Abstract
The giant cytosolic protease tripeptidyl peptidase II (TPPII) has been implicated in the regulation of proliferation and survival of malignant cells, particularly lymphoma cells. To address its functions in normal cellular and systemic physiology we have generated TPPII-deficient mice. TPPII deficiency activates cell type-specific death programs, including proliferative apoptosis in several T lineage subsets and premature cellular senescence in fibroblasts and CD8+ T cells. This coincides with up-regulation of p53 and dysregulation of NF-κB. Prominent degenerative alterations at the organismic level were a decreased lifespan and symptoms characteristic of immunohematopoietic senescence. These symptoms include accelerated thymic involution, lymphopenia, impaired proliferative T cell responses, extramedullary hematopoiesis, and inflammation. Thus, TPPII is important for maintaining normal cellular and systemic physiology, which may be relevant for potential therapeutic applications of TPPII inhibitors.
Keywords: apoptosis, senescence, T lymphocytes, tripeptidyl peptidase II
Tripeptidyl peptidase II (TPPII) forms the largest known protease complex (≈6 MDa) in eukaryotic cells. TPPII operates mostly downstream of proteasomes in cytosolic proteolysis (1–4). Several lines of evidence suggest that TPPII is important for proliferation and survival of malignant lymphoid cells, particularly under conditions of cellular stress. TPPII is up-regulated in EL4 lymphoma cells adapted to grow in the presence of proteasome inhibitors (3, 5). Conversely, overexpression of TPPII increases resistance of lymphoma cells against proteasome inhibitors (6). TPPII is up-regulated by c-Myc, as observed in Burkitt's lymphoma cells expressing high amounts of c-Myc. The semispecific TPPII inhibitor Ala-Ala-Phe-chloromethylketone (AAF-CMK) reduces proliferation and initiates apoptosis in Burkitt's lymphoma cells (7). TPPII is up-regulated in nutritionally starved lymphoma cells in vitro, as well as in vivo in advanced lymphomas (8). In addition to TPPII up-regulation, such cellular stress conditions are commonly associated with decreased proteasomal activity and up-regulation of deubiquitinating enzymes (7, 8). Under these conditions tumor cells share an antiapoptotic phenotype, to which TPPII may contribute (8). TPPII-transfected and TPPII-siRNA-treated tumor cells show accelerated and decelerated proliferation, respectively, and both show mitotic aberrations. TPPII overexpression permits tumor cell survival in the presence of mitotic inhibitors (9, 10). Together, these results provide evidence for the importance of TPPII in tumor cell proliferation and survival. In contrast, only scattered information exists on the role of TPPII in nonmalignant cells. For example, a role in the regulation of apoptosis in differentiated macrophages has been proposed (11). TPPII has also been implicated in MHC class I antigen processing (12).
We have generated TPPII-deficient mice and provide here genetic evidence that TPPII is important for proliferative survival of nonmalignant cells. Lack of TPPII activates cell death programs, including proliferative apoptosis in several T lineage subsets and premature senescence of CD8+ T cells and fibroblasts. In addition, lack of TPPII prematurely causes degenerative alterations at the level of the entire organism. These include abnormalities that coincide with symptoms characteristic of immunohematopoietic senescence and premature death of elderly knockout (KO) mice.
Results
Generation of TPPII-Deficient Mice.
To generate TPPII-deficient mice, we used the Cre-loxP and FLP-FRT systems [supporting information (SI) Fig. S1A]. Crossing of floxed mice to CMV-Cre-transgenic mice deleted exons 11 and 12, the latter bearing the active-site serine of TPPII's subtilisin-like catalytic triad (13). TPPII protein expression and proteolytic activity are completely abolished in cells from KO mice (Fig. S1 B and C). The overall cellular AAF-AMC-degrading activity is reduced by 80% in KO splenocytes or fibroblasts.
Young mice with a ubiquitous TPPII deletion are viable and grossly indistinguishable from WT littermates. Over 1 year of age KO mice show elevated mortality (Fig. S1D) associated with aged appearance, disheveled fur, large bald areas, reduced body mass, and loss of vigor.
Enhanced Apoptosis of Immature Thymocytes and Accelerated Thymic Involution in TPPII KO Mice.
A tightly regulated balance among cell proliferation, survival, and cell death is fundamental to normal thymocyte development. In addition, the thymus is known to involute with age. The DN1, DN2, DN3, and DN4 stages of early CD4− CD8− double-negative (DN) thymocytes are followed by the CD4+ CD8+ double-positive (DP) and the CD4+ CD8− or CD4− CD8+ single-positive (SP) stages. Maturation involves two waves of proliferative expansion [in DN2 (in newborn mice in DN1/DN2) and DN3/DN4] and two waves of apoptosis (in DN4 and DP) (14, 15).
Whereas young TPPII KO mice had normal thymic cellularity, elderly (>1 year) mice showed a more pronounced thymic involution compared with WT littermates (Fig. 1A). Thymic subpopulations showed a slight decrease in DP and slight increases in SP and DN cells of KO mice, respectively (Fig. 1B). Similar slight alterations in thymocyte subpopulations are found in very old WT mice and in strains with rapid thymic involution, in which increased apoptosis of DN thymocytes has been reported as well (16–18).
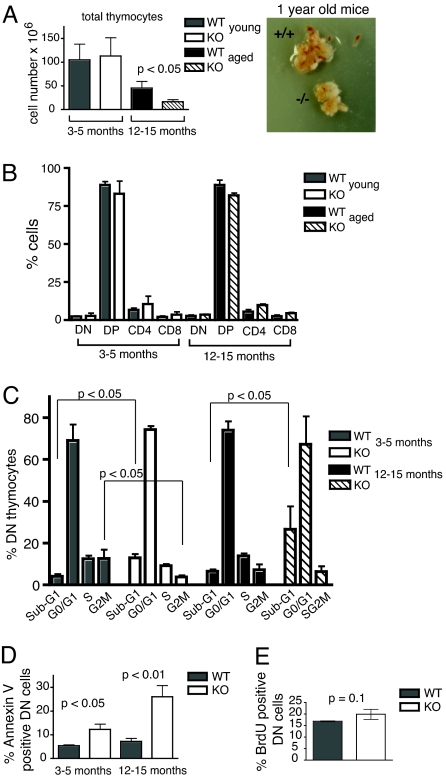
Fig. 1.
Increased DN thymocyte apoptosis and accelerated thymic atrophy in TPPII KO mice. (A) Total thymocyte numbers for young and elderly KO and WT mice (Left) and examples of thymi of 1-year-old littermates (Right). (B) Proportions of major thymocyte subsets determined by staining with anti-CD4 and anti-CD8. In A and B, n = 11 (for young) and n = 6 (for elderly) pairs of littermates, respectively. (C) Cell cycle distribution of DN thymocytes directly ex vivo. DNA staining was performed with propidium iodide. In elderly KO mice, an accurate assessment of S and G2M phase cells could not be made. (D) Annexin V staining of DN thymocytes directly ex vivo. In C and D, n = 3–4 experiments with pooled thymocytes from two or three mice each. (E) In vivo BrdU incorporation into DN thymocytes 1 h after injection of BrdU into 3- to 5-month-old WT or KO mice (n = 3–4 mice per group).
Cell cycle analyses (sub-G1 fraction) and annexin V labeling consistently revealed increased apoptosis of TPPII-deficient DN thymocytes directly ex vivo. This was seen for all DN subpopulations (Fig. S2) and animals of different ages (Fig. 1 C and D and Fig. S2). Importantly, the KO/WT difference in DN apoptosis was much stronger in elderly than in young adult mice (Fig. 1D). The normal total thymocyte numbers in young KO mice are likely due to a compensation of the small apoptotic difference by slightly increased DN cell proliferation. In vivo BrdU incorporation into KO DN thymocytes was slightly increased (Fig. 1E), although the difference did not reach high statistical significance. This is consistent with a slightly enhanced proportion of blastoid cells in total KO thymocytes, both newborn and aged (Fig. S3). The faster proliferation may be associated with altered cell cycle kinetics, as indicated by a relative abbreviation of the S/G2/M phases in DN cells of KO mice (Fig. 1C and Fig. S3). The marked decrease in G2/M phase cells may reflect, in addition, enhanced mitotic apoptosis, consistent with a proposed role of TPPII in mitosis (9, 10).
Enhanced T Cell Receptor (TCR)-Mediated Apoptosis of Peripheral T Lymphocytes and Lymphopenia in TPPII KO Mice.
Young KO adults already have reduced numbers of splenic T cells, for CD8+ cells more pronounced than for CD4+ cells (Fig. 2A Upper). Peripheral T cells further decreased for CD8+ cells to ≈50% of that of WT mice at around 1 year of age (Fig. 2A Lower). The proportions of memory CD8 T cells (CD44high) were increased in spleen and liver of most KO mice, indicating preferential decline in naive CD8+ cells (Fig. 2B).
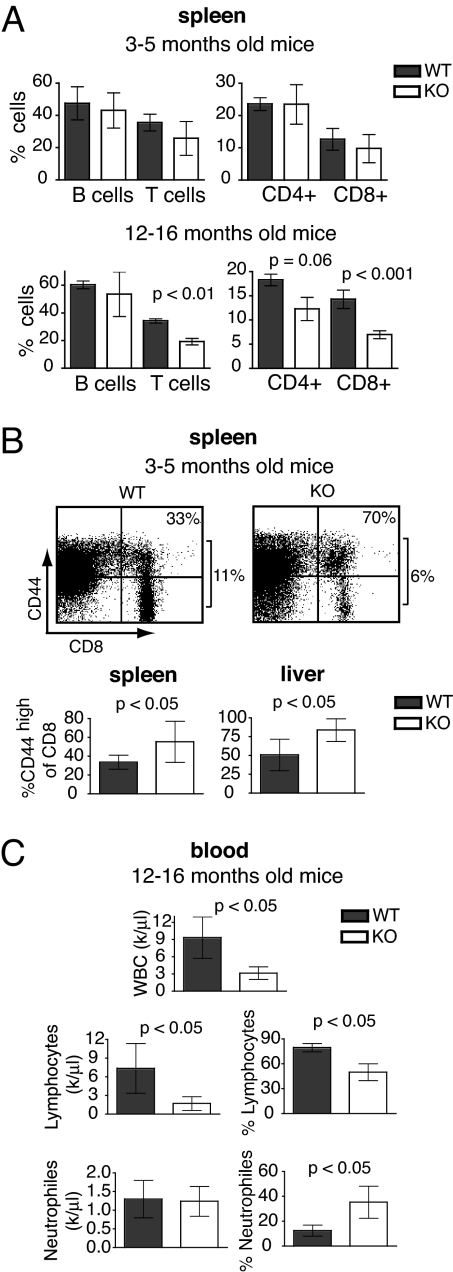
Fig. 2.
Progressive reduction of T lymphocytes in secondary lymphoid organs and blood lymphopenia in elderly TPPII KO mice. (A) Relative numbers of spleen lymphocyte subsets (n = 11 and n = 6 pairs of WT and KO littermates for young and old mice, respectively). (B) Increased proportions in memory CD8 T cells, which are CD44high, in spleen and liver of KO and WT mice (n = 9 pairs of mice). Dot plots show the percentage of CD8+ cells in total splenocytes on the right of each frame and that of CD44high cells in CD8+ cells in the upper right corner. (C) Strong reduction in WBC and lymphocyte counts in the blood of elderly KO mice (n = 16 KO and 14 WT mice).
Essentially all TPPII KO mice over 1 year of age displayed a strong lymphopenia. Absolute numbers of neutrophils in the blood were normal, with an increase in their percentage owing to the reduction of lymphocytes (Fig. 2C). The numbers of monocytes, platelets, and erythrocytes were normal as well (data not shown). The lymphopenia in blood was more pronounced than in lymphoid organs of KO mice.
The decrease in peripheral T lymphocytes could be caused by increased peripheral cell death. We therefore examined survival of splenic T cells upon TCR stimulation. When purified CD8+ and CD4+ cells were activated in anti-CD3-coated plates, cell recovery was reduced for CD8+ but not for CD4+ cells of KO mice (Fig. 3A). This was not caused by impaired T cell activation as judged by expression of the early activation marker CD69 on CD3+ cells (Fig. 3B). Carboxyfluorescein succinimidyl ester (CFSE) dimming showed that the numbers of cell divisions were not significantly different between surviving KO and WT CD3+ cells (Fig. 3B). These data suggested that many KO CD8+ cells die and disintegrate upon proliferation. Increased apoptosis of activated KO CD8+ cells was confirmed by staining with annexin V (Fig. 3C). The serine protease inhibitor AAF-CMK inhibited proliferation and survival of anti-CD3-stimulated WT T lymphocytes (Fig. S4 Left), with the strongest inhibition at doses that maximally inhibit purified TPPII complexes in vitro (3).
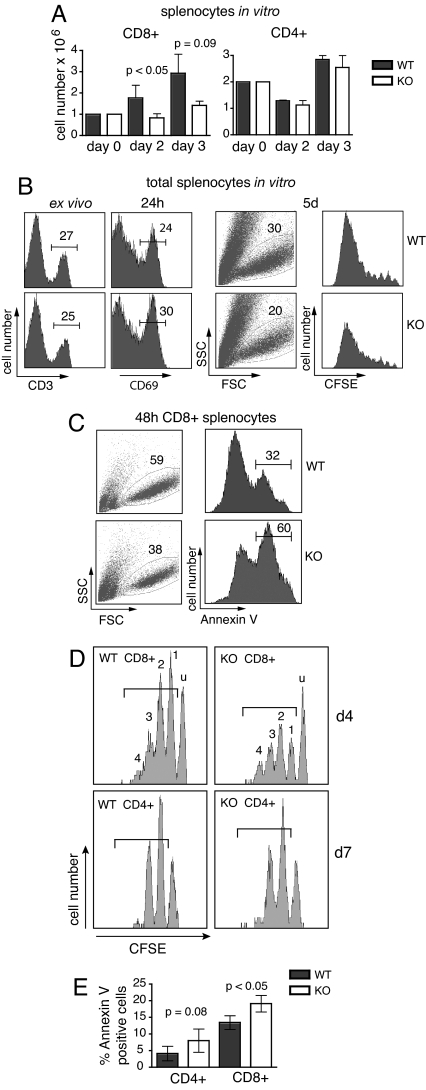
Fig. 3.
Compromised mitogenic and homeostatic proliferation of TPPII-deficient CD8+ T cells. (A–C) Mitogenic responses to anti-CD3 and IL-2 in vitro. (A) Reduced recovery of viable cells after stimulation of purified CD8+, but not CD4+, splenocytes. (B) Left to right, staining of WT and TPPII KO splenocytes with anti-CD3 ex vivo, with anti-CD69 after 24 h of stimulation, viable cells by FCS/SSC, and numbers of cell divisions by loss of CFSE after 5 days of stimulation. (C) Staining with annexin V after 48 h of in vitro stimulation of CD8 T cells. (D) Reduced numbers of proliferating CD8+, but not CD4+, KO T cells after adoptive transfer into sublethally irradiated B6 mice. Histograms show representative CFSE profiles of CD8+ or CD4+ lymph node cells at day 4 and day 7, respectively. Peak designation: u, undivided cells; 1, 2, 3, and 4, peaks containing cells that have undergone one to four cell divisions. (E) Apoptosis of homeostatically proliferating T cells. Determined was the percentage of annexin V-positive dividing cells. Cells that were gated are indicated by the brackets in D. Data are representative of experiments done at least three times independently, and data in A and C are representative of at least 10 experiments.
Pointing at enhanced T cell apoptosis in vivo, freshly prepared KO splenic T cells contained approximately twice as many dead cells as WT controls (Fig. S4 Right), and CTL responses to lymphocytic choriomeningitis virus were reduced in KO mice (12). To assess T cell survival upon homeostatic proliferation in vivo, purified CFSE-labeled CD8+ and CD4+ T cells were inoculated into irradiated B6 recipient mice, and CFSE profiles as well as annexin exposure were determined several days later (Fig. 3 D and E). Similar to anti-CD3-stimulated T cells subsets in vitro (Fig. 3 A and B), fewer CD8+ cells, but similar numbers of CD4+ cells, were proliferating in the KO compared with the WT inoculum. Because the numbers of cell divisions and the proportions of undivided cells were similar between WT and KO T cells, the results suggest that proliferative survival of KO CD8+, but not CD4+, cells was impaired in vivo. The shift toward cells with more divisions among proliferating KO vs. WT CD8+ cells indicates a slightly faster proliferation, similar to thymocytes (see above).
Inflammation Associated with Extramedullary Granulopoiesis.
Common signs of hematopoietic aging include increased proportions of blood myeloid cells, increased numbers of myeloid bone marrow (BM) progenitors, extramedullary hematopoiesis (19–22), and inflammation (23). All TPPII KO mice prematurely displayed some or all of these symptoms. Most pronounced were alterations typical of extramedullary hematopoiesis, including splenomegaly with enlargement of the red pulp (Fig. S5A) and elevated numbers of splenic granulocytes (Fig. S5 B and C). Whereas mature BM granulocytes were normal or moderately reduced (Fig. S5C), granulocyte progenitors in spleen and BM were increased in KO mice (Fig. S5D). Extramedullary hematopoiesis and increased myeloid BM progenitors may be attempts to compensate for inefficient hematopoiesis in the BM of TPPII-deficient mice.
During normal aging MHC expression is increased (23, 24) probably because of chronic inflammation, which is often associated with cellular senescence and ectopic granulopoiesis (25, 26). Consistently, MHC class I and II cell-surface levels were elevated on freshly isolated TPPII KO splenocytes but rapidly decreased on in vitro cultured cells (12). Consistent with local inflammation are also histopathologically detected liver infiltrates of lymphocytes and mature granulocytes (data not shown) and increased NF-κB activity in freshly isolated splenocytes (see below).
Premature Senescence of TPPII-Deficient Fibroblasts and CD8+ T Cells.
Primary skin fibroblasts of newborn KO mice displayed reduced growth rates (Fig. 4A) and considerably increased cell size compared with WT controls (Fig. 4 B, C, and F), associated with premature cellular senescence. Senescence is a state in which cells are irreversibly growth-arrested but remain alive and metabolically active for some time, secreting tissue-destructive enzymes and inflammatory cytokines. Premature senescence can be induced by various types of cellular stress, including DNA damage induced by oxygen radicals or aberrant mitogenic signaling, e.g., by activated oncogenes (26–28). At low passage numbers, 20–40% of TPPII KO but only 1–3% of WT fibroblasts showed signs of senescence, including an enlarged, flattened appearance of the cells and nuclei (Fig. 4 B and F), a granular appearance, and accumulation of β-gal (Fig. 4B Top) (28). Similar to some reports on senescent phenotypes (29, 30), up to 10% of the KO fibroblasts (corresponding to 30–40% of the β-gal-positive cells) exhibited two nuclei (Fig. 4D). This indicates inability to complete mitosis, in line with the increased percentage of cells with 4N DNA content among TPPII-deficient fibroblasts (Fig. 4E). Giant multinuclear cells were also observed, albeit infrequently (Fig. 4F). A senescence-like phenotype was also seen upon treating primary WT fibroblasts for several days with the TPPII inhibitor AAF-CMK (50 μM) (Fig. S6). Interestingly, a considerable proportion of CD8+, but not of CD4+, KO T cells stained positive for acidic β-gal after a few days of stimulation on anti-CD3. Stimulated WT T cells did not stain positive (Fig. 4G).
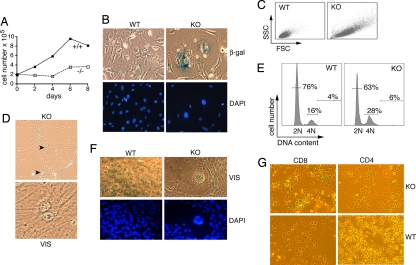
Fig. 4.
Premature senescence of TPPII-deficient fibroblasts and CD8+ T cells. (A) Proliferation of skin fibroblasts derived from newborn mice at passage 10. (B) Microscopic analysis revealed increased numbers of senescent KO cells. Note the flattened, rounded, and enlarged KO fibroblasts with increased expression of β-gal and the larger nuclei as visualized by DAPI staining. (C) Increased cell size of TPPII KO fibroblasts shown by forward scatter/side scatter (FSC/SSC) analysis. (D) Binucleated KO fibroblasts observed by microscopy. (E) Higher percentage of cells with 4N DNA content. (F) Giant multinucleated KO fibroblasts. In all panels, representative experiments including different cell lines at low passage numbers (passage 4–10) are shown. Four different pairs of skin fibroblast lines (three from newborn mice and one from adult mice) were analyzed. (G) Premature senescence of CD8+, but not CD4+, TPPII-deficient splenocytes visualized by staining of acidic β-gal after 4 days of stimulation on anti-CD3. A total of 28 ± 4% of CD8+ KO cells were β-gal+ (n = 3 experiments).
Deregulation of p53 and NF-κB in TPPII-Deficient Fibroblasts and T Cells.
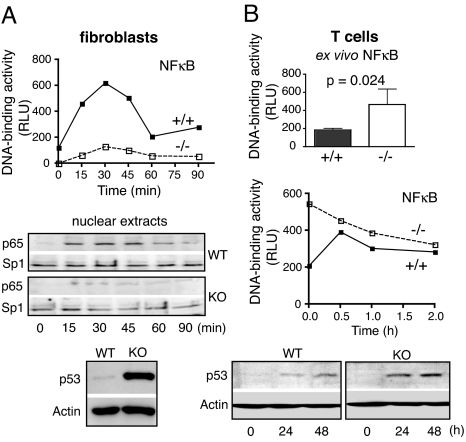
Although spontaneous and Fas-mediated apoptosis were similar between KO and WT fibroblasts (Fig. S7A), the sensitivity to TNF was enhanced in the former. Whereas WT fibroblasts are TNF-sensitive only when protein synthesis is inhibited, TPPII-deficient fibroblasts are inherently sensitive and the sensitivity is enhanced by cycloheximide (Fig. S7B). Dependency on blocked protein synthesis reflects that TNF-mediated apoptosis is normally counteracted by activation of NF-κB followed by the induction of antiapoptotic genes (31). Accordingly, NF-κB activation upon TNF addition was defective in TPPII-deficient fibroblasts, as seen by the greatly reduced nuclear accumulation of NF-κB p65 (Fig. 5A). In contrast, the antiproliferative tumor suppressor p53 was massively overexpressed (Fig. 5A). The increased TNF sensitivity of TPPII KO fibroblasts could be related to cellular senescence, in line with the up-regulation of p53 known to be an important senescence regulator (27, 28). p53 was induced in T cells after TCR stimulation (Fig. 5B), but differences between WT and KO cells were not as pronounced as in fibroblasts. Whereas basal DNA-binding activity of NF-κB was increased in KO T cells, activation upon TCR signaling was attenuated (Fig. 5B). This is consistent with chronic inflammation (see above) and indicates that activation of NF-κB p65 is not generally defective but dysregulated in KO T cells upon TCR stimulation.
Fig. 5.
Deregulation of p53 and NF-κB in TPPII-deficient fibroblasts and T cells. (A) Defective NF-κB activation in KO fibroblasts upon addition of TNF-α as shown by nuclear NF-κB p65 DNA binding activity (in relative light units in Top) and p65 protein levels in nuclear extracts (Middle). Protein levels of Sp1 are shown as control. Strongly increased p53 protein expression in cultures of TPPII KO fibroblasts is shown in Bottom. Actin expression is shown as control. (B) Higher basal NF-κB activity in TPPII-deficient splenic T cells directly ex vivo. Data are the mean ± SD for four mice of each genotype (Top). Dysregulated NF-κB p65 activation (Middle) and p53 protein expression (Bottom) in TCR-stimulated splenic T cells are shown. In each panel, representative results of at least two independent experiments with similar results are shown.
Discussion
We report here the generation and phenotypic characterization of mice lacking the giant protease TPPII and show that specific TPPII ablation is associated with cell-type-specific death programs such as aggravated T cell apoptosis and premature cellular senescence of fibroblasts and CD8+ T cells. These defects likely contribute to the overall phenotype of the KO mice, which is dominated by the premature appearance of immunosenescence-like symptoms and higher age-related mortality.
Apoptosis and senescence are both irreversible cell death programs, are important antitumor mechanisms, contribute to aging, and are affected by common signaling pathways (26–28). p53 is critical for control of both cell death programs. Under most circumstances, NF-κB has been shown to be antiapoptotic (31). TPPII deficiency is associated, as we show here, with alterations in p53 expression and NF-κB activation. The alterations in NF-κB activation, including decreased basal activity in fibroblasts, increased basal activity in other tissues (e.g., T cells), and attenuated activation by classical stimuli, have repeatedly been observed in association with cellular senescence and systemic aging (23, 32). However, we have so far no evidence for a direct molecular link between TPPII and p53 or NF-κB. Signal-induced degradation of the NF-κB inhibitor IκBα, which is mediated by proteasomes, was tested and was not found to be delayed in TPPII KO cells, either in TNF-treated fibroblasts or in anti-CD3-stimulated T cells (data not shown).
A common cause for the activation of apoptosis and senescence in TPPII-deficient cells may be mitotic errors. The reduction of TPPII-deficient thymocytes in the G2/M cell cycle phase together with increased apoptosis is consistent with problems in mitosis (Fig. S3). Similar to TPPII KO fibroblasts (see Fig. 4 D and F), incomplete mitoses with binucleated and multinucleated cells have been observed upon TPPII siRNA silencing in tumor cell lines (9, 10). Mitotic dysregulation with increased binucleation is also found in fibroblasts of old persons and progeria patients and seems to be causally related to systemic aging (33). Similar findings have been reported for senescence induced by Ras or by lack of the stress kinase MKK7 (29, 34).
Several lines of evidence suggest a relationship among mitotic errors, apoptosis induced by p53, and TPPII. Mitotic errors often provoke p53 up-regulation thus activating the apoptotic machinery (35). In cell lines, TPPII overexpression conferred resistance to apoptosis induced by p53 (10). TCR stimulation in primary T cells causes p53 up-regulation (see Fig. 5B) as do the various stimuli causing cellular senescence (26–28). p53 is also up-regulated at the DN thymocyte stage (36). TPPII may thus be particularly important for survival of proliferating cells during conditions that lead to up-regulation of p53, and lack of TPPII expression may aggravate the antiproliferative responses to p53.
The abnormalities of TPPII KO mice that coincide with symptoms of immunohematopoietic senescence in mice and humans are also found in part in mice lacking NF-κB pathway components (25) and mice with augmented p53 expression (37). These alterations include accelerated thymic atrophy associated with increased DN thymocyte apoptosis (16–18), T lymphopenia with particular reduction in naive CD8+ cells as found in human aging (23, 25, 37, 38), and impaired mitogen-induced and homeostatic T cell proliferation due to increased T cell apoptosis (23, 25, 39). Furthermore, these symptoms include greater proportions of blood myeloid cells accompanied by more myeloid BM progenitors and compensatory extramedullary hematopoiesis as found in aging mice (19–22) and chronic inflammation associated with MHC up-regulation (23, 24).
Inflammation may be sustained by secretion of proinflammatory cytokines by senescent cells (26, 28) or by extramedullary granulopoiesis (25) and may accelerate lymphoid degeneration in TPPII-deficient mice. Similarly, many degenerative processes during normal systemic aging are associated with chronic inflammation (23).
Because previous studies have suggested that TPPII is up-regulated in many tumors and therefore likely important for tumor growth, TPPII has been considered as a target for tumor therapy. This study shows that TPPII is required for normal cellular and organismic physiology, although TPPII KO mice are viable. The value of putative TPPII inhibitory drugs in tumor therapies will thus depend on the nature and severity of the predictable systemic side effects.
Materials and Methods
Generation of TPPII KO Mice.
Using genomic clones containing exons 11–15 of the TPPII gene, we constructed a targeting vector for homologous recombination and used it for homologous recombination in W4 embryonic stem cells to generate mutant mice (see SI Materials and Methods for a detailed description).
Fibroblast Lines, β-galactosidase Assays, and DAPI Staining.
Fibroblast lines were generated from the skin of newborn mice. β-gal activity was detected with the senescence β-gal staining kit (Cell Signaling), and nuclear DNA was visualized by adding DAPI (3 μg/ml) after permeabilization with 0.1% Triton X-100.
Cell Proliferation.
Cell proliferation was measured by counting viable cells after staining with trypan blue or flow cytometrically after labeling with CFSE (Fluka) at 1 μM concentration or after in vivo BrdU labeling (see SI Materials and Methods for details).
Cell Cycle Analysis.
DNA content was monitored flow cytometrically by using propidium iodide or 7-aminoactinomycin D staining.
Mitogenic Stimulation of Splenic T Cells.
Total or CD8+ splenic T cells were isolated with the Pan T Cell Isolation or the CD8 Negative Selection Kit (Miltenyi Biotec). A total of 1 × 106 cells were cultured per well in 24-well plates coated with anti-CD3 mAb (145-2C11; 1 μg/ml) in the presence of IL-2 (25 units/ml; eBioscience).
Apoptosis Assays.
Apoptotic cells were determined flow cytometrically after staining with FITC-labeled annexin V (Miltenyi Biotec) (see SI Materials and Methods for details).
Multicolor Flow Cytometry.
For the Abs used, see SI Materials and Methods. Analysis was carried out by using a CYTOMICS FC500 flow cytometer (Beckman Coulter).
NF-κB DNA-Binding Assay.
Nuclear extracts from fibroblasts or splenic T cells were analyzed with an NF-κB Family Transcription Factor Assay Kit (Chemicon) (for details see SI Materials and Methods).
Western Blotting.
Cells and isolated nuclei were lysed in RIPA buffer or nuclear extraction buffer, respectively, and proteins were quantified by using a BCA assay (Pierce). For the primary Abs used, see SI Materials and Methods. Proteins were detected by ECL (Amersham).
Methylcellulose Colony Assay.
The colony assay of single-cell suspensions of BM or spleen was done with MethoCult M3534 (Stem Cell Technologies) with the cytokines mouse stem cell factor, mouse IL-3, and human IL-6.
Peripheral Blood Counts.
Complete hematological profiles were obtained from tail vein blood of age-matched mice by using an automated complete blood cell counter.
Histology.
Organs were fixed, paraffin-embedded, and then stained with hematoxylin/eosin.
Statistical Analysis.
All data are presented as mean ± SD and analyzed by a two-tailed Student t test with unequal variance. P < 0.05 was considered significant.
Acknowledgments.
We thank Prof. Hermann Frommhold for his generous support and Dr. Randy Cassada for critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft (NI 368/4-2), the Clotten Foundation, and Grant NIE346/04 from the Forschungskommission of the University of Freiburg Medical Faculty.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801413105/DCSupplemental.
References
- 1.Tomkinson B. Tripeptidyl peptidases: Enzymes that count. Trends Biochem Sci. 1999;24:355–359. doi: 10.1016/s0968-0004(99)01435-8. [DOI] [PubMed] [Google Scholar]
- 2.Rockel B, Peters J, Kuhlmorgen B, Glaeser RM, Baumeister W. A giant protease with a twist: The TPP II complex from Drosophila studied by electron microscopy. EMBO J. 2002;21:5979–5984. doi: 10.1093/emboj/cdf601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geier E, et al. A giant protease with potential to substitute for some functions of the proteasome. Science. 1999;283:978–981. doi: 10.1126/science.283.5404.978. [DOI] [PubMed] [Google Scholar]
- 4.Reits E, et al. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity. 2004;20:495–506. doi: 10.1016/s1074-7613(04)00074-3. [DOI] [PubMed] [Google Scholar]
- 5.Glas R, Bogyo M, McMaster JS, Gaczynska M, Ploegh HL. A proteolytic system that compensates for loss of proteasome function. Nature. 1998;392:618–622. doi: 10.1038/33443. [DOI] [PubMed] [Google Scholar]
- 6.Wang EW, et al. Integration of the ubiquitin-proteasome pathway with a cytosolic oligopeptidase activity. Proc Natl Acad Sci USA. 2000;97:9990–9995. doi: 10.1073/pnas.180328897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavioli R, Frisan T, Vertuani S, Bornkamm GW, Masucci MG. c-myc overexpression activates alternative pathways for intracellular proteolysis in lymphoma cells. Nat Cell Biol. 2001;3:283–288. doi: 10.1038/35060076. [DOI] [PubMed] [Google Scholar]
- 8.Hong X, Lei L, Glas R. Tumors acquire inhibitor of apoptosis protein (IAP)-mediated apoptosis resistance through altered specificity of cytosolic proteolysis. J Exp Med. 2003;197:1731–1743. doi: 10.1084/jem.20020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stavropoulou V, et al. Mitotic infidelity and centrosome duplication errors in cells overexpressing tripeptidyl-peptidase II. Cancer Res. 2005;65:1361–1368. doi: 10.1158/0008-5472.CAN-04-2085. [DOI] [PubMed] [Google Scholar]
- 10.Stavropoulou V, Vasquez V, Cereser B, Freda E, Masucci MG. TPPII promotes genetic instability by allowing the escape from apoptosis of cells with activated mitotic checkpoints. Biochem Biophys Res Commun. 2006;346:415–425. doi: 10.1016/j.bbrc.2006.05.141. [DOI] [PubMed] [Google Scholar]
- 11.Hilbi H, Puro RJ, Zychlinsky A. Tripeptidyl peptidase II promotes maturation of caspase-1 in Shigella flexneri-induced macrophage apoptosis. Infect Immun. 2000;68:5502–5508. doi: 10.1128/iai.68.10.5502-5508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Firat E, et al. Analysis of direct and cross-presentation of antigens in TPPII knockout mice. J Immunol. 2007;179:8137–8145. doi: 10.4049/jimmunol.179.12.8137. [DOI] [PubMed] [Google Scholar]
- 13.Hilbi H, Jozsa E, Tomkinson B. Identification of the catalytic triad in tripeptidyl-peptidase II through site-directed mutagenesis. Biochim Biophys Acta. 2002;1601:149–154. doi: 10.1016/s1570-9639(02)00468-5. [DOI] [PubMed] [Google Scholar]
- 14.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 15.Su DM, Manley NR. Stage-specific changes in fetal thymocyte proliferation during the CD4–8- to CD4+8+ transition in wild type, Rag1-/-, and Hoxa3, Pax1 mutant mice. BMC Immunol. 2002;3:12. doi: 10.1186/1471-2172-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrew D, Aspinall R. Il-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J Immunol. 2001;166:1524–1530. doi: 10.4049/jimmunol.166.3.1524. [DOI] [PubMed] [Google Scholar]
- 17.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, et al. Phenotype of genetically regulated thymic involution in young BXD RI strains of mice. Scand J Immunol. 2006;64:287–294. doi: 10.1111/j.1365-3083.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- 19.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 20.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janzen V, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 22.Haines DC, Chattopadhyay S, Ward JM. Pathology of aging B6;129 mice. Toxicol Pathol. 2001;29:653–661. doi: 10.1080/019262301753385988. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Agrawal A, Agrawal S, Su H, Gollapudi S. A paradox of immunodeficiency and inflammation in human aging: Lessons learned from apoptosis. Immun Ageing. 2006;3:5. doi: 10.1186/1742-4933-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assounga AG, Warner CM. Transcription of major histocompatibility complex class I (Kb) and transporter associated with antigen processing 1 and 2 genes is up-regulated with age. Immunology. 2004;113:378–383. doi: 10.1111/j.1365-2567.2004.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005;5:435–445. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 26.Campisi J. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 28.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 29.Mason DX, Jackson TJ, Lin AW. Molecular signature of oncogenic ras-induced senescen. Oncogene. 2004;23:9238–9246. doi: 10.1038/sj.onc.1208172. [DOI] [PubMed] [Google Scholar]
- 30.Eom YW, et al. Two distinct modes of cell death induced by doxorubicin: Apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene. 2005;24:4765–4777. doi: 10.1038/sj.onc.1208627. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 32.Helenius M, Hanninen M, Lehtinen SK, Salminen A. Changes associated with aging and replicative senescence in the regulation of transcription factor nuclear factor-kappa B. Biochem J. 1996;318:603–608. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ly DH, Lockhart DJ, Lerner RA, Schultz PG. Mitotic misregulation and human aging. Science. 2000;287:2486–2492. doi: 10.1126/science.287.5462.2486. [DOI] [PubMed] [Google Scholar]
- 34.Wada T, et al. MKK7 couples stress signalling to G2/M cell-cycle progression and cellular senescence. Nat Cell Biol. 2004;6:215–226. doi: 10.1038/ncb1098. [DOI] [PubMed] [Google Scholar]
- 35.Castedo M. Cell death by mitotic catastrophe: A molecular definition. Oncogene. 2004;23:2825–2837. doi: 10.1038/sj.onc.1207528. [DOI] [PubMed] [Google Scholar]
- 36.Haks MC, Krimpenfort P, van den Brakel JH, Kruisbeek AM. Pre-TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre-T cells. Immunity. 1999;11:91–101. doi: 10.1016/s1074-7613(00)80084-9. [DOI] [PubMed] [Google Scholar]
- 37.Tyner SD. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 38.Fagnoni FF, et al. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- 39.Gupta S, Su H, Bi R, Agrawal S, Gollapudi S. Life and death of lymphocytes: A role in immunesenescence. Immun Ageing. 2005;2:12. doi: 10.1186/1742-4933-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]