Abstract
The influence of the different phases of the menstrual cycle on skeletal muscle contractile characteristics was studied in 19 regularly menstruating women. Muscle function was measured when (i) oestrogen and progesterone concentrations were low (menstruation), (ii) oestrogen was elevated and progesterone was low (late follicular phase), and (iii) oestrogen and progesterone were both elevated (luteal phase).
Maximal isometric quadriceps strength, fatiguability and electrically stimulated contractile properties were measured. Isokinetic knee flexion and extension strength and fatiguability were also assessed as well as handgrip strength. Menstrual cycle phases were confirmed through measurement of oestrogen, progesterone, follicle stimulating hormone and luteinising hormone.
No significant changes were found in any of the muscle function parameters throughout the menstrual cycle (n = 15). The muscle function measurements showed no significant correlations with any of the female reproductive hormone concentrations.
These results suggest that the fluctuations in female reproductive hormone concentrations throughout the menstrual cycle do not affect muscle contractile characteristics.
During their reproductive years the hormone levels in women fluctuate due to the menstrual cycle. The four hormonal markers of the menstrual cycle (oestrogen, progesterone, follicle stimulating hormone (FSH) and luteinising hormone (LH)) change continuously throughout the cycle. These fluctuations in female steroid hormones affect the autonomic nervous system and metabolic functions (Florini, 1987). Therefore certain physiological parameters and athletic performance could change along with the menstrual cycle phases (Becker et al. 1982). However, the influence of the menstrual cycle phase on exercise performance, particularly muscle strength, is unclear.
Sarwar et al. (1996) tested skeletal muscle strength, relaxation rate and fatiguability of the quadriceps during the menstrual cycle. They found no changes in these parameters for women taking oral contraceptives. For women not taking oral contraceptives, however, the quadriceps were stronger, more fatiguable and had a longer relaxation time at mid-cycle (day 12-18). Phillips et al. (1996) reported a higher adductor pollicis strength during the follicular phase than during the luteal phase, with a rapid decrease in strength around ovulation. They suggested that oestrogen has a strengthening action on skeletal muscle, although the underlying mechanism is not clear. Greeves et al. (1999), however, reported the highest quadriceps strength during the mid-luteal phase and found a positive relationship between strength and progesterone concentration. Several other studies have found no changes in skeletal muscle strength over the menstrual cycle (DiBrezzo et al. 1991; Quadango et al. 1991; Lebrun et al. 1995; Gür, 1997).
The main problem in the measurement of maximum voluntary strength is ensuring that the contraction truly reflects the maximum force-generating capacity of the muscle. Even well-motivated subjects may not always reach full neural activation of their muscles (Rutherford et al. 1986). The extent of neural activation can be evaluated by applying a superimposed electrical stimulus to the muscle during the performance of a maximal voluntary contraction (MVC). When comparing strength over a period of time, such as in menstrual cycle research, it is especially important to ensure maximal neural activation during each test.
A further problem encountered in research on the influence of the menstrual cycle on physical performance is the timing of the testing. It is difficult to predict the exact phases of the menstrual cycle and the concurrent reproductive hormone concentrations. Counting days from the onset of bleeding and basal body temperature (BBT) charting can be used to estimate the different phases of the menstrual cycle. These methods, however, only provide predictions, and serum hormone level measurements of at least oestrogen and progesterone are necessary to confirm the menstrual cycle phase.
Most of the studies on muscle strength during the menstrual cycle have either not measured hormone levels or relied upon the assumption that voluntary strength measurements were maximal. These problems have been addressed in this investigation of the influence of the menstrual cycle phase on skeletal muscle strength, fatigue and contractile properties.
METHODS
Subjects
Nineteen healthy women with regular menstrual cycles volunteered to take part in this study. All subjects provided written informed consent and all procedures were approved by The University of Sydney Human Ethics Committee and conformed to the Declaration of Helsinki. Subjects were medically screened and were healthy and not taking any medication. They had not been taking oral contraceptives or hormone supplements for at least the preceding 6 months. Subjects with an irregular menstrual cycle history were excluded from this study. Seven subjects volunteered to continue the testing for a consecutive cycle.
Protocol
The muscle strength, fatiguability and contractile properties of the subjects were measured during different phases of the menstrual cycle. This study aimed to include the three distinctly different combinations of oestrogen and progesterone concentrations that occur during the menstrual cycle: firstly menstruation (day 1-3), during which both oestrogen and progesterone concentrations are relatively low; secondly the late follicular phase, when oestrogen is elevated and progesterone remains low; and finally the luteal phase, during which both oestrogen and progesterone are elevated. BBT patterns were used to estimate the different phases of the menstrual cycle (Vollman, 1977). The timing of the testing during the late follicular phase is difficult because the oestrogen peak only lasts approximately 3 days (Landgren et al. 1980). To increase the likelihood of testing during the late follicular oestrogen peak, two tests were conducted during this phase. The muscle function test coinciding with the highest oestrogen concentration and a low progesterone concentration was analysed as the late follicular phase test. During each test session isometric strength and fatiguability of the quadriceps were assessed first. After a 5 min rest period isokinetic knee flexion and extension strength and fatiguability were measured. This was followed by the handgrip strength assessment.
Basal body temperature
During the familiarization period the subjects were informed on how to measure their own BBT. Mercurial ovulation thermometers with scale steps of 0.05°C were used and BBT was measured orally for 5 min, before rising in the morning. The subjects continued to measure and record their BBT throughout the study. The pattern of the BBT was used to estimate the duration of the different phases of the cycle (Vollman, 1977) and determine the testing schedule. The subjects also completed a daily questionnaire about symptoms such as breast tenderness and fluid retention. Changes in these symptoms in the 2 weeks preceding the menstrual flow indicate an ovulatory cycle (Lebrun et al. 1995).
Hormone analysis
Before each muscle function testing session, blood samples were taken from an antecubital vein. The serum collection was standardized by taking a fasting blood sample at 08.00 h after the subjects had rested for 20 min. The serum concentrations of oestrogen, progesterone, FSH and LH were later measured using ELISA kits (DSL, TX, USA and Bioclone, Australia). A serum progesterone concentration greater than 16 nmol l−1 was required for confirmation of an ovulatory cycle (Landgren et al. 1980).
Isometric strength, fatigue and contractile properties
Prior to the strength testing all subjects performed a 10 min warm-up on a cycle ergometer at 50 W with a cadence of 60 r.p.m. To measure isometric quadriceps strength and contractile properties the subjects were seated in a steel-framed adjustable, straight-backed chair. The pelvis and trunk were tightly secured by adjustable webbing straps and the knee joint angle was set at 60 deg from full leg extension. The ankle was secured to a fixed force transducer from which the analog signal was amplified, digitized and collected using the data acquisition program ASYST. A high voltage stimulator (DS7, Digitimer Ltd, UK) programmed by a digital timer (D4030, Digitimer Ltd) was used to deliver percutaneous electrical pulses to the quadriceps via two electrodes placed proximally and distally on the anterolateral thigh. Prior to the maximal voluntary strength measurement a series of electrical stimuli was applied during which the current was increased in 50 mA steps. When the involuntary force created by the electrical stimuli reached a plateau, the current of the third stimulus in the plateau was defined as the supra-maximal response. Two types of electrical stimuli were used: (i) a twitch at 400 V and (ii) an 80 ms tetanus at 50 Hz (5 impulses) with 50 μs pulsed square waves at 400 V. The supra-maximal stimuli responses were analysed for time to peak tension (TPT), peak tension (PT), half-relaxation time (1/2RT), normalized peak rate of force development (normPRFD) and normalized peak rate of relaxation (normPRR). To monitor full neural activation during the strength measurements, a supra-maximal twitch was superimposed on each MVC. The central activation ratio (CAR) was used to quantify the extent of neural activation (Kent-Braun & Le Blanc, 1996). CAR = MVC/total force, in which total force = voluntary force + superimposed force. CAR = 1.0 indicates full voluntary activation of the muscle (Kent-Braun & Le Blanc, 1996). Contractions during which CAR was smaller than 1.0 were excluded from analysis. During each test session the subjects performed five MVCs of the quadriceps of their dominant leg. Each MVC was preceded by a 2 min rest period. The subjects were well motivated and received verbal encouragement during the performance of the MVC. The peak force recorded in each session was taken as the MVC. The electrically stimulated fatigue test consisted of 300 ms stimuli every second for 2 min at 20 Hz at a current that elicited at least 50% of the MVC force (modified from Burke et al. 1973).
Isokinetic strength and fatigue
An isokinetic dynamometer (Biodex Multi-Joint System II, Biodex Medical Systems Inc., USA) was used to record torques about the knee joint at constant angular velocities during MVCs of the knee flexors and extensors. The subjects performed five knee flexions and five knee extensions at a velocity of 60 deg s−1 and repeated this protocol at a velocity of 240 deg s−1 with a 2 min rest interval between each set of five contractions. The final isokinetic test was a voluntary fatigue test, which consisted of 60 knee flexions and extensions at a velocity of 240 deg s−1.
Handgrip strength
Dominant handgrip strength was measured using a force transducer set within an adjustable frame. The subjects stood with their arms beside their body and the elbow extended. As with the quadriceps, MVCs were performed with a 2 min rest interval between each contraction. The MVC was the highest score recorded over five consecutive trials.
RESULTS
Hormone analysis
The blood sample analysis for progesterone showed that four of the 19 subjects did not exceed the 16 nmol l−1 limit that confirms ovulation had occurred (Landgren et al. 1980). These four subjects were therefore excluded from the results. The mean ±s.d for age, height and weight for the remaining 15 subjects was 29.9 ± 8.0 years, 167 ± 7 cm and 61.4 ± 8.4 kg, respectively. The results for the serum hormone concentrations of oestrogen, progesterone, FSH and LH are shown in Table 1.
Table 1.
Serum hormone concentrations of oestrogen, progesterone, follicle stimulating hormone (FSH) and luteinising hormone (LH) throughout the menstrual cycle
| Hormone concentration | Menstruation | Late follicular | Luteal |
|---|---|---|---|
| Oestrogen (pmol l−1) | 170.1 ± 116.8 | 533.5 ± 289.6* | 369.7 ± 126.9*† |
| Progesterone (nmol l−1) | 6.53 ± 12.12 | 2.45 ± 1.30 | 40.63 ± 19.17*† |
| FSH (i.u. l−1) | 5.18 ± 2.31 | 7.56 ± 3.85* | 3.29 ± 3.38† |
| LH (i.u. l−1) | 6.01 ± 3.87 | 24.87 ± 21.12* | 4.76 ± 2.96† |
Data are shown as means ± S.D., n = 15.
Significantly different from Menstruation (P < 0.05).
Significantly different from Late follicular (P < 0.05).
As expected, repeated measures ANOVA showed significant changes for the hormone concentrations throughout the menstrual cycle. Tests of within-subject contrasts showed that the oestrogen level during menstruation was lower than that during both the late follicular and the luteal phase (both P < 0.01). The oestrogen concentration during the late follicular phase was also higher than during the luteal phase (P < 0.05). For progesterone, the concentration during the luteal phase was significantly higher than during the rest of the menstrual cycle (P < 0.01 for both menstruation and follicular phase). The FSH and LH concentrations were significantly higher during the late follicular phase than during menstruation (P < 0.05 for FSH and P < 0.01 for LH) and the luteal phase (P < 0.01 for FSH and LH). All these findings agree well with the hormone concentration pattern of a regular ovulatory menstrual cycle. As can be seen in Table 1, there was a very large variation in serum hormone levels throughout the menstrual cycle, but also within each menstrual cycle phase.
Strength, fatiguability and contractile properties
Repeated measures ANOVA showed no significant changes over the phases of the menstrual cycle for isometric quadriceps strength with superimposed electrical stimulation (see Table 2). All subjects reached a CAR equal to or greater than 1.0 during the quadriceps contractions with superimposed electrical stimulation. No significant changes in the extent of neural activation were found throughout the menstrual cycle. The twitch and tetanus characteristics (TPT, PT, 1/2RT, normPRFD, normPRR) and the electrically stimulated fatigue test also did not show any change over the menstrual cycle (see Table 3). No significant changes over the menstrual cycle were found for isokinetic strength and fatiguability of the knee flexors and extensors. The repeated measures ANOVA for handgrip strength also showed no significant changes throughout the menstrual cycle (see Table 2).
Table 2.
Muscle strength and fatigue parameters throughout the menstrual cycle
| Muscle function | Menstruation | Late follicular | Luteal |
|---|---|---|---|
| Isometric quadriceps (N) | 571 ± 114 | 551 ± 114 | 570 ± 109 |
| Isokinetic ext 60 deg s−1 (Nm) | 153.6 ± 33.0 | 148.9 ± 27.7 | 152.8 ± 27.8 |
| Isokinetic flex 60 deg s−1 (Nm) | 76.6 ± 13.7 | 71.1 ± 13.3 | 73.0 ± 12.0 |
| Isokinetic ext 240 deg s−1 (Nm) | 92.0 ± 17.9 | 88.8 ± 18.9 | 90.9 ± 20.6 |
| Isokinetic flex 240 deg s−1 (Nm) | 54.4 ± 9.0 | 51.9 ± 8.2 | 52.3 ± 8.0 |
| Fatigue index – ext | 0.470 ± 0.12 | 0.479 ± 0.09 | 0.471 ± 0.15 |
| Fatigue index – flex | 0.553 ± 0.19 | 0.476 ± 0.13 | 0.517 ± 0.17 |
| Handgrip (N) | 348 ± 43 | 341 ± 39 | 340 ± 44 |
Data are shown as means ± S.D., n = 15. Ext, knee extension; flex, knee flexion. There were no significant differences between menstrual cycle phases for any of the variables.
Table 3.
Electrically evoked contractile characteristics of the quadriceps throughout the menstrual cycle
| Contractile characteristics | Menstruation | Late follicular | Luteal |
|---|---|---|---|
| Fatigue index quadriceps | 0.877 ± 0.21 | 0.819 ± 0.15 | 0.870 ± 0.24 |
| Twitch PT (N) | 77.93 ± 26.65 | 79.86 ± 24.11 | 78.21 ± 27.20 |
| Twitch TPT (ms) | 85.55 ± 6.86 | 87.02 ± 11.74 | 85.04 ± 7.05 |
| Twitch 1/2RT (ms) | 74.64 ± 13.96 | 76.60 ± 18.9 | 73.64 ± 16.50 |
| Twitch norm PRFD (% ms−1) | 2.41 ± 0.19 | 2.36 ± 0.25 | 2.38 ± 0.20 |
| Twitch norm PRR (% ms−1) | −1.15 ± 0.24 | −1.15 ± 0.26 | −1.14 ± 0.32 |
| Tetanus PT (N) | 281.63 ± 54.67 | 269.33 ± 55.35 | 274.89 ± 56.37 |
| Tetanus TPT (ms) | 154.51 ± 12.76 | 155.12 ± 14.00 | 153.88 ± 9.60 |
| Tetanus 1/2RT (ms) | 63.86 ± 7.12 | 65.00 ± 10.87 | 64.12 ± 12.37 |
| Tetanus norm PRFD (% ms−1) | 1.74 ± 0.23 | 1.76 ± 0.14 | 1.80 ± 0.17 |
| Tetanus norm PRR (% ms−1) | −1.24 ± 0.15 | −1.21 ± 0.14 | −1.25 ± 0.17 |
Data are shown as means ± S.D., n = 15 for twitch and tetanus characteristics and n = 12 for Fatigue index PT, peak tension; TPT, time to peak tension; 1/2RT, half-relaxation time; norm PRFD, normalized peak rate of force development; norm PRR, normalized peak rate of relaxation. There were no significant differences between menstrual cycle phases for any of the variables.
Figure 1 shows the maximal isometric quadriceps strength with superimposed electrical stimulation throughout the menstrual cycle and the corresponding oestrogen concentrations. No correlations were found between any of the strength and fatigue parameters and the serum concentrations of oestrogen, progesterone, LH and FSH (see Table 4).
Figure 1. Maximal isometric quadriceps strength with superimposed stimulation and serum oestrogen concentration throughout the three phases of the menstrual cycle.
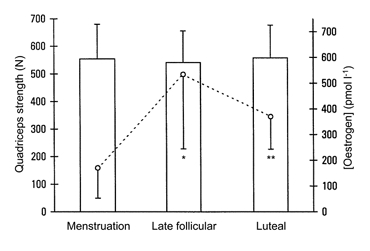
The columns represent the means of the quadriceps strength (+s.d.) and show no significant differences between menstrual cycle phases. The dotted line represents the means of the oestrogen concentration (-s.d.). *Significantly different from Menstruation (P < 0.01). **Significantly different from Menstruation (P < 0.01) and significantly different from Late follicular phase (P < 0.05).
Table 4.
Correlation coefficients between the muscle strength and fatigue parameters and the serum hormone concentrations of oestrogen, progesterone, FSH and LH
| Muscle function | Oestrogen | Progesterone | FSH | LH |
|---|---|---|---|---|
| Isometric quadriceps | 0.025 | 0.005 | −0.074 | 0.039 |
| (0.872) | (0.975) | (0.631) | (0.798) | |
| Fatigue index | −0.288 | 0.096 | 0.153 | −0.146 |
| quadriceps | (0.088) | (0.579) | (0.374) | (0.396) |
| Isokinetic ext | 0.120 | 0.004 | 0.032 | 0.045 |
| 60 deg s−1 | (0.438) | (0.980) | (0.836) | (0.770) |
| Isokinetic flex | 0.004 | 0.070 | 0.159 | 0.022 |
| 60 deg s−1 | (0.979) | (0.654) | (0.307) | (0.890) |
| Isokinetic ext | 0.122 | 0.016 | 0.069 | 0.119 |
| 240 deg s−1 | (0.429) | (0.916) | (0.654) | (0.442) |
| Isokinetic flex | 0.046 | −0.069 | 0.130 | 0.048 |
| 240 deg s−1 | (0.765) | (0.658) | (0.400) | (0.758) |
| Fatigue index – ext | 0.089 | 0.073 | −0.273 | 0.142 |
| (0.577) | (0.648) | (0.080) | (0.371) | |
| Fatigue index – flex | −0.289 | 0.119 | −0.170 | −0.114 |
| (0.063) | (0.452) | (0.283) | (0.473) | |
| Handgrip | −0.015 | −0.089 | −0.046 | −0.214 |
| (0.922) | (0.559) | (0.766) | 0.158 |
Data are shown as Pearson correlation coefficients with P values below. n =45 (15 subjects over 3 phases), except for the Fatigue index quadriceps (n =36) and the Fatigue index – extension and flexion n =42). Ext, knee extension; flex, knee flexion. There were no significant correlations between the strength and fatigue parameters and the serum hormone concentrations.
Repeatability
The test-retest repeatability of the strength parameters was demonstrated with an intra-class correlation coefficient (ICC) ranging from 0.88 to 0.96. Only handgrip strength showed a lower ICC of 0.73.
DISCUSSION
Muscle function was measured during three phases of the menstrual cycle with significantly different concentrations of circulating female reproductive hormones. The results showed no change over the menstrual cycle for any of the strength parameters, including maximal isometric quadriceps strength with superimposed electrical stimulation, isokinetic knee flexion and extension strength at 60 and 240 deg s−1 and handgrip strength. The quadriceps contractile properties, the electrically stimulated quadriceps fatigue and the isokinetic knee flexor and extensor fatigue also did not change throughout the menstrual cycle.
The present findings for the isometric quadriceps strength do not agree with the results reported by Sarwar et al. (1996). These authors also used superimposed electrical stimulation and found the highest quadriceps strength at mid-cycle, which was defined as day 12 to day 18. For most women ovulation would occur during these 6 days, so the female reproductive hormone concentrations would be expected to fluctuate significantly. Sarwar et al. (1996) did not measure hormone concentrations, but assumed that oestrogen levels would be high during mid-cycle and suggested that oestrogen increases muscle strength. To explain why strength did not increase during the luteal phase they suggested that progesterone might inhibit the proposed strength-enhancing effect of oestrogen.
Phillips et al. (1996) also suggested that oestrogen has a strengthening effect on skeletal muscle. They reported an increase in maximal voluntary adductor pollicis strength during the follicular phase and a drop in strength at ovulation. Hormone concentrations in a sub-group of subjects (n = 9) were assessed but did not show a correlation between strength and oestrogen concentration. Phillips et al. (1996) explain this lack of correlation by suggesting that the strengthening effect of oestrogen could have a delayed onset.
Contrary to the work by Phillips et al. (1996) and Sarwar et al. (1996), Greeves et al. (1997) showed in patients undergoing in vitro fertilization that supra-physiological levels of oestrogen did not cause any changes in strength of the first dorsal interosseus muscle. The present study found no changes in strength over the menstrual cycle and did not show a correlation between oestrogen concentration and strength. Based on these findings the suggested effect of oestrogen on strength (Phillips et al. 1996; Sarwar et al. 1996) is questionable.
The isokinetic findings from this study agree well with studies by Gür (1997) and Lebrun et al. (1995), who also did not find any changes over the menstrual cycle for maximal isokinetic knee flexion and extension strength. Both these studies suggested that menstrual cycle phase does not affect isokinetic strength, which is confirmed by the present results.
This study showed that fatiguability and electrically stimulated contractile properties of the quadriceps did not change over the menstrual cycle. This supports the findings of White & Weekes (1998) who found no changes in fatiguability and maximal electrically evoked contractile character of the triceps surae during the menstrual cycle. Sarwar et al. (1996) reported an increase in fatiguability and a slowing of the half-time of relaxation at mid-cycle (day 12-18), but did not measure hormone concentrations. Greeves et al. (1997), however, showed no changes with extreme oestrogen fluctuations, which supports the present findings and confirms the suggestion that oestrogen concentration does not affect skeletal muscle fatiguability and contractile properties.
In the present study a large variation in hormone concentrations was found throughout the menstrual cycle, but also within each menstrual cycle phase. Especially during the late follicular phase the variation in hormone levels was very large, with a range from 157 to 1038 pmol l−1 for oestrogen concentration. This large variation in hormone levels within each phase is partly due to the secretory pulses of these hormones (ultradian rhythm). Serum collection for the hormone analysis was standardized, but it should be noted that rapid fluctuations in the reproductive and pituitary hormones could occur at any time during the day throughout the menstrual cycle.
Besides the sudden daily fluctuations, the large variation in hormone concentrations reported was also likely to be caused by timing problems within each menstrual cycle phase. The days of testing for each subject were estimated based on the BBT pattern of their previous cycle. However, the length of the menstrual cycle can vary from cycle to cycle. The BBT charting does give an indication of whether or not ovulation took place, but is not accurate enough to predict the exact menstrual cycle phase. To increase the chance of testing during the oestrogen peak, two tests were conducted during the late follicular phase. The variation in hormone concentrations within each menstrual cycle phase was decreased by analysing only the test with the highest oestrogen concentration as the late follicular phase. This way a more accurate representation of the three phases of a regular menstrual cycle was given. Thus the chances of showing a relationship between hormone concentrations and muscle function were increased. The fact that even with this method no correlations between hormone concentrations and muscle function were found supports the suggestion that the fluctuations in female reproductive hormones throughout the menstrual cycle do not affect muscle strength, fatiguability and contractile properties.
It is important in menstrual cycle studies to measure the hormone concentrations on the days of testing. Four of the 19 volunteers in this study did not ovulate according to the hormone measurements, but these 4 subjects did appear to have a normal control cycle based on BBT pattern. De Souza et al. (1998) showed a high frequency of luteal phase deficiency and anovulation in recreational runners and a high inconsistency from one menstrual cycle to the next. The subjects in the present study were recreationally active, which might explain the high percentage of anovulation found. Given the present anovulation findings and those by De Souza et al. (1998) one cannot assume a consistent menstrual cycle with regular hormone concentrations for recreationally active women. To avoid inclusion of non-ovulatory menstrual cycles in research and to be able to draw valid conclusions about the effects of menstrual cycle phase on muscle strength and fatigue it is necessary to measure the actual hormone levels at the day of testing, as in this study.
In conclusion, no changes over the menstrual cycle were found for strength, fatigue and the twitch and tetanus characteristics (TPT, PT, 1/2RT, normPRFD, normPRR). Care was taken that the three tests represented the three different phases of the menstrual cycle with concurrent significantly different hormone (oestrogen, progesterone, FSH and LH) concentrations. The muscle function measures showed no correlations with any of the measured hormone concentrations. The results suggest that the fluctuations in female reproductive hormones throughout the menstrual cycle do not affect muscle strength, fatiguability and contractile properties.
References
- Becker D, Creutzfeldt OD, Schwibbe M, Wuttke W. Changes in physiological, EEG and psychological parameters in women during the spontaneous menstrual cycle and following oral contraceptives. Psychoneuroendocrinology. 1982;7:75–90. doi: 10.1016/0306-4530(82)90057-9. [DOI] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FED. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. Journal of Physiology. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, Lasley BL. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. Journal of Clinical Endocrinology and Metabolism. 1998;83:4220–4232. doi: 10.1210/jcem.83.12.5334. [DOI] [PubMed] [Google Scholar]
- DiBrezzo R, Fort IL, Brown B. Relationships among strength, endurance, weight and body fat during three phases of the menstrual cycle. Journal of Sports Medicine and Physical Fitness. 1991;31:89–94. [PubMed] [Google Scholar]
- Florini JR. Hormonal control of muscle growth. Muscle and Nerve. 1987;10:577–598. doi: 10.1002/mus.880100702. [DOI] [PubMed] [Google Scholar]
- Greeves JP, Cable NT, Luckas MJ, Reilly T, Biljan MM. Effects of acute changes in oestrogen on muscle function of the first dorsal interosseus muscle in humans. Journal of Physiology. 1997;500:265–270. doi: 10.1113/jphysiol.1997.sp022016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeves JP, Cable NT, Reilly T. 4th Annual Congress of the European College of Sport Science. Rome: Italy; 1999. The relationship between maximal muscle strength and reproductive hormones during the menstrual cycle; p. 189. [Google Scholar]
- Gür H. Concentric and eccentric isokinetic measurements in knee muscles during the menstrual cycle: a special reference to reciprocal moment ratios. Archives of Physical Medicine and Rehabilitation. 1997;78:501–505. doi: 10.1016/s0003-9993(97)90164-7. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle and Nerve. 1996;19:861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Landgren BM, Unden AL, Diczfalusy E. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinologica. 1980;94:89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- Lebrun CM, McKenzie DC, Prior JC, Taunton JE. Effects of menstrual cycle phase on athletic performance. Medicine and Science in Sports and Exercise. 1995;27:437–444. [PubMed] [Google Scholar]
- Phillips SK, Sanderson AG, Birch K, Bruce SA, Woledge RC. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. Journal of Physiology. 1996;496:551–557. doi: 10.1113/jphysiol.1996.sp021706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadango D, Faquin L, Gei-Nam L, Kuminka W, Moffatt R. The menstrual cycle: Does it affect athletic performance? Physician and Sports Medicine. 1991;19:121–124. [Google Scholar]
- Rutherford OM, Jones DA, Newham DJ. Clinical and experimental application of the percutaneous twitch superimposition technique for the study of human muscle activation. Journal of Neurology, Neurosurgery and Psychiatry. 1986;49:1288–1291. doi: 10.1136/jnnp.49.11.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar R, Niclos BB, Rutherford OM. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. Journal of Physiology. 1996;493:267–272. doi: 10.1113/jphysiol.1996.sp021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollman RF. The Menstrual Cycle. Philadelphia, PA, USA: W. B. Saunders Company; 1977. [Google Scholar]
- White MJ, Weekes C. No evidence for a change in the voluntary or electrically evoked contractile characteristics of the triceps surae during the human menstrual cycle. Journal of Physiology. 1998;506.P:119. P. [Google Scholar]


