Abstract
Rotifers of class Bdelloidea are common invertebrate animals with highly unusual characteristics, including apparently obligate asexuality, the ability to resume reproduction after desiccation at any life stage, and a paucity of transposable genetic elements of types not prone to horizontal transmission. We find that bdelloids are also extraordinarily resistant to ionizing radiation (IR). Reproduction of the bdelloids Adineta vaga and Philodina roseola is much more resistant to IR than that of Euchlanis dilatata, a rotifer belonging to the desiccation-intolerant and facultatively sexual class Monogononta, and all other animals for which we have found relevant data. By analogy with the desiccation- and radiation-resistant bacterium Deinococcus radiodurans, we suggest that the extraordinary radiation resistance of bdelloid rotifers is a consequence of their evolutionary adaptation to survive episodes of desiccation encountered in their characteristic habitats and that the damage incurred in such episodes includes DNA breakage that is repaired upon rehydration. Such breakage and repair may have maintained bdelloid chromosomes as colinear pairs and kept the load of transposable genetic elements low and may also have contributed to the success of bdelloid rotifers in avoiding the early extinction suffered by most asexuals.
Keywords: desiccation, DNA repair, asexual reproduction, anhydrobiosis
Rotifers of class Bdelloidea are common invertebrate animals inhabiting temporary freshwater pools, the surfaces of mosses and lichens, and other ephemerally aquatic habitats (1). Their success in such habitats depends on their extraordinary ability to survive desiccation at any stage of their life cycle (2). This ability sets them apart not only from the great majority of other animal species, which are incapable of anhydrobiosis at any life stage, but also from the few that survive desiccation only when in a special relatively dormant stage, such as the larvae of the chironomid insect Polypedilum vanderplanki, the embryonic cysts of brine shrimps, and the resting eggs of monogonont rotifers (3–5). Bdelloids are further distinguished from certain species of nematodes and tardigrades that also tolerate desiccation at any life stage by not producing trehalose, a nonreducing disaccharide that is thought to contribute to the protection of cellular constituents against desiccation damage (6–8).
We examined the effects of ionizing radiation (IR) on the reproduction of two species of rotifers representing different families within the putatively asexual class Bdelloidea and a rotifer belonging to the facultatively sexual, desiccation-intolerant class Monogononta, the sister class to the Bdelloidea. Reproduction of the bdelloids was found to be far more resistant to IR than that of the monogonont and all other animals for which we have found applicable data. We propose that the extraordinary resistance of bdelloid rotifers to IR, like that of the bacterium Deinococcus radiodurans, is a consequence of evolutionary adaptation to survive desiccation in ephemerally aquatic habitats and that such desiccation causes extensive DNA breakage, which they are able to repair.
Results
Reproduction.
The reproduction of Adineta vaga and Philodina roseola was much more resistant to IR than that of Euchlanis dilatata. The relative parental fecundity of E. dilatata was reduced 10-fold by a dose of ≈200 Gy, whereas a corresponding reduction in bdelloid fecundity required a dose five times greater. A similar difference between the monogonont and the bdelloids was seen in relative parental fertility. The reproductive effects of IR extended to the daughters of irradiated parents, with dose-dependent reductions in fecundity and fertility almost as great as seen for the parents. Even at the highest doses tested, irradiated rotifers remained active for the 1–2 weeks during which they were observed.
Table 1 presents data on parental and F1 reproduction of rotifers exposed to doses up to 280 Gy for the monogonont and 1,120 Gy for the bdelloids. For each dose group of 96 animals, including nonirradiated controls, Table 1 gives the number of animals that deposited eggs, the number that produced at least one daughter, the proportion of animals giving daughters normalized to the corresponding proportion for nonirradiated rotifers (relative parental fertility), the total number of eggs deposited, the total number of eggs that hatched, and the total number of hatched eggs normalized to the corresponding number produced by nonirradiated animals (relative parental fecundity). It also gives for each dose group the number of daughters, taking one from each fertile parent, that produced eggs, the number that produced granddaughters, the proportion of daughters that were fertile normalized to the corresponding proportion for daughters of nonirradiated rotifers (relative F1 fertility), and, for E. dilatata and P. roseola, the number of hatched eggs per daughter normalized to the number produced per daughter by daughters of nonirradiated rotifers (relative F1 fecundity). For E. dilatata, the number of hatched eggs was counted directly, whereas for the bdelloids it was taken as the number of progeny, as described in Materials and Methods.
Table 1.
Reproductive performance of bdelloid and monogont rotifers exposed to IR
| Species | Dose, h | Parental wells |
Daughter wells |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wells with eggs, n | Wells with F1, n | Total eggs, n | Total eggs hatched, n | Relative parental fertility | Relative parental fecundity | Wells with eggs, n | Wells with F2, n | Total eggs hatched, n | Relative F1 fertility | Relative F1 fecundity | ||
| E. dilatata | 0 | 81 | 68 | 290 | 249 | 1.00 | 1.00 | 58 | 58 | 1.00 | ||
| 1 | 82 | 26 | 272 | 35 | 0.38 | 0.14 | 9 | 6 | 0.27 | |||
| 2 | 78 | 0 | 266 | 0 | 0.00 | 0.00 | n/a | n/a | n/a | |||
| 3 | 84 | 0 | 278 | 0 | 0.00 | 0.00 | n/a | n/a | n/a | |||
| E. dilatata | 0 | 88 | 86 | 324 | 291 | 1.00 | 1.00 | 65 | 64 | 1.00 | ||
| 0.5 | 87 | 80 | 302 | 160 | 0.93 | 0.55 | 45 | 40 | 0.67 | |||
| 1 | 87 | 24 | 278 | 58 | 0.28 | 0.20 | 15 | 10 | 0.56 | |||
| 2 | 85 | 2 | 316 | 2 | 0.02 | 0.01 | 1 | 0 | 0.00 | |||
| E. dilatata | 0 | 83 | 83 | 613 | 588 | 1.00 | 1.00 | 78 | 78 | 1,085 | 1.00 | 1.00 |
| 0.25 | 86 | 86 | 577 | 461 | 1.04 | 0.78 | 74 | 72 | 977 | 0.89 | 0.87 | |
| 0.5 | 88 | 82 | 602 | 360 | 0.99 | 0.61 | 73 | 54 | 612 | 0.70 | 0.57 | |
| 1.5 | 82 | 14 | 538 | 24 | 0.17 | 0.04 | 4 | 1 | 9 | 0.08 | 0.05 | |
| A. vaga | 0 | 89 | 89 | 985 | 866 | 1.00 | 1.00 | 87 | 87 | 1.00 | ||
| 2 | 91 | 90 | 1,063 | 845 | 1.01 | 0.98 | 87 | 86 | 0.98 | |||
| 4 | 88 | 84 | 1,240 | 672 | 0.94 | 0.78 | 80 | 78 | 0.95 | |||
| 6 | 93 | 62 | 1,002 | 187 | 0.70 | 0.22 | 54 | 50 | 0.83 | |||
| 8 | 92 | 6 | 1,169 | 12 | 0.07 | 0.01 | 3 | 2 | 0.34 | |||
| P. roseola | 0 | 95 | 95 | 1,914 | 1,569 | 1.00 | 1.00 | 92 | 92 | 2,356 | 1.00 | 1.00 |
| 2 | 96 | 95 | 1,956 | 1,134 | 1.00 | 0.72 | 91 | 89 | 1,877 | 0.97 | 0.80 | |
| 4 | 93 | 76 | 1,873 | 638 | 0.80 | 0.41 | 73 | 67 | 1,201 | 0.91 | 0.64 | |
| 6 | 89 | 46 | 1,690 | 217 | 0.48 | 0.14 | 36 | 29 | 240 | 0.65 | 0.21 | |
| 8 | 89 | 9 | 1,711 | 13 | 0.09 | 0.01 | 3 | 1 | 5 | 0.11 | 0.02 | |
Rotifers were irradiated on ice for various times with a 137Cs source delivering 140 Gy per h. Parental and F1 relative fecundity and relative fertility are defined in Materials and Methods. The number of daughters transferred to new wells was in each case equal to the number of parental wells in which daughters were produced. For E. dilatata the number of hatched eggs was counted directly. For bdelloids it was taken as the number of progeny, as described in Materials and Methods.
The proportion of parental rotifers not depositing eggs averaged ≈5% for bdelloids and 12% for E. dilatata, independent of dose. Such animals were apparently infertile or had reached the end of their reproductive lifespan, ≈5 days for E. dilatata and 2 weeks for the bdelloids under our conditions. The total number of eggs deposited per individual averaged 12 and 19 for A. vaga and P. roseola, respectively, whereas the average for E. dilatata was ≈3 in the first two experiments and 6 in the third, a variation possibly reflecting differences in the age of the parents.
Relative parental and F1 fecundity and fertility are plotted as a function of dose in Fig. 1. The two bdelloid species are similar in their dose–response relations, with an indication that reproduction of P. roseola may be a little more radiosensitive than that of A. vaga. Parental fecundity, which measures the total number of daughters produced by irradiated animals, is only moderately more radiosensitive than parental fertility, which requires only the production of at least one daughter. Bdelloid parental and F1 fertility and fecundity display an extended shoulder in their dependence on dose, whereas only a slight shoulder is found for monogononts. Beyond their respective shoulders, reproduction falls off rapidly, with no indication of a resistant fraction. The reproduction of daughters of irradiated rotifers is also radiosensitive, with a dose–response relation similar to that of their parents but shifted to somewhat higher dose. Again, P. roseola appears to be a little more radiosensitive than A. vaga.
Fig. 1.
Dose dependence of bdelloid and monogonont reproduction. Data on reproduction are from Table 1. (A) Parental reproduction. (B) F1 reproduction. Shown are A. vaga (triangles), P. roseola (squares), and E. dilatata (circles). Relative fecundity (filled symbols), relative fertility (empty symbols), and DSBs per Mbp (empty diamonds) are indicated. Left vertical axis shows relative fecundity or fertility; right vertical axis shows DSBs per Mbp; horizontal axis shows dose in Gy.
DNA Breakage.
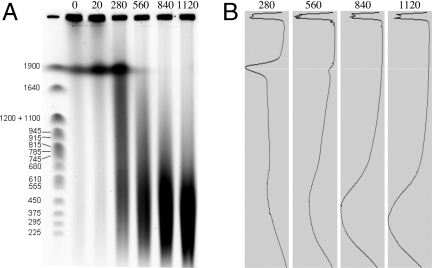
Fig. 2 shows the gel electrophoretic distribution of DNA found after exposing A. vaga to doses in the range of 0 to 1,120 Gy. The average molecular sizes at 560, 840, and 1,120 Gy are 350, 250, and 190 kbp, respectively, with an average of 0.005 double-strand breaks (DSBs) Gy−1·Mbp−1, a value within the range of 4 to 7 × 10−3 DSBs Gy−1·Mbp−1 measured by pulsed-field gel electrophoresis or sucrose sedimentation analysis for Escherichia coli, Saccharomyces cerevisiae, and diverse mammalian cell lines (9–12). Some of the DSBs we observed may have arisen from non-DSB lesions as a result of high-temperature digestion, an effect estimated to cause about a 30% overestimate in γ-irradiated mammalian cells (13).
Fig. 2.
DNA double-strand breakage by IR in A. vaga. (A) Pulsed-field gel electrophoresis. Yeast chromosome molecular weight markers are in kbp. Doses are in Gy. (B) Photometric scans of gel lanes.
Discussion
Reproduction of Irradiated Rotifers.
We find that reproduction of the bdelloid rotifers A. vaga and P. roseola is much more resistant to 137Cs γ-radiation than that of the monogonont rotifer E. dilatata. A dose of ≈200 Gy reduced the number of daughters produced by the monogonont by ≈90%, whereas a corresponding reduction in bdelloid fecundity required a dose five times higher. The principal difference between the bdelloid and monogonont dose–response relations lies in the extended shoulder over which bdelloid fertility and fecundity remain high before falling steeply, whereas only a much less extended shoulder is found for the monogonont. The sterilizing effect of IR extends to the daughters of irradiated parents, with dose–response relations shifted to somewhat higher doses. Taking the size of the A. vaga genome as ≈180 Mbp (R. Gregory, personal communication), the average molecular size of 350 kbp observed after a dose of 560 Gy, causing a reduction in fecundity of only 20%, corresponds to ≈500 DSBs per genome.
The reproduction of A. vaga and P. roseola is more resistant to IR than that of any other metazoan for which we have found relevant data. Such resistance to IR is likely to be characteristic of the Bdelloidea generally, as A. vaga and P. roseola represent families that diverged millions of years ago (14) and because of the probable association, discussed below, of bdelloid radiation resistance with anhydrobiosis. Doses of IR or high-energy electrons required to prevent reproduction have been measured for many arthropod species at various life stages, mainly for purposes of pest control. In a tabulation of such sterilizing doses for male arthropods, which are generally more radiosensitive than females, including 285 species representing 61 families of insects and other arthropods, irradiated mainly as pupae or nymphs and generally achieving at least 90% parental or F1 sterility, the average dose is ≈110 Gy (ref. 15 and www-ididas.iaea.org/ididas/). For the most radio-resistant species in the tabulation, the lepidopteran Spilosoma (Diacrasia) obliqua, a dose of 200 Gy to pupal or adult males, the most resistant sex, resulted in >99% F1 sterilization.§ Among other invertebrates, sensitivity to IR has been measured for X-irradiation of Caenorhabditis elegans eggs in buffer, for which ≈30 Gy reduced the number developing into adults by 90% and the snail Zonitoides arboreus, which produced only sterile eggs after exposure to 70 Gy (16, 17). Among the very few animal species known to be capable, like bdelloid rotifers, of anhydrobiosis at any life stage, resistance to IR has been studied in the tardigrades Richtersius coronifer and Milnesium tardigradum, which produced only sterile eggs after exposure to the lowest doses tested, 500 and 1,000 Gy, respectively (18, 19).
As we find for bdelloid and monogonont rotifers, the effect of IR on other animals generally extends beyond the irradiated parents to their progeny. F1 sterility induced by parental irradiation, widely made use of in insect control programs, is associated with chromosome aberrations, mutations, and possibly deleterious epigenetic effects expressed as impaired reproduction in generations beyond that which is irradiated.
The observation that rotifers rendered sterile by IR nevertheless remain active is consistent with the fact that somatic cell division is already complete at the time of hatching (20–23) and with the general observation that metabolic processes are less radiosensitive than cell division. Egg production, however, involves two maturation divisions starting from primary oocytes present in the hatched animal (24, 25) yet is also essentially unaffected even at the highest doses tested, suggesting either that egg production can proceed without maturation divisions or that the sterilizing effect of IR is manisfested only later in development.
Basis of Radiation Resistance.
The extraordinary resistance of bdelloid reproduction to IR cannot be attributed to an unusually small genome size, as the genome sizes of A. vaga and P. roseola are comparable to or larger than those of C. elegans, Drosophila melanogaster and many other animals that display no comparable radiation resistance (15, 21, 26, 27). Nor can bdelloid radiation resistance be explained by genetic redundancy or a multiplicity of templates for homologous repair. Bdelloid primary oocytes are in G1 (21, 27) and, as described in ref. 28, bdelloids appear to be degenerate tetraploids, with only two copies of most genes.
The possibility that bdelloid radiation resistance reflects a novel chromosome alignment that keeps homologous regions together, facilitating access of broken ends to homologous templates, has been proposed for D. radiodurans where it appears unlikely for a number of reasons (29). Moreover, neither chromosome alignment nor the novel variant of synthesis-dependent strand annealing proposed to facilitate DSB repair at high levels of DNA breakage (30), provide an explanation for the extended shoulder in bdelloid and D. radiodurans dose–response relations that distinguishes them from radiosensitive organisms.
It might be thought that the measured efficiency of IR breakage, 0.005 DSB per Gy per Mbp, refers only to somatic DNA and that bdelloid germ line DNA is somehow protected. We think this unlikely as no such resistance is seen in D. radiodurans or in other radiation-resistant prokaryotes, where the number of breaks made per Mbp per Gy, ≈0.004 (31, 32), is not significantly different from that for radiosensitive bacteria, fungi, and animals. More likely, the ability of bdelloids to remain fertile after extensive DNA breakage and other damage inflicted by IR derives from attributes of the systems that repair such damage or those that protect the repair systems, or both.
The finding that IR killing of D. radiodurans is paralleled by oxidative damage to its proteins has led to the proposal that the extraordinary radio resistance of D. radiodurans results from unusually effective protection of its proteins against toxic products of IR (33), a conclusion consistent with the ability of extracts of D. radiodurans to protect E. coli from radiation killing (34) and with the much higher scavenging ability for reactive oxygen species in extracts of D. radiodurans than in similarly prepared extracts of E. coli (35). Although protein damage in highly radiosensitive species may cause lethality even before significant DNA damage occurs (36, 37), this is clearly not the case for bdelloid rotifers, where hundreds of DSBs are made by a dose of IR that causes but little reduction in fecundity.
The picture that emerges from the above considerations is that a major defense against radiation damage in D. radiodurans and bdelloid rotifers, accounting for the distinctive shoulders in their dose–response relations, is an enhanced capacity for scavenging the reactive molecular species generated by IR and that the proteins and other cellular components thereby protected include those essential for the repair of broken DNA. This, however, leaves the question of why DNA, in both D. radiodurans and bdelloid rotifers, is not similarly protected but instead accumulates DSBs in direct proportion to dose and to the same extent per Gy per Mbp as in radio-sensitive organisms (Fig. 1). A possible answer lies in evidence that IR-induced DSBs result from closely spaced scission events on opposite chains of the DNA duplex that are caused by clusters of hydroxyl radicals or other reactive species generated in close association with DNA that are relatively immune or inaccessible to elimination by scavengers (38, 39).
IR and Desiccation.
Naturally occurring bacterial isolates that are highly resistant to IR are also resistant to desiccation, and mutations that diminish radiation resistance in D. radiodurans also reduce its resistance to desiccation. These observations and the low levels of IR in the natural habitats of D. radiodurans and other radioresistant bacteria indicate that their radiation resistance is a consequence of evolutionary adaptation to survive desiccation (40). Similarly, the extraordinary radiation resistance of bdelloid rotifers is almost certainly an adaptation to survive in their characteristic ephemerally aquatic habitats.
The association of resistance to IR with anhydrobiosis and the observation that desiccation, like exposure to IR, is accompanied by the production of reactive oxygen species (41) and protein oxidation in diverse biological systems and that proteins of desiccation-resistant bacteria are protected against such damage (33, 42) indicates that at least part of the damage caused by desiccation is the same as that caused by radiation. In D. radiodurans this includes DNA breakage, which increases with desiccation time and can reach a high level before there is appreciable killing (40). Although DNA breakage has not been investigated in desiccated bdelloid rotifers, their ability to resume reproduction as a function of desiccation time also exhibits an extended shoulder (2), and it seems likely that, as in D. radiodurans, desiccation is accompanied by extensive DNA breakage, implying that the evolution of bdelloid genomes has been accompanied by unusually frequent and extensive DNA breakage and repair.
Implications for Bdelloid Genome Structure and Evolution.
Accurate repair of a DSB requires the presence of a homologous template. Although bdelloid primary oocytes are in G1 and therefore lack sister chromatids, the requirement for homologous templates would be satisfied if bdelloid primary chromosomes were present as colinear pairs. In fact, this appears to be the case, as described in ref. 28, showing that bdelloids are probably degenerate tetraploids. Homology of colinear chromosomes sufficient for efficient repair could be maintained by selection against clones in which such homology becomes inadequate to support DSB repair by template-dependent repair processes. Local relaxation of the requirement for homology might occur, however, if sub- or neo-functionalization becomes established at particular sites and where the decreased efficiency of repair is more than offset by selection against clones in which such sites have been homogenized.
There are several ways in which the anhydrobiotic lifestyle of bdelloid rotifers may have contributed to their success in avoiding the early extinction that is the usual fate of asexuals. Desiccation can be of benefit to surviving bdelloids by facilitating their dispersal and freeing them from desiccation-sensitive competitors, parasites, pathogens, and predators. In addition to such ecological factors, bdelloid anhydrobiosis may have genetic benefits. In degenerate tetraploids, as in ordinary diploids, homogenization associated with the repair of desiccation-damaged DNA may significantly accelerate the appearance of clones homozygous for recessive beneficial mutations (43).
Finally, frequent DSB repair may act to keep the deleterious load of transposable elements low and account for the remarkable paucity in bdelloid genomes of transposable elements (TEs) of types that are not prone to horizontal transfer, namely long interspersed element LINE-like and other nonviral retrotransposons (44–46). TEs could be deleted or truncated by single-strand annealing mediated by microhomologies and, if not homozygous, by removal of nonhomologous 3′ ends in the course of synthesis-dependent strand annealing (47). Synergistic selection against TEs could occur, for example, if in the course of DSB repair an extended invading strand dissociates after copying a donor TE. Reinvasion and continued replication may then occur either at a eutopic site, leading to faithful repair, or at an ectopic TE, giving a lethal or semilethal nonreciprocal translocation (48). As the probability of a DSB being near a TE and the probability of ectopic reinvasion are both proportional to the density of homologous TEs, such template switching would cause synergistic selection against them. Nonreciprocal translocations, probably mediated by such template switching, accompany the repair of a substantial proportion of IR-induced DSBs in or near Ty retrotransposons in diploid S. cerevisiae (J.L. Argueso, J. Westmorland, P.A. Mieczkowski, M. Gawell, T.D. Petes, and M.A. Resnick, unpublished data). DNA damage and repair associated with anhydrobiosis may therefore have enabled bdelloids to become free of vertically transmitted TEs and limit their load of horizontally transmitted elements, avoiding the unchecked and ultimately lethal increase of deleterious TEs that may otherwise occur in asexuals (49, 50).
Materials and Methods
Rotifer Culture.
The bdelloid rotifers A. vaga and P. roseola were provided by Claudia Ricci (University of Milan, Milan, Italy) and purchased from Carolina Biological Supply, respectively. Our cultures of these species were established from single eggs and have been maintained continuously in spring water on a diet of E. coli for 11 (A. vaga) and 19 (P. roseola) years. The monogonont rotifer E. dilatata was provided by Elizabeth Walsh (University of Texas, El Paso, TX). Rotifers were cultured in MBL medium made up in spring water (51) and fed E. coli grown in tryptone broth (bdelloids) or Chlamydomonas reinhardtii (E. dilatata). C. reinhardtii was provided by Joel Rosenbaum (Yale University, New Haven, CT) and cultured with aeration in MBL medium with continuous illumination in a flask 20 cm from a 40-w cool-white (4100° K) spiral fluorescent lamp. Bacteria and algae were centrifuged and resuspended in distilled water twice, refrigerated, and used within 10 days. Rotifers were cultured in 150 × 25-mm plastic Petri dishes at room temperature, transferring ≈1,000 animals by pipette to a dish containing fresh medium every week or so. P. roseola and E. dilatata are free-swimming and may easily be collected by pipette. A. vaga generally crawls leech-like on surfaces from which it is not readily dislodged but also gathers in swarms from which animals may easily be collected by pipette.
Irradiation and Scoring Reproductive Performance.
Approximately 1,000 rotifers were transferred by pipette to a 5-cm plastic Petri dish containing 5 ml of half-strength MBL medium and kept 6–10 h at room temperature to clear the animals of food organisms. As rapid cooling was found to be lethal to E. dilatata, the dish was wrapped in a cloth towel, placed in a small cardboard box, and kept for ≈6 h in the cold room before being taken, on ice, to the irradiation facility, a procedure followed for both monogononts and bdelloids. After addition of 0.5 ml of freshly prepared 2 mM l-cysteine to prevent the possible accumulation of toxic radiation products in the medium, the dish was placed on a block of ice on a slowly rotating platform beneath a 137Cs source delivering a dose of 140 Gy/h at the position of the dish. As the reproductive lifespan of E. dilatata is only a few days (52), young monogononts, distinguished by their small size, were generally selected for irradiation.
For determination of reproductive performance, the dish was removed from the irradiator at specific intervals, and individual animals were transferred by pipette to each well of a 96-well microtiter dish containing 0.15 ml of MBL medium per well and a small amount of food. The dish was kept on ice during sampling, making it relatively easy to dislodge A. vaga by pipetting. Relative parental fertility was scored as the proportion of irradiated animals producing at least one active daughter, normalized to the corresponding proportion for nonirradiated animals. Each day or two until egg deposition ceased, ≈1 week for E. dilatata and 2 weeks for the bdelloids, any daughters present, distinguished from the parent by their smaller size, were removed to avoid including the eggs of daughters in counts of eggs deposited by their mothers. A daughter from each fertile parental well was placed in a well of another microtiter dish containing food and 0.15 ml of MBL medium, and every few days any granddaughters present were removed. Relative F1 fertility was scored as the proportion of daughters producing at least one granddaughter, normalized to the proportion produced by daughters of nonirradiated parents. For the reason explained below, bdelloid daughters were counted as they were removed from parental wells, as were bdelloid granddaughters removed from daughter wells.
Hatched and nonhatched eggs of E. dilatata and nonhatched bdelloid eggs were easily counted, but the transparency of hatched bdelloid eggs prevented their reliable identification. As inactive progeny were only rarely encountered, the number of hatched bdelloid eggs was therefore taken to be the same as the number of bdelloid progeny. At each dose, relative parental and F1 fecundity was taken as the total number of hatched eggs (monogononts) or progeny (bdelloids), normalized to the corresponding number from the nonirradiated animals. Three such experiments were performed with E. dilatata and one each with A. vaga and P. roseola.
Pulsed-Field Gel Electrophoresis.
For examination of DNA breakage ≈1,000 starved, prechilled rotifers were irradiated as described above for 0, 1/6, 2, 4, 6, or 8 h, harvested by centrifugation in the cold at ≈10,000 × g for 5 min, resuspended in 0.2 ml of cold 50 mM EDTA and 10 mM Tris (pH 8.0), and centrifuged again. After removing 0.16 ml of supernatant, tubes were placed in a 42°C water bath and supplemented with 40 μl of freshly melted 1% low melting point agarose (LMPA; NuSieve GTG) in 2× Tris-EDTA (200 mM EDTA, 100 mM Tris, pH 8.0) at 42°C, mixed well by pipetting, transferred to a plug mold, and left to solidify for 30 min in the cold room. Each plug and an additional plug containing S. cerevisiae chromosomes (New England BioLabs N0345S) was individually placed in 0.2 ml of digestion buffer (1× Tris-EDTA supplemented with 1% sarcosyl and 1 mg/ml freshly dissolved proteinase K) and kept for 1 h in the cold room and 18 h at 55°C. Plugs were then rinsed with 0.5× 40 mM Tris acetate, 1 mM EDTA, pH 8.5 (TAE), kept with gentle rocking for 3 h in 1.5 ml of 0.5× TAE at room temperature and embedded in a 5-mm deep slab of 0.7% LMPA in 0.5× TAE. Electrophoresis was done in a BioRad CHEF-DR III electrophoretic system at 14°C and 5.5 V/cm, with a switch angle of 120° and switch times of 50–250 s for 18 h.
After electrophoresis, the gel was placed in SYBR Gold (Invitrogen) freshly diluted 1:10,000 in water, gently rocked overnight, and scanned with a BioRad Molecular Imager FX with Quantity One quantitation software (SYBR Gold settings: 488-mμ exitation, 530-mμ band pass). Under the conditions used, signal intensity is a linear function of DNA concentration in the gel (53). After baseline subtraction, each photometric scan was divided into 33 equal intervals along the direction of migration and the number average molecular size of DNA was calculated as Σ(intensity)/Σ[(intensity)/(size)] by summation over intervals, taking molecular sizes from a plot of the migration distance of the yeast markers against their sizes.
Acknowledgments.
Karine VanDoninck participated in the early phase of this work. This work was supported by the Eukaryotic Genetics Program of the National Science Foundation.
Footnotes
The authors declare no conflict of interest.
Rahman R, Rahman MM, Islam S, Huque R, Food and Agriculture Organization/International Atomic Energy Agency Final Research Coordination Meeting, Evaluation of Population Suppression by Irradiated Lepidoptera and Their Progeny, May 28–30, 1998, Penang, Malasia.
References
- 1.Ricci C. Ecology of bdelloids: How to be successful. Hydrobiologia. 1987;147:117–127. [Google Scholar]
- 2.Ricci C. Anhydrobiotic capabilities of bdelloid rotifers. Hydrobiologia. 1998;387/388:321–326. [Google Scholar]
- 3.Kikawada T, Minakawa N, Watanabe M, Okuda T. Factors inducing successful anhydrobiosis in the African chironomid Polypedilum vanderplanki: Significance of the larval tubular nest. Integr Comp Biol. 2005;45:710–714. doi: 10.1093/icb/45.5.710. [DOI] [PubMed] [Google Scholar]
- 4.Clegg JS. Cryptobiosis: A peculiar state of biological organization. Comp Biochem Phys B. 2001;128:613–624. doi: 10.1016/s1096-4959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 5.Wallace RL, Snell TW, Ricci C, Nogrady T. Rotifera: Biology, Ecology, and Systematics. Vol 1. Leiden, The Netherlands: Backhuys; 2006. [Google Scholar]
- 6.Jonsson KI, Schill RO. Induction of Hsp70 by desiccation, ionizing radiation, and heat shock in the eutardigrade Richtersius coronifer. Comp Biochem Physiol B. 2007;146:456–460. doi: 10.1016/j.cbpb.2006.10.111. [DOI] [PubMed] [Google Scholar]
- 7.Shannon AJ, Browne JA, Boyd J, Fitzpatrick DA, Burnell AM. The anhydrobiotic potential and molecular pohylogenetics of species and strains of Panagrolaimus (Nematoda, Panagrolaimidae) J Exp Biol. 2005;208:2433–2445. doi: 10.1242/jeb.01629. [DOI] [PubMed] [Google Scholar]
- 8.Tunnacliffe A, Lapinski J, McGee B. A putative LEA protein, but no trehalose, is present in anhydrobiotic bdelloid rotifers. Hydrobiologia. 2005;546:315–321. [Google Scholar]
- 9.Bonura T, Smith KC. The involvement of indirect effects in cell killing and DNA double-strand breakage in γ–irradiated Escherichia coli K12. Int J Radiat Biol. 1976;29:293–296. doi: 10.1080/09553007614550331. [DOI] [PubMed] [Google Scholar]
- 10.Resnick MA, Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976;143:119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- 11.Krisch RE, Flick MB, Trumbore CN. Radiation chemical mechanisms of single- and double-strand break formation in irradiated SV40 DNA. Radiat Res. 1991;126:251–259. [PubMed] [Google Scholar]
- 12.Prise KM, et al. A review of dsb induction data for varying quality radiations. Int J Radiat Biol. 1998;74:173–184. doi: 10.1080/095530098141564. [DOI] [PubMed] [Google Scholar]
- 13.Sternlöw B, Karlsson KH, Cooper B, Rydberg B. Measurement of prompt DNA double-strand breaks in mammalian cells without including heat-labile sites. Rad Res. 2003;159:502–510. doi: 10.1667/0033-7587(2003)159[0502:mopdds]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Mark Welch D, Meselson M. Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science. 2000;288:1211–1215. doi: 10.1126/science.288.5469.1211. [DOI] [PubMed] [Google Scholar]
- 15.Bakri A, Mehta K, Lance DR. Sterilizing insects with ionizing radiation. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique, Principles, and Practice in Area-Wide Integrated Pest Management. Dordrecht, The Netherlands: Springer; 2005. pp. 233–268. [Google Scholar]
- 16.Hartman PS, Herman RK. Radiation-sensitive mutants of Caenorhabditis elegans. Genetics. 1982;102:159–178. doi: 10.1093/genetics/102.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollingsworth RG, Follett PA, Armstrong JW. Effects of irradiation on the reproductive ability of Zonitoides arboreus, a snail pest or orchid roots. Ann Appl Biol. 2003;143:395–399. [Google Scholar]
- 18.Jonsson KI, Harms-Ringdahl M, Torudd J. Radiation tolerance in the eutardigrade Richtersius coronifer. Int J Radiat Biol. 2005;81:649–656. doi: 10.1080/09553000500368453. [DOI] [PubMed] [Google Scholar]
- 19.Horikawa DD, et al. Radiation tolerance in the tardigrade Milnesium tardirgradum. Int J Radiat Biol. 2006;82:843–848. doi: 10.1080/09553000600972956. [DOI] [PubMed] [Google Scholar]
- 20.Boschetti C, Ricci C, Sotgia C, Fascio U. The development of a bdelloid egg: A contribution after 100 years. Hydrobiologia. 2005;546:323–331. [Google Scholar]
- 21.Pagani M, Ricci C, Redi CA. Oogenesis in Macrotrachela quadricornifera (Rotifera, Bdelloidea), I. Germarium eutely, karyotype, and DNA content. Hydrobiologia. 1993;255/256:225–230. [Google Scholar]
- 22.Pray FA. Studies on the early development of the rotifer Monostyla cornuta Müller. Trans Am Microscop Soc. 1965;84:210–216. [Google Scholar]
- 23.Bentfield ME. Studies of oogenesis in the rotifer Asplanchna. I. Fine structure of the female reproductive system. Z Zellforsh. 1971;115:165–183. doi: 10.1007/BF00391123. [DOI] [PubMed] [Google Scholar]
- 24.Hsu WS. Oogenesis in Habrotrocha tridens (Milne) Biol Bull. 1956;111:364–374. [Google Scholar]
- 25.Hsu Ws. Oogenesis in the Bdelloidea rotifer Philodina roseola Ehrenberg. Cellule. 1956;57:283–296. [Google Scholar]
- 26.Gregory TR, et al. Eukaryotic genome size databases. Nucleic Acids Res. 2007;35:D332–338. doi: 10.1093/nar/gkl828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mark Welch DB, Meselson M. Oocyte nuclear DNA content and GC proportion in the anciently asexual class Bdelloidea. Biol J Linn Soc. 2003;79:85–91. [Google Scholar]
- 28.Mark Welch DB, Mark Welch JL, Meselson M. Evidence for degenerate tetraploidy in bdelloid rotifers. Proc Natl Acad Sci USA. 2008;105:5145–5149. doi: 10.1073/pnas.0800972105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makarova KS, et al. Deinococcus geothermalis: The pool of extreme radiation resistance genes shrinks. PLoS ONE. 2007;2:e955. doi: 10.1371/journal.pone.0000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahradka K, et al. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature. 2006;443:569–573. doi: 10.1038/nature05160. [DOI] [PubMed] [Google Scholar]
- 31.Daly MJ, et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 32.Gerard E, Jolivet E, Prieur D, Forterre P. DNA protection mechanisms are not involved in the radioresistance of the hyperthermophilicic archaea Pyrococcus abyssi and P. furiosus. Mol Gen Genet. 2001;266:72–78. doi: 10.1007/s004380100520. [DOI] [PubMed] [Google Scholar]
- 33.Daly MJ, et al. Protein oxidation implicated as the primary determinant of bacterial radio resistance. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050092. 0769–0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein LS, Gersten DM, Bruce AK. Radio protection in E. coli by an agent from M. radiodurans. Int J Rad Biol. 1978;34:375–380. doi: 10.1080/09553007814551011. [DOI] [PubMed] [Google Scholar]
- 35.Tian B, et al. Chemiluminescence assay for reactive oxygen species scavenging activities and inhibition on oxidative damage of DNA in Deinococcus radiodurans. Luminescence. 2004;19:78–84. doi: 10.1002/bio.761. [DOI] [PubMed] [Google Scholar]
- 36.Du J, Gebicki JM. Proteins are major initial cell targets of hydroxyl free radicals. Int J Biochem Cell Biol. 2004;36:2334–2343. doi: 10.1016/j.biocel.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Qiu X, et al. Transcriptome analysis as applied to survival of Shewanella oneidensis MR-1 exposed to ionizing radiation. J Bacteriol. 2006;188:1199–1204. doi: 10.1128/JB.188.3.1199-1204.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milligan JR, et al. Effect of hydroxyl radical scavenging capacity on clustering of DNA damage. Rad Res. 1997;148:325–329. [PubMed] [Google Scholar]
- 39.Ayene IS, Koch CJ, Krisch RE. DNA strand breakage by bivalent metal ions and ionizing radiation. Int J Rad Biol. 2007;83:195–210. doi: 10.1080/09553000601146956. [DOI] [PubMed] [Google Scholar]
- 40.Mattimore V, Battista JR. Radioresistance of Deinococcus radiodurans: Functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol. 1996;178:633–637. doi: 10.1128/jb.178.3.633-637.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.França MB, Panek AD, Eleutherio EC. Oxidative stress and its effects during drying. Comp Biochem Physiol A Mol Interg Physiol. 2007;146:621–631. doi: 10.1016/j.cbpa.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 42.Fredrickson JK, et al. Protein oxidation: Key to bacterial desiccation resistance? ISME J. 2008 doi: 10.1038/ismej.2007.116. in press. [DOI] [PubMed] [Google Scholar]
- 43.Mandegar MA, Otto S. Mitotic recombination counteracts the benefits of genetic segregation. Proc R Soc Ser B. 2007;274:1301–1307. doi: 10.1098/rspb.2007.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arkhipova I, Meselson M. Transposable elements in sexual and ancient asexual taxa. Proc Natl Acad Sci USA. 2000;97:14473–14477. doi: 10.1073/pnas.97.26.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arkhipova IR, Meselson M. Diverse DNA transposons in rotifers of the class Bdelloidea. Proc Natl Acad Sci USA. 2005a;102:11781–11786. doi: 10.1073/pnas.0505333102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gladyshev E, Meselson M, Arkhipova IR. A deep-branching clade of retrovirus-like transposons in bdelloid rotifers. Gene. 2007;390:136–145. doi: 10.1016/j.gene.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paques F, Haber JE. Two pathways for removal of nonhomolgous DNA ends during double-strand break repair. Mol Cell Biol. 1997;17:6765–6771. doi: 10.1128/mcb.17.11.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith CE, Llorente B, Symington LS. Template switching during break-induced replication. Nature. 2007;447:102–105. doi: 10.1038/nature05723. [DOI] [PubMed] [Google Scholar]
- 49.Arkhipova IR, Meselson M. Deleterious transposable elements and the extinction of asexuals. BioEssays. 2005b;27:76–85. doi: 10.1002/bies.20159. [DOI] [PubMed] [Google Scholar]
- 50.Dolgin ES, Charlesworth B. The fate of transposable genetic elements in asexual populations. Genetics. 2006;174:817–827. doi: 10.1534/genetics.106.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stemberger RS. A general approach to the culture of planktonic rotifers. Can J Fish Aquat Sci. 1981;38:721–724. [Google Scholar]
- 52.King CE. Food, age, and the population dynamics of a laboratory population of rotifers. Ecology. 1967;48:111–128. [Google Scholar]
- 53.Malloy MJ, et al. Effective and robust plasmid topology analysis and the subsequent characterization of the plasmid isoforms thereby observed. Nucleic Acid Res. 2004;32:e129. doi: 10.1093/nar/gnh124. [DOI] [PMC free article] [PubMed] [Google Scholar]