Abstract
The yeast ABC transporter Pdr5 plays a major role in drug resistance against a large number of structurally unrelated compounds. Although Pdr5 has been extensively studied, many important aspects regarding its molecular mechanisms remain unresolved. For example, a striking degeneration of conserved amino acid residues exists in the nucleotide binding domains (NBDs), but their functional relevance is unknown. Here, we performed in vivo and in vitro experiments to address the functional asymmetry of NBDs. It became evident by ATPase activity and drug transport studies that catalysis at only one of the two NBD composite sites is crucial for protein function. Furthermore, mutations of the proposed “catalytic carboxylate” (E1036) and the “catalytic dyad histidine” (H1068) were characterized. Although a mutation of the glutamate abolished ATPase activity and substrate transport, mutation of H1068 had no influence on ATP consumption. However, the H1068A mutation abolished rhodamine transport in vivo and in vitro, while leaving the transport of other substrates unaffected. By contrast to mammalian P-glycoprotein (P-gp), the ATPase activity of yeast Pdr5 is not stimulated by the addition of substrates, indicating that Pdr5 is an uncoupled ABC transporter that constantly hydrolyses ATP to ensure active substrate transport. Taken together, our data provide important insights into the molecular mechanism of Pdr5 and suggest that not solely the transmembrane domains dictate substrate selection.
Keywords: ATPase activity, multidrug resistance, substrate recognition
The plasma membrane ABC transporter Pdr5 is a central element of the pleiotropic drug resistance (PDR) network in the yeast Saccharomyces cerevisiae (1). The phenomenon of PDR has received special attention as the Cdr1 orthologue overexpressed in azole-resistant Candida spp. hampers therapy of infections with opportunistic fungal pathogens affecting patients with impaired immune systems (2).
Numerous studies unraveled the complex nature of the yeast PDR network (3), which is composed of several stress response factors and transcriptional regulators, ultimately controlling expression of PDR5 and related drug pumps (4). Pdr5 is the most abundant ABC transporter in S. cerevisiae, capable of extruding hundreds of structurally unrelated hydrophobic compounds across the plasma membrane in an ATP-dependent manner (5–7). The discovery that mutations in the transcription factor Pdr1 lead to a dramatic overexpression of PDR5 has opened the possibility to study the function of this ABC transporter in vivo and in vitro (5–8). Characterization in a cellular context and/or the native membrane environment provide an excellent tool to assay the substrate specificity and the ATPase and transport activities without interference from solubilization and purification in detergents, which have also been proposed to be substrates of Pdr5 (9).
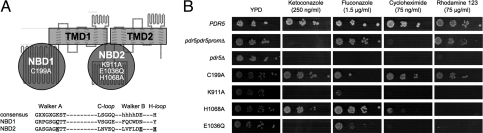
Like all functional ABC transporters, Pdr5 carries two transmembrane domains (TMDs) and two nucleotide binding domains (NBDs). The NBDs contain all characteristic sequence motifs of ABC proteins, such as the Walker A and B motifs; the C-loop, the hallmark of the ABC family; and the H- and D-loop sequences (10). However, the primary structure of Pdr5 reveals a profound asymmetry between the two NBDs (Fig. 1A). The highly conserved lysine of the Walker A motif and the histidine of the H-loop are missing in the N-terminal NBD (NBD1), whereas the C-loop sequence in the C-terminal NBD (NBD2) deviates from the canonical amino acids (LNVEQ instead of LSGGQ). The crystal structures of isolated NBDs (for a recent review, see ref. 11) and of fully assembled ABC transporters (for example, ref. 12), demonstrated a “head-to-tail” arrangement of NBDs that suggests a functional asymmetry in Pdr5 with one corrupted and one intact catalytic site. Here, the intact site, sandwiched between the Walker A motif of NBD2 and the C-loop of NBD1, will be referred to as the “NBD2 composite“ site. The corrupted site, the NBD1 composite site, includes the nonconsensus residues from the Walker A motif of NBD1 and the C-loop of NBD2.
Fig. 1.
Mutational analysis of Pdr5. (A) Schematic representation based on a membrane topology prediction of Pdr5 [supporting information (SI) Fig. S1]. Mutants within NBD1 or NBD2 used in this study are indicated. (B) Drug resistance assays with PDR5 variants. Serial dilutions of WT cells, isogenic pdr5pdr5promΔ and pdr5Δ null mutant cells, and cells expressing mutants of PDR5 were spotted on drug agar plates.
Furthermore, two opposing models of ATP hydrolysis in ABC transporters have been proposed, the “catalytic carboxylate” (13) and the “catalytic dyad” models (14). According to the first model, the glutamate C-terminal to the Walker B motif is essential for ATP hydrolysis. Interestingly, in Pdr5, this glutamate (E1036) is present only in NBD2 but not in NBD1 (Fig. 1A). The second, the catalytic dyad model, proposed an interaction between the glutamate and the histidine of the H-loop as a prerequisite for ATP hydrolysis. Again, the histidine is only found in NBD2 of Pdr5 (H1068) (Fig. 1A). Remarkably, both models are supported by experimental data (15, 16), and, currently, it is not even clear whether ATP hydrolysis occurs by a single universal mechanism in ABC transporters or whether certain subfamilies use different mechanisms.
A fascinating feature of ABC transporters is the cross-talk between substrate binding and ATP hydrolysis. Many ABC systems display a so-called “basal” ATPase activity in the absence of transport substrates, which, however, is stimulated in the presence of substrates. In the case of P-gp, basal and stimulated ATPase activity are thought to reflect two different modes of operation and are a prerequisite to ensure the proper function of this human drug efflux pump (17). In contrast, the substrate transport and ATP hydrolysis appear strictly coupled in the bacterial uptake systems for histidine and maltose (18, 19), because ATPase activity is only observed in presence of transport substrates in reconstituted systems. The existence of coupled and partially uncoupled systems raises the question of the evolutionarily benefit from an energy-wasting uncoupled mode.
Here, we report functional and mutational studies of Pdr5 in yeast cells and in highly enriched plasma membrane preparations. The results shed new light on the functional asymmetry of both NBDs and suggest that substrate selection in this eukaryotic multidrug transporter might be dictated not only by the TMDs, but presumably also by nucleotide-protein interactions.
Results
Functional Characterization of Pdr5.
We performed site-directed mutagenesis of the S. cerevisiae multidrug transporter Pdr5 to study its mechanism of ATP hydrolysis. The constructed mutants were characterized in vivo by analyzing their substrate specificities, and in vitro via ATPase and transport assays, using preparations of highly enriched plasma membranes.
Drug Resistance Phenotypes of PDR5 Mutants.
The most straightforward methods to analyze the functionality of Pdr5 are drug susceptibility assays either on drug agar plates (Fig. 1B) or in liquid culture (Fig. S2) (1). As expected, cells overexpressing PDR5 are highly resistant to ketoconazole (KA), fluconazole (FA), cycloheximide (CHX), and rhodamine 123 (R123) (Fig. 1B, PDR5) (5, 20, 21), whereas cells deleted of PDR5 were highly susceptible to all tested drugs (Fig. 1B, pdr5Δ). Because the presence of a removable N-terminal histidine-tag did not affect Pdr5-dependent drug resistance phenotypes or its expression levels (data not shown), we used only the tagged Pdr5 version in all subsequent experiments.
The disruption of the PDR5 promotor and PDR5 (for details, see SI Text) resulted in cells being more resistant against FA and R123 when compared with cells lacking only PDR5 (Fig. 1B; pdr5pdr5promΔ). Immuno-detection of Pdr5 in a crude cell extract confirmed the complete absence of Pdr5 (data not shown). Therefore, the observed drug resistance can depend only on the PDR5 promotor, not PDR5. Interestingly, the PDR5 promotor acts bidirectionally, also controlling expression of YOR152C, which may be involved in the phenomenon of drug resistance as well (4, 9).
We constructed mutant variants of Pdr5 and used cell-based resistance assays to address (i) the function of NBD1 and NBD2 and (ii) the mechanism of ATP hydrolysis. The asymmetry of NBD1 and NBD2, already evident from the primary structure, was assessed by mutation of highly conserved residues within the Walker A sequence motifs (Fig. 1A, C199A and K911A). Both residues may be essential for ATP hydrolysis and transport activity in related (22–24) and more distant ABC transporters (25, 26). In the case of Pdr5, mutation of the Walker A cysteine in NBD1 resulted in cells with a drug resistance phenotype undistinguishable from WT cells (Fig. 1B, C199A). The corresponding mutation in NBD2 (K911A) resulted in hypersensitive cells against all tested drugs with the only exception (Fig. 1B, K911A) of a very modest resistance to FA. This result was confirmed in an alternative drug susceptibility assay in liquid culture (Fig. S2), and may be due to alternative drug resistance mechanisms controlled by the expression of the YOR152C gene neighbouring PDR5 (see above).
Taken together, these data clearly demonstrate a functional nonequivalence of NBD1 and NBD2 in Pdr5. Consistent with previous studies, a catalytically active NBD1 composite site appears to be dispensable for protein function, whereas the NBD2 composite site is essential for drug transport.
To test, whether Pdr5 hydrolyzes ATP according to the catalytic carboxylate or the catalytic dyad model, the glutamate adjacent to the Walker B motif and the histidine of the H-loop in NBD2, individually proposed to be essential for catalytic activity, were mutated to glutamine and alanine, respectively (Fig. 1A, E1036Q and H1068A). Cells expressing the E1036Q mutant were highly sensitive against all tested drugs, with only residual resistance against FA (Fig. 1B, E1036Q). However, cells expressing the H1068A mutant are highly resistant to KA, FA, and CHX but exhibit a dramatic loss of resistance to R123 (Fig. 1B, H1068A). This was confirmed by liquid culture assays (Fig. S2). Therefore, this histidine is not essential for Pdr5 function, and ATP is most likely hydrolyzed by the catalytic carboxylate mechanism. However, the loss of resistance against R123 in the H1068A mutant raises the question for the role of the H-loop in the selection of substrates.
Isolation of S. cerevisiae Plasma Membranes.
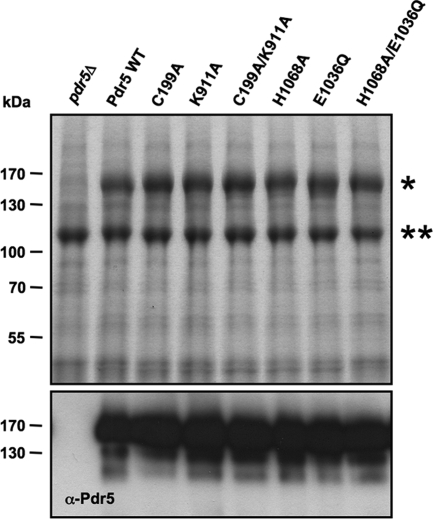
For a more detailed analysis of the constructed PDR5 mutants, we isolated plasma membranes from mutant cells according to a protocol pioneered by Goffeau and coworkers (7, 27). A specific band could be assigned to Pdr5 (asterisk in Fig. 2Upper) but was missing in control membranes from pdr5Δ cells. This was further confirmed by immunodetection of Pdr5 (Fig. 2 Lower). No immuno-reactivity was observed for plasma membranes isolated from pdr5Δ cells. The identity of the second prominent band in the Coomassie-stained SDS/PAGE (double asterisks in Fig. 2 Upper) was analyzed by mass spectrometry and identified as the plasma membrane ATPase Pma1 (data not shown).
Fig. 2.
Expression of Pdr5 variants. (Upper) Isolated plasma membrane fractions (15 μg/lane) were fractionated through a 7% SDS/PAGE and stained with Coomassie blue. The two major bands correspond to Pdr5 (*) and to the plasma membrane ATPase Pma1 (**). The positions of molecular mass markers are indicated at Left. (Lower) The identity of Pdr5 was verified by immunoblotting with Pdr5-specific antibodies.
ATPase Activity of Pdr5.
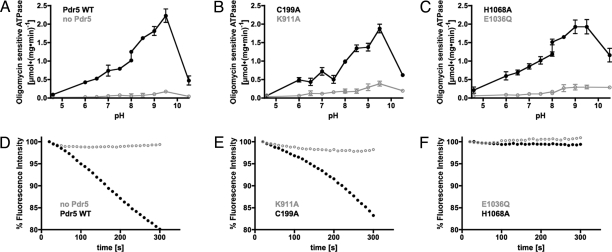
Isolated plasma membranes were subsequently used to characterize the Pdr5-specific ATPase activity. The ATPase activity of Pdr5 peaks over a relatively broad pH range (Fig. 3A, Pdr5 WT) and was reduced severely by the addition of 20 μg/ml oligomycin (OM). The maximal OM-sensitive ATPase activity of 2.2 ± 0.3 μmol·(mg·min)−1 was determined at pH 9.5. Control membranes lacking Pdr5 exhibited only background activity of 0.20 ± 0.02 μmol·(mg·min)−1 (Fig. 3A, no Pdr5).
Fig. 3.
Pdr5-specific ATPase activity and R6G transport. (A–C) OM-sensitive ATPase activities of plasma membrane preparations derived from cells overexpressing N-terminally 14-His-tagged Pdr5 (Pdr5 WT) and of control membranes derived from isogenic pdr5Δ cells (no Pdr5) (A) was assayed and plotted against pH values. The asymmetry of the NBDs was analyzed by using two mutants (C199A and K911A) (B). Furthermore, the role of the glutamate adjacent to the Walker B motif (E1036Q) and the histidine of the H-loop (H1068A) were assayed (C). (D–F) Isolated plasma membranes were incubated with R6G, and the fluorescence signal was recorded after the addition of Mg2+/ATP (t = 0 s). (D) A decrease in R6G fluorescence represents active transport and is only observed in plasma membranes containing N-terminally 14-His-tagged Pdr5 (Pdr5 WT) but not in control membranes derived from isogenic pdr5Δ cells (no Pdr5). (E) The asymmetric role of the NBDs for R6G transport was analyzed by the C199A and the K911A mutant (C199A and K911A). (F) The influence of the H1068A and the E1036Q mutation on ATPase activity was also analyzed.
How do the results from the drug susceptibility assays relate to ATPase activities observed for mutants of NBD1 and NBD2? As depicted in Fig. 3B, the C199A mutant (Walker A motif of NBD1) exhibited an ATPase activity identical to WT Pdr5 [1.9 ± 0.2 μmol·(mg·min)−1]. In contrast, the equivalent mutation K911A in NBD2 strongly impaired the ATPase activity [0.4 ± 0.1 μmol·(mg·min)−1]. These results mirror the observations of the drug susceptibility assays, which identified a crucial role of the NBD2 composite site for protein function.
Further insights into the mechanism of ATP hydrolysis were obtained from the analysis of the H1068A and E1036Q mutants. If the proposed catalytic carboxylate is mutated (E1036Q), no significant Pdr5-specific ATPase activity was detectable [0.3 ± 0.1 μmol·(mg·min)−1]. The H1068A mutant should exhibit, according to the catalytic dyad model, no Pdr5-specific ATPase activity. However, the steady-state ATPase activity of the H1068A mutant was identical to WT Pdr5 [2.0 ± 0.3 μmol·(mg·min)−1].
Transport Activity of Pdr5 and Its Mutant Variants.
In parallel to the ATPase activity, we analyzed the capability of Pdr5 and its mutant variants to transport rhodamine 6G (R6G) in vitro. Active transport of this fluorescent dye is monitored by an ATP-, Mg2+-, and Pdr5-dependent fluorescence quenching reaction, which reflects the concentration-dependent formation of nonfluorescent R6G excimers upon its redistribution between the leaflets of the plasma membrane bilayer (7). Full reversibility of R6G quenching was confirmed with several inhibitors, such as OM, vanadate, beryllium fluoride, FK506, or EDTA (Fig. S3).
Only membranes containing Pdr5 mediated dye transport (Fig. 3D, Pdr5 WT and no Pdr5). Mutants with abolished ATPase activity exhibited no active transport, demonstrating that ATPase activity is a prerequisite for transport. Analysis of the transport activities of Walker A variants (Fig. 3E, C199A and K911A) provided further proof for the functional asymmetry of NBD1 and NBD2. The K911A mutant in NBD2 abolished Pdr5-mediated dye transport, whereas the equivalent mutation in NBD1 (C199A) exhibited similar dye transport as WT Pdr5. Taken together, the presented in vivo and in vitro data provide compelling evidence that ATP hydrolysis and active drug transport are catalyzed exclusively by the NBD2 composite site.
The central role of a functional NBD2 for drug transport becomes even more evident, when the transport assay was performed with additional mutants. Significantly, the H1068A and E1036Q mutations (both located in NBD2) abolished active dye transport (Fig. 3F, H1068A and E1036Q). Numerous independent studies on different ABC transporters proposed a crucial role for these residues in ATP hydrolysis. However, the H1068A mutation impaired solely R6G transport and had no impact on the observed steady-state ATPase activity (Fig. 3C, H1068A). According to available crystal structures, this residue is most likely in close proximity to the γ-phosphate of ATP (14, 25, 28). If this holds true for Pdr5 as well, it implies that the absence of the histidine in the H1068A mutant directly or indirectly affects the R6G selection at the substrate binding site. To learn more about this intimate cross-talk, we determined the impact of several drugs on the Pdr5-specific ATPase activities.
ATPase Activity of Pdr5 Is Uncoupled from Drug Transport.
Many ABC transporters exhibit a severalfold stimulated ATPase activity in the presence of their transport substrate. However, many ABC transporters display also a so-called basal or “substrate-uncoupled” ATPase activity that was observed even in the absence of substrates (29). In the case of P-gp, the ATPase activity exhibits a biphasic response to transport substrates. At low concentrations of added drugs, the basal ATPase activity is stimulated severalfold (30), whereas it is inhibited at higher concentrations. Hence, we determined ATPase activities of Pdr5 in the presence or absence of well known substrates (FA, KA, CHX, and R6G) and an inhibitor of Pdr5-mediated drug resistance (FK506) at different concentrations ranging from picomolars to millimolars (see Fig. S4). Remarkably, no significant stimulation of ATPase activity above the basal level could be observed for all tested drugs. However, some of the tested substances, including R6G, inhibited the Pdr5-specific ATPase activity at high concentrations (Fig. S4). The data were fitted by using a model for ATPase inhibition by inhibitory drug concentrations (Eq. 1). The Ki values for this inactivation are summarized in Table 1. n represents the number of independent experiments. The F test was used to test the assumption that the Ki value of the H1068A mutant and the WT version were distinct. The assumption was only rejected for KA (P > 0.05). Thus, significantly higher concentrations of R6G, R123, and FK506 were required to inhibit the H1068A mutant compared with WT Pdr5. Smaller molecules like FA and CHX did not inhibit the ATPase activity of both Pdr5 versions (data not shown). Thus, consistent with data from systematic studies by Golin et al., it became evident that a strong size dependence determines the interaction between Pdr5 and its substrates (6, 31).
Table 1.
Drug mediated inhibition of Pdr5-specifc ATPase activity
| Drug | Ki, WT, μM | Ki, H1068A, μM | Ki, H1068A/Ki, WT | F test | n |
|---|---|---|---|---|---|
| R123 | 13.7 ± 3.6 | 50.3 ± 11.0 | 3.7 | P < 0.05 | 2 |
| R6G | 0.6 ± 0.1 | 1.8 ± 0.2 | 2.8 | P < 0.0001 | 5 |
| KA | 5.4 ± 0.8 | 4.2 ± 0.7 | equal | P > 0.05 | 2 |
| FK506 | 0.05 ± 0.01 | 0.12 ± 0.04 | 2.4 | P < 0.05 | 2 |
Discussion
Functional Asymmetry in Pdr5.
Our parallel in vitro and in vivo analysis of Pdr5 revealed nonequivalent functions of NBD1 and NBD2 (Figs. 1 and 3). ATP-hydrolysis at the NBD1 composite site is negligible, whereas ATP-hydrolysis at the NBD2 composite site is essential for drug transport. Consistent with our data, similar observations have been made in earlier studies of Pdr5 and the closely related multidrug transporter Cdr1 from Candida glabrata (5, 24). In sharp contrast, other studies indicated a critical role of the Walker A motif in the NBD1 of Cdr1 from C. albicans for ATPase activity and transport (22, 23). However, swapping experiments between NBD1 and NBD2 in Cdr1 support our interpretation that ATP hydrolysis at the NBD2 composite site is more critical for protein function (32). Such functional asymmetry has also been described for other related ABC transporters (25, 33, 34). Thus, the role of the NBD1 composite site could be architectural, by providing in its nucleotide bound form a platform for the interaction with the opposing NBD, or regulatory, as proposed for the N-terminal NBD of the cystic fibrosis transmembrane conductance regulator (33).
Residues involved in ATP hydrolysis.
Numerous studies in the field of ABC transporters were dedicated to the mechanism of ATP hydrolysis. Because of the complex and to some extent contradicting data, this issue remains under dispute (13–16, 25, 34). As already stated above, two mutually exclusive models have been proposed. Our simplistic approach in this study was to mutagenize two key residues, each of them individually proposed to be essential (Figs. 1B and 3C). The functional characterization of these mutant variants resulted in several major observations: First, the proposed catalytic carboxylate E1036 adjacent to the Walker B motif is essential for ATP hydrolysis. Second, the mutation of H1068, according to the catalytic dyad essential for ATP hydrolysis, did not have any impact on the observed steady-state ATPase activity. Third, the basal ATPase activity was not stimulated by any substrate tested, implying that ATPase and transport are uncoupled in Pdr5 (Fig. S4). Fourth, and most important, the mutation of H1068 selectively abolished transport of R6G by Pdr5 (Fig. 1B).
Not surprisingly, because the proposed catalytic carboxylate E1036 is crucial for ATP hydrolysis, it is essential for R6G transport and mediating drug resistance in living cells as well. Notably, and in contrast to other ABC systems (14, 35, 36), Pdr5 is the first ABC transporter for which a mutation of the H-loop histidine to alanine did not affect the steady-state ATPase activity. Strikingly, the observation that the H1068A mutation abolishes R6G transport without any impact on the observed ATPase activity clearly represents a novelty in the field of ABC transporters (Fig. 3).
Uncoupled ATPase activity of Pdr5.
We determined the steady-state ATPase activity of Pdr5 in the presence of different substrates (Table 1 and Fig. S4) to further investigate the communication between substrate binding and ATP hydrolysis. Because none of the substrates stimulated the steady-state ATPase activity of Pdr5 we concluded that Pdr5 is a strictly uncoupled transport system exhibiting only basal ATPase activity. Several substrates, however, even inhibited the ATPase activity of Pdr5, implying that substrate release from the transporter may be rate-limiting. Most likely, this inhibition is due to a substrate-mediated lock of the outward facing conformation. Taking into account the observations by Golin et al., this substrate-mediated inhibition appears to require a certain substrate size (6, 31).
Many ABC transporters have been shown to exhibit basal ATPase activity, which is stimulated in the presence of transport substrates. P-gp, for example, switches between these two modes: a basal uncoupled mode and a substrate-dependent coupled mode (17). Prerequisite for such behavior is an intense cross-talk between substrate binding and ATP hydrolysis. However, in the case of the bacterial importers for histidine and maltose, it has been demonstrated that substrate transport and ATP hydrolysis are strictly coupled (18, 19). Interestingly, the ATPase activity is strictly dependent on the H-loop histidine in these systems. In marked contrast, the uncoupled system Pdr5 exhibits only basal ATPase activity (Fig. S4), which is not influenced by mutation of the histidine of the H-loop (Fig. 3). Whether or not this is only coincidence remains to be established.
Does the basal, uncoupled ATPase activity of Pdr5 mean a great waste of energy and an evolutionarily drawback? Interestingly, also in the case of the closely related multidrug transporter Cdr1 from C. albicans, if at all, only a very minor stimulation (<50%) of the basal ATPase activity is observed in the presence of drugs (23). Obviously, the waste of energy by futile, basal ATP hydrolysis is evolutionarily conserved. First of all, this is no surprise because a strictly coupled multidrug transporter is extremely hard to envision. How can thousands of different inputs (binding of highly diverse substrates) give rise to a single specific answer (stimulated ATP hydrolysis and coupled transport)? As proposed for P-gp, the constant turnover of ATP may be necessary in Pdr5 and related fungal ABC transporters to induce conformational changes that always keep the cytosolic substrate binding sites accessible (17, 30). Thus, instead of being a waste of energy, the basal ATPase activity might represent a concept indispensable to life by keeping the protein in a transport-competent conformation, representing an open “guarding mode conformation” for rapid detoxification events.
Mutation of H-Loop Selectively Abolishes Transport of R6G.
Even though Pdr5 is a highly uncoupled transport system, some cross-talk between substrate binding and ATP hydrolysis appears to be conserved. Surprisingly, the H1068A mutation selectively abolished the transport of R6G despite of its expendability for steady-state ATPase activity. Mutant cells showed normal resistance against FA and KA, but were highly susceptible to R123 (Fig. 1). A complete loss of R6G transport activity was confirmed in vitro (Fig. 3). Because R6G is still capable to inhibit the activity of the H1068A mutant (Table 1), presumably by locking the outward facing conformation, we assume that this mutation rather affects the duration of the initial drug binding during nucleotide exchange, when Pdr5 is in the inward facing conformation.
Previously, the histidine of the H-loop has been designated a “linchpin” because of its intense interactions within a catalytic dyad with the γ-phosphate of bound ATP and the conserved D-loop to the opposing NBD (14). Mutation of this residue may have structural effects on the substrate binding site via this network of interactions and explains the changed substrate specificity. However, a much more likely interpretation is that substrate selection by Pdr5 is not only determined by static structural features but also by kinetics. Crucial to an understanding is that, even though it does not affect the steady-state ATPase activity, the H1068A mutant might have changed the kinetics of certain substeps of the catalytic cycle, such as nucleotide binding, hydrolysis, or release, which appear not to be rate-limiting. Manipulating the kinetics of nucleotide binding and release either by mutation (presumably H1068A) or by the use of different nucleotides (e.g., UTP instead of ATP) will change the equilibration time of transport substrates with the inward facing drug binding site. Because also each drug exhibits specific on- and off-rates upon binding and release, an altered duration of equilibration (and competition) between substrates and the drug binding site would result in an altered selection of substrates as well. Such kinetic substrate selection can also explain the somewhat mysterious observation that, even though it is efficiently hydrolyzed by Pdr5, UTP does not mediate R6G transport (7).
Remarkably, also the ATPase inhibition characteristics were altered by the H1068A mutation. In contrast to WT Pdr5, R6G is not efficiently transported to the outward facing drug binding site in the H1068A mutant. Therefore, this binding site is less populated in the H1068A mutant and higher drug concentrations are required for saturation, which then results in the inhibition of ATPase activity by a conformational lock. Consistent with this model, the Ki value for KA, which is efficiently transported by WT Pdr5 and the H1068A mutant, is identical for both variants. Thus, differences in inhibition are only a consequence of the altered substrate selection (Table 1).
The idea of a kinetic substrate selection is strongly supported by other mutations identified in the NBDs of Pdr5 (5, 37) and is consistent with the different inhibition of the Pdr5-specific ATPase and UTPase activities by diverse drugs (8). Very recently, it was proposed that GTP and ATP might be used as an energy source for substrate transport. Fully consistent with our proposed model, the Pdr5-specific ATPase and GTPase activities were differently inhibited by clotrimazole (38), implying that protein-nucleotide interactions dictate substrate selection and presumably as a consequence ATPase activity inhibition.
In summary, the lack of any observable substrate stimulation implies that Pdr5 is functioning as an uncoupled ABC transporter, i.e., that ATP is constantly hydrolyzed even in the absence of a transport substrate. The new observation that a H1068A mutation abolished R6G transport without affecting the steady-state ATPase activity contributes to the molecular understanding of the communication between substrate binding and ATP hydrolysis and will pave the way for future investigations related to the dynamics of substrate selection, using purified Pdr5 in reconstituted systems.
Materials and Methods
Materials.
All chemicals were reagent-grade and obtained from commercial sources. OM represents a mixture of components A, B, and C. Stock solutions of KA, FA, CHX, OM, and FK506 were prepared in dimethyl sulfoxide, and R6G and R123 were dissolved in ethanol.
Yeast Strains and Plasmid Mutagenesis.
Yeast strains were cultured either in rich medium (YPD) and synthetic medium supplemented with appropriate auxotrophic components. The following S. cerevisiae strains were used in this study: YALF-A1 (MATa; ura3-52 trp1-1 leu2-3,112 his3-11,15 ade2-1 PDR1-3), YHW-A5 (MATa ura3-52 trp1-1 leu2-3,112 his3-11,15 ade2-1 PDR1-3 pdr5Δ::TRP1) from the K.K. laboratory strain collection, and YRE1001 (MATa ura3-52 trp1-1 leu2-3,112 his3-11,15 ade2-1 pdr1-3 pdr5pdr5promΔ::TRP1). A detailed description of plasmid and strain construction can be found in SI Text. Site-directed mutagenesis of PDR5 was performed on plasmid pRE5 with the QuikChange II XL site-directed mutagenesis kit (Stratagene).
Drug Resistance Assays.
Drug resistance assays were performed essentially as described, by spotting serial dilutions of exponentially growing cell cultures from a fresh liquid YPD culture onto appropriate drug containing plates (5, 39).
Isolation of Plasma Membranes.
Cells were grown in YPD at 30°C. At an OD600 of 1.5, the nitrogen source was refreshed by addition of a 10th volume of 5× YP (50 g/liter yeast extract; 100 g/liter tryptone/peptone). Cells were harvested at OD600 = 3.5. The isolation of plasma membranes was performed essentially as described in ref. 7. Further information is provided in SI Text.
Rhodamine 6G Transport Assay.
Active R6G transport was recorded according to the protocol developed by Kolaczkowski et al., using a Fluorolog II fluorescence spectrometer (Horiba) (7). Isolated plasma membranes (30 μg of protein) were resuspended in 1 ml of transport buffer [50 mM Hepes (pH 7.0), 5 mM MgCl2, 150 nM R6G, and 10 mM azide] and incubated at 35°C. Transport was initiated by addition of 4 mM ATP. To stop transport reactions, 20 μg/ml OM was added to the solution.
ATPase Activity Assays.
OM-sensitive ATPase activity of plasma membrane fractions was measured by a colorimetric assay and performed in 96-well microtiter plates (27, 40, 41). Further information is provided in SI Text.
Drug Titrations.
Drug titrations of ATPase activities were fitted to a steady-state kinetic model (30). This model describes the observed inhibition of the basal Pdr5-specific ATPase activity by increased drug concentrations. The equation represents a simplification of the nonpartitioning model described for human P-glycoprotein (ABCB1) (30) and is given by
where v is the percentage of residual ATPase activity, [drug] is the drug concentration in mol/liter, and Ki is the inhibition constant.
Acknowledgments.
We thank Robin Klemm, Gergely Szakacs, Joost Holthuis, Gerrit van Meer, and Sander Smits for stimulating discussions and constant support. This work was supported by European Molecular Biology Organization Short-Term Fellowship ASFT 193-2004 (to R.E.), Deutsche Forschungsgemeinschaft Grant Sch1279/5-3 (to L.S.), Austrian Science Foundation Project FWF-SFB35-04 (to K.K.), a grant from the transnational SysMO program (Project MOSES) (to K.K.), and European Commission FP6 Marie Curie Training Network “Flippases” Grant MCRTN-CT-2004-005330 (to K.K.). C.M.K. was supported by a Marie-Curie Early Stage Training Fellowship through Flippases.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800191105/DCSupplemental.
References
- 1.Ernst R, Klemm R, Schmitt L, Kuchler K. Yeast ATP-binding cassette transporters: Cellular cleaning pumps. Methods Enzymol. 2005;400:460–484. doi: 10.1016/S0076-6879(05)00026-1. [DOI] [PubMed] [Google Scholar]
- 2.Sanglard D. Resistance of human fungal pathogens to antifungal drugs. Curr Opin Microbiol. 2002;5:379–385. doi: 10.1016/s1369-5274(02)00344-2. [DOI] [PubMed] [Google Scholar]
- 3.Sipos G, Kuchler K. Fungal ATP-binding cassette (ABC) transporters in drug resistance & detoxification. Curr Drug Targets. 2006;7:471–481. doi: 10.2174/138945006776359403. [DOI] [PubMed] [Google Scholar]
- 4.DeRisi J, et al. Genome microarray analysis of transcriptional activation in multidrug resistance yeast mutants. FEBS Lett. 2000;470:156–160. doi: 10.1016/s0014-5793(00)01294-1. [DOI] [PubMed] [Google Scholar]
- 5.Egner R, Rosenthal FE, Kralli A, Sanglard D, Kuchler K. Genetic separation of FK506 susceptibility and drug transport in the yeast Pdr5 ATP-binding cassette multidrug resistance transporter. Mol Biol Cell. 1998;9:523–543. doi: 10.1091/mbc.9.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golin J, Ambudkar SV, Gottesman MM, Habib AD, Sczepanski J, Ziccardi W, May L. Studies with novel Pdr5p substrates demonstrate a strong size dependence for xenobiotic efflux. J Biol Chem. 2003;278:5963–5969. doi: 10.1074/jbc.M210908200. [DOI] [PubMed] [Google Scholar]
- 7.Kolaczkowski M, et al. Anticancer drugs, ionophoric peptides, and steroids as substrates of the yeast multidrug transporter Pdr5p. J Biol Chem. 1996;271:31543–31548. doi: 10.1074/jbc.271.49.31543. [DOI] [PubMed] [Google Scholar]
- 8.Conseil G, et al. Prenyl-flavonoids as potent inhibitors of the Pdr5p multidrug ABC transporter from Saccharomyces cerevisiae. Biochemistry. 2000;39:6910–6917. doi: 10.1021/bi000040f. [DOI] [PubMed] [Google Scholar]
- 9.Schuller C, et al. Membrane-active compounds activate the transcription factors Pdr1 and Pdr3 connecting pleiotropic drug resistance and membrane lipid homeostasis in Saccharomyces cerevisiae. Mol Biol Cell. 2007;18:4932–4944. doi: 10.1091/mbc.E07-06-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt L, Tampé R. Structure and mechanism of ABC-transporters. Cur Opin Struc Biol. 2002;12:754–760. doi: 10.1016/s0959-440x(02)00399-8. [DOI] [PubMed] [Google Scholar]
- 11.Oswald C, Holland IB, Schmitt L. The motor domains of ABC-transporters. What can structures tell us? Naunyn Schmiedebergs Arch Pharmacol. 2006;372:385–399. doi: 10.1007/s00210-005-0031-4. [DOI] [PubMed] [Google Scholar]
- 12.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 13.Orelle C, Dalmas O, Gros P, Di Pietro A, Jault JM. The conserved glutamate residue adjacent to the Walker-B motif is the catalytic base for ATP hydrolysis in the ATP-binding cassette transporter BmrA. J Biol Chem. 2003;278:47002–47008. doi: 10.1074/jbc.M308268200. [DOI] [PubMed] [Google Scholar]
- 14.Zaitseva J, Jenewein S, Jumpertz T, Holland IB, Schmitt L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 2005;24:1901–1910. doi: 10.1038/sj.emboj.7600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst R, Koch J, Horn C, Tampe R, Schmitt L. Engineering ATPase activity in the isolated ABC cassette of human TAP1. J Biol Chem. 2006;281:27471–27480. doi: 10.1074/jbc.M601131200. [DOI] [PubMed] [Google Scholar]
- 16.Moody JE, Millen L, Binns D, Hunt JF, Thomas PJ. Cooperative, ATP-dependent association of the nucleotide binding cassettes during the catalytic cycle of ATP-binding cassette transporters. J Biol Chem. 2002;277:21111–21114. doi: 10.1074/jbc.C200228200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Shawi MK, Omote H. The remarkable transport mechanism of P-glycoprotein: A multidrug transporter. J Bionenerg Biomembr. 2005;37:489–496. doi: 10.1007/s10863-005-9497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson AL, Shuman HA, Nikaido H. Mechanism of maltose transport in Escherichia coli: Transmembrane signaling by periplasmic binding proteins. Proc Natl Acad Sci USA. 1992;89:2360–2364. doi: 10.1073/pnas.89.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CE, Liu PQ, Ames GF. Characterization of the adenosine triphosphatase activity of the periplasmic histidine permease, a traffic ATPase (ABC transporter) J Biol Chem. 1997;272:21883–21891. doi: 10.1074/jbc.272.35.21883. [DOI] [PubMed] [Google Scholar]
- 20.Balzi E, Wang M, Leterme S, Van Dyck L, Goffeau A. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J Biol Chem. 1994;269:2206–2214. [PubMed] [Google Scholar]
- 21.Bissinger PH, Kuchler K. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product. A yeast ABC transporter conferring mycotoxin resistance. J Biol Chem. 1994;269:4180–4186. [PubMed] [Google Scholar]
- 22.Jha S, et al. Purification and characterization of the N-terminal nucleotide binding domain of an ABC drug transporter of Candida albicans: Uncommon cysteine 193 of Walker A is critical for ATP hydrolysis. Biochemistry. 2003;42:10822–10832. doi: 10.1021/bi0345900. [DOI] [PubMed] [Google Scholar]
- 23.Shukla S, Rai V, Banerjee D, Prasad R. Characterization of Cdr1p, a major multidrug efflux protein of Candida albicans: Purified protein is amenable to intrinsic fluorescence analysis. Biochemistry. 2006;45:2425–2435. doi: 10.1021/bi0519147. [DOI] [PubMed] [Google Scholar]
- 24.Wada S, et al. Phosphorylation of candida glabrata ATP-binding cassette transporter Cdr1p regulates drug efflux activity and ATPase stability. J Biol Chem. 2005;280:94–103. doi: 10.1074/jbc.M408252200. [DOI] [PubMed] [Google Scholar]
- 25.Procko E, Ferrin-O'Connell I, Ng SL, Gaudet R. Distinct structural and functional properties of the ATPase sites in an asymmetric ABC transporter. Mol Cell. 2006;24:51–62. doi: 10.1016/j.molcel.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 26.Szabo K, et al. Drug-stimulated nucleotide trapping in the human multidrug transporter MDR1. Cooperation of the nucleotide binding domains. J Biol Chem. 1998;273:10132–10138. doi: 10.1074/jbc.273.17.10132. [DOI] [PubMed] [Google Scholar]
- 27.Goffeau A, Dufour JP. Plasma membrane ATPase from the yeast Saccharomyces cerevisiae. Methods Enzymol. 1988;157:528–533. doi: 10.1016/0076-6879(88)57101-x. [DOI] [PubMed] [Google Scholar]
- 28.Smith PC, et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol Cell. 2002;10:139–149. doi: 10.1016/s1097-2765(02)00576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauna ZE, Smith MM, Muller M, Kerr KM, Ambudkar SV. The mechanism of action of multidrug-resistance-linked P-glycoprotein. J Bionenerg Biomembr. 2001;33:481–491. doi: 10.1023/a:1012875105006. [DOI] [PubMed] [Google Scholar]
- 30.Al-Shawi MK, Polar MK, Omote H, Figler RA. Transition state analysis of the coupling of drug transport to ATP hydrolysis by P-glycoprotein. J Biol Chem. 2003;278:52629–52640. doi: 10.1074/jbc.M308175200. [DOI] [PubMed] [Google Scholar]
- 31.Golin J, Ambudkar SV, May L. The yeast Pdr5p multidrug transporter: How does it recognize so many substrates? Biochem Biophys Res Commun. 2007;356:1–5. doi: 10.1016/j.bbrc.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Jha S, Dabas N, Karnani N, Saini P, Prasad R. ABC multidrug transporter Cdr1p of Candida albicans has divergent nucleotide-binding domains which display functional asymmetry. FEMS Yeast Res. 2004;5:63–72. doi: 10.1016/j.femsyr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Payen LF, Gao M, Westlake CJ, Cole SP, Deeley RG. Role of carboxylate residues adjacent to the conserved core Walker B motifs in the catalytic cycle of multidrug resistance protein 1 (ABCC1) J Biol Chem. 2003;278:38537–38547. doi: 10.1074/jbc.M305786200. [DOI] [PubMed] [Google Scholar]
- 35.Davidson AL, Sharma S. Mutation of a single MalK subunit severely impairs maltose transport activity in Escherichia coli. J Bacteriol. 1997;179:5458–5464. doi: 10.1128/jb.179.17.5458-5464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofacker M, et al. Structural and functional fingerprint of the mitochondrial ATP-binding cassette transporter Mdl1 from Saccharomyces cerevisiae. J Biol Chem. 2007;282:3951–3961. doi: 10.1074/jbc.M609899200. [DOI] [PubMed] [Google Scholar]
- 37.Tutulan-Cunita AC, Mikoshi M, Mizunuma M, Hirata D, Miyakawa T. Mutational analysis of the yeast multidrug resistance ABC transporter Pdr5p with altered drug specificity. Genes Cells. 2005;10:409–420. doi: 10.1111/j.1365-2443.2005.00847.x. [DOI] [PubMed] [Google Scholar]
- 38.Golin J, et al. Complete inhibition of the Pdr5p multidrug efflux pump ATPase activity by its transport substrate clotrimazole suggests that GTP as well as ATP may be used as an energy source. Biochemistry. 2007;46:13109–13119. doi: 10.1021/bi701414f. [DOI] [PubMed] [Google Scholar]
- 39.Egner R, Bauer BE, Kuchler K. The transmembrane domain 10 of the yeast Pdr5p ABC antifungal efflux pump determines both substrate specificity and inhibitor susceptibility. Mol Microbiol. 2000;35:1255–1263. doi: 10.1046/j.1365-2958.2000.01798.x. [DOI] [PubMed] [Google Scholar]
- 40.Decottignies A, Kolaczkowski M, Balzi E, Goffeau A. Solubilization and characterization of the overexpressed PDR5 multidrug resistance nucleotide triphosphatase of yeast. J Biol Chem. 1994;269:12797–12803. [PubMed] [Google Scholar]
- 41.Wada S, et al. Candida glabrata ATP-binding cassette transporters Cdr1p and Pdh1p expressed in a Saccharomyces cerevisiae strain deficient in membrane transporters show phosphorylation-dependent pumping properties. J Biol Chem. 2002;277:46809–46821. doi: 10.1074/jbc.M207817200. [DOI] [PubMed] [Google Scholar]