCR are well studied, little is known about the biochemistry of digestive enzymes in adults. E-64 in the diet of adult WCR and JB was a potent inhibitor of azocasein proteolysis, inhibiting both total and cysteine proteinases in the gut of WCR (cysteine, 76.3%; P = 0.004; total, 84.3%; P = 0.0008) and JB (cysteine, 64.8%; P < 0.0001; total 63.4%; P = 0.0004) [supporting information (SI) Fig. S1], which is consistent with the role of cysteine proteinases as the primary digestion enzymes in these two species (10). Sequence analysis from WCR larval midguts revealed that, of 11 cysteine proteinases, 9 are cathepsin L-like enzymes and 2 are cathepsin B-like enzymes (27, 28). Although these gut cysteine proteinases of WCR were efficiently inhibited by the two wound-inducible soybean CystPIs (N2 and R1), the constitutive CystPI L1 (Fig. 3) had very low papain and digestive cysteine proteinase inhibitor activity (13, 14). In view of the fact that L1 CystPI was slightly induced in roots by MeJA (14), CystPI L1 may play a role in regulating cysteines involved in nodule senescence (29).
Discussion
Exploitation of plant defenses, such as CystPIs, is a component of integrated pest management programs, and the introduction of these foreign genes with inducible promoters has been proposed as a tool to confer resistance to crop pests in the future (15). However, such programs may be limited by the capacity of insects to adapt to CystPIs (12). If CO2 levels continue to increase unabated, by 2050, soybean will grow in an atmosphere with levels of CO2 ≈50% higher than today (1). This study suggests that, under such conditions, inducible CystPI will be down-regulated and defense against insects may be compromised.
Predicting the consequences of such down-regulation of defense in terms of future losses to herbivory is at best a risky enterprise due to gaps in understanding even present-day interactions between insects and crop plants. In this study, for example, we did not directly demonstrate the extent to which changes in the magnitude of the down-regulation of proteinase inhibitors actually alter insect performance (e.g., via effects on feeding preferences or consumption rates) or fitness (via effects on survival, growth, and reproduction). Nor was the capacity of soybeans with down-regulated defenses to compensate for losses to herbivory via enhanced growth measured. Previous work at SoyFACE demonstrated that Japanese beetles are longer lived and more fecund consuming soybean foliage grown elevated CO2 (21) and display a marked preference in choice tests for soybean foliage grown under elevated CO2 (20), consistent with the findings reported here. Moreover, the fact that in field studies soybean plants sustain higher losses to herbivores under conditions of elevated CO2 (20) also is consistent with the suggestion that the down-regulation of proteinase inhibitor defenses is likely to be the mechanism underlying increased susceptibility under conditions of elevated CO2.
Thus, increases in atmospheric CO2 at levels predicted to occur in the next half-century have the potential to increase soybean susceptibility to invasive coleopterans and reduce the predicted increase in productivity (3). Elevated CO2 may affect the expression of other soybean defense traits regulated by JA, including serine proteinase inhibitors (30), isoflavonoid content, or polyphenol oxidase activity (31, 32), potentially rendering plants more vulnerable to herbivores other than beetles and leading to even greater losses. As well, in other systems, elevated CO2 has had diverse effects on insects other than coevolved specialist herbivores. Elevated CO2 decreased the emission of JA-regulated terpene volatiles compounds in cabbage (Brassica oleracea), reducing the host-searching efficiency of the specialist parasitoid Cotesia plutellae (33). Impaired JA-signaling Nicotiana attenuata planted into native habitats was not only more vulnerable to adapted herbivores, but also was colonized at a higher rate by novel herbivore species, which fed and reproduced successfully (34). Thus, changes in defense-signaling systems of host plants produced by elevated CO2 can be expected to affect the entire trophic structure of agroecosystems, potentially exacerbating pest problems by multiple mechanisms.
Methods
Soybeans (Glycine max cultivar 93B15; Pioneer Hi-Bred) were grown at the SoyFACE facility established at University of Illinois at Urbana–Champaign (40°02′ N, 88°14′ W, 228 m above sea level; http://www.soyface.uiuc.edu). SoyFACE consisted of eight 20-m-diameter octagonal plots distributed within four randomized blocks of soybean (for extended site and operation description, see ref. 35). Within each block, one control plot was at the current ambient CO2 of 380 μmol mol−1 and one plot was fumigated to a target CO2 of 550 μmol mol−1. The experimental plots were separated by at least 100 m to prevent cross-contamination of CO2. The rate and position of gas release were automatically and continuously altered with wind speed and direction to maintain the desired enrichment within the plot. One-minute average CO2 was ± 20% of the target for >95% of the time. At current rates of anthropogenic emissions, the targets for CO2 represent predicted atmospheric levels in 2050 (1).
In each FACE plot, 38 days after emergence, 38 undamaged soybean plants at the vegetative stage were selected, and the uppermost fully expanded trifoliate leaf on each of eight plants was treated with 150 μg of MeJA (Sigma–Aldrich) in 20 μl of lanolin paste. An additional 10 plants each were infested with five adult JB; 10 plants each were infested with five adult WCR, and 10 plants served as controls. Half of the treatment and control leaves were harvested for analysis 1 day after infestation, and the other half were harvested 3 days after infestation. To ensure that the control leaves remained undamaged and that the insects remained where they were placed, leaves were enclosed in 1 × 4-mm plastic mesh. Adult JB and WCR were collected from the SoyFACE site from plants outside the rings 24 h before infestation.
One and 3 days after the insects were placed on leaves, infested and control leaves were collected and flash-frozen in liquid nitrogen and ground to a fine powder. Control leaves (four at each time point) and those from each of the treatments (five at each time point) were combined to form one sample from each FACE plot; the unit of replication for statistical analyses was the individual FACE plot (n = 4). To determine CystPI activity, leaf powder was extracted with 50 mM phosphate buffer (pH 7.2) containing 150 mM NaCl and 2.0 mM EDTA (4-ml extraction buffer g−1 fresh weight of tissue). The samples were extracted by vortexing for 10 s and centrifuging at 12,000 × g for 15 min. CystPI activity in the leaves was measured against papain by following the release of p-nitroaniline (pNA; 37°C for up to 20 min at 410 nm) after adding the synthetic substrate p-Glu-Phe-Leu-pNA (36). Briefly, 30 μl of 28 μg/ml papain was incubated in a 96-microplate with 0–10 μl of supernatant of plant extracts at 37°C for 10 min before addition of the substrate. Protein concentrations were measured (37) by using BSA as a standard. The molar concentration of active papain in the commercial preparation (Sigma–Aldrich) was determined by titrating a known concentration of the inhibitor E-64 (1–100 μl of 2 μM) against papain until all activity had been inhibited (38). The inhibitory activity of extracted protein was lost after heating at 100°C for 30 min (39), indicating that no other compounds were involved in papain inhibition.
To determine the expression of genes related to CystPI and the signaling hormones jasmonic acid and ethylene in foliage from the treatments, total RNA was extracted from an aliquot of powder from each leaf with a guanidine thiocyanate-acid phenol-based method (http://cropsci.uiuc.edu/faculty/clough/protocol.htm). Total RNA (1 μg from each sample) was converted to cDNA by using a SuperScript first-strand synthesis system for RT-PCR according to the manufacturer's instructions (Invitrogen). PCRs were carried out in four replicates of cDNA for all primers. Two concentrations of cDNA (viz., 50× and 250× dilutions of original cDNA) derived from control and induced leaves were used as a template to amplify the respective cDNA fragment with the right primer combinations and standardized independently for each pair of primers. After denaturing cDNAs at 94°C for 3 min, PCR was carried out for 25 cycles of 94°C for 60 s, followed by annealing at 56°C for 30 s and then extension at 72°C for 1.5 min. The amplified cDNA fragments were purified from agarose gel by using a GFX gel elution kit (Amersham). The intensity of the spots in the gel was determined with image analysis software (Photoshop 7.0; Adobe Systems). Actin and two non-wound-inducible genes, lox6 and L1, in leaves served as internal standards to determine equal amplification of cDNA. The gene names, accession numbers, primer sequences, and size of amplified cDNA fragments are provided in Table S1.
After 3 days of feeding on leaves grown under ambient or elevated CO2, JB and WCR were removed for analysis of total gut proteinase activity and cysteine proteinase activity. For each species, midguts were removed from the five beetles on each leaf and combined with the midguts from beetles on the five replicate leaves to create one composite sample for each FACE plot. Midguts were stored at −20°C.
The effect of a synthetic cysteine inhibitor on gut proteinase activity was examined in a separate experiment. JB and WCR were fed an artificial diet of casein and wheat germ modified from ref. 40 with or without the synthetic cysteine inhibitor E-64 (50 nmol). A group of 15 JB or WCR was fed the control diet or the artificial diet with E-64. The midguts were removed, combined to form a composite sample for each species and treatment, and stored at −20°C. The experiment was repeated three times to generate three composite samples for the control and the treatment diet, respectively.
The composite samples of midguts of beetles from each field plot or from the artificial diet experiment were pulverized in liquid nitrogen with a mortar and pestle. Proteinases from midguts were extracted by homogenizing tissue with 30 mM Tri-K citrate (pH 6.0) 1:1 and incubated on ice for 30 min. The suspension was centrifuged at 12,000 × g for 15 min at 4°C, and the resulting supernatant was used as a source of either JB or WCR gut proteinase activity.
We used azocasein as a substrate to estimate total protease activity. Briefly, 10 μl of 2× diluted enzyme [gut proteinase in 30 mM Tri-K citrate and 125 μM dithioerythreitol (pH 6.0)] was added to 180 μl of 1% azocasein [in 30 mM Tri-K citrate (pH 6.0)] and incubated at 37°C for 2.5 h. The reaction was terminated by adding 300 μl of 10% trichloroacetic acid. After centrifuging at 10,000 × g for 10 min, an equal volume of 1 M NaOH was added to the supernatant, and absorbance was measured at 450 nm in both samples and controls. One protease unit was defined as the amount of enzyme that increases absorbance by 1 OD/min.
Cysteine proteinase activity was estimated by using the chromogenic substrate p-Glu-Phe-Leu-pNA (36). Then 10 μl of the 18× diluted enzyme was added to 20 μl of 0.38 mM p-Glu-Phe-Leu-pNA [in 0.1 M NaPhosphate, 0.3 M KCl, 0.1 mM EDTA, and 3 mM dithioerythreitol (pH 6.0)] and incubated at 37°C. Absorbance at 410 nm from wells on the microtiter plate was measured at 20-s intervals for 20 min with JB enzymes and for >30 min with WRC enzymes. Initial rates of hydrolysis were estimated from the slopes of the resulting absorbance versus time graphs. Assays were linear over the assay period. One cysteine activity unit was defined as the amount of enzyme required to produce 1 mM 4-nitroaniline per minute at 37°C using p-Glu-Phe-Leu-pNA as a substrate under given assay conditions.
Data were analyzed with Stat View, version 5.0 (SAS Institute). The intensity values of spots from the semiquantitative RT-PCR and CystPI activity values were analyzed with a 2 × 2 (time × treatment) repeated measures ANOVA, followed by Fisher's protected LSD post hoc comparisons in all experiments. Cysteine proteinase activity values were analyzed by ANOVA, followed by Fisher's protected LSD post hoc comparisons in all experiments.
Acknowledgments.
We thank F. Xu and D. Bilgin for technical assistance in the laboratory, B. O'Neill and T. Mies for help with field experiments, and Richard Lindroth and Gary Felton for careful and constructive review of the manuscript. This work was supported by the Office of Science (Biological and Environmental Research) and U.S. Department of Energy Grant DE-FG02-04ER63849.
Footnotes
The authors declare no conflict of interest.
This article contains www.pnas.org/cgi/content/full/0800568105/DCSupplemental supporting information online at www.pnas.org/cgi/content/full/0800568105/DCSupplemental.
References
- 1.Prather KA, Guazzotti SA, Suess DT, Pastor SH, Coffee K. New insights into the role of aerosols in affecting pollution and global climate change. Abstr Papers Am Chem Soc. 2001;221:U458–U458. [Google Scholar]
- 2.Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: Plants FACE the future. Annu Rev Plant Biol. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- 3.Long SP, Ainsworth EA, Leaky ADB, Nosberger J, Ort DR. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- 4.Ziska LH, Bunce JA. Predicting the impact of changing CO2 on crop yields: Some thoughts on food. New Phytol. 2007;175:607–618. doi: 10.1111/j.1469-8137.2007.02180.x. [DOI] [PubMed] [Google Scholar]
- 5.Ainsworth EA, et al. A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Global Change Biol. 2002;8:695–709. [Google Scholar]
- 6.Zvereva EL, Kozlov MV. Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: A meta-analysis. Global Change Biol. 2006;12:27–41. [Google Scholar]
- 7.Coviella CE, Trumble JT. Effects of elevated atmospheric carbon dioxide on insect–plant interactions. Conservation Biol. 1998;13:700–712. [Google Scholar]
- 8.Birk Y. Plant Protease Inhibitors; Significance in Nutrition, Plant Protection, Cancer Prevention and Genetic Engineerin. Berlin: Springer; 2003. [Google Scholar]
- 9.Murdock IL, et al. Cysteine digestive proteinases in Coleoptera. Comp Bichem Physiol. 1987;87B:783–787. [Google Scholar]
- 10.Kim JH, Mullin CA. Impact of cysteine proteinase inhibition in midgut fluid and oral secretion on fecundity and pollen consumption of Western Corn Rootworm (Diabrotica virgifera virgifera) Arch Insect Biochem Physiol. 2003;52:139–154. doi: 10.1002/arch.10074. [DOI] [PubMed] [Google Scholar]
- 11.Fabrick J, et al. Effects of potato cysteine proteinase inhibitor on midgut proteolytic enzyme activity and growth of the southern corn rootworm, Diabrotica undecimpunctata howardi (Coleoptera: Chrysomelidae) Insect Biochem Mol Biol. 2002;32:405–415. doi: 10.1016/s0965-1748(01)00117-5. [DOI] [PubMed] [Google Scholar]
- 12.Bolter C, Jongsma MA. Colorado potato beetles (Leptinotarsa decemlineata) adapt to proteinase inhibitors induced in potato leaves by methyl jasmonate. J Insect Physiol. 1995;41:1071–1078. [Google Scholar]
- 13.Zhao Y, et al. Two wound-inducible soybean cysteine proteinase inhibitors have greater insect digestive proteinase inhibitory activities than a constitutive homolog. Plant Physiol. 1996;111:1299–1306. doi: 10.1104/pp.111.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Botella MA, et al. Differential expression of soybean cysteine proteinase inhibitor genes during development and in response to wounding and methyl jasmonate. Plant Physiol. 1996;112:1201–1210. doi: 10.1104/pp.112.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koiwa H, et al. A plant defensive cystatin (soyacystatin) targets cathepsin L-like digestive cysteine proteinases (DvCALs) in the larval midgut of western corn rootworm (Diabrotica virgifera virgifera) FEBS. 2000;471:67–70. doi: 10.1016/s0014-5793(00)01368-5. [DOI] [PubMed] [Google Scholar]
- 16.Lalitha S, et al. Effectiveness of recombinant soybean cysteine proteinase inhibitors against selected crop pests. Comp Biochem Physiol. 2005;140:227–235. doi: 10.1016/j.cca.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Mabry TR, Spencer JL. Survival and oviposition of a western corn rootworm variant feeding on soybean. Ent Exp App. 2003;109:113–121. [Google Scholar]
- 18.Potter DA, Held DW. Biology and management of the Japanese beetle. Annu Rev Entomol. 2002;47:175–205. doi: 10.1146/annurev.ento.47.091201.145153. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder JB, Gray ME, Ratcliffe ST, Estes RE, Long SP. Effects of elevated CO2 and O3 on a variant of the Western corn rootworm (Coleoptera: Chrysomelidae) Environ Entomol. 2006;35:637–644. [Google Scholar]
- 20.Hamilton JG, et al. Anthropogenic changes in tropospheric composition increase susceptibility of soybean to insect herbivory. Environ Entomol. 2005;34:479–485. [Google Scholar]
- 21.O'Neill BF, Zangerl AR, DeLucia EH, Berenbaum MR. Longevity and fecundity of Japanese beetle, Popillia japonica (Newman), on foliage grown under elevated CO2. Environ Entomol. 2008 doi: 10.1603/0046-225x(2008)37[601:lafojb]2.0.co;2. in press. [DOI] [PubMed] [Google Scholar]
- 22.Bunker TW, et al. Sink limitations induces the expression of multiple soybean vegetative lipoxygenase mRNAs while the endogenous jasmonic acid level remains low. The Plant Cell. 1995;7:1319–1331. doi: 10.1105/tpc.7.8.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y-F, Etheridge N, Schaller GE. Ethylene signal transduction. Ann Bot (London) 2005;95:901–915. doi: 10.1093/aob/mci100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saravitz DM, Siedow JN. The differential expression of wound-inducible lipoxygenase genes in soybean leaves. Plant Physiol. 1996;110:287–299. doi: 10.1104/pp.110.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldwin IT, et al. Quantification, correlation and manipulations of wound-induced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta. 1997;201:397–404. [Google Scholar]
- 26.McCloud ES, Baldwin IT. Herbivory and caterpillar regurgitants amplify the wound-induced increases in jasmonic acid but not nicotine in Nicotiana sylvestris. Planta. 1997;203:430–435. [Google Scholar]
- 27.Siegfried BD, Waterfield N, Ffrench-Constant RH. Expressed sequence tags from Diabrotica virgifera virgifera midgut identify a coleopteran cadherin and diversity of cathepsins. Insect Mol Biol. 2005;14:137–143. doi: 10.1111/j.1365-2583.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 28.Bown DP, Wilkinson HS, Jongsma MA, Gatehouse JA. Characterization of cysteine proteinases responsible for digestive proteolysis in guts of larval western corn rootworm (Diabrotica virgifera) by expression in the yeast Pichia pastoris. Insect Mol. Biol. 2004;34:305–320. doi: 10.1016/j.ibmb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Groten K, et al. Redox regulation of peroxyredoxin and proteinases by ascorbate and thiols during pea root nodule senescence. FEBS. 2006;580:1269–1276. doi: 10.1016/j.febslet.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 30.da Silva Fortunato F, et al. Lipoxygenase-induced defense of soybean varieties to the attack of the velvetbean caterpillar (Anticarsia gemmatalis Hübner) J Pest Sci. 2007;80:241–247. [Google Scholar]
- 31.Felton GW, Summers CB, Mueller AJ. Oxidative responses in soybean foliage to herbivory by bean leaf and three-cornered alfalfa hopper. J Chem Ecol. 1994;20:639–650. doi: 10.1007/BF02059604. [DOI] [PubMed] [Google Scholar]
- 32.Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 33.Vuorinen T, Nerg AM, Ibrahim MA, Reddy GVP, Holopainen JK. Emission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol. 2004;135:1984–1992. doi: 10.1104/pp.104.047084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kessler A, Halitschke R, Baldwin IT. Silencing the jasmonate cascade: Induced plant defenses and insect populations. Science. 2004;305:665–668. doi: 10.1126/science.1096931. [DOI] [PubMed] [Google Scholar]
- 35.Ainsworth EA, Rogers A, Nelson R, Long SP. Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated CO2 in the field with single gene substitutions in Glycine max. Agric Forest Meteorol. 2004;122:85–94. [Google Scholar]
- 36.Filipova IY, Lysogorskaya EN, Oksenoit ES, Rudenskaya GN, Stepenov VM. L-pyroglutamyl-L-phenylalanyl-L-leucine-p-nitroanilide: A chromogenic substrate for thiol proteinase assays. Anal Biochem. 1984;143:293–297. doi: 10.1016/0003-2697(84)90665-1. [DOI] [PubMed] [Google Scholar]
- 37.Bradford MM. A rapid sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 38.Zucker S, Buttle DJ, Nicklin MJH, Barrett AJ. The proteolytic activities of chymopapain, papain and papaya proteinase III. Biochem Biophys Acta. 1985;828:196–204. doi: 10.1016/0167-4838(85)90057-3. [DOI] [PubMed] [Google Scholar]
- 39.Hines ME, Osuala CI, Nielsen SS. Isolation and partial characterization of a soybean cystatin cysteine proteinase inhibitor of coleopteran digestive proteolytic activity. J Agric Food Chem. 1991;39:1515–1520. [Google Scholar]
- 40.Marrone PG, Ferri FD, Mosley TR, Meinke LJ. Improvement in laboratory rearing of the southern corn rootworm Diabrotica undecimpunctata howardi Barther (Coleoptera: Chrysomelidae), on artificial diet and corn. J Econ Entomol. 1985;78:290–293. [Google Scholar]