Abstract
Nifurtimox and benznidazole are the front-line drugs used to treat Chagas disease, the most important parasitic infection in the Americas. These agents function as prodrugs and must be activated within the parasite to have trypanocidal effects. Despite >40 years of research, the mechanism(s) of action and resistance have remained elusive. Here, we report that in trypanosomes, both drugs are activated by a NADH-dependent, mitochondrially localized, bacterial-like, type I nitroreductase (NTR), and that down-regulation of this explains how resistance may emerge. Loss of a single copy of this gene in Trypanosoma cruzi, either through in vitro drug selection or by targeted gene deletion, is sufficient to cause significant cross-resistance to a wide range of nitroheterocyclic drugs. In Trypanosoma brucei, loss of a single NTR allele confers similar cross-resistance without affecting growth rate or the ability to establish an infection. This potential for drug resistance by a simple mechanism has important implications, because nifurtimox is currently undergoing phase III clinical trials against African trypanosomiasis.
Keywords: activation, nitroheterocyclic drugs, Trypanosoma brucei, Trypanosoma cruzi
The protozoan parasites Trypanosoma cruzi, Trypanosoma brucei, and Leishmania are the causative agents of Chagas disease, African sleeping sickness, and leishmaniasis, respectively. Over 20 million people are infected by these pathogens, and >500 million are at risk. Because there is no immediate prospect of vaccines, chemotherapy is of importance. Nitroheterocyclic drugs such as nifurtimox and benznidazole have been used for >40 years against Chagas disease. However, their use is problematic. They can have side effects, and some strains are refractory to treatment, the basis for which has yet to be elucidated (1). In addition, medication is expensive with, for example, nifurtimox regimes requiring 10 mg per kg of body weight in three or four doses per day over a 60- to 120-day period. Because of these problems, the recommended course of treatment is often not completed, resulting in considerable scope for the development of resistance. Plans to extend the use of nifurtimox in combinational therapies with eflornithine to African sleeping sickness are being evaluated (2). This, in conjunction with reports that several new nitroheterocycles have trypanocidal activities with no/low toxicity, has led to a renewed interest in the use of these compounds as antiparasitic agents (3, 4).
Nitroheterocyclic compounds are characterized by a nitrogroup linked to an aromatic ring (5). They include the broad-spectrum nitrofuran and nitroimidazole antibiotics, which are effective against a variety of bacterial and parasitic infections (5, 6). These agents function as prodrugs and must undergo enzyme-mediated activation within the pathogen to have cytotoxic effects, reactions, which are catalyzed by nitroreductases (NTRs). Based on oxygen sensitivity, NTRs are divided into two groups (7). Type I NTRs are oxygen-insensitive, contain FMN as a cofactor, and function via a series of two-electron reductions of the conserved nitro-group, leading to moieties that promote DNA damage (8, 9). This class of NTR is characteristically bacterial. The only trypanosomal enzyme shown to mediate this type of activity is prostaglandin F2α synthase (also known as “old yellow enzyme”) (10, 11), although only under anaerobic conditions. Type II NTRs are ubiquitous oxygen-sensitive FAD- or FMN-containing enzymes that mediate a one-electron reduction of the nitro-group generating an unstable nitro-radical. In the presence of oxygen, this radical undergoes futile cycling to produce superoxide, with the subsequent regeneration of the parent nitro-compound (12, 13). In trypanosomes, type II activity has been proposed as the main activation mechanism (14, 15). However, the only direct link between drug-induced reactive oxygen species formation and trypanocidal activity stems from functional studies on the iron-dependent superoxide dismutase SODB1. Parasites lacking this gene are more sensitive to nifurtimox and benznidazole (16). Analysis of other components of the oxidative defense system indicates they do not play a major role in protecting trypanosomes against nitroheterocyclic drugs (17–22).
To resolve how nitroheterocyclic drugs are activated by trypanosomes, we used two approaches: the first involved in vitro selection to generate nifurtimox-resistant parasites, and the second entailed functional analysis of a recently identified trypanosomal type I NTR. Here, we demonstrate that this type I NTR has the capacity to metabolize a wide range of nitroheterocyclic drugs, and that a reduction in this activity in both T. cruzi and T. brucei confers resistance to these trypanocidal agents.
Results
Selection of Nifurtimox-Resistant T. cruzi.
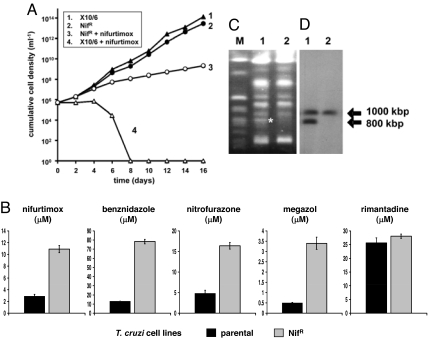
To investigate the mechanism of nifurtimox resistance, we cultured T. cruzi in the presence of 10 μM nifurtimox for 8 months (Materials and Methods). This drug concentration inhibits parasite growth by >99%. Several drug-resistant parasite populations were generated independently, and these were cloned by limited dilution (designated NifR). We first investigated the growth properties of three NifR clones (Fig. 1A). In the absence of nifurtimox, their doubling time was comparable to the parental line. When NifR parasites were grown in medium containing nifurtimox (10 μM), the doubling time increased 2-fold; by comparison, there was no growth in the parental line beyond 1 week. We next determined the extent of nifurtimox resistance by establishing the IC50. All clones were 4-fold more resistant to nifurtimox than controls (Fig. 1B). When the studies were expanded to include other nitroheterocyclic drugs, cross-resistance was observed to benznidazole, megazol, and nitrofurazone (Fig. 1B). The phenotype was specific to this class of compound, because NifR clones had the same sensitivity to the nonnitro-compound rimantadine as the parental line.
Fig. 1.
Nifurtimox-resistant T. cruzi obtained by in vitro culture are cross-resistant to other nitroheterocyclic drugs and have lost a copy of a TcNTR-containing chromosome. (A) Cumulative cell density of parental (X10/6) and laboratory-generated nifurtimox-resistant (NifR) lines in medium containing 10 μM nifurtimox. Two other NifR clones analyzed in parallel displayed similar growth properties. (B) The drug concentrations that inhibited T. cruzi growth by 50% were established. The data are means from three experiments ± standard deviation (SD). Differences observed in susceptibility were statistically significant (P < 0.01), as assessed by Student's t test. Rimantadine was used as a drug control. (C) Ethidium bromide-stained CHEFE gel containing genomic DNA from T. cruzi. The asterisk (*) identifies an 800-kbp chromosome present in X10/6 (lane 1), but missing in NifR clones (lane 2). M corresponds to yeast DNA markers. (D) Autoradiograph of T. cruzi chromosomal DNA separated by CHEFE and hybridized with TcNTR gene probe (lane order as in B).
In vitro drug selection has been shown to give rise to parasites with an altered karyotype (11, 23). To investigate whether this had occurred here, we analyzed the chromosomal profiles from parental and NifR parasites and found that an 800-kbp band present in parental cells was missing from NifR clones (Fig. 1C). This correlated with a reduction in nifurtimox reducing activity in NifR parasites compared with that of controls (2.79 ± 0.20 vs. 6.73 ± 1.18 nmol NADH oxidized min− 1·mg−1 protein). Because nifurtimox and benznidazole are prodrugs, we postulated that the observed resistance might result from a reduction in the copy number of the gene encoding the physiological activator, and that it could be present on this chromosome. Intriguingly, in the parental T. cruzi line, a putative type I NTR gene is located on chromosome homologues of 1,000 and 800 kbp (Fig. 1D).
Trypanosomes Express a Functional Type I NTR.
Type I NTRs are a family of bacterial FMN-containing oxidoreductases, many of unknown function (24). There is a single type I NTR-like gene in both T. cruzi (TcNTR; GenBank accession no. XP_810645) and T. brucei (TbNTR; GenBank accession no. XP_846343). Both enzymes contain a predicted N-terminal mitochondrial-targeting signal and a nitroreductase domain with several residues that may interact with FMN (25) [supporting information (SI) Fig. S1]. To investigate whether TcNTR could function as an oxidoreductase, the catalytic domain was expressed as a recombinant protein in bacteria. During purification, fractions containing the recombinant enzyme were yellow, suggestive of a flavoprotein. TcNTR activity was then assessed in the presence of various nitroaromatic compounds (Fig. 2A). This demonstrated that the enzyme could metabolize a range of nitroheterocycles including nitrofurans (such as nifurtimox, nitrofurantoin, and nitrofurazone) and nitroimidazoles (such as benznidzazole and megazol) using NADH as reductant; no activity was observed when NADPH was used as electron donor (Fig. 2B). A Hanes plot revealed a linear relationship from which the apparent Km for nifurtimox was determined (0.35 ± 0.08 μM) (Fig. 2C). Further analysis gave an apparent Vmax value of 496 ± 13 nmol NADH oxidized min−1·mg−1 with a catalytic specificity (Kcat/Km) of 7.1 × 105 (Fig. 2D). These data confirmed that TcNTR could function as a NADH-dependent nitroreductase and could reduce several trypanocidal agents.
Fig. 2.
Biochemical properties of TcNTR. (A) Postulated scheme for the reduction of nitroheterocyclic compounds by NTRs; “red” represents the reduced form of drug, whereas “oxid” represents the oxidized form. (B) TcNTR activity was monitored by following the oxidation of NADPH or NADH (100 μM) in the presence of TcNTR (0.2 μg) and nifurtimox (100 μM). (C) TcNTR activity was monitored after the oxidation of NADH (100 μM) in the presence of TcNTR (0.2 μg) and nifurtimox (0.5–100 μM). All reactions were initiated by the addition of the recombinant enzyme. TcNTR activities are expressed as nanomoles NADH oxidized min−1·mg−1 of enzyme. (D) TcNTR metabolizes a range of nitroheterocyclic compounds.
TcNTR Null Mutants Are Cross-Resistant to Nitroheterocyclic Drugs.
To assess whether the drug-resistance phenotype observed in NifR cells was specifically due to a reduction in the NTR copy number, we insertionally inactivated one TcNTR gene in the T. cruzi genome (Fig. S2). DNA fragments corresponding to the 5′ and 3′ regions of TcNTR were amplified and cloned sequentially on either side of a cassette containing a puromycin resistance marker. The construct was then used to transform T. cruzi epimastigotes and clones selected using puromycin. Southern hybridization of genomic DNA from heterozygous parasites (TcNTR+/−) showed that one TcNTR allele could be disrupted (Fig. S2), with no obvious effect on parasite growth (Fig. 3A). To evaluate whether a reduction in the TcNTR copy number affected resistance to nitroheterocyclic drugs, TcNTR+/− parasites were grown in the presence of nifurtimox and the IC50 determined (Fig. 3B). Heterozygotes were 2.5-fold more resistant compared with controls. A similar level of cross-resistance was observed when these studies were expanded to include nitrofurazone and megazol (Fig. 3B). Interestingly, when the growth inhibitory property of benznidazole was examined, the heterozygous cell lines exhibited a higher level of resistance (5-fold) (Fig. 3B).
Fig. 3.
Phenotypic effect of deleting NTR from the T. cruzi genome. (A) T. cruzi null mutants have a growth defect. Mean cell density was determined from three independent epimastigote cultures. TcNTR+/− and TcNTR−/− represent the TcNTR heterozygous and null-mutant cell lines, respectively. (B) Growth inhibitory effect of nitroheterocyclic drugs on NTR-deficient T. cruzi epimastigotes. Data are means from fourexperiments ± SD, and the differences in susceptibility were statistically significant (P < 0.01), as assessed by Student's t test. G418 was used as a drug control.
To determine the importance of TcNTR to T. cruzi and examine the full extent of nitroheterocyclic drug resistance due to inactivation of the corresponding gene, TcNTR+/− cells were transformed with a blasticidin knockout construct (Fig. S2). Southern hybridization of genomic DNA from recombinant parasite clones showed that in T. cruzi epimastigotes, both alleles could be disrupted to generate a TcNTR null mutant line (TcNTR−/−) (Fig. S2). To evaluate whether inactivation of both copies of TcNTR resulted in an increased resistance to nitrofurans and nitroimidazoles, TcNTR−/− cells were grown in the presence of the nitroheterocycles (Fig. 3B). When exposed to nifurtimox, nitrofurazone, and megazol, the null-mutant lines were 4-fold more resistant than controls, whereas with benznidazole, the TcNTR−/− cells exhibited an even higher level of resistance (10-fold) (Fig. 3B). Thus, the sequential depletion of TcNTR is accompanied by a stepwise increase in resistance to a range of nitroheterocyclic drugs.
In contrast to heterozygous cells, TcNTR null mutants displayed a growth defect having a doubling time twice that of controls (Fig. 3A). Additionally, the maximal cell density attained with these cultures was consistently 4- to 5-fold lower (1 × 107 ml−1 for TcNTR−/− compared with 5 × 107 ml−1 for controls), and we could detect no development of the needle-shaped infective trypomastigotes, with the stumpy epimastigotes generally having reduced motility. The inability of TcNTR−/− to differentiate was borne out by infection studies; unlike parental and heterozygous lines, TcNTR null mutant parasites could not infect cultured Vero cells. This indicates that TcNTR is essential for differentiation of noninfectious T. cruzi epimastigotes into metacyclic trypomastigotes, the stage of the life cycle infective to humans.
Functional Analysis of TbNTR in Bloodstream Form T. brucei.
Nitroheterocyclic compounds have shown promise in the treatment of African sleeping sickness, with a nifurtimox/eflornithine combination demonstrating significant potential in phase III clinical trials (2). To determine whether NTR plays a role in resistance toward nitroheterocycles in T. brucei, we deleted one copy of TbNTR from the genome of bloodstream form (BSF) parasites. DNA fragments corresponding to the 5′ and 3′ flanks of TbNTR were amplified and cloned sequentially on either side of a cassette containing blasticidin or puromycin resistance markers (Fig. S3). The constructs were used to transform BSF T. brucei and clones selected. Southern hybridization confirmed that one TbNTR allele could be deleted from the genome, with either drug resistance cassette, generating heterozygous parasites (TbNTR+/−PAC or TbNTR+/−BLA) (Fig. S3). This had no effect on growth or the ability to generate a lethal infection in mice (data not shown). To evaluate whether deletion of one copy of TbNTR altered sensitivity to nitroheterocyclic drugs, heterozygous cell lines were grown in the presence of nifurtimox and the IC50 determined (Fig. 4A). Cells containing a single NTR replacement were 3-fold more resistant than controls. When these studies were expanded to include other nitroheterocyclic drugs, a similar level of cross-resistance was observed to benznidazole, megazol and nitrofurazone (Fig. 4A). This phenotype was specific to nitroheterocycles, because heterozygous cells had the same sensitivity to melarsoprol as the parental line.
Fig. 4.
Phenotypic effect of altering the TbNTR copy number in T. brucei. (A) Deletion of one copy of TbNTR confers cross-resistance on T. brucei as judged by IC50. (B) Overexpression of TbNTR (TbNTRRV) confers hypersensitivity to nitroheterocyclic drugs. Data in A and B are means from four experiments ± SD, and the differences in susceptibility were statistically significant (P < 0.01), as assessed by Student's t test. Melarsoprol was used as a drug control. (C) Cumulative cell density of T. brucei BSF cells grown in the absence or presence of tetracycline. Two other TbNTRRV−/− clones analyzed in parallel displayed similar growth properties. On the fifth and subsequent days, we observed an outgrowth of viable parasites. This type of reversion is frequently observed in T. brucei (21).
If activation of nitroheterocycles by NTR is a major determinant of trypanocidal activity, and deletion of the corresponding gene gives rise to drug resistance, it is implicit that overexpression should result in hypersensitivity. TbNTR was cloned in-frame with the 9E10 epitope from the human c-myc protein in a vector that facilitated tetracycline-inducible gene expression (26). The construct was used to transform a T. brucei BSF line, generating parasite clones capable of overexpressing TbNTR (designated TbNTRRV). To demonstrate that inducible expression was occurring, extracts from cells treated with tetracycline (1 μg ml−1) for 24 h were examined on Western blots by using a monoclonal antibody against the 9E10 epitope (Fig. S3). Lysates derived from parasites expressing tagged TbNTR contained a band of the expected size (≈35 kDa). We then examined whether elevated levels of TbNTR affected sensitivity to nitroheterocyclic compounds. T. brucei cells with an elevated level of TbNTR (TbNTRRV + tetracycline) were shown to be >10-fold more sensitive to all nitroaromatic drugs than controls (Fig. 4B). This phenotype was shown to be specific to nitroheterocycles as cells overexpressing TbNTR had the same sensitivity as controls to melarsporol (Fig. 4B).
As shown, one TbNTR allele could be readily deleted from BSF parasites. Attempts (16 independent transformations) to generate a TbNTR double-knockout failed, leading us to speculate that the protein is essential. To investigate this further, we deleted both copies of the nitroreductase in TbNTRRV cells. When deleting the second copy of the gene, tetracycline was added to the growth medium every 3 days to ensure expression of the gene from the “rescue” locus. In the presence of tetracycline, both endogenous copies of TbNTR could be deleted to generate a conditional null-mutant cell line (TbNTRRV−/−) (Fig. S3). To determine whether TbNTR is essential for BSF growth, the effect of withdrawing tetracycline from the growth medium was examined (Fig. 4C). Under these conditions, three independent clones stopped dividing after 3 days, whereas parasites cultured in the presence of tetracycline grew at the same rate as controls. This clearly demonstrated that in the infective stage, TbNTR is essential for growth.
NTR Is Targeted to the Mitochondrion in T. brucei.
To investigate whether NTR is mitochondrial, the 5′ end of TbNTR was amplified and ligated in-frame and upstream of the gene encoding the enhanced GFP. The cloning was performed in a vector that facilitated tetracycline inducible gene expression (26). The resultant construct was used to transform BSF T. brucei and parasite clones selected. To induce expression, cells were incubated in the presence of tetracycline for 24 h, then fixed and examined by confocal microscopy. For parasites expressing TbNTR-GFP, fluorescence was restricted to a network that spread throughout the cell, a pattern reported for trypanosomal proteins localized to the large single mitochondrion (Fig. 5). To confirm this, cells were costained with the mitochondrial dye, MitoTracker. When the images were superimposed, the pattern of colocalization indicated that TbNTR-GFP was located in the same compartment as MitoTracker.
Fig. 5.
Localization of TbNTR in bloodstream form T. brucei. Parasites expressing TbNTR-GFP (green) were costained with DAPI (DNA; blue) and MitoTracker (mitochondrion; red). The cells were examined by confocal microscopy. The pattern of colocalization (yellow) is shown in the merged image. (A) Expression of TbNTR-GFP in a population of cells. (B) Single cell at higher resolution.
Discussion
This article demonstrates that trypanosomes express a type I NTR and a reduction in the level of its activity plays a major role in resistance to nitroheterocyclic drugs. We also show that this mitochondrially targeted protein is essential for establishing infections in the mammalian host for both T. cruzi, an intracellular parasite, and T. brucei, a bloodstream parasite.
Drug resistance is a major problem associated with a number of infections. This occurs by several different mechanisms that can broadly be divided into two groups: those that affect the drug (such as reduced uptake, detoxification, or sequestration) or those that impinge on the drug target (such as target modification or amplification). Nitroheterocyclic compounds are prodrugs and require activation to mediate cytotoxic activity. Therefore, factors that reduce/prevent this process can lead to resistance. In bacteria, a link between resistance to nitrobased drugs and activation was established >35 years ago after the observation that nitrofuran resistant Escherichia coli had reduced NTR activity, a phenotype later shown to be due to the acquisition of mutations in their type I NTR complement (8, 27). This mechanism has been largely overlooked for the trypanocidal agents nifurtimox and benznidazole, because type I NTR activity was believed to be specific to bacteria. Instead, it was proposed that in trypanosomes, these drugs mediate parasite killing by inducing oxidative stress (12, 14, 15). This hypothesis gained credence as trypanosomes were reported to have a limited ability to metabolize reactive oxygen species (28). However, it is now clear that trypanosomes possess an array of enzyme-mediate antioxidant defense pathways, often distinct from those in the host (17–22, 29). To investigate whether trypanosomes possess a type I NTR and have potential to activate nitroheterocycles by a bacterial-like mechanism, we used an E. coli type I NTR sequence to screen the T. cruzi and T. brucei genome databases and identified a single hit in both cases. Initial studies using the T. cruzi recombinant enzyme demonstrated it could metabolize a range of nitrofuran and nitroimidazole drugs, with no specific substrate preference. Therefore, in vitro, this NTR has the potential to mediate the activation step (Fig. 2). To conclusively demonstrate this, we generated T. cruzi and T. brucei lines with reduced or elevated levels of enzyme. If NTR has a role in drug activation, we predicted that reduction in NTR levels would be associated with resistance to nitroheterocyclic compounds, whereas overexpression would result in hypersensitivity. This is what we observed (Figs. 3 and 4): nifurtimox and benznidazole are activated in trypanosomes by a type I NTR, and reduced expression results in drug resistance.
In parallel, we performed in vitro selection. By continuous culturing of T. cruzi epimastigotes in the presence of nifurtimox, we generated parasite cell lines that displayed cross-resistance to a variety of nitroheterocyclic compounds (Fig. 1). This has worrying implications, because in a clinical context, nifurtimox and benznidazole need to be administered for several months, and in many cases, the treatment is not completed. This suggests considerable scope for the development of drug resistance in the field. Indeed, strains refractory to treatment are common, with 20–25% of cases being resistant to nifurtimox and/or benznidazole (1, 30).
The endogenous function of NTR is essential to invasive trypanosomes; TbNTR could not be deleted from BSF T. brucei, and TcNTR null mutants displayed an inability to differentiate to infectious metacyclic trypomastigotes and could not infect mammalian cells. Therefore, it is implicit that NTR activity in trypanosomes is required to establish and maintain infection. Although the precise biological function of the trypanosomal NTRs remains unknown, they are similar to NADH dehydrogenases, enzymes that catalyze the transfer of electrons from NADH to ubiquinone-generating ubiquinol (QH2) (31). In trypanosomes, several distinct NADH dehydrogenases activities have been reported, including respiratory chain complex I, a 33-kDa FMN-containing protein and a 54-kDa FAD-containing protein (32–34). These activities have been observed specifically in the insect form of T. brucei. In the BSF, ubiquinone reduction is reported to only occur via mitochondrial glycerol-3 phosphate dehydrogenase activity (35), because this life-cycle stage does not possess a functionally intact respiratory chain. Instead, they utilize an alternative electron sink, the trypanosome alternative oxidase (TAO), to maintain redox balance within the cell (36). TAO mediates electron transfer from QH2 to reduce oxygen generating water. In most organisms, ubiquinone is an integral component of the respiratory chain, and QH2 functions as an antioxidant either directly by inhibiting protein and lipid oxidation in cell membranes or indirectly by transferring reducing equivalents to vitamin E (37). The type I NTRs identified here may correspond to the previously reported 33-kDa FMN-containing NADH dehydrogenase activity or could represent an additional class of ubiquinone-reducing enzyme whose activity is essential in mammalian stage trypanosomes.
Our data identify a major mechanism that results in activation of nitroheterocyclic drugs in trypanosomes. This has important implications for drug policy and could account for the high levels of T. cruzi infections which are refractory to current drug regimes. However, as we have shown, NTR is essential in BSF T. brucei and for differentiation of T. cruzi. Therefore, selective pressure to retain some type I NTR activity appears to restrict drug resistance to an upper limit of ≈5-fold. At this stage though, we cannot exclude the possibility that specific mutations in NTR could generate versions of the enzyme that maintain the endogenous function but lose the drug-activating capacity, thus resulting in even higher levels of drug resistance. This is a scenario that will require close surveillance at the level of both the laboratory and the field. One way forward, suggested by our data, would be to develop drugs targeted at NTR. A dual therapy could then be used: initial treatment that relies on prodrug activation by parasite type I NTR, and if this fails, the targeting of the NTR activity itself.
Materials and Methods
Parasites.
T. cruzi epimastigotes (clone MHOM/BR/78/Sylvio- X10/6) were grown as described (38). Transformed T. cruzi were maintained in the same medium with 5 μg ml−1 puromycin and/or 10 μg ml−1 blasticidin. T. cruzi amastigotes were obtained from African green monkey kidney (Vero) cells infected with metacyclic trypomastigotes. BSF T. brucei (Lister 427; clone 221a) were grown in HMI-9 medium (39). Transformed T. brucei were maintained in medium with 2.5 μg ml−1 blasticidin, 2 μg ml−1 puromycin, or 2.5 μg ml−1 hygromycin. T. brucei BSF lines (2T1) that constitutively express the tetracycline repressor protein (26) were grown in medium containing 1 μg ml−1 phleomycin. Tetracycline free FCS (Autogen Bioclear) was used in the media. Genomic DNA was isolated by using the phenol/chloroform method, and intact trypanosomal chromosomes for contour clamped homogenous field electrophoresis (CHEFE) analysis were extracted by using an agarose-embedding technique (40) and separated by using a BioRad CHEFE mapper.
Biochemical Properties.
T. cruzi NTR was amplified from genomic DNA, the fragment digested with BamHI/HindIII then cloned into the corresponding sites of pTrcHis-C. E. coli BL-21, transformed with the plasmid pTrcHis-TcNTR, were grown in NZCYM broth containing 50 μg ml−1 ampicillin and protein expression induced by IPTG. His-tagged TcNTR was affinity purified on a Ni-NTA column (Qiagen). Cell lysis, column wash and elution steps were carried out in the presence of protease inhibitors (Roche). Fractions were analyzed by SDS/PAGE and protein concentrations determined by BCA protein assay system (Pierce).
TcNTR activity was measured by following the change in absorbance at 340 nm due to NADH oxidation (41). A reaction mixture (1 ml) containing 50 mM Tris·Cl, pH 7.0, 100 μM NADH and nitroheterocycle was incubated at 22°C for 5 min. The background rate of NADH oxidation was determined and the reaction initiated by addition of 0.2 μg of TcNTR. As control, activity of any potentially copurified E. coli proteins was also examined. Enzyme activity was calculated by using ε of 6,220 M−1·cm−1. Data were analyzed by fitting to a rectangular hyperbola (Kinenort program, A. G. Clark, University of Wellington, Wellington, New Zealand).
Construction of Trypanosomal Vectors and Parasite Transformation.
The vectors used to delete TcNTR or TbNTR were generated as follows. For TcNTR knockout, primers were designed to amplify 435- or 410-bp fragments from the 5′ and 3′ regions of the gene, respectively. These were cloned sequentially either side of a puromycin (PAC) or blasticidin (BLA) resistance cassette. The constructs were linearized (SacI/KpnI for the PAC vector or SacII/KpnI for the BLA vector) then electroporated into T. cruzi epimastigotes (42). For TbNTR knockout, a similar strategy was used, except the 5′ and 3′ flanking regions were amplified (825 and 698 bp, respectively). Constructs were linearized (SacI/KpnI for the PAC vector or MluI/KpnI for the BLA vector) then electroporated into T. brucei BSFs (43). The vector used for overexpression was generated as follows: the TbNTR ORF, lacking the stop codon, was amplified from genomic DNA. The resultant fragment was digested with HindIII/XbaI and cloned into the vector pLEW-tagC (D.H., unpublished data). The ligation was performed such that an epitope (9E10) derived from the human c-myc protein was added to the C terminus of TbNTR. The TbNTR-9E10 fusion was amplified, digested with HindIII/BglII and cloned into pRPaC-GFP (26), such that the insert DNA replaced the GFP cassette. The AscI digested construct was electroporated into T. brucei 2T1 cells. The vector used for localization was generated as follows: a 255-bp DNA sequence corresponding to the N-terminal of TbNTR (amino acids 1–87) was amplified from genomic DNA and digested with HindIII/XbaI. This was ligated into the HindIII/XbaI sites of vector pRPaC-GFP (26). The cloning was carried out such that the gene coding for the GFP was inserted in-frame at the 3′ end of the TbNTR-derived DNA fragment. The AscI digested construct was electroporated into T. brucei 2T1 parasites.
Localization.
BSF trypanosomes expressing TbNTR-GFP were suspended at 5 × 106 cells ml−1 in medium containing 100 nM MitoTracker Red (Molecular Probes) and incubated at 37°C for 5 min. Cells were washed twice in PBS, fixed in 2% paraformaldehyde/PBS, then washed again in PBS. Aliquots of the cell suspension (105 cells) were then air-dried onto microscope slides. Parasite DNA was stained with 200 pM DAPI (Sigma) in 50% glycerol/PBS and slides were viewed by using a Zeiss LSM 510 confocal microscope.
Selection of Laboratory-Generated Nifurtimox-Resistant T. cruzi.
T. cruzi epimastigotes were seeded at 1 × 105 ml−1 in medium containing 10 μM nifurtimox. After 2 months growth at 27°C, the parasites were subcultured. This was repeated every 3–4 weeks for a further 6 months before cloning by limited dilution. Nifurtimox (10 μM) was maintained throughout the selection.
Susceptibility Experiments.
T. cruzi epimastigotes were seeded at 5 × 105 ml−1 in 200 μl of growth medium containing different concentrations of nifurtimox, benznidazole, megazol, nitrofurazone, rimantidine, or G418. After incubation at 27°C for 2 days, 20 μl of Alamar blue (Biosource U.K. Ltd.) was added to each well and the plates incubated for a further 10 days. Cell densities were determined by monitoring the fluorescence of each culture using a Gemini Fluorescent Plate reader (Molecular Devices) at an excitation wavelength of 530 nm, emission wavelength of 585 nm, and a filter cutoff at 550 nm, and the drug concentration that inhibits parasite growth by 50% (IC50) established. The reduction of Alamar blue is proportional to the number of live cells, which was established after production of a standard curve.
T. brucei BSF parasites were seeded at 1 × 103 ml−1 in 200 μl of growth medium containing different concentrations of nifurtimox, benznidazole, megazol, nitrofurazone, or melarsoprol. Where appropriate, induction was carried out by adding tetracycline (1 μg ml−1). After incubation at 37°C for 3 days, 20 μl of Alamar blue was added to each well and the plates incubated for a further 16 h. The cell density of each culture was determined as described above and the IC50 established.
Acknowledgments.
We thank members of the trypanosomatid genome projects for sequencing data. This work was supported by The Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711014105/DCSupplemental.
References
- 1.Murta SM, Gazzinelli RT, Brener Z, Romanha AJ. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol Biochem Parasitol. 1998;93:203–214. doi: 10.1016/s0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]
- 2.Priotto G, et al. Three drug combinations for late-stage Trypanosoma brucei gambiense sleeping sickness: A randomized clinical trial in Uganda. PLoS Clin Trials. 2006;1:e39. doi: 10.1371/journal.pctr.0010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart ML, et al. Trypanocidal activity of melamine-based nitroheterocycles. Antimicrob Agents Chemother. 2004;48:1733–1738. doi: 10.1128/AAC.48.5.1733-1738.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliani A, et al. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. J Med Chem. 2005;48:5570–5579. doi: 10.1021/jm050177+. [DOI] [PubMed] [Google Scholar]
- 5.Grunberg E, Titsworth EH. Chemotherapeutic properties of heterocyclic compounds: monocyclic compounds with five-membered rings. Annu Rev Microbiol. 1973;27:317–346. doi: 10.1146/annurev.mi.27.100173.001533. [DOI] [PubMed] [Google Scholar]
- 6.Raether W, Hanel H. Nitroheterocyclic drugs with broad spectrum activity. Parasitol Res. 2003;S1:S19–S39. doi: 10.1007/s00436-002-0754-9. [DOI] [PubMed] [Google Scholar]
- 7.Peterson FJ, Mason RP, Hovsepian J, Holtzman JL. Oxygen-sensitive and -insensitive nitroreduction by Escherichia coli and rat hepatic microsomes. J Biol Chem. 1979;254:4009–4014. [PubMed] [Google Scholar]
- 8.McCalla DR, Reuvers A, Kaiser C. Breakage of bacterial DNA by nitrofuran derivatives. Cancer Res. 1971;31:2184–2188. [PubMed] [Google Scholar]
- 9.Streeter AJ, Hoener BA. Evidence for the involvement of a nitrenium ion in the covalent binding of nitrofurazone to DNA. Pharm Res. 1988;5:434–436. doi: 10.1023/a:1015988401601. [DOI] [PubMed] [Google Scholar]
- 10.Kubata BK, et al. A key role for old yellow enzyme in the metabolism of drugs by Trypanosoma cruzi. J Exp Med. 2002;196:1241–1451. doi: 10.1084/jem.20020885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murta SM, et al. Deletion of copies of the gene encoding old yellow enzyme (TcOYE), a NAD(P)H flavin oxidoreductase, associates with in vitro-induced benznidazole resistance in Trypanosoma cruzi. Mol Biochem Parasitol. 2006;146:151–162. doi: 10.1016/j.molbiopara.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Docampo R, Mason RP, Mottley C, Muniz RP. Generation of free radicals induced by nifurtimox in mammalian tissues. J Biol Chem. 1981;256:10930–10933. [PubMed] [Google Scholar]
- 13.Moreno SN, Mason RP, Docampo R. Reduction of nifurtimox and nitrofurantoin to free radical metabolites by rat liver mitochondria. Evidence of an outer membrane-located nitroreductase. J Biol Chem. 1984;259:6298–6305. [PubMed] [Google Scholar]
- 14.Docampo R. Sensitivity of parasites to free radical damage by antiparasitic drugs. Chem Biol Interact. 1990;73:1–27. doi: 10.1016/0009-2797(90)90106-w. [DOI] [PubMed] [Google Scholar]
- 15.Viode C, et al. Enzymatic reduction studies of nitroheterocycles. Biochem Pharmacol. 1999;57:549–557. doi: 10.1016/s0006-2952(98)00324-4. [DOI] [PubMed] [Google Scholar]
- 16.Prathalingham SR, Wilkinson SR, Horn D, Kelly JM. Deletion of the Trypanosoma brucei superoxide dismutase gene Tbsodb1 increases sensitivity to nifurtimox and benznidazole. Antimicrob Agents Chemother. 2007;51:755–758. doi: 10.1128/AAC.01360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly JM, Taylor MC, Smith K, Hunter KJ, Fairlamb AH. Phenotype of recombinant Leishmania donovani and Trypanosoma cruzi, which over-express trypanothione reductase. Sensitivity towards agents that are thought to induce oxidative stress. Eur J Biochem. 1993;218:29–37. doi: 10.1111/j.1432-1033.1993.tb18348.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson SR, Temperton NJ, Mondragon A, Kelly JM. Distinct mitochondrial and cytosolic enzymes mediate trypanothione-dependent peroxide metabolism in Trypanosoma cruzi. J Biol Chem. 2000;275:8220–8225. doi: 10.1074/jbc.275.11.8220. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson SR, et al. The Trypanosoma cruzi enzyme TcGPXI is a glycosomal peroxidase and can be linked to trypanothione reduction by glutathione or tryparedoxin. J Biol Chem. 2002;277:17062–17071. doi: 10.1074/jbc.M111126200. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson SR, Obado SO, Mauricio IL, Kelly JM. Trypanosoma cruzi expresses a plant-like ascorbate-dependent hemoperoxidase localized to the endoplasmic reticulum. Proc Natl Acad Sci USA. 2002;99:13453–13458. doi: 10.1073/pnas.202422899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson SR, Horn D, Pathalingham R, Kelly JM. RNAi identifies two hydroperoxide metabolising enzymes that are essential to the bloodstream form of the African trypanosome. J Biol Chem. 2003;278:31640–31646. doi: 10.1074/jbc.M303035200. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson SR, et al. Functional characterisation of the iron superoxide dismutase gene repertoire in Trypanosoma brucei. Free Radic Biol Med. 2006;40:198–209. doi: 10.1016/j.freeradbiomed.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Nozaki T, Engel JC, Dvorak JA. Cellular and molecular biological analyses of nifurtimox resistance in Trypanosoma cruzi. Am J Trop Med Hyg. 1996;55:111–117. doi: 10.4269/ajtmh.1996.55.111. [DOI] [PubMed] [Google Scholar]
- 24.McCalla DR, Kaiser C, Green MH. Genetics of nitrofurazone resistance in Escherichia coli. J Bacteriol. 1978;133:10–16. doi: 10.1128/jb.133.1.10-16.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkinson GN, Skelly JV, Neidle S. Crystal structure of FMN-dependent nitroreductase from Escherichia coli B: a prodrug-activating enzyme. J Med Chem. 2000;43:3624–3631. doi: 10.1021/jm000159m. [DOI] [PubMed] [Google Scholar]
- 26.Alsford S, Kawahara T, Glover L, Horn D. Tagging a T. brucei RRNA locus improves stable transfection efficiency and circumvents inducible expression position effects. Mol Biochem Parasitol. 2005;144:142–148. doi: 10.1016/j.molbiopara.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCalla DR, Reuvers A, Kaiser C. Mode of Action of nitrofurazone. J Bacteriol. 1970;104:1126–1134. doi: 10.1128/jb.104.3.1126-1134.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnieri EVS, Moreno SNJ, Docampo R. Trypanothione-dependent peroxide metabolism in Trypanosoma cruzi different stages. Mol Biochem Parasitol. 1993;61:79–86. doi: 10.1016/0166-6851(93)90160-y. [DOI] [PubMed] [Google Scholar]
- 29.Nogoceke E, Gommel DU, Kiess M, Kalisz HM, Flohe L. A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biol Chem. 1997;378:827–836. doi: 10.1515/bchm.1997.378.8.827. [DOI] [PubMed] [Google Scholar]
- 30.Filardi LS, Brener Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans R Soc Trop Med Hyg. 1987;81:755–759. doi: 10.1016/0035-9203(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 31.Brandt U. Energy converting NADH:quinone oxidoreductase (complex I) Annu Rev Biochem. 2006;75:69–92. doi: 10.1146/annurev.biochem.75.103004.142539. [DOI] [PubMed] [Google Scholar]
- 32.Fang J, Wang Y, Beattie DS. Isolation and characterization of complex I, rotenone-sensitive NADH: ubiquinone oxidoreductase, from the procyclic forms of Trypanosoma brucei. Eur J Biochem. 2001;268:3075–3082. doi: 10.1046/j.1432-1327.2001.02205.x. [DOI] [PubMed] [Google Scholar]
- 33.Fang J, Beattie DS. Novel FMN-containing rotenone-insensitive NADH dehydrogenase from Trypanosoma brucei mitochondria: isolation and characterization. Biochemistry. 2002;41:3065–3072. doi: 10.1021/bi015989w. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, Beattie DS. Identification of a gene encoding a 54 kDa alternative NADH dehydrogenase in Trypanosoma brucei. Mol Biochem Parasitol. 2003;127:73–77. doi: 10.1016/s0166-6851(02)00305-5. [DOI] [PubMed] [Google Scholar]
- 35.Guerra DG, Decottignies A, Bakker BM, Michels PA. The mitochondrial FAD-dependent glycerol-3-phosphate dehydrogenase of Trypanosomatidae and the glycosomal redox balance of insect stages of Trypanosoma brucei and Leishmania spp. Mol Biochem Parasitol. 2006;149:155–169. doi: 10.1016/j.molbiopara.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Chaudhuri M, Ott RD, Hill GC. Trypanosome alternative oxidase: from molecule to function. Trends Parasitol. 2006;22:484–491. doi: 10.1016/j.pt.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Crane FL. Discovery of ubiquinone (coenzyme Q) and an overview of function. Mitochondrion S. 2007;1:S2–S7. doi: 10.1016/j.mito.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Kendall G, Wilderspin AF, Ashall F, Miles MA, Kelly JM. Trypanosoma cruzi glycosomal glyceraldehyde-3-phosphate dehydrogenase does not conform to the ‘hotspot’ topogenic signal model. EMBO J. 1992;9:2751–2758. doi: 10.1002/j.1460-2075.1990.tb07462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirumi H, Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J Parasitol. 1989;75:985–989. [PubMed] [Google Scholar]
- 40.Obado SO, Taylor MC, Wilkinson SR, Bromley EV, Kelly JM. Functional mapping of a trypanosome centromere by chromosome fragmentation identifies a 16-kb GC-rich transcriptional “strand-switch” domain as a major feature. Genome Res. 2005;15:36–43. doi: 10.1101/gr.2895105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zenno S, et al. Biochemical characterization of NfsA, the Escherichia coli major nitroreductase exhibiting a high amino acid sequence homology to Frp, a Vibrio harveyi flavin oxidoreductase. J Bacteriol. 1996;178:4508–4514. doi: 10.1128/jb.178.15.4508-4514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly JM, Taylor MC, Rudenko G, Blundell PA. In: Methods in Molecular Biology volume 47. Electoporation Protocols for Microorganisms. Nickoff JS, editor. Totowa, NJ: Humana; 1995. [Google Scholar]
- 43.Ingram AK, Cross GA, Horn D. Genetic manipulation indicates that ARD1 is an essential N (infinity)-acetyltransferase in Trypanosoma brucei. Mol Biochem Parasitol. 2000;111:309–317. doi: 10.1016/s0166-6851(00)00322-4. [DOI] [PubMed] [Google Scholar]