Abstract
The relaxing effect of extracellular ATP on renal glomeruli has been investigated by applying ATP and its analogues to suspensions of angiotensin II-precontracted rat renal glomeruli. Based on changes of glomerular [3H]inulin space (GIS) the relaxation of glomeruli was analysed in the presence of agonists: ATP, ADP, AMP, UTP, 2-methylthio-ATP (P2Y agonist), β,γ-methylene-ATP (P2X agonist) and adenosine.
ATP, 2-methylthio-ATP, ADP and UTP induced concentration-dependent relaxation whereas AMP, β,γ-methylene-ATP and adenosine had no effect. The rank order of relaxation potency was 2-methylthio-ATP > ATP > ADP > UTP.
An inhibitor of constitutive nitric oxide synthase (NOS), Nω-nitro-L-arginine (NNA) prevented the ATP-induced increased accumulation of L-citrulline and the relaxation effect of ATP. An inhibitor of the neuronal isoform of NOS, 7-nitroindazole, had no effect on the relaxation effect of ATP.
The relaxing effect of ATP was prevented in the presence of inhibitors of cyclic guanylyl cyclase: methylene blue (MB) and the more specific inhibitor 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ). ATP stimulated an accumulation of cGMP that was diminished in the presence of MB.
We indicated that extracellular ATP may relax the glomeruli via activation of P2Y receptors with the subsequent activation of the endothelial isoform of nitric oxide synthase and soluble guanylyl cyclase. We suggest that, based on the described mechanism, extracellular ATP may increase the filtration surface which, in turn, may influence the glomerular filtration rate.
Extracellular ATP plays an important role in the control of renal haemodynamics. It has been shown that extracellular ATP influences the function of renal vascular beds through activation of P2 receptors which are located on plasma membranes (Harvey, 1964; Inscho et al. 1992; Kugelgen et al. 1995; Rump et al. 1998). Among P2 purinoceptors two main groups are distinguished: a ligand-gated ion channel group, P2X, and a G protein-coupled receptor group, P2Y. Both groups have multiple subtypes (Ralevic & Burnstock, 1998). The effect of ATP action on vascular beds depends on the type of activated purinoceptors and their location. ATP interacts with P2X receptors located on smooth muscle cells resulting in vasoconstriction (Kugelgen et al. 1995) due to activated calcium entry into the cells (Inscho et al. 1995). However, activation of vascular P2Y receptors located on endothelial cells results in vasorelaxation (Ralevic & Burnstock, 1996; Yunping et al. 1997). This process is due to increased intracellular calcium concentration and activation of the constitutive nitric oxide synthase (NOS) and subsequent production of NO (Long & Stone, 1985; Marsden et al. 1990; Briner & Kern, 1994). The generated NO leads to accumulation of intracellular cGMP, a mediator of vasorelaxation. Moreover, ATP may induce NO-independent vasorelaxation through formation of arachidonic acid metabolites (Bolger et al. 1978; Carter et al. 1988; De Nucci et al. 1988).
Despite the number of reports concerning ATP action on the renal vascular system only limited information about its action on glomerular microvasculature (which takes part in forming a glomerular filter) is available. Due to the fact that P2Y receptors are located on the cells of the glomerulus, e.g. mesangial, smooth muscle-like cells (Pfeilschifter et al. 1990; Pavenstadt et al. 1993; Schulze-Lohoff et al. 1995) and endothelial cells (Briner & Kern, 1994), it is possible that extracellular ATP in glomeruli may change the surface of the filter which may be reflected by changes of glomerular filtration rate.
Taken together, it seems possible that extracellular ATP through activation of P2 receptors may change the intracapillary volume of glomeruli. Hence, it may influence glomerular haemodynamics and subsequently the glomerular filtration rate. Thus, our study analysed the effects of ATP and its analogues on isolated renal rat glomeruli precontracted with angiotensin II. We present data showing that ATP is a potent dilator compound of glomerular capillaries and that this effect is mediated via signalling pathway P2Y receptors-NOS activity-cGMP accumulation.
METHODS
Isolation of renal glomeruli
Glomeruli were isolated from the renal cortex of adult male Wistar rats (200-250 g). Rats were decapitated under ether anaesthesia and kidneys were removed and placed in ice-cold Dulbecco’s phosphate-buffered saline (PBS) containing (mM): 137 NaCl, 2.7 KCl, 8.1 Na2HPO4, 1.5 KH2PO4, 0.9 CaCl2, 0.49 MgCl2 and 5.6 glucose at pH 7.4. The renal capsule was removed and the cortex was minced with a razor blade to a paste-like consistency. The minced cortex was then squeezed through a graded nylon sieve (with pore size in sequence: 250, 120 and 70 μm). The final suspension consisted of decapsulated glomeruli devoid of afferent and efferent arterioles. The assessed tubule contamination was less then 5 %. The entire procedure was carried out in an ice bath and took no more than 1.5 h.
Determination of glomerular inulin space
Glomerular inulin space (GIS) was measured according to previously described methods (Savin & Terreros, 1981; Fujiwara et al. 1989) with our own modifications (Szczepa´nska-Konkel et al. 1991; Kalinowski et al. 1997). Briefly, about 2000 glomeruli were suspended in 200 μl ice-cold PBS containing 1 % bovine serum albumin. Samples were preincubated with 0.5 μCi [3H]inulin for 30 min at 37°C in a shaking water bath (1.7 Hz). Incubation was continued with 1 μM Ang II or solvent alone (PBS) for 5 min and then with nucleotides (ATP, ADP, AMP, UTP), ATP analogues (2-methylthio-ATP, β,γ-methylene-ATP) or adenosine for the indicated time. Some experiments were done in the presence of inhibitors of soluble guanylyl cyclase (50 μM methylene blue, 10 μM 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one) and nitric oxide synthase (100 μM Nω-nitro-L-arginine, 10 μM 7-nitroindazole). Moreover, one set of experiments was done in the presence of a P2 receptor antagonist, reactive blue 2 (30 μM). Reactions were terminated by centrifugation in the following manner: 200 μl of suspension was transferred to a microtube containing 100 μl of ice-cold silicone oil (Wecker Silicone) and centrifuged for 5 s at 5000 g. Glomeruli were spun through the oil, forming a pellet on the bottom of the tube with the incubation medium remaining behind. The tip of the microtube with the glomerular pellet was cut off and the contents were resuspended in 500 μl 0.3 % Triton X-100. Supernatant (20 μl) was taken from the medium above the oil and also transferred to the scintillation vial. After solubilization, the radioactivity of samples was measured in a liquid scintillation counter. Glomerular inulin space (GIS) of a single glomerulus was calculated as follows:
where [3H]pellet is measured in counts per minute (c.p.m.), and [3H]supernatant in counts per minute divided by picolitres. Each GIS determination was carried out in quadruplicate samples. The results are expressed as picolitres per glomerulus or as a percentage of basal GIS value (641 ± 21pl per glomerulus).
Determination of cGMP accumulation in isolated glomeruli
Glomeruli were suspended in PBS (9000/450 μl) and then preincubated with 3-isobutyl-1-methylxanthine (1 mM) for 30 min at 37°C in a shaking water bath (1.7 Hz). Incubation was initiated by addition of ATP to the suspension (final concentration, 1 μM). In other experiments, glomeruli were preincubated for 10 min with 50 μM methylene blue prior to the addition of ATP. Incubation was terminated after the indicated time by adding ice-cold HClO4 (final concentration, 5 %). After centrifugation, the supernatant was neutralized, immediately frozen and kept at -20°C for analysis. For determination of the protein concentration the pellet was dissolved in 0.2 n NaOH/0.2 % SDS. cGMP was determined in triplicate samples by radioimmunoassay using rabbit anti-cGMP antibodies and [8,5′-3H]cGMP as a radioligand (Lewko et al. 1997).
Determination of nitric oxide synthase activity
The activity of nitric oxide synthase (NOS) was determined by measuring the conversion of L-[3H]arginine to L-[3H]citrulline and the subsequent separation of these amino acids by anion exchange chromatography (Bredt & Snyder, 1989). Glomeruli were suspended in PBS (2000/500 μl) and preincubated in the absence and presence of Nω-nitro-L-arginine (100 μM) for 20 min (water bath at 37°C) with 1.5 μCi L-[3H]arginine (final concentration, 1 mM). Incubation was initiated by addition of ATP (final concentration, 100 μM) to the suspension and was stopped 30 s later by centrifugation (5 s, 5000 g) of the whole suspension in a microtube containing 100 μl of ice-cold silicone oil. The pellet was transferred to 1 ml of 1 M trichloroacetic acid. Samples were sonicated for 2 min and centrifuged (20 min, 10000 g). Supernatant (0.95 ml) was removed and extracted three times with 2 ml diethyl ether. Extract (0.5 ml) was neutralized with 2.0 ml 20 mM Hepes (pH 6.0) and the total volume was applied to a 2 ml column of pre-equilibrated Dowex AG 50W-X8 (Na+ form). The column was eluted with 4 ml of water; L-[3H]arginine was retained on the column whereas L-[3H]citrulline was recovered in the eluate. Using a mixture of L-[3H]arginine and L-[14C]citrulline, the amount of Dowex AG 50W-X8 used in the present determination completely trapped the L-[3H]arginine (Tack et al. 1997). Therefore, the radioactivity of the eluate consisted of only L-[3H]citrulline. The quantity of L-[3H]citrulline was determined by liquid scintillation counting.
Other analytical methods
Protein concentration was determined by the method of Lowry et al. (1951), using bovine serum albumin as a standard.
Materials
2-Methylthio-ATP (2-MeSATP), β,γ-methylene-ATP (β,γ-meATP) and 7-nitroindazole were purchased from RBI (Natick, MA, USA). [3H]Inulin and L-[3H]arginine were obtained from Du Pont NEN Products (Boston, MA, USA). All nucleotides were purchased from Boehringer (Mannheim, Germany). Angiotensin II, Nω-nitro-L-arginine (NNA), methylene blue (MB), 1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one (ODQ) and adenosine were obtained from Sigma (St Louis, MO, USA). Dowex AG 50WX-8 was obtained from Serva (Heidelberg, Germany) and reactive blue 2 from Aldrich Chemical Company Inc. (Milwaukee, USA). All other agents were purchased from POCh (Gliwice, Poland).
Statistics
Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Dunnett’s t test to determine significance. P values < 0.05 were considered to be significant.
RESULTS
Exposure of the isolated renal glomeruli to angiotensin II (Ang II) reduces the intracapillary volume of glomeruli. This process is reflected by a decreased glomerular inulin space, GIS. In our experiments (Fig. 1), 1 μM Ang II induced reduction of the basal GIS value (641 ± 21 pl per glomerulus) by about 16 ± 0.7 %. The reduction of the GIS did not change significantly during 10 min of incubation.
Figure 1. Time-dependent relaxing effect of ATP on Ang II-precontracted glomeruli.
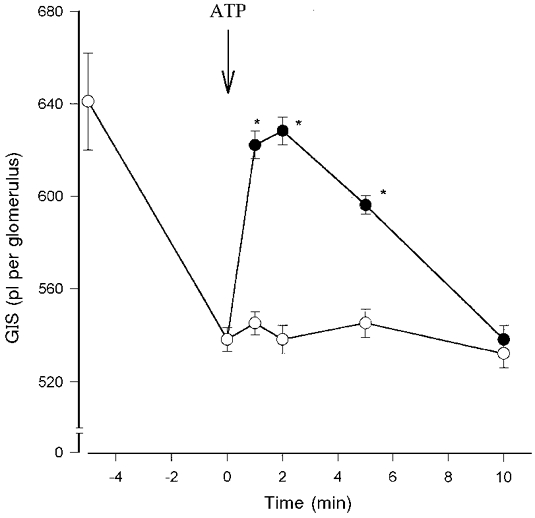
Glomeruli were preincubated with 1 μM angiotensin II (Ang II) for 5 min. Incubation was continued with (•) or without (^) 1 μM ATP for the indicated time. Results are expressed as mean values of GIS (pl per glomerulus). Data points are means ±s.e.m. (n = 7%); *P < 0.05 ATP + Ang II vs. Ang II alone (control).
As shown in Fig. 1, incubation of Ang II-precontracted glomeruli with 1 μM ATP generated transient relaxation of glomeruli. The GIS value of Ang II-precontracted glomeruli (539 ± 5 pl per glomerulus) in the presence of ATP increased to the basal value (622 ± 3 pl per glomerulus) in the 2nd minute of incubation and returned to the initial value (545 ± 3 pl per glomerulus) in the 10th minute of incubation. The relaxation of Ang II-precontracted glomeruli was dependent on ATP concentration with an EC50 of about 0.1 μM (Fig. 2). The maximal relaxation of glomeruli observed was at 1 μM and this was prevented by the presence of the P2Y receptor antagonist reactive blue 2 (30 μM). To provide additional confirmation that the relaxation effect of ATP was due to activation of P2Y receptors we used specific P2 receptor agonists: 2-MeSATP (P2Y receptor agonist) and β,γ-meATP (P2X receptor agonist), non-metabolized analogues of ATP. As shown in Fig. 2, 0.01 μM 2-MeSATP induced complete relaxation of the Ang II-precontracted glomeruli but 100 μM β,γ-meATP had no relaxing effect. Other nucleotides such as ADP and UTP also induced relaxation of Ang II-precontracted glomeruli in a concentration-dependent manner but AMP (100 μM) and adenosine (10 μM) had no relaxing effect.
Figure 2. Concentration-dependent relaxing effects of nucleotides, ATP analogues and adenosine on Ang II-precontracted glomeruli.

Glomeruli were preincubated with 1 μM angiotensin II (Ang II) for 5 min. Incubation was continued with various concentrations of nucleotides: ATP (•), ADP (^), 100 μM AMP (▴), UTP (▴), 0.01 μM 2-MeSATP (□), 100 μM β,γ-meATP (▪) or 10 μM adenosine (⋄) for 2 min. Results are expressed as a percentage of basal value. Data points are means ±s.e.m. (n = 6-8); *P < 0.05 nucleotides + Ang II vs. ineffective dose of nucleotides.
In order to investigate the involvement of the endothelial isoform of nitric oxide synthase (NOS) in glomerular relaxation induced by ATP, the change of GIS values was determined in the presence of Nω-nitro-L-arginine (NNA), a non-selective inhibitor of the constitutive nitric oxide synthases. As shown in Fig. 3, NNA (100 μM) prevented the relaxing effect of 1 μM ATP. In order to exclude the possible involvement of the neuronal isoform of nitric oxide synthase (nNOS) the relatively selective nNOS inhibitor 7-nitroindazole (7-NI) was used. ATP in the presence of 7-NI (10 μM) induced complete relaxation of the glomeruli (Fig. 3). Next, the effect of ATP on NOS activity on the basis of L-citrulline accumulation in the glomeruli was evaluated. As demonstrated in Fig. 4, ATP (100 μM) increased the level of L-citrulline accumulation from 6.9 ± 0.2 to 8.5 ± 0.3 pmol min−1 (mg protein)−1 and this effect was prevented in the presence of NNA (100 μM).
Figure 3. Effect of NOS inhibitors on ATP-induced relaxation of Ang II-precontracted glomeruli.

Glomeruli were preincubated with 100 μM Nω-nitro-L-arginine (NNA), 10 μM 7-nitroindazole (7-NI) or PBS (Con) for 30 min, followed by 1 μM Ang II for 5 min. Incubation was then continued with 1 μM ATP (filled bars) or PBS (open bars) for 2 min. Results are expressed as a percentage of basal value. Columns indicate means ±s.e.m. (n = 6%); *P < 0.05 ATP vs. PBS.
Figure 4. Effect of ATP on L-citrulline accumulation in the glomeruli in the presence of Nω-nitro-L-arginine.

Glomeruli were preincubated with 1 mM L-[3H]arginine and 100 μM Nω-nitro-L-arginine (NNA) or PBS (Con) for 30 min. Incubation was continued with 100 μM ATP (filled bars) or PBS (open bars) for 30 s. Columns indicate means ±s.e.m. (n = 4%); *P < 0.05 ATP vs. PBS.
To answer the question whether ATP induces relaxation of glomeruli in a cGMP-dependent manner the changes of GIS were measured in the presence of the inhibitors of soluble guanylyl cyclase methylene blue (MB) and 1H-[1,2,4]oxa diazolo-[4,3-a]quinoxalin-1-one (ODQ). As shown in Fig. 5, both MB (50 μM) and the more specific inhibitor ODQ (10 μM) completely prevented the relaxing effect of ATP. Moreover, MB reduced ATP-induced accumulation of cGMP in the glomeruli. ATP (1 μM) in the absence of MB increased the cGMP accumulation from a basal value of 4.3 ± 0.2 to 10.8 ± 0.7 pmol min−1 (mg protein)−1 but in the presence of MB only to 6.8 ± 1.1 pmol min−1 (mg protein)−1 (Fig. 6).
Figure 5. Effect of cyclic guanylyl cyclase inhibitors on ATP-induced relaxation of Ang II-precontracted glomeruli.
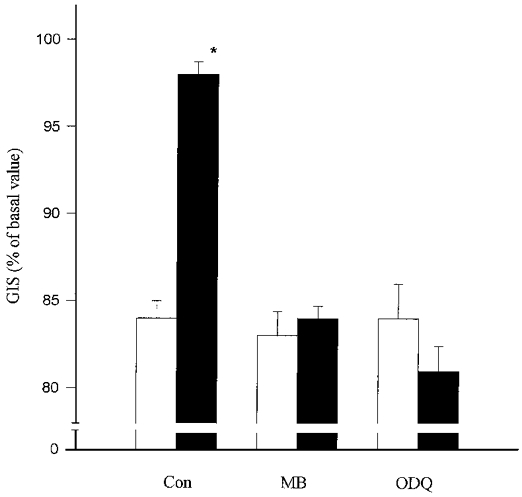
Glomeruli were preincubated with 50 μM methylene blue (MB), 10 μM ODQ or PBS (Con) for 20 min and with 1 μM Ang II for a further 5 min. Incubation was continued with 1 μM ATP (filled bars) or PBS (open bars) for 2 min. Results are expressed as a percentage of basal value. Columns indicate means ±s.e.m. (n = 5%); *P < 0.05 ATP vs. PBS.
Figure 6. Effect of ATP on cGMP accumulation in the glomeruli in the presence of methylene blue.
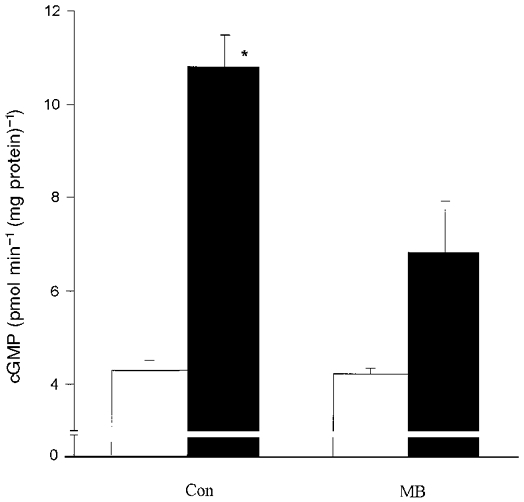
Glomeruli were preincubated with 50 μM methylene blue or PBS (Con) for 20 min. Incubation was continued with 1 μM ATP (filled bars) or PBS (open bars) for 1 min. Columns indicate means ±s.e.m. (n = 4%); *P < 0.05 ATP vs. ATP + MB.
These results suggest that ATP induces relaxation of isolated renal glomeruli via P2Y receptors and that this effect resulted from the increased activity of the endothelial isoform of nitric oxide synthase and the subsequent accumulation of cGMP in glomerular cells.
DISCUSSION
In the present study we have investigated the relaxation effect of extracellular ATP on angiotensin II-precontracted glomeruli. We analysed the relaxation and contraction of glomeruli by using [3H]inulin (the agent penetrating extracellular space only) and measuring the changes of glomerular inulin space (GIS). It has been shown that most of the extracellular space of decapsulated glomeruli is the intracapillary space (Savin & Terreros, 1981) and an increase or decrease of GIS reflects a relaxation or contraction of glomeruli, respectively (Arriba et al. 1988; Fujiwara et al. 1989). The isolated renal glomeruli suspended in buffer are fully relaxed and are spherical or ellipsoid in shape (Savin & Terreros, 1981). Thus, it is widely accepted that in vitro investigation of renal glomeruli relaxation, similar to blood vessels, is carried out only in the presence of a vasoconstrictor agent. In our experiments to investigate relaxation of glomeruli we preincubated the glomeruli with angiotensin II.
The basal GIS value of glomeruli isolated from rats weighing 200-250 g was calculated as 641 ± 21 pl per glomerulus. This value was comparable with others (Fujiwara et al. 1989) as well as our previous estimation (Szczepa´nska-Konkel et al. 1991; Kalinowski et al. 1997). However, GIS values found in the current experiments were smaller than those in one report (Savin & Terreros, 1981) but in which the body weight of rats was not indicated.
Our results show for the first time that rat renal glomeruli precontracted with angiotensin II underwent relaxation in the presence of nucleotides such as ATP, ADP and UTP, but not in the presence of AMP and adenosine. The effect of ATP was concentration dependent and transient with a maximum at 2 min. In the extracellular space ATP is rapidly catabolized apparently at the surface of the endothelium via ectonucleotidases to yield adenosine (Meghji et al. 1995) which may bind to specific receptors (Ralevic & Burnstock, 1998) and induce vasorelaxation. This mechanism of vasorelaxation, i.e. via adenosine as a ATP breakdown product, has been observed by Rump et al. (1998). They have presented data which show that extracellular ATP and adenosine produced very similar patterns of vasorelaxation of perfused rabbit and human renal arteries and that these effects were attenuated in the presence of antagonists of P1 receptors, i.e. 8-p-(sulphophenyl)theophylline (Rump et al. 1998). In our experiments neither adenosine nor the precursor of adenosine AMP relaxed glomeruli, which suggests that in rat renal glomeruli the dilatory effect of exogenous ATP mainly results from activation of P2 receptors.
The existence of P2 receptors, mainly P2Y, on glomerular endothelial cells (Briner & Kern, 1994; Pavenstadt et al. 1997), mesangial cells (Pfeilschifter et al. 1990; Schulze-Lohoff et al. 1995) and epithelial cells (Pavenstadt et al. 1992) has been confirmed pharmacologically. Using different analogues of ATP we tried to study whether P2Y receptors are involved in the relaxation of glomeruli. Earlier studies showed that 2-MeSATP and β,γ-meATP are most specific agonists of P2Y and P2X receptors, respectively (Dalziel & Westfall, 1994). Moreover UTP activates P2Y receptors (Ralevic & Burnstock, 1998). In the present studies we showed that both 2-MeSATP and UTP induced relaxation of the glomeruli whereas β,γ-meATP did not (Fig. 2). Furthermore, the antagonist of P2Y receptors reactive blue 2 blocks the effect of ATP. Thus, our results suggest that the relaxation effect of ATP on glomeruli is probably mediated via activation of P2Y receptors.
It is well documented that activation of P2Y receptors leads to an intracellular rise of [Ca2+] (Briner & Kern, 1994). An increase of [Ca2+]i leads to activation of nitric oxide synthase (NOS), both endothelial (eNOS) and neuronal (nNOS) isoforms of NOS and subsequently an increased production of NO (Long & Stone, 1985; Cattell & Cook, 1993; Hill-Kapturczak et al. 1995). To provide confirmation that relaxation of glomeruli is due to activation of NOS we used an inhibitor of this enzyme, Nω-nitro-L-arginine. As shown in Fig. 3, Nω-nitro-L-arginine prevents the relaxation effect of ATP. To exclude the involvement of nNOS, located in macula densa cells (Wilcox et al. 1992; Wu et al. 1999), in relaxation of glomeruli, we used a relatively selective nNOS inhibitor 7-nitroindazole, which did not show any influence on the relaxing effect of ATP (Fig. 3). Furthermore, in the present studies the activity of NOS has been determined by measuring the conversion of L-arginine to L-citrulline. Incubation of the glomeruli with ATP induced a significant increase of L-citrulline accumulation. In order to confirm that the L-arginine to L-citrulline conversion was due to NOS activity, we used the arginine analogue Nω-nitro-L-arginine, which has been demonstrated to have inhibitory action on NOS activity. As shown in Fig. 4, conversion of L-arginine to L-citrulline is inhibited by Nω-nitro-L-arginine. Additionally, we have shown that preincubation of the glomeruli with Nω-nitro-L-arginine induced a decrease of basal accumulation of L-citrulline. This observation is in agreement with another study (Wang et al. 1996) which clearly shows that NO is released from endothelial cells in culture even in the absence of any stimulation. We believe that ATP induces relaxation of Ang II-precontracted glomeruli via activation of eNOS with a subsequent increased production of NO in the glomeruli. The involvement of NO in ATP-induced relaxation of glomeruli was documented by experiments in which sodium nitroprusside (SNP), a donor of NO, caused a relaxation of Ang II-precontracted glomeruli (Lewko et al. 1997).
It has been shown that glomerular endothelial cells release a factor possessing the pharmacological properties of NO, which increases the cGMP level in the glomerular mesangial cell via activation of cytoplasmatic guanylyl cyclase (Marsden et al. 1990; Yuen et al. 1990). We have shown that accumulation of cGMP increases in a parallel manner to ATP-induced glomeruli relaxation. In the presence of methylene blue, an inhibitor of soluble guanylyl cyclase, the relaxing effect of ATP was abolished and the accumulation of cGMP was markedly reduced. Since methylene blue has been ascribed not only with the inhibition of guanylyl cyclase but also to the scavenging of NO (Mayer et al. 1993), we used the specific inhibitor of guanylyl cyclase, ODQ, which completely prevented the relaxing effect of ATP. These findings suggest that ATP induces relaxation of Ang II-precontracted glomeruli in a cGMP-dependent manner.
Mechanisms other than cGMP-dependent ones should be considered when analysing the relaxing effect of ATP. It has also been shown that activation of P2Y receptors located on mesangial cells leads to an increase of intracellular cAMP accumulation (Schulze-Lohoff et al. 1995). Moreover, the stimulation of cAMP accumulation in mesangial cells has been shown to inhibit contraction induced by vasoactive agents (Mene & Dunn, 1988). Therefore, it is possible that the relaxation of glomeruli in the presence of ATP is partially mediated by the ATP-induced cAMP increase in the glomerulus. Further studies are needed to determine the extent of cAMP-dependent relaxation with respect to ATP action.
There are several physiological conditions which may lead to release of ATP from the cytoplasm (concentration of ATP about 10−3 M) or intracellular stores (about 1 M ATP). Although ATP is hydrolysed quickly by ectonucleotidases the extracellular concentration of ATP may transiently increase to a level sufficient to activate P2 receptors. One of the large sources of nucleotides are blood cells: thrombocytes and erythrocytes (Goetz et al. 1971; Born & Kratzer, 1984; Gordon, 1986). Calculation indicates that, following complete degranulation of thrombocytes in response to aggregation agents, the serum concentration of ATP and ADP will transiently reach values in the 20-50 μM range (Dubyak et al. 1993). Moreover, changes in shape of erythrocytes during passage through capillary vessels leads to release of ATP (Sprague et al. 1996). Another physiological relevant source of extracellular ATP is neuronal endings (Burnstock, 1990). Finally, the release of ATP from different cells without compromising viability may be induced by auto/paracrine factors, e.g. bradykinin, serotonin (Yang et al. 1994), ATP per se (Bodin & Burnstock, 1996) or the vasoactive drugs (Capecchi et al. 1995). ATP which is released into the extracellular space may contribute to target cells and function via activation of purinoceptors (Motte et al. 1995). In the case of glomerular cells, which take part in forming the glomerular filter, the activation of P2 receptors may change glomerular blood flow and subsequently the glomerular filtration rate.
In summary, these studies have shown that extracellular ATP induces relaxation of glomeruli, an action occurring via activation of the P2Y receptor subtype. The activation of these receptors leads to an increased activity of eNOS and subsequently of soluble guanylyl cyclase. The present data raise important questions regarding the physiological importance of extracellular ATP in the regulation of glomerular volume. We suggest that extracellular ATP may antagonize the vasoconstrictor agents and thus may regulate the glomerular filtration rate.
Acknowledgments
This study was supported by the Polish Committee for Scientific Research (grant no. 4P05A 036 16). We acknowledge the help of Professor Paul Tomboulian (Department of Chemistry, Oakland University, Rochester, MI, USA) in reviewing the manuscript.
References
- Arriba LJ, Bario V, Olivera A, Rodriguez-Puyol D, Lopez-Novoa JM. Atrial natriuretic peptide inhibits angiotensin II-induced contraction of isolated glomeruli and cultured glomerular mesangial cells of rats: the role of calcium. Journal of Laboratory and Clinical Medicine. 1988;111:466–474. [PubMed] [Google Scholar]
- Bodin P, Burnstock G. ATP-stimulated release of ATP by human endothelial cells. Journal of Cardiovascular Pharmacology. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- Bolger PM, Eisner GM, Ramwell PW, Slotkoff LM. Renal actions of prostacyclin. Nature. 1978;271:467–469. doi: 10.1038/271467a0. [DOI] [PubMed] [Google Scholar]
- Born GVR, Kratzer MAA. Source and concentration of extracellular adenosine triphosphate during homeostasis in rats, rabbits and man. The Journal of Physiology. 1984;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proceedings of the National Academy of Sciences of the USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briner VA, Kern F. ATP stimulates Ca2+ mobilization by a nucleotide receptor in glomerular endothelial cells. American Journal of Physiology. 1994;266:F210–217. doi: 10.1152/ajprenal.1994.266.2.F210. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Noradrenaline and ATP as cotransmitters in sympathetic nerves. Neurochemistry International. 1990;17:357–368. doi: 10.1016/0197-0186(90)90158-p. [DOI] [PubMed] [Google Scholar]
- Capecchi PL, Pasini FL, Sodi N, Chiaventta M, Sensi S, De Lalla A, Volpi L, Di Perri T. Increase in plasma levels of adenosine and adenine nucleotides after intravenous infusion of buflomedil in humans. Journal of Cardiovascular Pharmacology. 1995;25:35–39. doi: 10.1097/00005344-199501000-00007. [DOI] [PubMed] [Google Scholar]
- Carter TD, Hallam TJ, Cusack NJ, Pearson JD. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. British Journal of Pharmacology. 1988;95:1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell V, Cook HT. Nitric oxide: Role in the physiology and pathology of the glomerulus. Experimental Nephrology. 1993;1:265–280. [PubMed] [Google Scholar]
- Dalziel HH, Westfall DP. Receptors for adenine nucleotides and nucleosides: Subclassification, distribution and molecular characterization. Pharmacological Reviews. 1994;46:449–466. [PubMed] [Google Scholar]
- De Nucci G, Gryglewski RJ, Warner TD, Vane JR. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proceedings of the National Academy of Sciences of the USA. 1988;85:2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. American Journal of Physiology. 1993;265:C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Kitamura E, Ueda N, Fukunaga M, Orita Y, Kamada T. Mechanism of action of angiotensin II on isolated rat glomeruli. Kidney International. 1989;36:985–991. doi: 10.1038/ki.1989.291. [DOI] [PubMed] [Google Scholar]
- Goetz U, Da Prada M, Pletscher A. Adenine-, guanine- and uridine-5′-phosphonucleotides in blood platelets and storage organelles of various species. Journal of Pharmacology and Experimental Therapeutics. 1971;178:210–215. [PubMed] [Google Scholar]
- Gordon JL. Extracellular ATP: effects, sources and fate. Biochemical Journal. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RB. Effects of adenosine triphosphate on autoregulation of renal blood flow and glomerular filtration rate. Circulation Research. 1964;14(suppl. I):178–182. [PubMed] [Google Scholar]
- Hill-Kapturczak N, Kapturczak MH, Malinski T, Gross P. Nitric oxide and nitric oxide synthase in the kidney: Potential roles in normal renal function and in renal dysfunction. Endothelium. 1995;3:253–299. [Google Scholar]
- Inscho EW, Ohishi K, Cook AK, Belott P, Navar LG. Calcium activation mechanism in the renal microvascular response to extracellular ATP. American Journal of Physiology. 1995;268:F876–884. doi: 10.1152/ajprenal.1995.268.5.F876. [DOI] [PubMed] [Google Scholar]
- Inscho EW, Ohishi K, Navar LG. Effects of ATP on pre- and postglomerular juxtamedullary microvasculature. American Journal of Physiology. 1992;263:F886–893. doi: 10.1152/ajprenal.1992.263.5.F886. [DOI] [PubMed] [Google Scholar]
- Kalinowski L, Szczepa´nska-Konkel M, Jankowski M, Angielski S. Modulation by low sodium intake of glomerular response to cicletanine and atrial natriuretic factor. British Journal of Pharmacology. 1997;121:635–642. doi: 10.1038/sj.bjp.0701160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelgen I, Krumme B, Schaible U, Schollmeyer PJ, Rump LC. Vasoconstrictor responses to the P2X-purinoceptor agonist β,γ-methylene-L-ATP in human cutaneous and renal blood vessels. British Journal of Pharmacology. 1995;116:1932–1936. doi: 10.1111/j.1476-5381.1995.tb16685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewko B, Wendt U, Szczepa´nska-Konkel M, St¸epiñski J, Drewnowska K, Angielski S. Inhibition of endogenous nitric oxide synthesis activates particulate guanylyl cyclase in the rat renal glomeruli. Kidney International. 1997;52:654–659. doi: 10.1038/ki.1997.379. [DOI] [PubMed] [Google Scholar]
- Long CJ, Stone TW. The release of endothelium-derived relaxation factor is calcium-dependent. Blood Vessels. 1985;22:205–208. doi: 10.1159/000158602. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Marsden PA, Brock TA, Ballermann BJ. Glomerular endothelial cells respond to calcium-mobilizing agonists with release of EDRF. American Journal of Physiology. 1990;258:F1295–1303. doi: 10.1152/ajprenal.1990.258.5.F1295. [DOI] [PubMed] [Google Scholar]
- Mayer B, Brunner F, Schmidt K. Novel actions of methylene blue. European Heart Journal. 1993;14:22–26. [PubMed] [Google Scholar]
- Meghij P, Pearson D, Slakey LL. Kinetics of extracellular ATP hydrolysis by microvascular endothelial cells from rat heart. Biochemical Journal. 1995;308:725–731. doi: 10.1042/bj3080725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mene P, Dunn MJ. Eicosanoids and control of mesangial cell contraction. Circulation Research. 1988;62:916–925. doi: 10.1161/01.res.62.5.916. [DOI] [PubMed] [Google Scholar]
- Motte S, Communi D, Pirotton S, Boeynaems JM. Involvement of multiple receptors in the action of extracellular ATP: the example of vascular endothelial cells. International Journal of Biochemistry and Cell Biology. 1995;27:1–7. doi: 10.1016/1357-2725(94)00059-x. [DOI] [PubMed] [Google Scholar]
- Pavenstadt H, Gloy J, Leipziger J, Klar B, Pfeilschifter J, Schollmeyer P, Greger R. Effect of extracellular ATP on contraction, cytosolic calcium activity, membrane voltage and ion currents of rat mesangial cells in primary culture. British Journal of Pharmacology. 1993;109:953–959. doi: 10.1111/j.1476-5381.1993.tb13713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavenstadt H, Henger A, Briner V, Fischer KG, Huber-Lang M, Schollmeyer P, Greger R. Agonist-induced activation of a non-selective ion current in glomerular endothelial cells. Kidney International. 1997;52:157–164. doi: 10.1038/ki.1997.315. [DOI] [PubMed] [Google Scholar]
- Pavenstadt H, Spath M, Schlunck G, Nauck M, Fischer R, Wanner C, Schollmeyer P. Effect of nucleotides on the cytosolic free calcium activity and inositol phosphate formation in human glomerular epithelial cells. British Journal of Pharmacology. 1992;107:189–195. doi: 10.1111/j.1476-5381.1992.tb14485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J. Comparison of extracellular ATP and UTP signalling in rat renal mesangial cells. No indications for the involvement of separate purino- and pyrimidino-ceptors. British Journal of Pharmacology. 1990;272:469–472. doi: 10.1042/bj2720469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Relative contribution of P2U- and P2Y-purinoceptors to endothelium-dependent vasodilatation in the golden hamster isolated mesenteric arterial bed. British Journal of Pharmacology. 1996;117:1797–1802. doi: 10.1111/j.1476-5381.1996.tb15357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Rump LC, Oberhauser V, Kugelgen I. Purinoceptors mediate renal vasodilatation by nitric oxide dependent and independent mechanism. Kidney International. 1998;54:473–481. doi: 10.1046/j.1523-1755.1998.00002.x. [DOI] [PubMed] [Google Scholar]
- Savin VJ, Terreros DA. Filtration in single isolated mammalian glomeruli. Kidney International. 1981;20:188–197. doi: 10.1038/ki.1981.121. [DOI] [PubMed] [Google Scholar]
- Schulze-Lohoff E, Bitzer M, Ogilvie A, Sterzel RB. P2U-purinergic receptor activation mediates inhibition of cAMP accumulation in cultured renal mesangial cells. Renal Physiology and Biochemistry. 1995;18:219–230. doi: 10.1159/000173919. [DOI] [PubMed] [Google Scholar]
- Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of pulmonary circulation. American Journal of Physiology. 1996;271:H2717–2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- Szczepa´nska-Konkel M, Redlak M, Angielski S. Glibenclamid-sensitive K+ channels are responsible for angiotensin II hypersensitive contraction and atrial natriuretic factor refractoriness of glomeruli in low-sodium rats. Biochemical and Biophysical Research Communications. 1991;181:871–876. doi: 10.1016/0006-291x(91)91271-d. [DOI] [PubMed] [Google Scholar]
- Tack I, Castano EM, Pecher C, Praddaude F, Bascands JL, Bompart G, Ader JL, Girolami JP. Endothelin increases NO-dependent cGMP production in isolated glomeruli but not in mesangial cells. American Journal of Physiology. 1997;272:F31–39. doi: 10.1152/ajprenal.1997.272.1.F31. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shin WS, Kawaguchi H, Inukai M, Kato M, Sakamoto A, Uehara Y, Miyamoto M, Shimamoto N, Korenaga R, Ando J, Toyo-Oka T. Contribution of sustained Ca2+ elevation for nitric oxide production in endothelial cells and subsequent modulation of Ca2+ transient in vascular smooth muscle cells in coculture. Journal of Biological Chemistry. 1996;271:5647–5655. doi: 10.1074/jbc.271.10.5647. [DOI] [PubMed] [Google Scholar]
- Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proceedings of the National Academy of Sciences of the USA. 1992;89:11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Park F, Cowley AW, Jr, Mattson DL. Quantification of nitric oxide synthase activity in microdissected segments of the rat kidney. American Journal of Physiology. 1999;276:F874–881. doi: 10.1152/ajprenal.1999.276.6.F874. [DOI] [PubMed] [Google Scholar]
- Yang S, Cheek DJ, Westfall DP, Buxton ILO. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circulation Research. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- Yuen PST, Potter LR, Garbers DL. A new form of guanylyl cyclase is preferentially expressed in rat kidney. Biochemistry. 1990;29:10872–10878. doi: 10.1021/bi00501a002. [DOI] [PubMed] [Google Scholar]
- Yunping Y, Johnson TD, Childres WF, Bryan RM. Endothelial-mediated dilatations of rat middle cerebral arteries by ATP and ADP. American Journal of Physiology. 1997;373:H1472–1477. doi: 10.1152/ajpheart.1997.273.3.H1472. [DOI] [PubMed] [Google Scholar]


