Abstract
The relationship between species diversity and ecosystem functioning has been debated for decades, especially in relation to the “macroscopic” realm (higher plants and metazoans). Although there is emerging consensus that diversity enhances productivity and stability in communities of higher organisms; however, we still do not know whether these relationships apply also for communities of unicellular organisms, such as phytoplankton, which contribute ≈50% to the global primary production. We show here that phytoplankton resource use, and thus carbon fixation, is directly linked to the diversity of phytoplankton communities. Datasets from freshwater and brackish habitats show that diversity is the best predictor for resource use efficiency of phytoplankton communities across considerable environmental gradients. Furthermore, we show that the diversity requirement for stable ecosystem functioning scales with the nutrient level (total phosphorus), as evidenced by the opposing effects of diversity (negative) and resource level (positive) on the variability of both resource use and community composition. Our analyses of large-scale observational data are consistent with experimental and model studies demonstrating causal effects of microbial diversity on functional properties at the system level. Our findings point at potential linkages between eutrophication and pollution-mediated loss of phytoplankton diversity. Factors reducing phytoplankton diversity may have direct detrimental effects on the amount and predictability of aquatic primary production.
Keywords: biodiversity, carbon cycle, ecosystem functioning
The relationship between species diversity and ecosystem functioning is a central issue in ecology, one fundamental question being the effect of diversity on community dynamics (“diversity–stability debate”; see ref. 1). It is commonly thought that diversity has stabilizing effects on ecosystem functioning (2), but early modeling work cast doubt on the validity of this belief (3). However, although single populations within diverse communities may indeed exhibit unstable dynamics, there is emerging consensus that diversity increases stability on the level of communities and ecosystems (1).
In view of globally-increasing species losses, the importance of diversity for ecosystem processes, such as resource use and productivity, have recently received considerable attention. Studies on terrestrial plants were the first to show that levels of primary production generally increase with increasing diversity and that more diverse communities are more resistant to extreme events (4). Meanwhile, there is accumulating evidence that this positive effect on productivity exists across various habitats and trophic levels (5–7). Cardinale et al. (8) and Gross and Cardinale (9) have shown that this positive effect may largely be attributed to more efficient resource use in more diverse communities.
Phototrophic bacteria and protists in the surface layers of lakes and oceans (phytoplankton) account for ≈50% of the global primary production, making this polyphyletic group a major component of the global carbon cycle (10). A few milliliters of sea water may contain tens to hundreds of species from very different taxonomic groups. Despite their obvious importance, however, our knowledge about the functional role of phytoplankton diversity (and microbial diversity in general) is very limited (11, 12). In addition to conceptual problems regarding the diversity of unicellular organisms (such as the species concept in organisms with largely asexual reproduction), it is generally unknown whether microbial diversity relates to ecosystem functioning in ways similar to those demonstrated for higher organisms.
The enormous dispersal potential of microbes and many species' being found across vast spatial scales (13, 14) have been the basis for the assumption that the spatial distribution of unicellular organisms is not dispersal limited, which should imply that the number of species present in a phytoplankton community becomes only a matter of local environmental conditions. With respect to ecosystem functioning, it has been proposed that the “local species richness [of microbes] is always sufficient to drive ecosystem functions” (13).
An increasing number of studies question the view of a global distribution of microbes (15, 16). Microbes show biogeographical patterns that do not always correlate to environmental gradients (e.g., refs. 17 and 18). For example, the number of diatom species found in a given lake correlates with the number of surrounding lakes (19). The existence of spatial diversity patterns alone cannot prove, however, that ecosystem function is related to microbial diversity.
Experiments with artificial communities of phytoplankton and other microbes indicate that processes carried out by microbes might be related to diversity (20–22) and that the relationships are similar to those known from the macroscopic realm. Such artificial communities, however, usually consist of arbitrary assemblages taken from easily cultivatable taxa and contain far fewer species than those found in natural assemblages, raising a serious concern as to what extent such “synthetic” communities exhibit natural responses.
Here, we investigate whether the variation in natural phytoplankton diversity spans a range that affects ecosystem functions and whether such patterns are comparable to those found for higher organisms. In particular, we test (i) whether phytoplankton diversity enhances resource use efficiency and (ii) whether diversity dampens variability in resource use and community composition. We use >3,000 phytoplankton samples from Scandinavian lakes and from the Baltic Sea. [See supporting information (SI) for a map with the sampling stations.]
Phosphorus is generally considered the limiting resource for phytoplankton primary production in temperate lakes (23, 24) and frequently represents the (co-) limiting nutrient in the Northern Baltic Sea (25). Moreover, phosphorus is rarely available in excess even in nitrogen limited systems (26, 27). Therefore, we use total phosphorus (TP) as a proxy for potential system productivity and express phytoplankton resource use efficiency (RUE) by the ratio between phytoplankton biomass and TP. To increase robustness of results, we use two independent measures of algal biomass, namely chlorophyll-a and algal carbon content as derived from microscopical cell counts, yielding two measures of resource use efficiency (RUEchl and RUEcarb). As a proxy for phytoplankton diversity, we use genus richness (G). Because both diversity and system productivity may affect system stability (28), we include TP as a covariable into our analysis.
Results
Diversity as a Predictor of RUE.
Diversity (G) was strongly and positively connected to RUE in all datasets (Fig. 1 and Table 1). A log–log transformation gave the best fit among several different transformations. This indicates a proportional rather than an additive dependency between G and RUE.
Fig. 1.
Resource use efficiency (RUE) as a function of diversity [genus richness (G)]. (Top) RUE in terms of chlorophyll-a per unit phosphorus (RUEChl). (Bottom) The same for algal carbon per unit phosphorus (RUECarb). (Left) Raw data with a fit for all observations. (Right) Fits for the individual datasets, corresponding to coefficients given in Table 1. The horizontal box plots show the diversity distribution for each dataset. Color codes refer to the single datasets. FI, Finland; NO, Norway; SE, Sweden; BS, Baltic Sea.
Table 1.
Regression coefficients for G and TP in different regression models predicting RUE
| Region | RUE | n | ln(RUE) = a + b × ln(G) |
ln(RUE) = a + b × ln(G) + c × ln(TP) |
|||
|---|---|---|---|---|---|---|---|
| a | b | a | b | c | |||
| All | RUEchl | 2,122 | 0.88 (<0.001) | 0.49 (<0.001) | 0.52 (<0.001) | 0.57 (<0.001) | −0.1 (<0.001) |
| All | RUEcarb | 2,535 | −0.96 (<0.001) | 1.12 (<0.001) | −0.79 (<0.001) | 1.09 (<0.001) | 0.06 (0.006) |
| BS | RUEchl | 99 | 0.28 (0.362) | 0.55 (0.01) | −0.74 (0.116) | 0.76 (<0.001) | −0.9 (0.002) |
| BS | RUEcarb | 512 | −0.32 (0.208) | 0.93 (<0.001) | −0.57 (0.067) | 0.97 (<0.001) | −0.26 (0.039) |
| Fi | RUEchl | 378 | 1.11 (0.001) | 0.4 (<0.001) | 1.26 (<0.001) | 0.37 (<0.001) | 0.08 (0.024) |
| Fi | RUEcarb | 378 | 2.17 (0.002) | 0.25 (0.082) | 2.47 (<0.001) | 0.2 (0.106) | 0.17 (0.004) |
| NO | RUEchl | 1,400 | 0.03 (0.452) | 0.79 (<0.001) | −0.7 (0.002) | 0.95 (<0.001) | −0.18 (<0.001) |
| NO | RUEcarb | 1,400 | −1.85 (<0.001) | 1.4 (<0.001) | −1.96 (<0.001) | 1.43 (<0.001) | −0.03 (0.212) |
| SE | RUEchl | 245 | 1.02 (0.005) | 0.47 (<0.001) | 1.09 (0.005) | 0.46 (0.002) | 0.02 (0.392) |
| SE | RUEcarb | 245 | 1.8 (0.034) | 0.43 (0.026) | 2.72 (0.003) | 0.24 (0.164) | 0.24 (<0.001) |
Coefficients were estimated from 1,000 bootstrap replications. Significance levels (in parentheses) refer to the probability of a coefficient being either positive or negative.
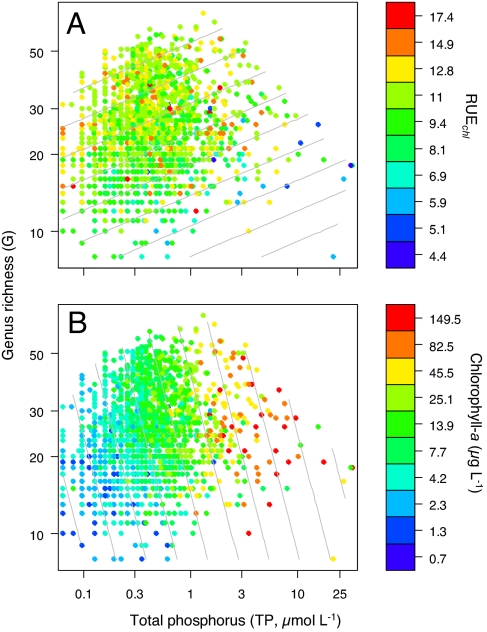
When RUE was analyzed as a function of G and TP, the positive relationship between G and RUE persisted, whereas there was no consistent relationship between RUE and TP (Table 1). Interestingly, the dependency of RUE on G was strongest in the datasets with, on average, low G (compare with box plots in Fig. 1): The estimated coefficients for the G effect for Norwegian lakes and the Baltic Sea range from 0.76 to 1.43, whereas they fall between 0.2 and 0.45 in the Finnish and Swedish lakes (Table 1). Fig. 2A shows that G generally increases with TP (Spearman's ρ = 0.16, P < 0.001). RUEchl is consistently high along the upper flank of this relationship but variable where high TP is met by low G (Fig. 2A).
Fig. 2.
RUEchl (A) and chlorophyll-a (B) as functions of total phosphorus (TP, x axis) and diversity (G, y axis). Contour lines in A indicate the fitted model for RUEchl given in Table 1. The contour lines in B correspond to the same model with RUEchl being replaced by chlorophyll-a (see Diversity as a Predictor of RUE).
A positive effect of G on RUE implies that, for a given level of TP, algal biomass increases with G. This can be seen when plotting chlorophyll-a instead of RUEchl as a function of TP and G (Fig. 2B). Because ln(RUEchl) equals ln(chlorophyll-a:TP), adding ln(TP) to both sides of the model displayed in Fig. 2A gives a model predicting ln(chlorophyll-a) (contour lines in Fig. 2B).
Variability of RUE and Community Composition.
Temporal and spatial variability of RUE were analyzed separately. In a heteroscedasticity analysis (see Materials and Methods), we tested whether G and TP predict variability of RUE among sites (i.e., spatial variability). In the combined dataset and in most of the separate datasets, error variances showed significant negative relationships with G (Table 2). Moreover, although TP did not have a consistent effect on absolute RUE (Table 1), the heteroscedasticity analysis revealed that variability of RUE increased with TP in the Norwegian, Swedish, and combined datasets (Table 2).
Table 2.
Heteroskedasticity analysis for the error variance in the regression ln(RUE) = a + b × ln(G) + c × ln(TP)
| Region | RUE | R2 | P | b | c |
|---|---|---|---|---|---|
| All | RUEchl | 0.04 | <0.001 | −0.9 (<0.001) | 0.51 (<0.001) |
| All | RUEcarb | 0.03 | <0.001 | −0.56 (<0.001) | 0.43 (<0.001) |
| BS | RUEchl | 0.03 | 0.23 | −0.71 (0.355) | −0.87 (0.276) |
| BS | RUEcarb | 0.02 | 0.01 | −0.58 (0.021) | −0.37 (0.122) |
| FI | RUEchl | 0.01 | 0.13 | −0.76 (0.068) | 0.19 (0.214) |
| FI | RUEcarb | 0.05 | <0.001 | −1.6 (<0.001) | −0.18 (0.228) |
| NO | RUEchl | 0.05 | <0.001 | −0.59 (0.004) | 0.58 (<0.001) |
| NO | RUEcarb | 0.06 | <0.001 | −0.49 (0.015) | 0.61 (<0.001) |
| SE | RUEchl | 0.11 | <0.001 | 0.44 (0.45) | 0.93 (<0.001) |
| SE | RUEcarb | 0.06 | <0.001 | −1.01 (0.068) | 0.6 (<0.001) |
Regression coefficients (b and c) and significance levels (P, in parentheses) refer to the regression on error variance (v): v = a + b × ln(G) + c × ln(TP). Number of observations as given in Table 1. Note that these models explain residual variance, i.e., R2 is expected to be low.
Using lakes with multiple observations per year, we analyzed temporal dynamics of RUE and community composition. Variability of both parameters increased with TP, but decreased with G (Fig. 3 and Table 3). The low number of observations in Finland and Sweden made a countrywise analysis meaningless, but the observations fit well into the trend seen in the combined dataset (Fig. 3). Overall, the data indicate that phytoplankton RUE and community composition are stabilized by G but destabilized by TP.
Fig. 3.
Results from time-series analysis. Partial residuals (Rp) for linear regressions predicting community turnover σs (A and B) and standard deviation (SD) of RUEchl (C and D) as functions of mean annual diversity (G) and total phosphorus (TP) (see Table 3). Each dot represents 1 lake year. Lines indicate the mean effect of a predictor together with confidence intervals.
Table 3.
Results from time series analysis, where community turnover (σs) and variability of RUE over time (expressed as its SD) were analyzed as functions of mean annual G and TP, respectively
| Response variable | R2 | n | P | G, mean | TP, mean |
|---|---|---|---|---|---|
| Community turnover σs | 0.37 | 449 | <0.001 | −0.01 (<0.001) | 0.02 (<0.001) |
| SD(ln(RUEchl)) | 0.09 | 435 | <0.001 | −0.20 (<0.001) | 0.09 (<0.001) |
| SD(ln(RUEcarb)) | 0.20 | 449 | <0.001 | −0.26 (<0.001) | 0.16 (<0.001) |
Given are the model summaries and estimated coefficients for G and TP, with corresponding P values in parentheses.
Discussion
Both measures of resource use efficiency (RUEchl, RUEcarb) show similar positive relationships with phytoplankton diversity (G), indicating that systematic biases, which could arise from microscopic counts or from variations in cellular chlorophyll content, were of minor importance. The general importance of diverse communities for RUE is also supported by the finding that both the absolute RUE and its predictability increase with diversity.
The patterns appear to be consistent among the different datasets. Moreover, despite considerable differences in regional diversity and environmental gradients (brackish vs. freshwater and continental vs. oceanic climate), diversity was the best predictor for RUE in all datasets (see Materials and Methods and SI). This indicates a general and strong dependency between phytoplankton diversity and resource use. The positive relationship between diversity and RUE is much more pronounced in areas with a low average diversity (Norwegian lakes and the Baltic Sea) compared with those with higher diversity (Finnish and Swedish lakes), indicating that diversity effects are stronger for less diverse communities.
Although species richness is often analyzed as a function of productivity (e.g., 29, 30), the direction of causality has been seriously challenged (9, 8). Experimental studies in which species richness is manipulated give considerable support that species richness enhances resource use and thus productivity (20–22, 31). Moreover, a recent mathematical model explicitly shows that positive correlations between productivity and diversity may not be interpreted such that productivity drives diversity (9).
A causal relationship between diversity and RUE cannot be established based on observational data alone. The relationships between diversity and RUE shown here, however, are very consistent with those known from higher organisms (4, 6), and supports the view of general scaling rules for microbes and higher organisms (15). The data are also in good agreement with experimental data from artificial microbial communities (see above) but represent the first of its kind from natural species-rich microbial communities that have not been subject to experimental manipulation. Moreover, stabilizing effects of diversity and destabilizing effects of enrichment or trophic state (“paradox of enrichment;” ref. 28) have been hitherto reported independently from each other. To the best of our knowledge, however, this is the first study showing that stabilizing effects of diversity interact with resource levels in natural systems.
Our analysis indicates that RUE and community composition are both stabilized by diversity. A coupling between fluctuations in RUE and fluctuations of community composition seems plausible, because fluctuating RUE implies temporal windows where resources are not used efficiently. Such situations provide available niche-space for new species to become established in the system, pointing to higher risk of species invasion and/or resource monopolization (algal blooms) in species-poor communities. The inverse relationship between community turnover and diversity reported here (Fig. 3) is consistent with previous results for communities of higher organisms, including fast dispersing groups, such as macroalgae and zooplankton (32, 33). Therefore, we suggest that the stabilizing effect of diversity may be a universal rule.
A tight coupling between microbial diversity and ecosystem function implies that factors impairing microbial diversity are likely to affect efficiency and predictability of ecosystem processes. For example, pollution stress, especially from toxic substances, is often manifested as biodiversity loss. Our results indicate that pollution-mediated losses of microbial diversity may have direct effects on ecosystem processes, pointing to important linkages between toxic pollutants, nutrient enrichment, and biodiversity. This conclusion is supported by two recent studies on microbial communities in soils (34, 35) in which diversity of natural communities was artificially reduced by toxic compounds, and the metabolic rates were measured. A reduction of microbial diversity lowered community function and made the communities less resistant to further stress (34, 35).
We still know very little about the diversity of phytoplankton and other microbial communities and how it affects ecosystem functioning. This is due in part to methodological difficulties in assessment of microbial diversity (12). General ecological theories have only recently been applied to microbial communities, but the emerging patterns encourage a closer look into diversity of microbial assemblages as a driver of ecosystem function.
Materials and Methods
Datasets.
We tested our hypotheses by using >3,000 phytoplankton samples from lakes in Fenno–Scandia (535 from Finland, 1,668 from Norway, and 299 from Sweden), and 512 phytoplankton samples from the Baltic Sea from altogether 550 different sampling locations (see map in the SI). The lake phytoplankton data originated from various sampling programs in these three Nordic countries in the period from 1989 to 2003. The Baltic Sea phytoplankton data came from the Finnish coastal monitoring program between 1980 and 2003 from stations ranging from the Bay of Bothnia to the Gulf of Finland. We only used observations where total phosphorus (TP) and phytoplankton biomass [chlorophyll-a (Chl)] were measured on the same sample. To exclude observations from extreme habitats, we excluded acidified lakes (pH <6.0) and localities located north of 65° from the analysis (see SI). Diversity was defined as number of genera present in a given sample. Reducing the taxonomic resolution to genus level made the dataset more homogeneous and more robust to differences in skill and effort among the individual phytoplankton analysts.
For all datasets, only surface samples from the period between July and September were used to limit seasonal variation and to exclude periods with unfavorable weather conditions except for the time series analysis. Most lakes with multiple observations per year were sampled monthly; thus, we had to use samples from May to September to make this analysis meaningful. We excluded observations with TP concentrations <2 μg·liter−1 (n = 15) because they are close to the detection limit, resulting in very imprecise measurements.
Abundances and biovolumes of phytoplankton species and genera were estimated with the Utermöhl method. Phytoplankton carbon biomass (PPC) was estimated from biovolumes, applying a constant conversion factor of 14% of algal wet weight (36, 37).
Phytoplankton biomass divided by the limiting resource (total phosphorus) gave the yield or resource use efficiency (RUE) for the community. We estimated RUE both in terms of chlorophyll-a (RUEchl = chlorophyll-a:TP) and carbon biomass (RUEcarb = PPC:TP) (see also Validation of Underlying Assumptions). For the Baltic Sea, chlorophyll-a data were missing for many samples, causing a considerably lower number of observations for RUEchl (n = 99) compared with RUEcarb (n = 512).
Data Analysis.
Regression analysis of RUE.
Initial analyses indicated nonconstant error variances in regression models predicting RUE. We applied nonparametric bootstrapping (1,000 replications) to obtain robust estimates for linear regression model parameters (38). Statistical inferences on regression coefficients were based on their bootstrap distributions.
To avoid bias due to varying number of observations per locality, observations from localities with multiple observations were down-weighted by the inverse of the corresponding number of observations. Furthermore, for lakes with >10 observations, 10 observations were selected randomly from the total of observations.
Heteroscedasticity of the regression models was analyzed by using the Breusch–Pagan test (38), which tests whether the variability of the response variable scales with the predictors, i.e., whether the predictors affect the predictability of the relationship (38). Using the regression coefficients estimated from the bootstrapped regressions (see above), we calculated the log-transformed absolute residuals (v) and analyzed heteroscedasticity by v = a + b ln(G) + c ln(TP).
Species turnover and temporal variability of RUE.
Variability in community composition and RUE were analyzed for all lakes and years (“lake year”) with a minimum of four observations per year. We calculated Bray–Curtis dissimilarity σ for all pairs of observations within a lake year, using square-root transformed genus-level data. Within a lake year, species turnover between two samples was a saturating function of the corresponding time interval (Δt). Dividing σ by ln(Δt) gave a standardized σs, which was not correlated with time. We calculated the averages of σs, ln(G), ln(TP), and the standard deviation (SD) of ln(RUE) for each lake year. Using these data, we then estimated the average effects of G and TP on community turnover (σs) and on variability (SD) of RUE in linear regressions. We did not estimate turnover rates for stations in the Baltic Sea, because sampling stations in an open system do not represent distinct systems, making changes in species composition influenced by horizontal advection rather than “local” dynamics.
Validation of Underlying Assumptions.
There is a risk of confounding true effects with spurious correlations when analyzing relationships between two parameters (RUE and diversity) that both reflect cellular growth. Environmental factors acting upon phytoplankton growth might synchronize patterns seen in productivity and diversity. To minimize such effects, we excluded sites with extreme conditions from the analysis (see above). Using multiple regressions with diversity and important environmental factors as predictors of RUE, we double-checked that effects of diversity seen in the analyses do not represent spurious correlations driven by environmental factors. These regressions revealed that diversity is the best predictor of RUE in our datasets, whereas the explanatory power of other factors, such as pH, temperature, salinity, and lake morphometry, is rather poor (see SI).
Our definition of RUE depends on phosphorus being either directly limiting or at least not being in excess compared with the actual limiting nutrient. We are aware that phytoplankton is rarely limited by phosphorus alone, particularly at high TP-levels (24). Elser et al. (26) recently showed how synergistic effects of both phosphorus and nitrogen additions fuel the pelagic productivity of lakes in a consistent manner across a large number of studies. Because phosphorus is rarely available in great excess compared with nitrogen (27), TP should give a reasonable surrogate for potential productivity even in nitrogen limited systems. This assumption may, however, be questioned for eutrophic systems with predominant light limitation. To safeguard against potential biases that might emerge from including eutrophic sites in our analysis, we performed the analysis shown in Table 1 for the same dataset with observations >0.5 μmol of TP per liter being excluded (n = 1570 of 2535; temperate lakes with TP-levels <0.5 μmol of P per liter were shown to be predominantly P-limited (24)). The results of this analysis compare very well to the results shown for the entire dataset in the SI.
Acknowledgments.
We thank H. Hillebrand, R. F. Wright, and two anonymous reviewers for their helpful comments. Data compilation and parts of the analysis were supported by European Union research projects REBECCA (Contract SSPI-CT-2003-502158) and THRESHOLDS (Contract GLOBAL-IP-02-0257).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708328105/DCSupplemental.
References
- 1.McCann KS. The diversity–stability debate. Nature. 2000;405:228–233. doi: 10.1038/35012234. [DOI] [PubMed] [Google Scholar]
- 2.Elton CS. Ecology of Invasions by Animals and Plants. London: Chapman & Hall; 1958. [Google Scholar]
- 3.May RM. Stability and Complexity in Model Ecosystems. Princeton: Princeton Univ Press; 1973. [PubMed] [Google Scholar]
- 4.Tilman D, Wedin D, Knops J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature. 1996;379:718–720. [Google Scholar]
- 5.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 6.Loreau M, et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 7.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 8.Cardinale BJ, et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature. 2006;443:989–992. doi: 10.1038/nature05202. [DOI] [PubMed] [Google Scholar]
- 9.Gross K, Cardinale BJ. Does species richness drive community production or vice versa? Reconciling historical and contemporary paradigms in competitive communities. Am Nat. 2007;170:207–220. doi: 10.1086/518950. [DOI] [PubMed] [Google Scholar]
- 10.Falkowski PG, Barber RT, Smetacek V. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–206. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- 11.Hughes JB, et al. Microbial biogeography: Putting microorganisms on the map. Nat Microb Rev. 2006;4:107–112. doi: 10.1038/nrmicro1341. [DOI] [PubMed] [Google Scholar]
- 12.Horner-Devine MC, Carney KM, Bohannan BJM. An ecological perspective on bacterial biodiversity. Proc R Soc London Ser B. 2004;271:113–122. doi: 10.1098/rspb.2003.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlay BJ. Global dispersal of free-living microbial eukaryote species. Science. 2002;296:1061–1063. doi: 10.1126/science.1070710. [DOI] [PubMed] [Google Scholar]
- 14.Fenchel T, Finlay BJ. The ubiquity of small species: Patterns of local and global diversity. BioScience. 2004;54:777–784. [Google Scholar]
- 15.Green JL, Bohannan B. Spatial scaling of microbial biodiversity. Trends Ecol Evol. 2006;21:501–507. doi: 10.1016/j.tree.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Lachance MA. Here and there or everywhere? BioScience. 2004;54:884. [Google Scholar]
- 17.Foissner W. Biogeography and dispersal of microorganisms: A review emphasizing protists. Acta Protozool. 2006;45:111–136. [Google Scholar]
- 18.Soininen J, McDonald R, Hillebrand H. The distance decay of similarity in ecological communities. Ecography. 2006;29:1–10. [Google Scholar]
- 19.Vyverman W, et al. Historical processes constrain patterns in global diatom diversity. Ecology. 2007;88:1924–1931. doi: 10.1890/06-1564.1. [DOI] [PubMed] [Google Scholar]
- 20.McGrady-Steed J, Harris PM, Morin PJ. Biodiversity regulates ecosystem predictability. Nature. 1997;390:162–164. [Google Scholar]
- 21.Bell T, Newman JA, Silverman BW, Turner SL, Lilley AK. The contribution of species richness and composition to bacterial services. Nature. 2005;436:1157–1160. doi: 10.1038/nature03891. [DOI] [PubMed] [Google Scholar]
- 22.Steiner C, Long Z, Krumins J, Morin PJ. Temporal stability of aquatic food webs: Partitioning the effects of species diversity, species composition and enrichment. Ecol Lett. 2005;8:819–828. [Google Scholar]
- 23.Vollenweider RA. In: Global Freshwater Quality—A First Assessment. Meybeck M, Chapman D, editors. Geneva: WHO; 1989. [Google Scholar]
- 24.Guildford SJ, Hecky RE. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol Oceanorgr. 2000;45:1213–1223. [Google Scholar]
- 25.Tamminen T, Andersen T. Seasonal phytoplankton nutrient limitation patterns as revealed by bioassays over Baltic Sea gradients of salinity and eutrophication. Mar Ecol Prog Ser. 2007;340:121–138. [Google Scholar]
- 26.Elser JJ, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett. 2007;10:1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 27.Davidson EA, Howarth RW. Environmental science: Nutrients in synergy. Nature. 2007;449:1000–1001. doi: 10.1038/4491000a. [DOI] [PubMed] [Google Scholar]
- 28.Rosenzweig ML. Paradox of enrichment: Destabilization of exploitation ecosystems in ecological time. Science. 1971;171:385–387. doi: 10.1126/science.171.3969.385. [DOI] [PubMed] [Google Scholar]
- 29.Mittelbach GG, et al. What is the observed relationship between species richness and productivity? Ecology. 2001;82:2381–2396. [Google Scholar]
- 30.Irigoien X, Huisman J, Harris RP. Global biodiversity patterns of marine phytoplankton and zooplankton. Nature. 2004;429:863–867. doi: 10.1038/nature02593. [DOI] [PubMed] [Google Scholar]
- 31.Naeem S, Li SB. Biodiversity enhances ecosystem reliability. Nature. 1997;390:507–509. [Google Scholar]
- 32.White EP, et al. A comparison of the species-time relationship across ecosystems and taxonomic groups. Oikos. 2006;112:185–195. [Google Scholar]
- 33.Shurin JB, et al. Diversity−Stability relationship varies with latitude in zooplankton. Ecol Lett. 2007;10:127–134. doi: 10.1111/j.1461-0248.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- 34.Griffiths BS, et al. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: An examination of the biodiversity−ecosystem function relationship. Oikos. 2000;90:279–294. [Google Scholar]
- 35.Tobor-Kapłon MA, Bloem J, Romkens PFAM, de Ruiter PC. Functional stability of microbial communities in contaminated soils near a Zinc smelter (Budel, the Netherlands) Ecotoxicology. 2006;15:187–197. doi: 10.1007/s10646-005-0050-4. [DOI] [PubMed] [Google Scholar]
- 36.Rocha O, Duncan A. The relationship between cell carbon and cell volume in freshwater algal species used in zooplankton studies. J Plankton Res. 1985;7:279–294. [Google Scholar]
- 37.Vadstein O, Jensen A, Olsen Y, Reinertsen H. Growth and phosphorus status of limnetic phytoplankton and bacteria. Limnol Oceanogr. 1988;33:489–503. [Google Scholar]
- 38.Fox J. Applied Regression, Linear Models, and Related Methods. Thousand Oaks, CA: Sage; 1997. [Google Scholar]