Abstract
This study was conducted to investigate whether the dose of estradiol (E) administered acutely, or chronic delivery of one dose of E impacts acquisition and subsequent cocaine self-administration in ovariectomized (OVX) female rats. Five groups of female rats were compared: OVX females treated with 0, 1, 2, or 5 µg 17 β-E, 30 min prior to the self-administration session, and OVX rats that received a 1.5 mg E pellet (designed to chronically release 25 µg E/d X 60 d) implanted 1 week before cocaine self-administration initiation. Rats were tested in 1 hr sessions on a FR1 schedule with the dose of cocaine increasing every week (testing occurred 5 day/wk; doses: 0.2, 0.3, 0.4, 0.5 and 0.75 mg/kg/infusion). We report that OVX rats treated with 2 µg E acquired self-administration more rapidly than all of the other groups, and animals that received 1 or 2 µg E self-administered significantly more cocaine compared to OVX+vehicle at 0.3 and 0.4 mg/kg/infusion. In contrast, OVX rats given 5 µg E acutely, or chronic E via slow-release pellets did not take more cocaine than the OVX+vehicle group at any time point. Physiological serum concentrations of E were seen with 1 or 2 µg E, but 5 µg E and the E pellet produced supra-physiological concentrations. These results suggest an inverted U-shaped dose-response curve for the effect of E on acquisition of cocaine self-administration.
Keywords: acute estradiol, chronic estradiol, drug abuse, psychomotor stimulants, sex differences
1. Introduction
Cocaine abuse by women has increased rapidly in the last decade, and it is estimated that approximately 600,000 of the 2 million estimated cocaine users are now women (Substance Abuse and Mental Health Service Administration; SAMHSA, 2003). Women are more likely to use cocaine at an earlier age and with a greater frequency (Lynch et al., 2002; Johnston et al., 2006). After their first use of cocaine, women tend to take less time to become addicted, they enter treatment sooner and when they enter treatment their cocaine habit is more severe than is usually seen in men seeking treatment (Griffin et al., 1989; Kosten et al., 1993; Mendelson et al., 1999; Carroll et al., 2004). Women also reported stronger cravings in response to cocaine cues than do men (Robbins et al., 1999), and the hormones of the menstrual cycle modulate the subjective value of psychomotor stimulants (Justice et al., 1999; Justice et al., 2000).
Similar to women, female rats are more sensitive to the psychomotor activating effects of psychostimulants than are males. Female rats show greater behavioral sensitization after repeated cocaine injections than do male rats (Glick et al., 1984; van Haaren et al., 1991; Chin et al., 2002; Hu et al., 2003). They also acquire cocaine self-administration more readily than male rats (Lynch et al., 1999; Lynch et al., 2001; Carroll et al., 2002; Hu et al., 2004).
Estradiol (E) is thought to modulate the reinforcing effects of cocaine (Roberts et al., 1989; Grimm et al., 1997; Lynch et al., 2000; Lynch et al., 2001; Carroll et al., 2002; Hu et al., 2004; Jackson et al., 2006). For example, female rats will work harder for cocaine during estrus than during other phases of the estrous cycle (Roberts et al., 1989; Carroll et al., 2002) and E treatment given to ovariectomized (OVX) rats enhances the motivation to self-administer cocaine (Becker et al., 2007). When various doses of cocaine are available, female rats choose higher doses of cocaine during estrus than during other phases of the estrous cycle (Lynch et al., 2000). OVX females given E replacement acquire cocaine self-administration faster, and at lower doses of cocaine, than do OVX females or male rats treated with vehicle (Hu et al., 2004). Finally, E does not enhance cocaine self-administration in male rats (Jackson et al., 2006).
In most previous studies, only a single dose of E has typically been used, and the doses of E used by various laboratories can be quite different. The current study was conducted to investigate whether the dose of E given acutely vs. chronic E delivery impacts acquisition of cocaine self-administration and subsequent cocaine taking behavior in OVX female rats.
2. Materials and Methods
2.1. Subjects
Adult female Sprague-Dawley rats weighed 250–300 g at the beginning of the experiment (Harlan Sprague-Dawley, Indianapolis, IN). Animals were housed individually under a 14:10 reverse light:dark cycle at a constant temperature of 20–21°C, and received a phytoestrogen-free rodent chow (2014 Teklad Global 14% protein rodent maintenance diet, Harlan Teklad, Madison, WI); carbon filtered tap water (to remove environmental contaminants) was continuously available. All the procedures were carried out according to a protocol approved by the University of Michigan Use and Care of Animals Committee.
2.2. Surgery
Approximately 1 week after arrival, all rats were ovariectomized (OVX) under isoflurane anesthesia as described previously (Hu et al., 2003). Four days later animals underwent daily vaginal lavage for 10 consecutive days to examine the cells of the vaginal epithelium and determine if there had been cessation of the ovarian cycle. All animals were confirmed to have complete ovariectomy.
Approximately 2–3 weeks after OVX surgery, rats were prepared with indwelling intravenous jugular catheters connected to a back port. Jugular catheter construction and implantation were based on previously described procedures (Weeks, 1972; Kalivas et al., 1993; Crombag et al., 2000). Briefly, catheters were constructed by gluing silastic tubing (0.51 mm ID, 0.94 mm OD; Dow Corning, Midland, MI.) to external guide cannulae. The cannulae were then glued to polypropylene mesh with cranioplastic cement. Animals were anesthetized with combination of ketamine (45 mg/kg, i.p.) and medetomidine hydrochloride (0.3 mg/kg, i.p.). The free end of the silastic tubing was inserted into the right jugular vein and secured with 4.0 silk sutures around the venous tissue. The catheters exited dorsally on the animal’s back. Dummy stylets were inserted into the catheters when rats were not connected to infusion pumps. Catheters were flushed twice daily with 0.1 ml heparinized saline (30 U/ml, in sterile saline buffered at pH=7.4) to prevent occlusions, and 0.1 ml gentamicin (0.08 mg/ml) to circumvent microbial buildup in the catheter. For each self-administration session, catheters were flushed with 0.1 ml of saline before sessions began and with 0.08 ml gentamicin after each session.
2.3. Self-administration
Five days after surgery rats were placed into standard operant chambers (Med Associates, Inc., Georgia, VT) and were allowed to nose poke to obtain an i.v. infusion of cocaine on a Fixed Ratio (FR)1 schedule of reinforcement during a 1-h session each day. Animals were not pretrained to nose poke or given cocaine non-contingently during the session. Animals were connected to an infusion syringe and tethered via a steel cable to a swivel, which was mounted on a counterbalanced arm. This allowed the animal to move freely in the test cage. Each response in the active hole produced a compound stimulus consisting of a white stimulus light, a tone (85 dB), and an intravenous injection of 50 µl of cocaine HCl in saline delivered over 2.8 s. The compound stimulus occurred simultaneously with the cocaine injection and was followed by a 5 s time out period, during which time further responses had no programmed consequences but nose pokes were recorded. Nose pokes in the inactive hole were also recorded, but had no programmed consequences.
As described previously (Hu et al., 2004), on the scheduled testing day, animals received 0.1 ml of peanut oil (OVX group and OVX+E Pellet group) or 17 β-estradiol (E) treatment in 0.1 ml peanut oil (s.c.) 30 min before the self-administration testing session (doses are given below for the acute E treatment group). Daily 1-hour sessions of self-administration testing were given for five consecutive days followed by two days off for five weeks. Hormones were not administered during the two days off. The two days without treatment was designed to prevent dopamine receptor super sensitivity, which develops with chronic elevation of serum E (Di Paolo et al., 1981).
Responses at the 'active' hole resulted in the administration of the following doses of cocaine HCl (mg/kg/infusion): week #1 - 0.2; week #2 - 0.3; week #3 - 0.4; week #4 - 0.5; and week #5 - 0.75. An animal could receive a maximum of 50 infusions on any test day. This procedure allowed us to determine the effect of dose on the acquisition of cocaine self-administration and subsequent cocaine taking behavior as a function of treatment condition. At the beginning and end of each week, the catheters were tested for patency by injecting intravenously 0.1 ml of the short-acting Pentothal (thiopental sodium, 20 mg/ml in sterile water). Rats that did not become ataxic within 5 s were excluded from the analysis.
Five groups of rats were given the opportunity to self-administer cocaine: (1) OVX + vehicle (OVX; N=11); (2) OVX+ 1 µg 17β-estradiol (OVX+1 µg E; N=14); (3) OVX+ 2 µg 17β-estradiol (OVX+2 µg E; N=13); (4) OVX+5 µg 17β-estradiol (OVX+5 µg E; N=14); 5) OVX+ 1.5 mg 17β-estradiol pellet (OVX+E pellet; N=12). The pellet was implanted in the rat (s.c.) 1 week before self-administration testing, and was designed to chronically release 25 µg E /day × 60 days (Innovative Research of America, Sarasota, Florida). Release rate was empirically evaluated (see below).
Laboratories use a variety of criteria for defining acquisition of drug self-administration. Based on previous work from this laboratory, acquisition was defined as three consecutive sessions in which the response in the active hole for was 2X greater than the mean responding on the inactive hole for all groups during the first week (which was determined to be 6 nose pokes). Thus, if a rat attained 12 infusions per day for three consecutive test sessions, it was considered to have acquired self-administration behavior. The day of acquisition was considered to be the first day of the 3 consecutive days an animal met the criterion.
2.4. Serum E analysis
Serum E concentrations were determined using a sensitive immunofluorimetric assay in separate groups of animals. Separate groups were used in order to be able to control the time from hormone administration to time of blood collection, and so that trunk blood could be collected during the time when self-administration was taking place. All rats were assigned to one of the following groups: OVX treated with Oil; 1 hr post-injection of 1µg E, 2 µg E or 5 µg E2; 24 hr post-injection of 1µg E, 2 µg E or 5 µg E2; 1 hr after daily injections, for 5 consecutive days, of 1µg E, 2 µg E or 5 µg E2; 24 hr after 5 consecutive daily injections of 1µg E, 2 µg E or 5 µg E2; 7 days after subcutaneous implant of a 1.5 mg E pellet (see table one). At the scheduled time, the rat was killed by decapitation, blood was collected by exsanguination, the blood was allowed to clot on ice, the serum was filtered and then frozen at −80° C. The 17β-E immunofluorimetric assay was developed on an ACS-180 system equipped with the manufacturer’s software and using E2-6 reagents supplied by Bayer Diagnostics as described previously (England et al., 2002). The reporting range for the assay is 1–200 pg/mL. The ACS Estradiol-6 Master Curve standards are manufactured and evaluated by GC-MS. We report the following: Inter-assay coefficient of variation: 13.78%, Intra-assay coefficient of variation: 8.51%. All E2 samples were measured at the same time, in the same assay.
Table 1.
Serum concentrations of E (pg/ml, mean ± SEM)) after hormone administration
| Group: | 1 µg | 2 µg | 5 µg |
|---|---|---|---|
| 1 hr after 1 injection1 | 244.7±51.7* N=4 | 407.5±58.5* N=5 | 1146.2±107.8*** N=5 |
| 24 hr. after 1 injection | 55.8±8.0 N=4 | 35.3±4.2 N=5 | 73.5±14.7 N=4 |
| 1 hr. after 5 consecutive daily injections | 265.9±30.9* N=4 | 577.9±35.1** N=5 | 1025.0±99.8*** N=5 |
| 24 hr. after 5 consecutive daily injections | 42.0±5.0 N=5 | 56.1±3.9 N=4 | 102.4±23 N=4 |
| OVX | 36.7±3.0 (N=5) | ||
| E pellet | 1541.5±156.5* (N=4) |
Significantly greater than serum E in OVX rats (p<0.05).
Significantly greater than serum E in OVX rats and rats that received 1 µg of E (p<0.05).
Significantly greater than serum E in OVX rats and rats that received 1 or 2 µg of E (p<0.05).
Results at 1 hour reported in Yang et al (Yang et al., 2007) and repeated here for completeness.
2.5. Statistical Analyses
All data were analyzed using the computer program Prism 4 for the Macintosh. Comparisons were made by analysis of variance (ANOVA) with repeated measures and subsequent post-hoc comparisons with Bonferroni’s Multiple Comparison test or Fisher’s Least Significant Difference Test. Acquisition of self-administration was evaluated by survival curve testing and chi-square analysis. Data were deemed significant when p<0.05.
3. Results
3.1. Serum E concentrations
As shown in Table 1, the injections of E induced a dose-dependent increase in serum E concentrations. The serum E concentrations achieved 1 hour after one s.c. injection with 1 or 2 µg E were in the physiological range for the proestrus rat (Butcher et al., 1974; Henderson et al., 1977a; Henderson et al., 1977b), and returned to OVX levels within 24 hours. There was no difference in the serum E concentration seen one hour after the fifth injection of 1 or 2 µg E (over five consecutive days) when compared with serum values after one injection (Table 1). After one or five consecutive injections of 5 µg E, the serum concentrations of E were significantly higher than the physiological levels for E in the proestrus rat (Table 1). Furthermore, 24 hours post-injection of 5 µg E, serum concentrations were significantly greater than seen for OVX animals (p<0.05), suggesting that there was an accumulation of E with this dose of E, although this could also be due to variability across individual animals. In OVX rats that received a 1.5 mg E pellet implanted 1 week before self-administration testing, the serum concentrations of E were 1541.5±156.5 pg/ml (see Table 1).
3.2. Effect of acute E on acquisition of cocaine self-administration
Acute E enhanced the acquisition of cocaine self-administration. Figure 1 shows the percentage of rats that met criteria for cocaine self administration in the 5 groups across the 25 testing sessions. Survival curve testing was conducted for data across the first three weeks (after which all but eight animals had acquired). There was a significant effect of E treatment on the percentage of rats that acquired cocaine self-administration (Chi square = 9.726, DF = 4, p=0.05). The OVX+2 µg E animals acquired cocaine self-administration in fewer days than the OVX (p<0.0058) and OVX+E pellet groups (p<0.0051); and there was a trend for the group to acquire faster than OVX+5 µg E (p<0.063). There were no significant differences among other groups in the day of acquisition.
Figure 1.
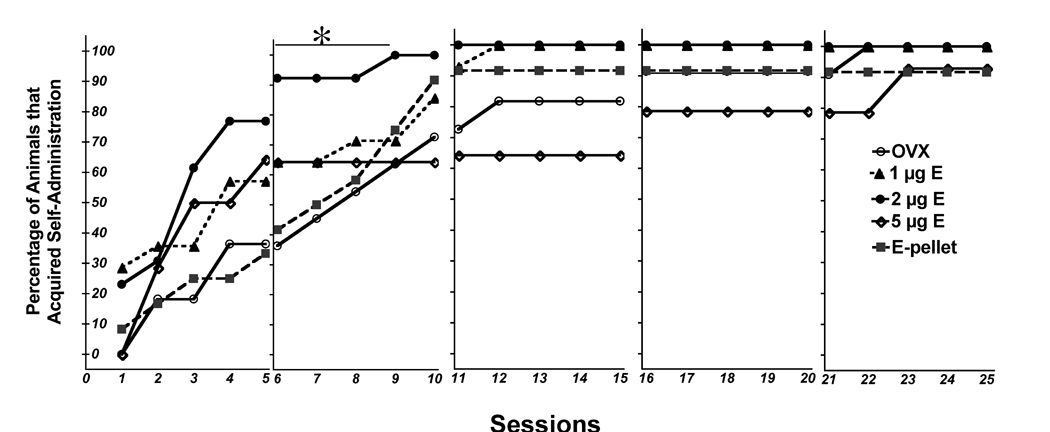
The percentage of animals that met criteria for acquisition of cocaine self-administration each day. OVX rats treated with estradiol or vehicle were tested 5 days a week for 5 weeks with increasing doses of cocaine each week.
Open circles - OVX; closed triangles - 1 µg E; closed circles - 2 µg E; open diamonds - 5 µg E; closed squares - E-pellet.
*There was a significant effect of 2 µg treatment on the percentage of rats that acquired self-administration during week 2 relative to the OVX and OVX+pellet groups (p<0.01).
Figure 2 provides a summary of the mean (+SEM) number of cocaine infusions for each group during each of the 5 weeks of testing. There was a main effect of treatment group, F4,16=10.55, p<0.0001; and an effect of dose of cocaine, F4,296=9.25, p<0.0001; with no interaction. The OVX group took fewer infusions at 0.2 mg/kg/inf than they did at 0.4 or 0.5 mg/kg/inf (p<0.05). At 0.3 mg/kg/inf the OVX group took fewer infusions than they did at 0.5 mg/kg/inf (p<0.05). The OVX+ 1 or 5 µg E groups did not differ in the number of infusions received throughout the 5 weeks of testing. The OVX+2 µg E group received more infusions when the dose was 0.3 mg/kg/inf than they did when the dose was 0.2 or 0.75 mg/kg/inf (p<0.05) and more infusions at 0.4 mg/kg/inf than with 0.75 mg/kg/inf (p<0.05). The E-pellet group received fewer infusions when the dose was 0.2 mg/kg/inf than when the dose was 0.4 or 0.5 mg/kg/inf (p<0.05). When groups were compared, the OVX+2 µg E group received more infusions than the OVX group at 0.3 mg/kg/inf (p<0.05).
Figure 2.
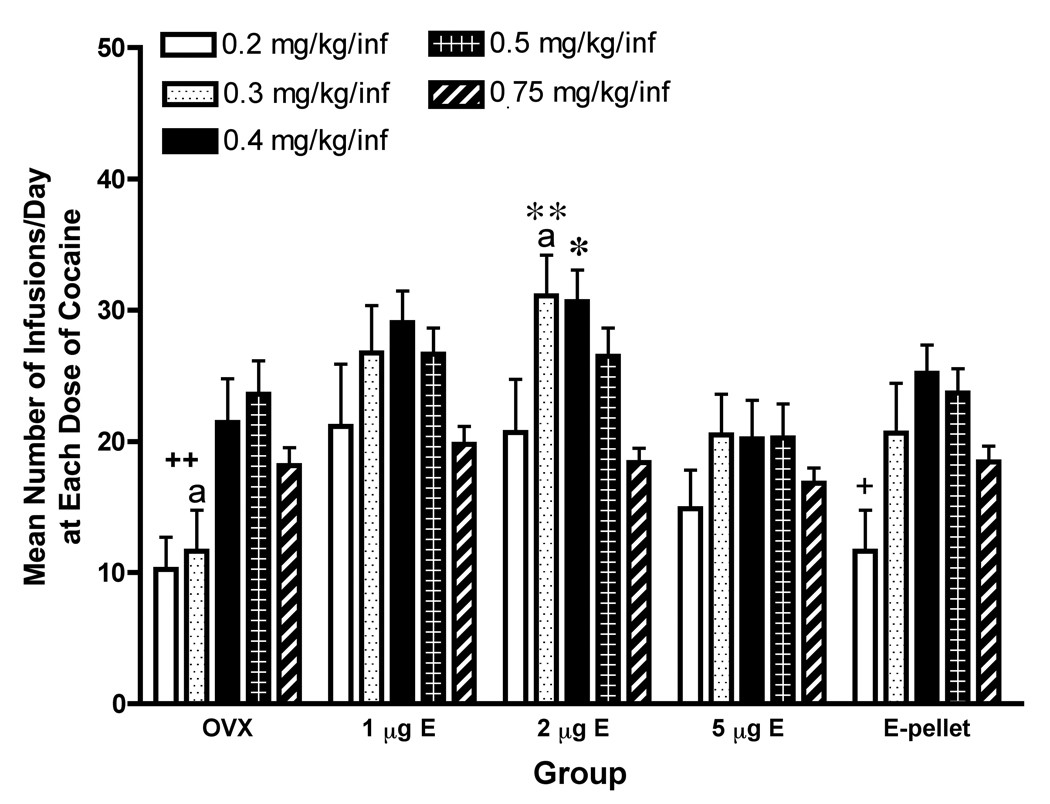
Mean number (+SEM) of infusions/day for each group/week. Bars represent different doses of cocaine; legend is on the figure.
++ The OVX group took fewer infusions at 0.2 than they did at 0.4 or 0.5 mg/kg/inf and fewer infusions at 0.3 than with 0.5 mg/kg/inf (p<0.05).
** The OVX+2µg E group received more infusions at 0.3 than with 0.2 or 0.75 mg/kg/inf (p<0.05).
* The OVX+2µg E group received more infusions at 0.4 than with 0.75 mg/kg/inf (p<0.05).
+ The OVX E-pellet group received fewer infusions at 0.2 than they did at 0.4 or 0.5 mg/kg/inf (p<0.05).
a – Indicates that these two groups are different (p<0.05).
3.3. Effect of acute E on total cocaine intake
There was a significant effect of dose of cocaine and dose of estradiol in a two-way ANOVA (effect of dose of estradiol F4,296=7.76, p<0.0001; effect of dose of cocaine F4,296=93.26, p<0.0001; no interaction). Daily cocaine intake (mg/kg) was analyzed for each dose of cocaine using an one-way ANOVA. At 0.2 mg/kg/infusion, animals were still acquiring cocaine self-administration and there was no effect of dose of estradiol. During the second week, when rats received 0.3 mg/kg/infusion of cocaine, there was a significant effect of hormone treatment (F4,59=2.52, p<0.05). Post-hoc pairwise comparisons indicated that the OVX control group received less cocaine than the OVX+1 µg E2 (p<0.05) or OVX+2 µg E (p<0.01) groups (Figure 3). During the third week, when rats received 0.4 mg/kg/infusion of cocaine, there was a significant effect of hormone treatment (F4,59=3.005, p=0.0252). Post-hoc pairwise comparisons indicated that OVX+2 µg E rats took more cocaine than OVX (p<0.05). OVX +1 µg E rats took more cocaine than OVX (p<0.05). There were no differences among the groups at 0.5 mg/kg/infusion or 0.75 mg/kg/infusion.
Figure 3.
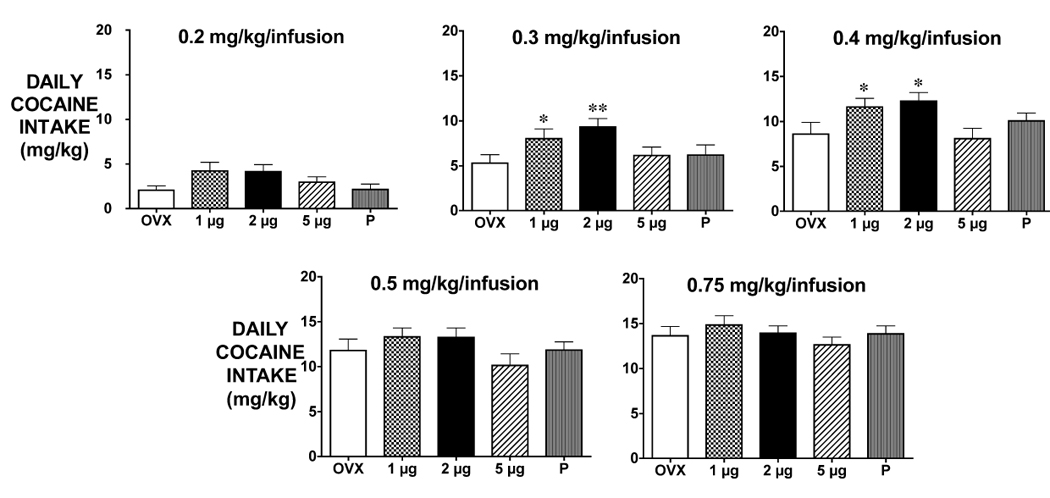
Mean cocaine intake (mg/kg) per day at each dose of cocaine (+SEM). There was a significant effect of E treatment on the total cocaine intake when rats received 0.3 and 0.4 mg/kg/infusion of cocaine; legend is on the figure.
* Indicates p<0.05 relative to OVX; ** indicates p<0.01 relative to OVX.
4. Discussion
In the present study, we replicated our earlier observation that acute E facilitates the acquisition of cocaine self-administration behavior in OVX rats (Hu et al., 2004). More importantly, we find that the effect of acute E on acquisition of cocaine self-administration has an inverted U-shaped dose-response curve with an apparent peak effect around 400 pg/ml E in serum (estimated from serum concentrations in separate groups of rats). In contrast, OVX rats with E pellets that deliver a high dose of E were not different from OVX. These results indicate that acute E enhances acquisition of cocaine-taking behavior in female rats, while chronic treatment with E, at least at this dose, does not.
When considering the data from the three figures together, it is apparent that the OVX group did not readily acquire self-administration at the 2 lower doses of cocaine (70% did not meet criteria for acquisition in week one at 0.2 mg/kg/inf, 30% did not meet criteria for acquisition in week two at 0.3 mg/kg/inf), and 20% of animals never met the criterion at any dose. Interesting, the animals that did not meet the criterion were exhibiting steady rates of nose pokes in the active hole, but at a low frequency. In the groups receiving 1 or 2 µg E, 100% of animals reach criteria for acquisition by early in week 3, and maintained responding at a steady rate thereafter. Conversely, in the 5 µg E and E-pellet groups not all animals met the criterion for acquisition of cocaine self-administration, but as was seen in the OVX animals some maintained a low rate of responding throughout testing. The differences among the groups reported here are primarily due to differences rates of responding for cocaine at 0.3 and 0.4 mg/kg/infusion, with physiological doses of E resulting in more avid responding than vehicle or high/chronic doses of E.
In many studies of the effects of E in OVX rats, serum concentrations of E are not reported. In the present study, plasma E concentrations after gonadectomy and E replacement were analyzed in different groups of rats. As expected, OVX animals had low serum concentrations of E, although the values reported here are somewhat higher than those obtained from radioimmunoassays conducted with commercial kits [e.g., (Stupka et al., 2001; Walker et al., 2001). Serum E concentrations produced by the 2 lower doses of E used in this study were within the high physiological range for E in the proestrus rat (Butcher et al., 1974), although there is considerable variability in the values reported for proestrus when only one or two time points are taken (Walker et al., 2001; Haim et al., 2003). The differences in values may be due to different strains of rat used, light cycle differences, or assay differences.
Commercially produced E pellets or 5 µg E resulted in higher E levels in serum than would be considered to be physiological, and serum concentrations were elevated for prolonged periods of time. The fact that the commercial pellets produced such high serum E concentrations one week after implantation suggests that the release of E from these pellets does not reach a low physiological steady state within 48 hours as indicated by the manufacturer. A high rate of E release from these pellets during the first 2 weeks has been reported by others (Haim et al., 2003; Theodorsson et al., 2005), so caution is needed when using and evaluating the effect of these pellets.
In previous studies, chronic elevation of E has been reported to have no effect or even to attenuate cocaine self-administration maintained under fixed ratio or progressive ratio schedules of reinforcement (Grimm et al., 1997). The results presented here may help to reconcile reports that report E facilitates cocaine self-administration with results that failed to find an enhancement of self-administration after treatment with high doses of E or chronic E treatment.
The effect of E on acquisition of cocaine self-administration may be mediated by its well-characterized actions on dopamine (DA) neurotransmission (Becker et al., 1981; Di Paolo et al., 1985; Becker et al., 1986; Becker, 1990b, a; Di Paolo, 1994; Thompson et al., 1995; Xiao et al., 1997; Becker et al., 1999; Becker et al., 2001). Acute E increases stimulated DA release in striatum and nucleus accumbens (Becker, 1990b, a; Becker et al., 1999; Becker et al., 2001) and enhances DA reuptake in nucleus accumbens (Thompson et al., 1995). Importantly, there is an inverted U-shaped curve dose-response for the effect of E on amphetamine-stimulated DA release in vitro, where physiological doses enhance DA release and supra-physiological doses do not (Becker, 1990a). Given that these mesotelencephalic dopamine systems are thought to mediate psychostimulant-induced behavioral sensitization and reward (Wise, 1987; Robinson et al., 2000), it is likely that the ability of E to facilitate dopaminergic activity contributes to its enhancement of cocaine self administration.
In contrast, extremely high doses of E or chronic treatment with physiological doses of E have been shown to induce down regulation of presynaptic DA activity (Di Paolo et al., 1982; Di Paolo et al., 1983; Morissette et al., 1993a) and produce DA receptor supersensitivity (Hruska et al., 1980; Di Paolo et al., 1981; Hruska et al., 1982; Hruska, 1986; Morissette et al., 1992; Morissette et al., 1993b, a). These effects of chronic E on DA functional activity may have contributed to discrepant findings on the effects of E on cocaine self-administration.
In conclusion, the effects of circulating E on cocaine self-administration behavior described here may be related to the growing evidence for sex differences in drug use and abuse in humans discussed above. Effects of circulating ovarian hormones may facilitate the acquisition of a cocaine habit for women. Incorporating considerations of gender and hormonal status is critical to designing appropriate prevention, intervention, and treatment strategies for women.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990a;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990b;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2007 doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204:361–372. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: A microdialysis study. Pharmacology Biochemistry and Behavior. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Molenda H, Hummer DL. Annals of the New York Academy of Sciences. Vol. 937. 2001. Gender differences in the behavioral responses to cocaine and amphetamine Implications for mechanisms mediating gender differences in drug abuse; pp. 172–187. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, Prolactin, Progesterone and Estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Carroll M, Lynch W, Roth M, Morgan A, Cosgrove K. Sex and estrogen influence drug abuse. Trends in Pharmacological Sciences. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HBK, Burrell S, Lu D, Jenab S, Perrotti LI, Quinones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Research. 2002;945:123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behavioural Brain Research. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Poyet P, Labrie F. Effect of chronic estradiol and haloperidol treatment on striatal dopamine receptors. Eur J Pharmacol. 1981;73:105–106. doi: 10.1016/0014-2999(81)90153-9. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Rouillard C, Bedard P. 17 beta-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117:197–203. doi: 10.1016/0014-2999(85)90604-1. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Diagle M, Picard V, Barden N. Effect of acute and chronic 17 beta-estradiol treatment on serotonin and 5-hydroxyindole acetic acid content of discrete brain nuclei of ovariectomized rat. Exp Brain Res. 1983;51:73–76. doi: 10.1007/BF00236804. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Bedard PJ, Dupont A, Poyet P, Labrie F. Effects of estradiol on intact and denervated striatal dopamine receptors and on dopamine levels: a biochemical and behavioral study. Can J Physiol Pharmacol. 1982;60:350–357. doi: 10.1139/y82-050. [DOI] [PubMed] [Google Scholar]
- England B, Parsons G, Possley R, McConnell D, Midgley A. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clinical Chemistry. 2002;48:1584–1586. [PubMed] [Google Scholar]
- Glick SD, Hinds PA. Sex differences in sensitization to cocaine-induced rotation. Eur J Pharmacol. 1984;99:119–121. doi: 10.1016/0014-2999(84)90442-4. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Lange U. A comparison of male and female cocaine abuse. Arch Gen Psychiatry. 1989;46:122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiology & Behavior. 1997;61:755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- Haim S, Shakhar G, Rossene E, Taylor AN, Ben-Eliyahu S. Serum levels of sex hormones and corticosterone throughout 4- and 5-day estrous cycles in Fisher 344 rats and their simulation in ovariectomized females. J Endocrinol Invest. 2003;26:1013–1022. doi: 10.1007/BF03348201. [DOI] [PubMed] [Google Scholar]
- Henderson SR, Baker C, Fink G. Oestradiol-17β and pituitary responsiveness to luteinizing hormone releasing factor in the rat: a study using rectangular pulses of estradiol-17β monitored by non-chromatographic radioimmunoassay. J Endocrinol. 1977a;73:441–453. doi: 10.1677/joe.0.0730441. [DOI] [PubMed] [Google Scholar]
- Henderson SR, Baker C, Fink G. Effect of oestradiol-17beta exposure on the spontaneous secretion of gonadotrophins in chronically gonadectomized rats. J Endocrinol. 1977b;73:455–462. doi: 10.1677/joe.0.0730455. [DOI] [PubMed] [Google Scholar]
- Hruska RE. Elevation of striatal dopamine receptors by estrogen: dose and time studies. J Neurochem. 1986;47:1908–1915. doi: 10.1111/j.1471-4159.1986.tb13106.x. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Ludmer LM, Silbergeld EK. Characterization of the striatal dopamine receptor supersensitivity produced by estrogen treatment of male rats. Neuropharmacology. 1980;19:923–926. doi: 10.1016/0028-3908(80)90095-7. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Ludmer LM, Pitman KT, De Ryck M, Silbergeld EK. Effects of estrogen on striatal dopamine receptor function in male and female rats. Pharmacol Biochem Behav. 1982;16:285–291. doi: 10.1016/0091-3057(82)90162-9. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. (06-5883 NPN, ed) Bethesda, MD: National Institute on Drug Abuse; 2006. Monitoring the Future national survey results on drug use 1975–2005: Volume I, Secondary school students. [Google Scholar]
- Justice AJH, de Witt H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJH, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacology Biochemistry and Behavior. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time Course of Extracellular Dopamine and Behavioral Sensitization to Cocaine .1. Dopamine Axon Terminals. Journal of Neuroscience. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. Journal of Substance Abuse Treatment. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacology Biochemistry and Behavior. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J Neurochem. 1993a;60:1876–1883. doi: 10.1111/j.1471-4159.1993.tb13415.x. [DOI] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993b;58:16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- Morissette M, Garcia SL, Belanger A, Di Paolo T. Changes of rat striatal neuronal membrane morphology and steroid content during the estrous cycle. Neuroscience. 1992;49:893–902. doi: 10.1016/0306-4522(92)90365-9. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- SAMHSA. (Services DoHaH, ed) Substance Abuse & Mental Health Services Administration; 2003. Results of the 2003 National Survey on Drug Use & Health. [Google Scholar]
- Stupka N, Tiidus PM. Effects of ovariectomy and estrogen on ischemia-reperfusion injury in hindlimbs of female rats. J Applied Physiol. 2001;91:1828–1835. doi: 10.1152/jappl.2001.91.4.1828. [DOI] [PubMed] [Google Scholar]
- Theodorsson A, Hilke S, Rugarn O, Linghammar D, Theodorsson E. Serum concentrations of 17b.beta-estradiol in ovariectomized rats during two times six weeks crossover treatment by daily injections in comparison with slow-release pellets. Scandinavian Journal of Clinical and Laboratory Investigation. 2005;65:699–706. doi: 10.1080/00365510500375206. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. In vivo stimulated dopamine release in the nucleus accumbens: modulation by the prefrontal cortex. Brain Res. 1995;686:93–98. doi: 10.1016/0006-8993(95)00429-t. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer M. Sex differences in the locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Walker QD, Francis R, Cabassa J, Kuhn CM. Effect of ovarian hormones and estrous cycle on stimulation of the hypothalamo-pituitary-adrenal axis by cocaine. Pharmacology and Experimental Therapeutics. 2001;297:291–298. [PubMed] [Google Scholar]
- Weeks JR. Long-term intravenous infusions. In: Meyers RD, editor. Methods in Psychobiology. London: Academic Press; 1972. pp. 155–168. [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Steroid-specific effects of estrogen agonists and antagonists on amphetamine-induced striatal dopamine released from superfused striatal tissue. Soc Neurosci Abst. 1997;23:403. [Google Scholar]


