Abstract
CD4+ helper T cells contribute to the induction and maintenance of antigen-specific CD8+ T cells. Their absence results in short-lived antigen-specific CD8+ T cells and defective secondary CD8+ T cell responses because of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. Here, we show that IL-15 codelivered with vaccines can overcome CD4+ T cell deficiency for promoting longevity of antigen-specific CD8+ T cells and avoidance of TRAIL-mediated apoptosis. In both priming and secondary responses, IL-15 down-regulates proapoptotic Bax, an intermediate in TRAIL-mediated apoptosis, and increases anti-apoptotic Bcl-XL in CD8+ T cells. Thus, IL-15 is sufficient to mimic CD4+ T cell help. Antigen-specific CD4+ T cells induce dendritic cells (DCs) to produce IL-15. IL-15 is also necessary for optimal help, because helper cells do not deliver effective help through IL-15−/− DCs. Therefore, IL-15 codelivered with vaccines can overcome CD4+ helper T cell deficiency for induction of functionally efficient CD8+ T cells and maintenance of CD8+ cytotoxic T lymphocytes (CTLs), and IL-15 is probably one of the natural mediators of help. These findings suggest new vaccine strategies against infections and cancers, especially in individuals with CD4-deficiency.
Keywords: cytotoxic T lymphocytes, T cell help
The longevity and activity of antigen-specific CD8+ T cells are vitally important in establishment of protective immunity against viral infections and cancer. Further, the ability of CD8+ T cells to expand on secondary encounter with antigens is essential for immunity (1, 2).
Antigen-specific CD8+ T cells can be programmed in the immune induction phase (3–5). CD4+ help is needed for induction of long-lived memory CD8+ T cells (6, 7). The results of early programming are more profound in secondary responses: CD8+ T cells primed in the absence of CD4+ helper T cells undergo tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis and show poor long term memory (8, 9). Thus, understanding the mechanisms by which help from CD4+ T cells prevents CD8+ T cell death and enhances the maintenance of memory CD8+ T cells is critical for developing new vaccines.
In addition to the maintenance of CD8+ memory T cells (10–15), we previously found that additional IL-15 provided by a vaccine vector only during priming induced long-lived memory CD8+ T cells (5) and contributed to the long-term avidity maturation of CD8+ T cells (16). These findings suggest that IL-15 contributes to programming of CD8+ T cells to be long-lived cells that can further expand on reexposure to antigen. Therefore, we hypothesized that CD8+ T cells programmed with IL-15 only during priming, even in the absence of CD4+ helper T cells, may overcome TRAIL-mediated apoptosis during secondary responses and persist longer. We also hypothesized that one mechanism by which CD4+ helper T cells help CD8+ T cells is to stimulate IL-15 production by the antigen presenting cells (APCs), including dendritic cells (DCs).
Here, we show that IL-15 provided by the vaccine can replace CD4+ helper T cells in inducing long-lasting antigen-specific CD8+ T cells that can efficiently respond to secondary responses. Conversely, help was less effective when DCs did not produce IL-15. Thus, we conclude that IL-15 codelivered with vaccines can overcome CD4+ helper T cell deficiency and that IL-15 may be one mediator of help. These new findings will be important for developing new vaccines against cancers and viral infections, especially those such as HIV that result in diminished CD4+ T cell help.
Results
IL-15 Substitutes for CD4+ Help in CD8+ T Cell Priming for Long-Lived Memory.
To test whether IL-15 can substitute for CD4+ T cells in the induction of longer-lived antigen-specific CD8+ T cells, animals, either CD4-depleted or undepleted, were immunized with recombinant vaccinia viruses expressing HIVgp160 (vPE16) or HIVgp160 and IL-15 (vPE16-IL-15).
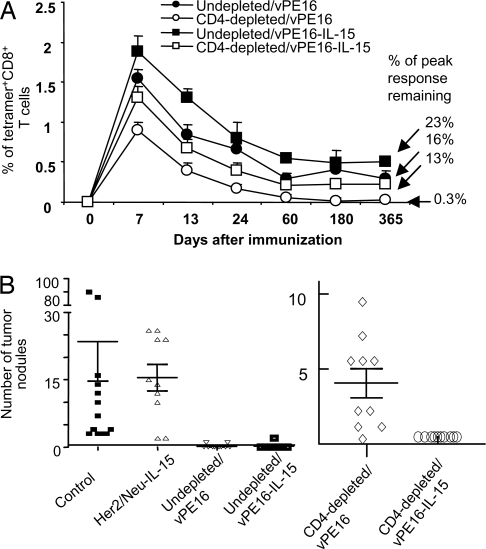
CD4+ T cell-undepleted animals immunized with vPE16 maintained P18-I10-specific CD8+ T cells (measured directly ex vivo without restimulation) for 1 year (Fig. 1A). Animals immunized with vPE16-IL-15 had a higher percentage of long-lasting memory CD8+ T cells than those without IL-15 (percentage of peak response remaining, 23% vs. 16%), indicating that IL-15 provided by the vaccine vector contributes to the enhanced induction of long-lasting memory CD8+ CTL in WT animals and that endogenous IL-15 may be limiting. In contrast, in CD4-depleted animals, the majority of the CD8+ T cells induced by vPE16 disappeared shortly after the primary response, with <1% of the peak response remaining at 1 year. Less than 0.01% of total CD8+ T cells from unimmunized animals were tetramer positive overall (data not shown). However, CD4-depleted animals immunized with vPE16-IL-15 maintained specific CD8+ T cells over 1 year at a level similar to that of intact animals immunized with vPE16 in the presence of CD4+ T cells, 13% vs. 16% of the peak level, suggesting that IL-15 can provide “help” for the induction of long lasting memory CD8+ T cells in the absence of CD4+ help. The CD4-depleted animals immunized with vPE16-IL-15 had significantly more antigen-specific CD8+ T cells than those immunized with vPE16 at all time points (P < 0.05). In parallel, lytic activity was measured 1 week after restimulation [supporting information (SI) Fig. S1]. In both undepleted and CD4-depleted animals, vPE16-IL-15 resulted in a higher percentage of the peak response remaining at 1 year after the immunization than vPE16 did. Furthermore, the lytic activity of CTL induced by vPE16-IL-15 in the absence of CD4+ helper T cells was similar to the activity of CTL induced by vPE16 in the presence of CD4+ helper T cells and significantly greater than that of CTL in the depleted animals immunized with vPE16 at all time points (P < 0.05) (Fig. S1). The CTL in animals immunized with vPE16 in the CD4-depleted state fell to background levels after approximately 2 months. Thus, IL-15 is sufficient to replace CD4+ help for induction of long-lived CTL.
Fig. 1.
IL-15 codelivered with antigen can substitute for CD4+ helper T cells to induce long lasting memory CD8+ T cells that are functional in vivo. (A) Intact or CD4-depleted mice were immunized s.c. with 2–4 × 106 pfu of vPE16 or vPE16-IL-15. Immunization dates were staggered so that all time points could be assayed on the same day for comparison. The frequency of P18-I10-specific CD8+ T cells measured over time by staining pooled spleen cells of 4–5 mice with anti-CD8 and H-2Dd-P18-I10 tetramer (mean ± SD of two experiments with similar results). As controls, in splenocytes from unimmunized animals, the frequency of tetramer positive CD8+ T cells was <0.01%. (B) IL-15 codelivered with vaccines can substitute for CD4 help to induce CD8+ T cells that prevent tumor growth in vivo. CD4-depleted and undepleted female BALB/c mice were immunized s.c. with 2–4 × 106 pfu of vPE16 or vPE16-IL-15. Four weeks after priming, 10 mice in each group received 2 × 105 15–12RM tumor cells per mouse iv, and the number of tumor nodules in the lungs was counted 30 days after the challenge. The CD4-depleted/vPE16 group showed significantly more lung nodules than the undepleted mice immunized with vPE16 (P < 0.0008; Wilcoxon test) and the CD4-depleted mice given vPE16-IL-15 (P < 0.0002), which were completely protected. Two independent experiments showed similar results.
This effect of IL-15 expressed by the recombinant vaccinia vector is not due to persistence of the vector producing IL-15 in CD4-depleted mice, which may not make anti-vaccinia antibodies, maintaining CD8+ T cell memory. We excluded this explanation by measuring the survival of IL-15-producing vaccinia in CD4-depleted or undepleted mice in lung, spleen, and ovary (where this virus tends to replicate). Both vPE16 and vPE16-IL15 were completely undetectable in both depleted and undepleted mice by day 11 in all three tissues. This is consistent with clearance of vaccinia being dependent on IFNγ (17, 18), which can be made in large quantity by NK cells, which are increased by IL-15 independent of CD4+ T cell help.
IL-15 Substitutes for CD4+ T Cell Help to Induce a Protective in Vivo Response Against Tumor Challenge.
To address whether the sustained CD8+ T cell response measured ex vivo and in vitro correlated with efficacy in vivo, we tested protective efficacy against tumor. Mice were challenged i.v. with 15–12RM fibrosarcoma tumor cells 4 weeks after priming, and tumors in the lung were enumerated on day 21 after challenge (Fig. 1B). Immunization with vPE16 protected undepleted mice but not mice depleted of CD4+ T cells before immunization (P < 0.0008) (Fig. 1B). In contrast, all 10 CD4-depleted mice immunized with vPE16-IL-15 were completely protected (P < 0.0002 compared with depleted mice without IL-15). This difference was reproducible in a second experiment (data not shown). In CD4-depleted animals, IL-15 made a life-or-death difference. The effect was not a nonspecific effect of vaccinia expressing IL-15, because a control recombinant vaccinia virus expressing IL-15 and the irrelevant antigen Her-2/neu had no significant effect. Thus, IL-15 codelivered at the time of priming can overcome CD4+ T cell deficiency for induction and maintenance of functionally efficacious CD8+ T cells that can prevent tumor growth in vivo.
CD8+ CTL Induced with CD4+ Helper T Cells or Codelivered IL-15 Express Lower Levels of Membrane-Bound TRAIL than CTL Induced in the Absence of CD4+ Helper T Cells.
Despite controversy regarding the role of TRAIL in antigen-specific CD8+ CTL (9, 19), TRAIL-mediated apoptosis has been one of the mechanisms for activation-induced death of antigen-specific CD8+ T cells (20). CD8+ CTL induced in the absence of CD4+ helper T cells undergo TRAIL-mediated apoptosis during secondary responses (8, 9). To determine whether the effect of IL-15 to substitute for CD4+ T cell help in the induction of longer-lived CTL was due to the same mechanism as that of helper T cells, we tested whether CD8+ CTL primed with CD4+ helper T cells or codelivered IL-15 express decreased levels of TRAIL, and this could explain the different rates of CTL contraction (Fig. 1).
We confirmed the specificity of the anti-TRAIL antibody used, characterized (21, 22) by measuring binding of the antibody after blockade with the DR5-Fc (TRAIL receptor) fusion protein. Purified splenic CD8+ T cells were cultured in plates coated with anti-CD3 antibody overnight, incubated with DR5-Fc, and stained with anti-TRAIL. CD8+ T cells stimulated with anti-CD3 express membrane-bound TRAIL, which could be blocked 91.5% (reduction in geometric mean fluorescence) by DR5-Fc fusion protein, suggesting that anti-TRAIL antibody used in this study is specific for TRAIL expressed on the cell surface (Fig. S2).
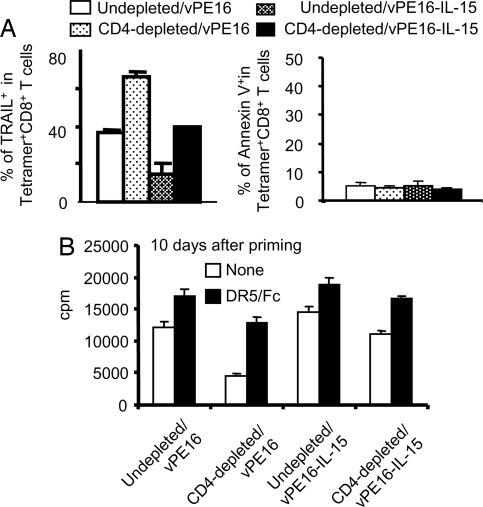
Using this approach, we measured expression levels of membrane-bound TRAIL on antigen (P18-I10)-specific CD8+ CTL during the contraction phase. More than 60% of the CTL induced with vPE16 in CD4-depleted animals express membrane-bound TRAIL directly ex vivo on day 5 after priming, compared with 39% of those induced with vPE16-IL-15 in the CD4-depleted animals (P < 0.03) (Fig. 2A). In undepleted animals, IL-15 also decreased the number of TRAIL positive cells. This direct ex vivo result suggested that more CTL induced without CD4+ help express TRAIL in vivo than CTL induced with CD4+ help. IL-15 codelivered by the vaccine vector during priming decreased the expression levels of membrane-bound TRAIL in CD8+ CTL and may contribute to the increased CD8+ CTL frequency in animals immunized with IL-15. Thus, consistent with Fig. 1, CD4+ help plays an important role in modulating the CTL contraction phase, and IL-15 codelivered with the vaccines played a similar role, apparently by a similar mechanism. However, we were not able to detect significantly different numbers of antigen-specific and Annexin V positive CD8+ CTL ex vivo, possibly because of the rapid disappearance of dead cells in vivo or loss of apoptotic cells during ex vivo experiments.
Fig. 2.
Antigen-specific CD8+ T cells induced by the vaccines codelivered with IL-15 express less TRAIL. (A) CD4+ T cell-depleted and undepleted animals were immunized s.c. with 2–4 × 106 pfu of vPE16 or vPE16-IL-15. On day 5–7 after priming, pooled spleen cells of 4–5 mice were stained with anti-CD8, H-2Dd-P18-I10 tetramer, and anti-TRAIL antibody. TRAIL+ or annexin V+ cells were assessed on gated tetramer+ CD8+ T cells. Three repeat experiments showed similar results. (B) Antigen-specific CD8+ T cells were purified from the pooled spleens of 4–5 mice on day 10 after the immunization. 2 × 105 CD8+ T cells were stimulated with 4 × 105 syngeneic splenocytes pulsed with 0.001 μM P18-I10. On day 3, 1 μCi per well 3H-Thymidine was added overnight before harvesting. Five micrograms per well of anti-DR5/Fc fusion protein or control Fc was added into the 96-well culture plates where indicated. Data are mean ± SD of triplicate assays, and two repeat experiments showed similar results. The differences between the DR5/Fc and control Fc-untreated groups in the CD4-depleted animals were significant (P < 0.002) by a Student's t test.
CD8+ CTL Induced with IL-15 Codelivered by Vaccines Resist TRAIL-Mediated Apoptosis During Secondary Responses.
To determine whether IL-15 acts by the same mechanism as help, we tested whether CD8+ CTL induced with IL-15 in the absence of CD4+ help can resist TRAIL-mediated apoptosis during secondary responses. Purified H-2Dd-P18-I10 tetramer+ CD8+ CTL from different groups of animals were restimulated in vitro, and their responsiveness was tested by measuring carboxyfluorescein (diacetate) succinimidyl ester (CFSE) dilution (Fig. S3). On days 7 and 24 after the immunization, antigen-specific tetramer+ CD8+ CTL from the undepleted animals immunized with vPE16 or vPE16-IL-15 proliferated efficiently in response to peptide-pulsed APCs. However, the tetramer+ CD8+ CTL induced in the CD4-depleted animals immunized with vPE16 proliferated poorly; although we could see some proliferation on day 7, on day 24, very few cells survived. In contrast, tetramer+ CD8+ CTL induced in the CD4-depleted animals immunized with vPE16-IL-15 proliferated efficiently in response to the peptide antigen with a frequency similar to that in undepleted animals immunized with vPE16 and significantly greater than that in CD4-depleted animals immunized with vPE16 (P < 0.001). Thus, IL-15 provided by the vaccine vector contributed to the induction of antigen-specific CD8+ CTL that efficiently responded to secondary stimulation in vitro. The effect was even more dramatic 24 days after priming, suggesting that the greater sensitivity of antigen-specific CD8+ cells primed without help to apoptosis on restimulation (9) is overcome by the presence of IL-15 during priming.
To see whether the mechanism was the same as that described for lack of help (9), involving TRAIL-mediated apoptosis on restimulation in vitro, we examined 3H-thymidine incorporation after restimulation with specific epitope peptide and the effect of soluble DR5-Fc (TRAIL receptor) (Fig. 2B), which competitively inhibits TRAIL activity, on this antigen-specific CD8+ T cell proliferation (Fig. 2B). There was an ≈3-fold decrease in antigen-induced thymidine uptake in the group primed in the absence of help, but this was largely reversed in the presence of DR5-Fc (P < 0.002). Thus, the decrease in thymidine uptake was due to TRAIL-mediated apoptosis, suggesting that TRAIL is the major effector of CD8+ CTL death during the secondary response as described in ref. 9. What is new here is that the proliferation of CD8+ T cells from the CD4-depleted mice immunized with vPE16-IL-15 was almost as great as that of the control undepleted group (Fig. 2B), even without TRAIL blockade by DR5-Fc, indicating that expression of IL-15 by the vaccine vector overcomes lack of CD4+ help and prevents TRAIL-mediated death on secondary stimulation.
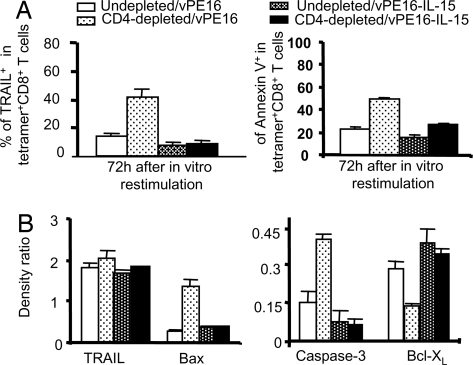
Accordingly, we measured the expression levels of membrane-bound TRAIL during secondary responses. Substantially more CD8+ CTL from the CD4-depleted animals immunized with vPE16 expressed TRAIL (>40%) (P < 0.001) and were Annexin V positive (50%), whereas TRAIL expression in the group immunized with vPE16-1L-15 was no greater than that of the control group (Fig. 3A). Overall, we conclude that IL-15, like CD4+ T cell help, prevents the up-regulation of TRAIL and annexin V, and the induction of TRAIL-mediated apoptosis that would otherwise occur on secondary stimulation of CD8+ T cells primed in the absence of help.
Fig. 3.
CD4+ help and IL-15 both induce longer-lived CD8+ T cells that escape apoptosis on secondary stimulation by up-regulating Bcl-XL and down-regulating Bax and surface TRAIL. (A) On day 10–12 after priming, purified antigen-specific CD8+ T cells from pooled spleen cells of 4–5 mice were restimulated with 0.001 μM P18-I10-pulsed splenocytes for 3 days in vitro. Data are mean ± SE of two independent experiments. CD8+ CTL from CD4-depleted mice immunized with vPE16-IL-15 expressed significantly less TRAIL (P < 0.001) and annexin V (P < 0.02) than those from mice immunized with vPE16. (B) Ten days after priming, mice were boosted with 2 × 106 pfu vPE16 and P18-I10-specific CD8+ T cells from individual groups were purified. Cell lysates were analyzed by Western blot. Data show the density ratios from two separate experiments with similar results (mean ± SD). The difference in TRAIL expression between A and B reflects the difference between surface TRAIL in A and total TRAIL in B.
IL-15 Substitutes for Help in Preventing the Up-Regulation of Proapoptotic Bax and Caspase 3 and Down-Regulation of Anti-Apoptotic Bcl-XL.
To determine the effect of IL-15 on other anti- and proapoptotic molecules, we tested whether CD8+ CTL programmed with IL-15 in CD4-depleted animals express more anti-apoptotic Bcl-XL and less proapoptotic Bax (a downstream mediator of TRAIL) during secondary responses as in the priming phase (Fig. 3B and Fig. S4). It is known that the TRAIL-initiated cell death pathway requires Bax (23) and that Bcl-XL blocks TRAIL-mediated apoptosis (24). To test this ex vivo, not in vitro, 10 days after priming, animals were boosted with vPE16, and P18-I10-specific CD8+ T cells were purified. Western blot mean data in Fig. 3B show that the total cellular expression levels of TRAIL are similar in CD8+ T cells from all four different groups of animals, in contrast to the surface levels. However, CD8+ CTL induced in CD4-depleted animals immunized with vPE16 express significantly higher levels of Bax and caspase 3 and lower levels of Bcl-XL than the other groups, whereas this effect is reversed in the CD4-depleted groups given vPE16-IL-15.
We also confirmed the same process in vitro. Purified CD8+ T cells were stimulated with anti-CD3 in the presence or absence of IL-15, and then the levels of Bcl-XL and Bax were measured by Western blot analyses. CD8+ T cells activated with IL-15 expressed dramatically more Bcl-XL and less Bax (Fig. S4).
CD4+ T Cells Providing Help Induce APCs to Express IL-15.
We next asked whether IL-15 might be a natural mediator of such CD4+ T cell help. If so, then CD4+ T cells, which cannot make IL-15, might be able to induce APCs, such as DCs, to make IL-15.
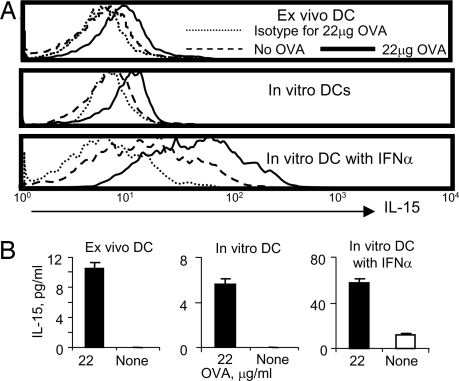
To test this hypothesis, we needed to use a population of uniformly antigen-specific helper T cells, and so we had to use TCR-transgenic CD4+ helper T cells. Thus, we turned to the OT-II TCR-transgenic mice specific for ovalbumin (OVA). Splenocytes from OT-II mice immunized with OVA were stimulated with OVA in vitro overnight, and then CD11c+ cells were stained with anti-IL-15 (Fig. 4A Top). DCs expressed membrane-bound IL-15 in an OVA-dependent manner at a level similar to that seen in the study in ref. 25, indicating that IL-15 detected in DCs is the result of antigen-specific interaction between CD4+ helper T cells and APCs. Likewise, purified bone-marrow-derived DCs pulsed with OVA and incubated with purified CD4+ T cells from OT-II mice also expressed IL-15 (Fig. 4A Middle), and the expression level of IL-15 was significantly enhanced in the presence of IFNα, which up-regulates IL-15Rα (Fig. 4A Bottom). As further confirmation of the ability of helper T cells responding to antigen to induce the APCs to secrete IL-15, culture supernatants from the experiments were analyzed for IL-15, and significant amounts of soluble IL-15 were also produced in all three groups only when the DCs were presenting the specific antigen (Fig. 4B). Overall, it is clear that CD4+ helper T cells deliver signals to APCs to induce production of IL-15.
Fig. 4.
CD4+ T cells induce DCs to produce IL-15. (A) OT-II class II MHC restricted OVA-TCR transgenic mice were immunized with OVA in adjuvant. Splenocytes (5 × 105 per well) were cultured in the presence or absence of 22 μg/ml of OVA for 16 h and stained with anti-CD11c and anti-IL-15 antibodies. IL-15 expression on CD11c positive cells was measured (Top). 5 × 104 per well in vitro-cultured DCs loaded with 22 μg/ml of OVA (or not) were cultured with purified 5 × 105 per well CD4+ T cells from OT-II transgenic mice without (Middle) or with (Bottom) 100 ng/ml IFNα. (B) IL-15 in the culture supernatants from the same three groups was measured by ELISA (bar graphs). Two repeated experiments showed similar results, and data in B represent mean ± SE.
IL-15 Production by DCs Presenting Antigen Is Also Necessary for Helper T Cells to Deliver Help Optimally.
We next asked whether IL-15 was indeed a natural mediator of help by determining whether such IL-15 production by DCs was also necessary for helper function in CD8+ T cell priming. To address this question, we turned to a different antigen system, because IL-15 −/− mice are available only on a C57BL/6 background. Therefore, we used the OVA immunodominant epitope SIINFEKL as our target antigen in such C57BL/6 mice. If help depends on the ability of helper T cells to induce DCs to make IL-15, then, if the DCs presenting antigen cannot make IL-15, even normal helper T cells in WT C57BL/6 mice should not be able to provide help. DCs from WT C57BL/6 or IL-15 −/− mice were pulsed with SIINFEKL and FCS as a source of helper epitopes, and WT C57BL/6 mice were then immunized with these peptide-loaded WT or knock-out DCs. Responses were measured in spleen cells on days 7 and 14 both by staining CD8+ T cells with H-2Kb-SIINFEKL tetramer (Fig. 5Left) and by lytic activity after a 1-week restimulation with antigen (Fig. 5 Right). By both measures, the mice immunized with IL-15−/− DCs manifested a slightly reduced response at day 7 (P < 0.03 in Fig. 5 Left, P < 0.05 for Fig. 5 Right), but a markedly reduced response on day 14 (P < 0.001 in Fig. 5 Left, P < 0.001 in Fig. 5 Right). Thus, the CD8+ T cells induced in vivo in the absence of IL-15 production by the DCs presenting antigen were comparatively short-lived, despite the availability of normal helper T cells in these undepleted mice (as confirmed by the response using the WT DC). These results support the conclusions that IL-15 produced by the DCs presenting antigen is important for induction of long-lived CD8+ T cells and that one important role of CD4+ helper T cells in the induction of robust CD8+ T cell responses is to induce IL-15 production by APCs. Although it was not feasible to include CD4-depleted mice to compare quantitatively, the qualitative effect of using IL-15−/− DCs is similar to that observed in the experiments above in CD4-depleted mice. To exclude critical biological differences between WT and IL-15−/− DCs other than IL-15 expression that could result in altered immune responses, we determined that bone-marrow-derived DCs from both strains produced similar levels of other cytokines, expressed similar levels of costimulatory molecules, and showed similar kinetics of activation (S.O., L.P., J.A.B., and T.A.W., unpublished data), in contrast to DCs studied ex vivo from IL-15−/− mice (26). Thus, overall, this study closes the circle and shows that IL-15 is not only sufficient to substitute for help but is also necessary for optimal help. Therefore, IL-15 appears to be at least one of the natural mediators of such help to induce CD8+ T cells, without which help is suboptimal.
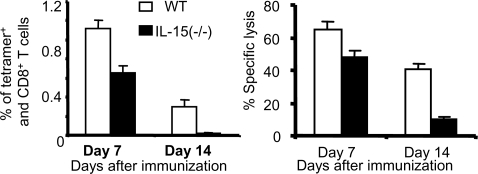
Fig. 5.
The ability of DCs presenting antigen to produce IL-15 is necessary for optimal delivery of CD4+ help to CD8+ T cells. Bone marrow-derived DCs from WT and IL-15−/− C57BL/6 mice were pulsed with SIINFEKL peptide (10 μM) and FCS as a source of helper epitopes and used to immunize WT C57BL/6 mice. On days 7 and 14 after priming, the frequency of peptide-specific CD8+ T cells in the pooled spleen of 3–5 mice was measured by tetramer staining (Left). CD8+ T cells at the same time points were also stimulated with syngeneic splenocytes pulsed with 0.001 μM peptide for 1 week, and lytic activity was measured against EL-4 target cells pulsed with 10 μM peptide in a 5h 51Cr release assay (Right).
Discussion
Discovering approaches to program CTL for improved survival and function in the absence of CD4+ help is critical for developing therapeutic vaccines for patients defective in CD4+ help, such as HIV-infected or cancer patients. We previously found that IL-15 during priming of immunocompetent mice results in long-lasting high avidity CD8+ CTL (5, 16). We now hypothesized that one mechanism by which CD4+ helper cells may induce such greater CTL longevity is by inducing IL-15 expression by the same APC that is presenting antigen to the CD8+ CTL. Therefore, we also hypothesized that in CD4-deficient animals, supplementing IL-15 by the vaccine vector might also allow CD8+ T cells to expand efficiently during the secondary responses, overcoming the defect of “helpless” CD8+ T cells. Here, we confirmed our hypothesis by showing that IL-15 during the priming could substitute for help inducing CTL persisting similarly to those induced in the presence of CD4+ help. Most CTL induced with vPE16 in CD4-depleted animals disappeared one month after the immunization, but CTL induced with vPE16/IL-15 in similarly CD4-depleted animals persisted a year, like CTL induced with vPE16 in the presence of CD4+ help. This was demonstrated both by the absolute number of antigen-specific CD8+ CTL and by the in vitro and in vivo functional activity of the CTL, including the protection in vivo against growth of tumors. Thus, IL-15 is sufficient even in the absence of CD4+ helper T cells to induce long-lived CD8+ CTL and can substitute for T help.
IL-15 induces a mechanism similar to help. Although CTL induced in the absence of CD4+ helper T cells could respond to the antigen and proliferate to a certain degree at least by day 7 after priming (Fig. 1B and Fig. S1), by day 24, most CTL induced with vPE16 in CD4-depleted animals were unable to proliferate on secondary antigen stimulation and underwent TRAIL-mediated apoptosis unless IL-15 was provided at priming. Thus, IL-15 is sufficient to substitute for CD4+ help to prevent TRAIL-mediated apoptosis on secondary stimulation. Further, IL-15 production by DCs during priming is also necessary for optimal induction of long-lived CD8+ T cells even in the presence of help. Thus, because helper cells do induce production of both secreted and membrane-bound IL-15 by APCs presenting antigen (Fig. 4 A and B), and this IL-15 is both necessary and sufficient to replace CD4+ T cell help, it is likely that IL-15 is one of the critical natural mediators of this helper effect, although other cytokines may contribute as well (27).
We were not able to examine whether IL-15 is absolutely necessary for help by using IL-15 knockout mice in vivo because of the concern that both CD8+ T cells and DCs developing from early ontogeny in the absence of IL-15 may not behave normally (26). Nevertheless, our conclusions are in part consistent with and supported by studies employing IL-15−/− and IL-15Rα−/− animals (10, 28). Schluns et al. (10) found that generation of memory CD8+ T cells in response to VSV infection was substantially diminished in IL-15−/− or IL-15Rα−/− mice, although a small population of IL-15-independent memory cells persisted. Likewise, Wherry et al. (28) found that both knockout animals infected with LCMV had a somewhat weaker initial response and then a more rapid attrition of antigen-specific CD8+ T cells (compare with Fig. 1). In those studies, the attrition was not as rapid or as complete as we observe in CD4-depleted mice, possibly because, in IL-15−/− mice that lack IL-15 from before birth, the CD8+ T cells still present had to learn to survive using other cytokines that may substitute for IL-15, including possibly IL-7 or IL-2. Both IL-15−/− and IL-15Rα−/− mice that lack IL-15 or its receptor throughout ontogeny may also have internal differences in both T cells and APCs from the WT animals used in this study (26). However, those studies did not examine secondary stimulation, so it is not clear whether such T cells would have undergone TRAIL-mediated apoptosis if restimulated.
There has been no previous evidence demonstrating that IL-15 is a major mediator of CD4+ help in determining the long-term fate of CTL, although a recent study showed that IL-15 could increase the magnitude of the short-term CTL response in the partial absence of CD4+ help (29). Although consistent with our findings, this study did not examine the ability of IL-15 to induce long-lived CD8+ T cell memory in the absence of CD4 help or to resist TRAIL-mediated death. However, a number of studies have suggested that the interaction between CD40 on APCs and CD40L on CD4+ T cells plays a central role in mediating CD4+ T cell function in the provision of help for CD8+ T cells (30–32). Furthermore, CD40 ligation on DCs induces IL-15 production (33) (S.O., L.P., J.A.B., and T.A.W., unpublished data). These results all suggest that helper T cells induce APCs to make IL-15, but, to confirm that they can do so, we have now tested this directly by showing that APCs presenting antigen to antigen-specific helper T cells are induced to express both membrane and secreted IL-15 in an antigen-dependent manner (Fig. 4).
Besides just up-regulation of TRAIL expression, levels of Bax and Bcl-XL also correlated with avoidance of TRAIL-mediated death and may play a critical role. Inverse changes in these two molecules may contribute synergistically to the survival of CTL during their activation and may complement the decrease in TRAIL expression afforded by CD4+ help or by IL-15. IL-15 can also block TNFα-mediated apoptosis by giving signals through IL-15Rα (34). Thus, in addition to expressing less TRAIL, CTL programmed with IL-15 are resistant to TRAIL-mediated apoptosis probably also because of increased Bcl-XL and decreased Bax during the secondary response.
Overall, we conclude that CTL induced with IL-15 are programmed for greater longevity and to respond efficiently to the secondary challenge without undergoing TRAIL-mediated apoptosis. IL-15 is both necessary and sufficient for optimal CD4+ help and operates through a similar mechanism. These results may provide a plausible mechanistic basis for the requirement for CD4+ T cell help to induce long-lasting CTL memory and suggest new vaccine strategies against viral infections and cancer, particularly in individuals deficient in CD4+ T cell numbers or function, such as during HIV infection or after some chemotherapy.
Materials and Methods
Viruses, Animals, and Immunization.
Recombinant vaccinia viruses, vPE16 expressing HIVgp160, vPE16-IL-15 expressing HIVgp160, and IL-15 were described in refs. 5 and 35. A recombinant vaccinia virus expressing Her2/neu and IL-15 was generated by standard procedures, using pSC11 (36).
Female BALB/c and C57BL/6 (Animal Production Colonies, Frederick Cancer Research Facility, National Institutes of Health, Frederick, MD) and IL-15−/− (The Jackson Laboratory), OVA TCR Tg OT-II (provided by Fumi Miyagawa, National Cancer Institute, Bethesda, MD) mice were studied at 6–8 weeks of age under National Cancer Institute Animal Committee-approved protocols. Mice were immunized s.c. in the tail base with 2–4 × 106 pfu of the viruses. Animals were also immunized s.c. in the tail base with 1 × 105 DCs pulsed with peptide. CD4+ T cells were depleted by i.p. injection of 200 μg per mouse anti-CD4 antibody (GK1.5) daily for 4 days before immunization. Depletion was verified by FACScan (Becton Dickinson) analysis of peripheral blood cells to be >98% depleted and only 5–10% reappeared by 1 month (data not shown). OVA TCR Tg OT-II mice were immunized s.c. with 10 μg of OVA in incomplete Freund's adjuvant (Sigma).
Tumor Challenge.
CD4-depleted or undepleted BALB/c mice were immunized with the viruses. 2 × 105 15–12RM cells per mouse were injected i.v. 4 weeks after the immunization, and the number of tumor nodules in lungs was counted 21 days after the challenge.
Antibodies, Tetramer, and Flow Cytometry.
See reagents in SI Materials and Methods. For intracellular IFN-γ staining, cells were stained following the manufacturer's protocol (BD–PharMingen).
Western Blot Analysis.
Purified P18-I10-specific CD8+ T cells were lysed in M2 buffer [20 mM Tris (pH 7.0), 0.5% Nonidet P-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 20 mM β-glycerol phosphate, 1 mM sodium vanadate, and 1 μg/ml leupeptin). When tetramer-positive CD8+ T cells were diminished in numbers, cells from larger groups of mice were pooled to obtain the same number of cells for Western blot analysis. Cell lysate (50 μg) from each sample was fractionated by SDS/PAGE and analyzed by Western blot. The proteins were visualized by enhanced chemiluminescence (Amersham Biosciences) instructions.
Peptides, Media, and Cells.
P18-I10 (RGPGRAFVTI) and SIINFEKL peptides were synthesized by NeoMPS. P815, EL4, and 15–12RM cells were maintained in complete RPMI medium 1640 (5) with 100 μg/ml geneticin for 15–12RM cells. DCs were prepared by culturing bone marrow cells (1 × 106/ml) in the media containing GM-CSF (100 ng/ml) and IL-4 (50 ng/ml) (R&D Systems). On days 2 and 4, the same amounts of these cytokines were added.
Proliferation and CTL Assay.
To assay proliferation, positively purified (Miltenyi Biotec) spleen CD8+ T cells were stimulated with peptide-pulsed splenocytes, and [3H]thymidine uptake was measured as described in ref. 5. To block TRAIL-mediated CD8+ T cell death, 5 μg/ml DR5-Fc fusion protein (R&D Systems) was added. Alternatively, CD8+ T cells were labeled with CFSE and then stimulated. Cell proliferation was measured by FACS analysis. Lytic activity of CD8+ CTLs was measured by a 5-h 51Cr release assay as described in ref. 5.
Acknowledgments.
We thank Drs. Richard Hodes and Alfred Singer for critical reading of the manuscript and very helpful suggestions. This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801003105/DCSupplemental.
References
- 1.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 3.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8+ T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 4.Van Stipdonk MJ, et al. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 5.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 7.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 9.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 10.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 11.Goldrath AW, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker TC, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 15.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 16.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15R alpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugin AW, Flexner C, Moss B. Clearance of recombinant vaccinia virus expressing IL-2: role of local host immune responses. Cell Immunol. 1993;152:499–509. doi: 10.1006/cimm.1993.1307. [DOI] [PubMed] [Google Scholar]
- 18.Perera LP, Goldman CK, Waldmann TA. Comparative assessment of virulence of recombinant vaccinia viruses expressing IL-2 and IL-15 in immunodeficient mice. Proc Natl Acad Sci USA. 2001;98:5146–5151. doi: 10.1073/pnas.081080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badovinac VP, Messingham KA, Griffith TS, Harty JT. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 20.Schneider P, et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-kappaB. Immunity. 1997;7:831–836. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- 21.Cretney E, et al. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 22.Zhang XR, et al. Reciprocal expression of TRAIL and CD95L in Th1 and Th2 cells: Role of apoptosis in T helper subset differentiation. Cell Death Differ. 2003;10:203–210. doi: 10.1038/sj.cdd.4401138. [DOI] [PubMed] [Google Scholar]
- 23.LeBlanc H, et al. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 24.Almasan A, Ashkenazi A. Apo2L/TRAIL: Apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 25.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 26.Ohteki T, Suzue K, Maki C, Ota T, Koyasu S. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat Immunol. 2001;2:1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 27.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wherry EJ, et al. Homeostatic proliferation but not the generation of virus specific memory CD8 T cells is impaired in the absence of IL-15 or IL-15Ralpha. Adv Exp Med Biol. 2002;512:165–175. doi: 10.1007/978-1-4615-0757-4_22. [DOI] [PubMed] [Google Scholar]
- 29.Kutzler MA, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175:112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 30.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 31.Bennett SRM, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 32.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 33.Ostrowski MA, et al. The role of CD4(+) T cell help and CD40 ligand in the in vitro expansion of HIV-1-specific memory cytotoxic CD8(+) T cell responses. J Immunol. 2000;165:6133–6141. doi: 10.4049/jimmunol.165.11.6133. [DOI] [PubMed] [Google Scholar]
- 34.Bulfone-Pau SS, et al. Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. FASEB J. 1999;13:1575–1585. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- 35.Earl PL, Hugin AW, Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990;64:2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: Coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]