Abstract
Posttranscriptional regulation is an important step in the regulation of gene expression. In this article, we show an unexpected connection between two proteins that participate in different processes of posttranscriptional regulation that ensures the production of functional mRNA molecules. Specifically, we show that the A-to-I RNA editing protein adenosine deaminase that acts on RNA 1 (ADAR1) and the human Upf1 (hUpf1) protein involved in RNA surveillance are found associated within nuclear RNA-splicing complexes. A potential functional role for this association was revealed by RNAi-mediated down-regulation of ADAR1, which was accompanied by up-regulation of a number of genes previously shown to undergo A-to-I editing in Alu repeats and to be down-regulated by hUpf1. This study suggests a regulatory pathway by a combination of ADAR1 A-to-I editing enzyme and RNA degradation presumably with the aid of hUpf1.
Keywords: RNAi, supraspliceosomes, posttranscriptional regulation, nuclear complexes, cross-linking
RNA editing catalyzed by the adenosine deaminase that acts on RNA (ADAR) family of proteins involves the conversion of adenosines to inosines (A to I) and is one of the pre-mRNA processing activities. Of the three identified human ADAR proteins, ADAR1 and ADAR2 are expressed ubiquitously and have different isoforms resulting from alternative splicing, whereas ADAR3 is expressed at low levels only in the brain (reviewed in refs. 1 and 2). ADAR1 exists in two major forms expressed from two distinct promoters. The IFN-induced longer one is present both in the nucleus and cytoplasm, whereas the constitutively expressed shorter one is present in the nucleus (3, 4).
ADARs act on double-stranded RNA and deaminate adenosines at specific sites. Because inosines are generally base-paired to cytidines, specific A-to-I editing can change the coding potential within an ORF, or change splice sites and other control elements (1, 2). A functional significance of the specific editing by ADARs is exemplified by the dramatic decrease of Ca2+ permeability of the AMPA channel by editing of the Q/R site of GluR-B subunit (5). Significant changes in the G protein coupling efficiency of 5-HT2CR have also been reported (6, 7). However, in many other substrates, A-to-I editing was found clustered in noncoding regions mainly in Alu repetitive elements (8–11), as expected from the identification of a large number of inosines in mRNA molecules (12). The functional significance of the latter editing is not yet fully understood. Some of the editing in noncoding regions was suggested as part of a protection mechanism of mRNA molecules against RNAi-like degradation (13). ADARs were also shown to bind siRNA and were thus proposed to protect mRNA molecules from RNAi-like degradation (14). However, double-stranded RNA molecules with repeating U-I base pairs undergo degradation mediated by Tudor, one of the RNA-induced silencing complex (RISC) components (15). Pan-editing by the IFN-induced ADAR1 was proposed as part of the antiviral protection mechanisms (3). Also pan-editing by ADARs can lead to nuclear retention of the RNA molecule (16, 17).
Another posttranscriptional regulatory process involves the RNA surveillance mechanism nonsense-mediated mRNA decay (NMD). This mechanism identifies RNA transcripts harboring premature translation termination codons (PTC) and brings about their degradation such that their potential toxic effect is reduced (18). NMD in human cells involves the hUpf proteins (hUpf1, hUpf2, and hUpf3), which together provide substrate specificity for the recruitment of mRNA into the NMD pathway (19, 20). hUpf1 also participates in a mechanism of degradation, termed Staufen1-mediated decay (SMD), which is independent of hUpf2 and hUpf3. In SMD, the protein Staufen1 binds a 3′-UTR bearing a stop codon and recruits hUpf1, leading to the degradation of the mRNA (21). The hUpf1 protein was also shown to be involved in the RNA surveillance mechanism nonsense-associated alternative splicing (NAS) (20, 22). Knockdown of hUpf1 by RNAi and microarray analysis of expressed genes revealed a large number of genes that are down-regulated by hUpf1 (23).
The present study was motivated by our finding (reported here) that hUpf1 is an integral component of the supraspliceosome. This large 21-MDa nuclear ribonucleoprotein complex (24) has been proposed to constitute the machine where RNA splicing occurs in living cells. In addition to its splicing activity (25), the supraspliceosome harbors other pre-mRNA processing components including the editing enzymes ADAR1 and ADAR2 and the A-to-I editing activity associated with them (26, 27). We therefore asked whether the hUpf and the ADAR proteins, which are involved in apparently distinct RNA processing functions, interact within the supraspliceosome. In this study, we show that ADAR1 and hUpf1 coexist in supraspliceosomes and in additional nuclear complexes. Our studies suggest a functional link between ADAR1 and hUpf1 in affecting the level of a subgroup of edited RNA Pol II transcripts.
Results
hUpf1 Is Associated with Supraspliceosomes.
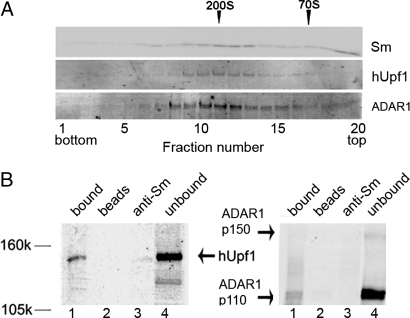
Nuclear pre-mRNAs together with all pre-mRNA processing components are packaged in supraspliceosomes that represent the native pre-mRNA processing machine (26–29). These complexes contain all five spliceosomal U small nuclear ribonucleoproteins (snRNPs) (25), as well as splicing factors such as the SR protein family (30), and the ADAR A-to-I RNA editing enzymes (26). Because hUpf1 protein was shown to be involved in NAS (20), we reasoned that it might be associated with supraspliceosomes. To search for such an association HeLa cells nuclear supernatant (NS) enriched for supraspliceosomes was fractionated in a sucrose gradient as previously described in refs. 28 and 29, and supraspliceosomes sedimenting at the 200S region of the gradient were collected and refractionated in a second gradient. We then checked by Western blotting for the presence of hUpf1 in fractions across the gradient. As shown in Fig. 1A, hUpf1 sediments at the 200S region of the gradient together with Sm proteins, which were previously shown to be associated with supraspliceosomes by cosedimentation analysis (25, 31) and by coimmunoprecipitation (co-IP) assays (31). ADAR1 also sediments at the 200S region of this gradient as previously shown (26). Because hUpf1 was shown to be in a complex with hUpf2 and hUpf3 (19), we searched for their presence in the supraspliceosome region. However, neither could be detected cosedimenting with supraspliceosomes in this gradient, whereas they could be detected in NS by the respective antibodies [supporting information (SI) Fig. 8].
Fig. 1.
hUpf1 is associated with supraspliceosomes. (A) hUpf1 sediments with supraspliceosomes. Supraspliceosomes prepared from HeLa cell nuclei as described in refs. 28 and 29 were fractionated in a sucrose gradient. The 200S fractions were refractionated in a second sucrose gradient. Aliquots from each fraction were run on an SDS/PAGE and were Western blotted with anti-Sm, anti-hUpf1, and anti-ADAR1 antibodies. The sedimentation of 200S TMV and 70S bacterial ribosomes size markers are indicated above the top row. (B) Indirect IP of hUpf1 and ADAR1 from supraspliceosomes. Supraspliceosomes were immunoprecipitated by anti-Sm antibodies, and the precipitated and unbound proteins were analyzed by SDS/PAGE and probed by anti-hUpf1 (Left) and ADAR1 (Right) antibodies (lanes 1 and 4, respectively). As controls, we show the reaction without the antibody (lane 2) and the reaction with antibodies and buffer instead of the sample (lane 3).
To demonstrate that hUpf1 is an integral component of supraspliceosomes, we performed indirect immunoprecipitation (IP) of supraspliceosomes with monoclonal antibodies against Sm proteins (Fig. 1B). The precipitated proteins and the unbound fraction were electrophoresed by SDS/PAGE and Western blotted by anti-hUpf1 antibodies (Fig. 1B Left, lanes 1 and 4, respectively). As controls, we show a mock reaction without the antibody (lane 2), and a mock reaction without the sample (lane 3). We found that hUpf1 was indeed specifically precipitated by the anti-Sm antibodies (Fig. 1B Left; IP yield of 16%), thus confirming the association of hUpf1 with supraspliceosomes. Notably, probing the same blot with antibodies against ADAR1 revealed that ADAR1 is also associated with supraspliceosomes (Fig. 1B Right).
hUpf1 and ADAR1 Coexist in Nuclear Complexes.
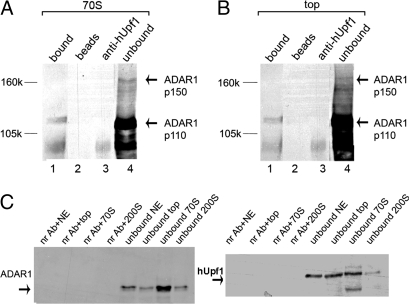
ADAR1 was previously shown to be associated not only with supraspliceosomes, but also with nuclear complexes sedimenting at the 70S region of the gradient (26), where native spliceosomes sediment (25, 32). The finding that hUpf1 also appears with complexes at the 70S fraction of the first sucrose gradient (data not shown), raised the question whether hUpf1 is associated with ADAR1 in these complexes. ADAR1 (26) and hUpf1 (data not shown) were also found at the top fraction of the gradient, where small complexes or individual components sediment. We therefore performed additional IP experiments, this time on the 70S and top fractions of a first gradient, and found that in IP with anti-hUpf1 antibodies ADAR1 was precipitated from the 70S fraction (14%) and to a lower extent (7%) from the top fraction (Fig. 2 A and B, respectively). These results indicate an association between the ADAR1 and hUpf1 proteins. Co-IP experiments on supraspliceosomes performed with anti-hUpf1 antibodies were not efficient enough to be conclusive, most probably because of hindrance of the hUpf1 antigenic determinants within the 21-MDa supraspliceosome. As a control for the IP experiments, we also performed IP with a nonrelevant antibody (preimmune anti-rabbit IgG) on the 200S, 70S, and top fractions of a first gradient, and on a HeLa cell nuclear extract (NE). The results show that IP with antibodies against ADAR1, hUpf1, and Sm were specific (Fig. 2C).
Fig. 2.
hUpf1 is associated with nuclear complexes together with ADAR1. (A) Nuclear complexes sedimenting at 70S were immunoprecipitated by anti-hUpf1 antibodies, and the precipitated and unbound proteins were analyzed by SDS/PAGE and probed by anti-ADAR1 antibodies (lanes 1 and 4, respectively). Controls: lane 2, no antibody; lane 3, no sample. (B) Nuclear complexes sedimenting at the top of the gradient were immunoprecipitated as described in A (lanes in B as in A). (C) HeLa cell NE and nuclear complexes sedimenting at the top of the gradient at 70S and at 200S were immunoprecipitated by a nonrelevant antibody, preimmune anti-rabbit IgG, and were Western blotted by anti-ADAR1 (Left) and anti-hUpf1 (Right) antibodies.
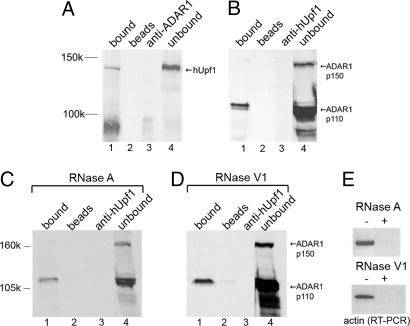
To further test the association of ADAR1 and hUpf1, we performed IP on a HeLa cell NE by using antibodies against ADAR1. Fig. 3A shows that hUpf1 was precipitated with a yield of 23%. In a reciprocal experiment, in which antibodies against hUpf1 were used for IP and anti-ADAR1 antibodies were used for Western blotting, ADAR1 was immunoprecipitated with a yield of 20% for the 110-kDa form and of 1% for the 150-kDa form (Fig. 3B). Both antibodies are specific and no cross-reactivity was observed. We can therefore conclude that hUpf1 is associated with ADAR1.
Fig. 3.
ADAR1 and hUpf1 are associated in HeLa cell NE. (A) HeLa cell NE was immunoprecipitated by anti-ADAR1 antibodies, and the precipitated and unbound proteins were analyzed by SDS/PAGE and probed by anti-hUpf1 antibodies (lanes 1 and 4, respectively). Controls: lane 2, no antibody; lane 3, no sample. (B) In a complementary experiment, we show IP of HeLa cell NE by anti-hUpf1 antibodies and Western blotting by anti-ADAR1 antibodies. (C) HeLa cell NE was subjected to RNase A treatment and was then immunoprecipitated by anti-hUpf1 antibodies and Western blotted by ADAR1. The lanes correspond to those in A. (D) The same as in C, except that the NE was treated with RNase V1. (E) RT-PCR of actin RNA extracted from the HeLa cell NE treated and untreated with RNase A (Upper) and RNase V1 (Lower) shown in C and D.
We next tested whether this association depends on RNA, by analyzing the effect of RNase treatment on the association as seen by IP. First, we incubated a HeLa cell NE with RNase A, which cleaves single-stranded RNA, and then performed IP by using antibodies against hUpf1 (Fig. 3C). An analogous experiment was performed with RNase V1, which cleaves double-stranded RNA (Fig. 3D). Extraction of RNA followed by analysis of actin RNA by RT-PCR showed that practically all RNA was degraded by the respective RNase treatment (Fig. 3E). Because both RNase treatments did not interfere with the precipitation of ADAR1 by the hUpf1 antibodies (Fig. 3 C and D), we can conclude that RNA is not required for the ADAR1-hUpf1 association.
Association of ADAR1 and hUpf1: Cross-Linking Experiments.
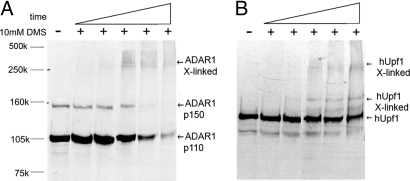
To further support the interaction between ADAR1 and hUpf1, we incubated a HeLa cell NE with the chemical cross-linker dimethyl suberimidate (DMS) and analyzed the protein products by SDS/PAGE and Western blotting. Incubation of the NE with the cross-linker for increasing time caused the disappearance of the monomeric forms of ADAR1 (Fig. 4A) and hUpf1 (Fig. 4B), and the concomitant appearance of higher oligomeric forms containing ADAR1 and hUpf1. A high-molecular-mass oligomeric form, which seems to contain both ADAR1 and hUpf1 (Fig. 4 A and B), has an apparent molecular mass of ≈350 kDa (as determined by molecular mass markers in the range of 75–500 kDa). The cross-linking experiment was also performed after RNase treatment (either RNase A or RNase V1). In both cases, even though the RNase treatment was effective, the cross-linking pattern did not change (SI Fig. 9).
Fig. 4.
Chemical cross-linking results in formation of oligomers containing ADAR1 and hUpf1. HeLa cell NE was cross-linked by DMS for increasing lengths of time (5–60 min). Aliquots were Western blotted with either anti-ADAR1 antibodies (A) or anti-hUpf1 antibodies (B). Non-cross-linked samples (−) are shown for comparison.
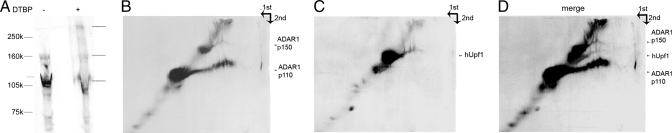
To further characterize the high-molecular-weight cross-linked forms that contain ADAR1 and hUpf1, we used a reversible chemical cross-linker and 2D gel analysis. We incubated a HeLa cell NE with dimethyl-3,3′-dithiobispropionimidate (DTBP) and analyzed the sample on an SDS/PAGE. We then reversed the cross-linking by reducing the disulfide bond of DTBP with β-mercaptoethanol, and electrophoresed the sample on a second dimension SDS/PAGE, followed by Western blotting with anti-ADAR1 and anti-hUpf1 antibodies. This way, proteins that are not cross-linked run the same way in both gels and create a diagonal, whereas cross-linked proteins run as such on the first gel but as non-cross-linked products on the second gel (because of the cleavage of the cross-linker) and are therefore found off the diagonal. Proteins that had been cross-linked together appear off diagonal on the same vertical line. As can be seen in Fig. 5, there are several off-diagonal bands corresponding to both ADAR1 (Fig. 5B) and hUpf1 (Fig. 5C), which were released from the cross-linked species by the reducing agent. For comparison, we show Western blots with ADAR1 of a first-dimension gel of a non-cross-linked NE, and a cross-linked NE without treatment by the reducing agent (Fig. 5A). From the merge of Fig. 5 B and C (Fig. 5D), it can be seen that both ADAR1 and hUpf1 were cross-linked to generate a complex with an apparent molecular mass of ≈350 kDa. On reversal of the cross-linking, both ADAR1 and hUpf1 appear on the same vertical line, indicating that they have been released from the same high-molecular-weight complex. We also show that running a 2D gel as described above without pretreatment of the sample with DTBP does not result in off-diagonal signals (SI Fig. 10).
Fig. 5.
Association of ADAR1 and hUpf1 is revealed by reversible cross-linking and analysis on 2D gels. HeLa cell NE was cross-linked by the reversible cross-linker DTBP for 20 min. (A) First-dimension SDS/PAGE analysis of untreated NE (−) and cross-linked NE (+). (B and C) The gel was soaked in 3% β-mercaptoethanol and electrophoresed on a second identical gel. Western blotting was performed with anti-ADAR1 and anti-hUpf1 as marked on the right. (D) A merge of B and C. The arrows mark the direction of migration of the first and second dimension of the gel.
Knockdown of ADAR1 Results in Up-Regulation of Genes Edited by ADARs and Regulated by hUpf1.
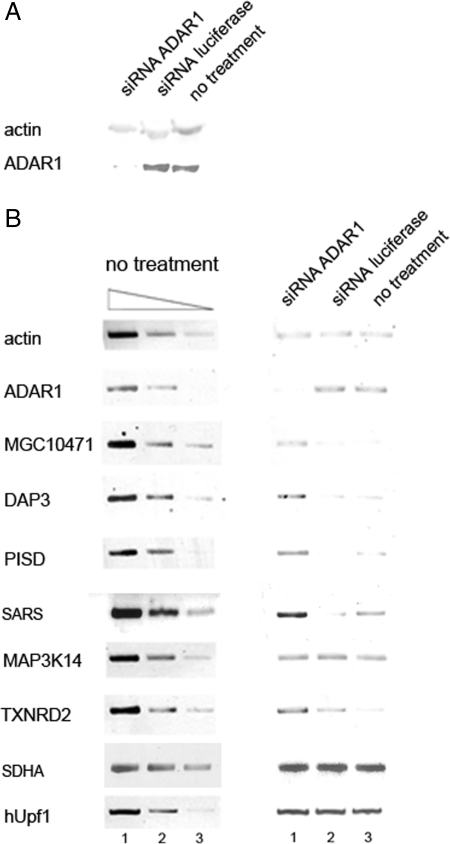
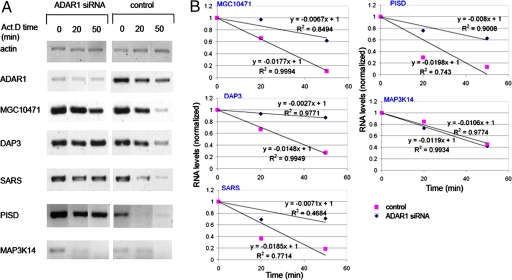
We then asked whether the apparent association between ADAR1 and hUpf1 has functional consequences. A clue for such a connection came from the observations that hUpf1 can also participate in an RNA degradation pathway distinct from NMD (21) and that its down-regulation results in up-regulation of the expression of many genes as detected by microarray analysis (23). We therefore searched for candidate genes that can potentially be degraded by a pathway involving both ADAR1 and hUpf1. For this aim, we compared the list of genes that are up-regulated after the knockdown of hUpf1 (23), with the list of genes that undergo editing according to bioinformatic analyses (8). Of the 13 genes that appeared in both lists, we chose the following six: MAP3K14 (mitogen-activated protein kinase kinase kinase), TXNRD2 (thioredoxin reductase), MGC10471 (hypothetical protein), PISD (phosphatidylserine decarboxylase), DAP3 (death-associated protein 3), and SARS (seryl-tRNA synthetase). RNAi of hUpf1 performed according to Mendell et al. (23), confirmed that the above six genes are down-regulated by hUpf1 (data not shown). We then performed RNAi of ADAR1 in HeLa cells, by using the siRNA duplexes described in ref. 33. As controls, we analyzed untreated cells and cells transfected with siRNA duplex against firefly luciferase, as described in ref. 34. Western blot and RT-PCR analyses (Fig. 6 A and B, respectively) show that the siRNA treatment down-regulated the level of ADAR1 protein to ≈20% and that of the RNA almost to completion. We used endogenous actin as an internal standard. Of the six candidate genes we found four (PISD, MGC10471, DAP3, and SARS) (Fig. 6B) that were clearly up-regulated after RNAi treatment of ADAR1, compared with the level of RNA expressed in control cells (≈3-fold compared with the level in cells treated with RNAi of luciferase). TXNRD2 was also up-regulated after RNAi of ADAR1. At present, it is not added to the list, because a mild increase in RNA level was seen after treatment with RNAi of luciferase (Fig. 6B). MAP3K14 did not seem to be affected (Fig. 6B). As controls, we show that the RNA level of transcripts of two genes that are not known to be affected by RNAi of ADAR1, namely SDHA and hUpf1, was not changed (Fig. 6B). These results suggest that ADAR1 participates in an RNA degradation mechanism, possibly with the aid of hUpf1. To strengthen the above results, we performed half-life experiments by using actinomycin D (Act.D). HeLa cells were treated with either siRNA against ADAR1 or mock-treated. After 72 h, Act.D was added to the medium and RNA was extracted at time points as indicated. RT-PCR analyses show that the gene transcripts of PISD, MGC10471, DAP3, and SARS, which were seen to be down-regulated by ADAR1 by semiquantitative RT-PCR analyses (Fig. 6), were also degraded more rapidly in the presence of ADAR1 than when it was down-regulated (Fig. 7). Furthermore, the transcript of MAP3K14, which was not affected by the ADAR1 siRNA treatment, did not show any difference in its degradation pattern between control and ADAR1 knocked-down cells (Fig. 7).
Fig. 6.
Up-regulation of MGC10471, DAP3, PISD, and SARS mRNAs by RNAi of ADAR1. HeLa cells were either transfected with siRNA against ADAR1, siRNA against firefly luciferase as a negative control or not transfected at all. After 72 h, total RNA and total proteins were prepared. (A) Western blot analyses of a total protein preparation extracted from the above treated cells show specific down-regulation of ADAR1 (≈20%). (B) (Right) RT-PCR analyses of actin, ADAR1, MGC10471, DAP3, PISD, SARS, MAP3K14, TXNRD2, SDHA, and hUpf1 RNAs extracted from HeLa cells treated with siRNA against ADAR1 (lane 1), luciferase (lane 2), and from untreated cells (lane 3). (Left) The image represents 3-fold serial dilutions of RNA from untreated cells to demonstrate that the RT-PCR is semiquantitative.
Fig. 7.
Increase in half-lives of MGC10471, DAP3, PISD, and SARS mRNAs after RNAi of ADAR1. HeLa cells were either mock-transfected or transfected with siRNA against ADAR1. After 72 h, Act.D was added to the cells for the indicated time. RT-PCR of actin, ADAR1, MGC10471, DAP3, SARS, PISD, and MAP3K14 RNAs was performed after RNA extraction. (A) RT-PCR analyses. (Left) ADAR1 siRNA. (Right) Mock transfection. (B) cDNA levels of the different transcripts were normalized to actin levels and then were normalized to the normalized level at time 0 (y axis). The equations of the linear functions are given, as is the R2. Results are representative of three independent experiments. The standard deviation of the slopes ranges from 0.003 to 0.007.
Discussion
ADAR1 Is Associated with hUpf1 in the Nucleus.
By performing cross-linking and co-IP experiments of nuclear complexes fractionated in density gradients and of HeLa cell nuclear extract, we showed that the RNA editing enzyme ADAR1 is associated with the RNA surveillance protein hUpf1. This association is not dependent on RNA, because treatment with either a single- or double-stranded specific RNase (RNase A or RNase V1, respectively) did not affect it. ADAR1 and hUpf1 are found associated in the nucleus within supraspliceosomes, as well as within 70S nuclear complexes, which correspond to the native spliceosomes described by Azubel et al. (25, 32). The cross-linking experiments (Figs. 4 and 5) show that on reversing the cross-links ADAR1 and hUpf1 are released from an oligomeric complex of an apparent molecular mass of at least 350 kDa. Although the accurate mass of the apparent ADAR1–hUpf1 complex has yet to be determined, the fact that ADAR1 was previously shown to form dimers (35, 36), is consistent with a complex containing at least one copy of hUpf1 and one of ADAR1, or possibly, an ADAR1 dimer. These experiments indicate that hUpf1 and ADAR1 are likely to interact with one another.
A Potential Functional Role for the Association of ADAR1 and hUpf1.
The finding that ADAR1 and hUpf1 coexist in nuclear complexes is interesting in the sense that, although both ADARs and hUpfs are known to be involved in the processing of pre-mRNAs to functional mRNA molecules, the pathways in which they participate have not been connected so far.
A clue for a possible functional role of the association of ADAR1 and hUpf1 emerges from the observations that hUpf1 is responsible for the down-regulation of many mRNA molecules (23), and that it is also involved in an mRNA degradation pathway independent of NMD (21). We have thus reasoned that ADAR1 and hUpf1 could participate in concert in a mechanism of RNA degradation. To test this possibility, we searched for genes that are both down-regulated by hUpf1 (23) and undergo A-to-I editing by ADAR enzymes (8). We found 13 such genes [6.6% of the smaller list (23)] and tested the effect of RNAi of ADAR1 on the expression of six of them: DAP3, PISD, MGC10471, SARS, MAP3K14, and TXNRD2. These genes undergo editing in introns or in UTRs in Alu elements (8), short interspersed repetitive elements of ≈280 bp, widespread in the human genome (≈106 copies per haploid genome) and mainly found in introns (37). Our results showed that DAP3, PISD, SARS, and MGC10471 were up-regulated and had longer half-lives after RNAi of ADAR1, whereas MAP3K14 was not affected by down-regulation of ADAR1, neither in its expression level nor in its half-life. These results are therefore in support of a mechanism by which a subgroup of A-to-I edited genes are down-regulated by ADAR1, possibly with the aid of hUpf1.
Interestingly, testing target genes by using the AceView gene models with alternative splicing database (National Center for Biotechnology Information), we found that the edited sites of DAP3, PISD, and MGC10471 transcripts are located within, or next to, a putative alternatively spliced RNA with an ORF having a stop codon. We did not find such a potential stop codon in MAP3K14. This observation, by analogy to NMD and SMD, may provide a possible explanation for the down-regulation by ADAR1 of only a subset of ADAR substrates. Further analyses are required to test this hypothesis, and other explanations cannot be excluded at this stage.
In conclusion, we show a connection between ADAR1 and hUpf1 and a down-regulation of genes by ADAR1. Our finding thus demonstrates once more how closely integrated are the apparently independent pathways that ensure the production of mRNA molecules that are adequate for translation of proteins.
Materials and Methods
Preparation of Splicing Complexes and Supraspliceosomes.
Supraspliceosomes were prepared from HeLa cells (CILBIOTECH) as described in refs. 28 and 29. For details, see SI Materials and Methods.
Western Blot Analysis.
For Western blot analysis, aliquots were applied directly to the wells of a 8 or 6% SDS/PAGE gel and analyzed as described in ref. 38, using antibodies against hUpf1 [kindly provided by J. Lykke-Andersen (University of Colorado, Boulder, CO), and J. Mendell (Johns Hopkins University, Baltimore, MD)], ADAR1 [mAb 15.8.6 kindly provided by K. Nishikura (University of Pennsylvania, Philadelphia, PA)], Sm proteins (mAb Y12) (39), and actin (I19; Santa Cruz). For details, see SI Materials and Methods.
Chemical Cross-Linking.
HeLa cell NE (CILBIOTECH) was mixed with aliquots of the cross-linker dimethyl suberimidate dihychloride (DMS) (Pierce) to final concentrations of 13% vol/vol and 10 mM, respectively, in 0.2 M triethanolamine, pH 8, and incubated at 20°C for 5, 10, 20, 40, and 60 min. The reaction was stopped by the addition of 1.5 vol of SDS/PAGE sample buffer (50 mM Tris·HCl, pH 6.8, 200 mM DTT, 4% SDS, 0.1% bromophenol blue, and 10% glycerol) and incubation on ice. The samples were then boiled for 5 min and run on an SDS/PAGE. The molecular mass markers (75–500 kDa) used were as follows: Rainbow RPN 800 (Amersham Biosciences), HiMark LC5699 (Invitrogen), and PageRuler SM0671 (Fermentas).
Reversible Cross-Linking and 2D Gel Analysis.
To HeLa cell NE (95% vol/vol final concentration), aliquots of the solution of the reversible cross-linker DTBP (Pierce) in 0.2 M triethanolamine, pH 8, were added to a final concentration of 5 mM, and the solution was incubated at 20°C for 20 min. The reaction was stopped by the addition of 1.1 vol of gel sample buffer (as above, but lacking DTT) and incubation on ice. The sample was then boiled for 5 min and run on an SDS/PAGE. For reversal of the cross-linking, by the reduction of the disulfide bond of DTBP, the analyzed lane was cut from the rest of the gel, and incubated for 15 min at 65°C with 3% (vol/vol) β-mercaptoethanol in 50 ml of the SDS/PAGE running buffer (25 mM Tris, 250 mM glycine, 0.1% SDS) adjusted to pH 6.8. Next, the treated lane was incubated for 30 min at 20°C in 50 ml of the SDS/PAGE running buffer adjusted to pH 6.8, placed horizontally on top of a second gel, and run on an SDS/PAGE for the second dimension.
Immunoprecipitations.
Indirect IPs were performed as described in ref. 30 by using anti-ADAR1 WI9 antibodies (kindly provided by K. Nishikura), anti-hUpf1 antibodies, or anti-Sm mAb Y12 or a nonrelevant antibody, preimmune anti-rabbit IgG (see SI Materials and Methods). IP yield is expressed as the ratio between the intensity of the immunoprecipitated band [minus background of control lanes (lanes 2 and 3)] and the sum of this intensity and that of the corresponding band in the unbound lane. Intensities were measured by the Image Gauge program.
RNase Assays.
HeLa cell NE was incubated with either RNase A at 2 μg/ml for 1 h at 4°C, or with RNase V1 (Ambion) at 0.01 units/μl for 18 h at 4°C. RNA was extracted from 10-μl aliquots of treated and untreated NEs, and the remaining reaction mixtures were taken either for cross-linking experiments or for immunoprecipitation as described above (cross-linking time was 20 min).
RNAi of ADAR1.
siRNAs targeted to (i) the long and the short forms of ADAR1 (33), and (ii) to the firefly's luciferase mRNA (34) were purchased from Dharmacon and transfected into HeLa cells as described in ref. 40, with some modifications. Cells grown in 6-cm plates were transfected with 125 nM siRNA by using 0.23% oligofectamine (Invitrogen). After 72 h, total proteins and RNA were extracted. The results are representative of three independent experiments. The percentage of ADAR1 protein and the effect of ADAR1 siRNA on the levels of the tested RNAs was calculated after normalization to the level of actin and then to the levels in the siRNA luciferase-transfection lane. Band intensities were measured by using the Image Gauge program.
RT-PCR.
RT-PCR was performed as described in ref. 40. Details and primer list are published as SI Materials and Methods.
Half-Life Experiments.
HeLa cells were either treated with siRNA against ADAR1 or mock-treated as described above. After 72 h, Act.D was added to 1 μg/ml medium. RNA was extracted at time points as indicated. RNA was also extracted from cells that were not treated with Act.D. RT-PCR analyses were performed as described above. Band intensities were measured by using the Image Gauge program.
Supplementary Material
Acknowledgments.
We thank Aviva Pecho for excellent technical assistance; Erez Levanon for helpful information; and Drs. Joshua Mendell, Kazuko Nishikura, and Jens Lykke-Andersen for antibodies. This work was supported in part by grants from the Israel Science Foundation (to R.S.) and from The Helen and Milton Kimmelman Center for Biomolecular Structure and Assembly at The Weizmann Institute of Science (to J.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710576105/DC1.
References
- 1.Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valente L, Nishikura K. ADAR gene family and A-to-I RNA editing: Diverse roles in posttranscriptional gene regulation. Prog Nucleic Acid Res Mol Biol. 2005;79:299–338. doi: 10.1016/S0079-6603(04)79006-6. [DOI] [PubMed] [Google Scholar]
- 3.Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: Evidence for two forms of the deaminase. Mol Cell Biol. 1995;15:5376–5388. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George CX, Samuel CE. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc Natl Acad Sci USA. 1999;96:4621–4626. doi: 10.1073/pnas.96.8.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67:11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 6.Burns CM, et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, et al. Altered G protein-coupling functions of RNA editing isoform and splicing variant serotonin 2C receptors. J Neurochem. 2000;74:1290–1300. doi: 10.1046/j.1471-4159.2000.741290.x. [DOI] [PubMed] [Google Scholar]
- 8.Levanon EY, et al. Systematic identification of abundant A-to-I editing sites in the human transcriptome. Nat Biotechnol. 2004;22:1001–1005. doi: 10.1038/nbt996. [DOI] [PubMed] [Google Scholar]
- 9.Athanasiadis A, Rich A, Maas S. Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome. PLoS Biol. 2004;2:e391. doi: 10.1371/journal.pbio.0020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DD, et al. Widespread RNA editing of embedded alu elements in the human transcriptome. Genome Res. 2004;14:1719–1725. doi: 10.1101/gr.2855504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morse DP, Aruscavage PJ, Bass BL. RNA hairpins in noncoding regions of human brain and Caenorhabditis elegans mRNA are edited by adenosine deaminases that act on RNA. Proc Natl Acad Sci USA. 2002;99:7906–7911. doi: 10.1073/pnas.112704299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul MS, Bass BL. Inosine exists in mRNA at tissue-specific levels and is most abundant in brain mRNA. EMBO J. 1998;17:1120–1127. doi: 10.1093/emboj/17.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight SW, Bass BL. The role of RNA editing by ADARs in RNAi. Mol Cell. 2002;10:809–817. doi: 10.1016/s1097-2765(02)00649-4. [DOI] [PubMed] [Google Scholar]
- 14.Yang W, et al. ADAR1 RNA deaminase limits short interfering RNA efficacy in mammalian cells. J Biol Chem. 2005;280:3946–3953. doi: 10.1074/jbc.M407876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scadden AD. The RISC subunit Tudor-SN binds to hyper-edited double-stranded RNA, promotes its cleavage. Nat Struct Mol Biol. 2005;12:489–496. doi: 10.1038/nsmb936. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Carmichael GG. The fate of dsRNA in the nucleus: A p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell. 2001;106:465–475. doi: 10.1016/s0092-8674(01)00466-4. [DOI] [PubMed] [Google Scholar]
- 17.Prasanth KV, et al. Regulating gene expression through RNA nuclear retention. Cell. 2005;123:249–263. doi: 10.1016/j.cell.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Maquat LE. Nonsense-mediated mRNA decay. Curr Biol. 2002;12:R196–R197. doi: 10.1016/s0960-9822(02)00747-9. [DOI] [PubMed] [Google Scholar]
- 19.Lykke-Andersen J, Shu MD, Steitz JA. Human Upf proteins target an mRNA for nonsense-mediated decay when bound downstream of a termination codon. Cell. 2000;103:1121–1131. doi: 10.1016/s0092-8674(00)00214-2. [DOI] [PubMed] [Google Scholar]
- 20.Mendell JT, ap Rhys CM, Dietz HC. Separable roles for rent1/hUpf1 in altered splicing and decay of nonsense transcripts. Science. 2002;298:419–422. doi: 10.1126/science.1074428. [DOI] [PubMed] [Google Scholar]
- 21.Kim YK, Furic L, Desgroseillers L, Maquat LE. Mammalian Staufen1 recruits Upf1 to specific mRNA 3′UTRs so as to elicit mRNA decay. Cell. 2005;120:195–208. doi: 10.1016/j.cell.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Hamilton JI, Carter MS, Li S, Wilkinson MF. Alternatively spliced TCR mRNA induced by disruption of reading frame. Science. 2002;297:108–110. doi: 10.1126/science.1069757. [DOI] [PubMed] [Google Scholar]
- 23.Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nat Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 24.Muller S, Wolpensinger B, Angenitzki M, Engel A, Sperling J, et al. A supraspliceosome model for large nuclear ribonucleoprotein particles based on mass determinations by scanning transmission electron microscopy. J Mol Biol. 1998;283:383–394. doi: 10.1006/jmbi.1998.2078. [DOI] [PubMed] [Google Scholar]
- 25.Azubel M, Habib N, Sperling J, Sperling R. Native spliceosomes assemble with pre-mRNA to form supraspliceosomes. J Mol Biol. 2006;356:955–966. doi: 10.1016/j.jmb.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 26.Raitskin O, Cho DS, Sperling J, Nishikura K, Sperling R. RNA editing activity is associated with splicing factors in lnRNP particles: The nuclear pre-mRNA processing machinery. Proc Natl Acad Sci USA. 2001;98:6571–6576. doi: 10.1073/pnas.111153798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raitskin O, Angenitzki M, Sperling J, Sperling R. Large nuclear RNP particles—the nuclear pre-mRNA processing machine. J Struct Biol. 2002;140:123–130. doi: 10.1016/s1047-8477(02)00541-5. [DOI] [PubMed] [Google Scholar]
- 28.Sperling R, Sperling J. In: RNP Particles, Splicing and Autoimmune Diseases. Schenkel J, editor. Berlin: Springer; 1998. pp. 29–47. [Google Scholar]
- 29.Spann P, Feinerman M, Sperling J, Sperling R. Isolation and visualization of large compact ribonucleoprotein particles of specific nuclear RNAs. Proc Natl Acad Sci USA. 1989;86:466–470. doi: 10.1073/pnas.86.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yitzhaki S, Miriami E, Sperling R, Sperling J. Phosphorylated Ser/Arg-rich proteins: Limiting factors in the assembly of 200S large nuclear ribonucleoprotein particles. Proc Natl Acad Sci USA. 1996;93:8830–8835. doi: 10.1073/pnas.93.17.8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperling R, Spann P, Offen D, Sperling J. U1, U2, and U6 small nuclear ribonucleoproteins (snRNPs) are associated with large nuclear RNP particles containing transcripts of an amplified gene in vivo. Proc Natl Acad Sci USA. 1986;83:6721–6725. doi: 10.1073/pnas.83.18.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azubel M, Wolf SG, Sperling J, Sperling R. Three-dimensional structure of the native spliceosome by cryo-electron microscopy. Mol Cell. 2004;15:833–839. doi: 10.1016/j.molcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Wong SK, Lazinski DW. Replicating hepatitis delta virus RNA is edited in the nucleus by the small form of ADAR1. Proc Natl Acad Sci USA. 2002;99:15118–15123. doi: 10.1073/pnas.232416799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 35.Cho DS, Yang W, Lee JT, Shiekhattar R, Murray JM, et al. Requirement of dimerization for RNA editing activity of adenosine deaminases acting on RNA. J Biol Chem. 2003;278:17093–17102. doi: 10.1074/jbc.M213127200. [DOI] [PubMed] [Google Scholar]
- 36.Gallo A, Keegan LP, Ring GM, O'Connell MA. An ADAR that edits transcripts encoding ion channel subunits functions as a dimer. EMBO J. 2003;22:3421–3430. doi: 10.1093/emboj/cdg327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurka J. Evolutionary impact of human Alu repetitive elements. Curr Opin Genet Dev. 2004;14:603–608. doi: 10.1016/j.gde.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Yitzhaki S, Sperling J. Concentrating dilute protein solutions for gel electrophoresis. Biotechniques. 1998;24:762–764. 766. doi: 10.2144/98245bm15. [DOI] [PubMed] [Google Scholar]
- 39.Lerner EA, Lerner MR, Janeway CA, Jr, Steitz JA. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci USA. 1981;78:2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wachtel C, Li B, Sperling J, Sperling R. Stop codon-mediated suppression of splicing is a novel nuclear scanning mechanism not affected by elements of protein synthesis and NMD. RNA. 2004;10:1740–1750. doi: 10.1261/rna.7480804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.