Abstract
To determine the ability of strong-binding myosin cross-bridges to activate the myocardial thin filament, we examined the Ca2+ dependence of force and cross-bridge interaction kinetics at 15°C in the absence and presence of a strong-binding, non-force-generating derivative of myosin subfragment-1 (NEM-S1) in chemically skinned myocardium from adult rats.
Relative to control conditions, application of 6 μM NEM-S1 significantly increased Ca2+-independent tension, measured at pCa 9.0, from 0.8 ± 0.3 to 3.7 ± 0.8 mN mm−2. Furthermore, NEM-S1 potentiated submaximal Ca2+-activated forces and thereby increased the Ca2+ sensitivity of force, i.e. the [Ca2+] required for half-maximal activation (pCa50) increased from pCa 5.85 ± 0.05 to 5.95 ± 0.04 (change in pCa50 (ΔpCa50) = 0.11 ± 0.02). The augmentation of submaximal force by NEM-S1 was accompanied by a marked reduction in the steepness of the force-pCa relationship for forces less than 0.50 Po (maximum Ca2+-activated force), i.e. the Hill coefficient (n2) decreased from 4.72 ± 0.38 to 1.54 ± 0.07.
In the absence of NEM-S1, the rate of force redevelopment (ktr) was found to increase from 1.11 ± 0.21 s−1 at submaximal [Ca2+] (pCa 6.0) to 9.28 ± 0.41 s−1 during maximal Ca2+ activation (pCa 4.5). Addition of NEM-S1 reduced the Ca2+ dependence of ktr by eliciting maximal values at low levels of Ca2+, i.e. ktr was 9.38 ± 0.30 s−1 at pCa 6.6 compared to 9.23 ± 0.27 s−1 at pCa 4.5. At intermediate levels of Ca2+, ktr was less than maximal but was still greater than values obtained at the same pCa in the absence of NEM-S1.
NEM-S1 dramatically reduced both the extent and rate of relaxation from steady-state submaximal force following flash photolysis of the caged Ca2+ chelator diazo-2.
These data demonstrate that strongly bound myosin cross-bridges increase the level of thin filament activation in myocardium, which is manifested by an increase in the rate of cross-bridge attachment, potentiation of force at low levels of free Ca2+, and slowed rates of relaxation.
The regulation of myocardial contraction is mediated by the binding of Ca2+ to a low affinity Ca2+-specific binding site on troponin C (TnC) (Johnson et al. 1980), which alters the interactions of thin filament proteins and allows myosin to bind to actin (Solaro & Rarick, 1998). In skeletal muscle, several studies (reviewed by Lehrer, 1994) have shown that Ca2+ alone is unable to fully activate the thin filament, i.e. maximal activation of steady force and the rate of force development results from synergistic actions of Ca2+ and strong-binding myosin cross-bridges. While similar mechanisms are thought to contribute to the activation of heart muscle (Tobacman, 1996), the roles played by strong-binding cross-bridges in the regulation of myocardial contraction are not known.
McKillop & Geeves (1993) have proposed a generally applicable model for the regulation of muscle contraction in which the thin filament exists in three distinct states: a blocked state that cannot easily bind myosin cross-bridges, a closed state that weakly binds cross-bridges and an open state in which cross-bridges bind strongly and generate force. In this model, Ca2+ shifts the thin filament from the blocked to the closed state, whereas strong binding of myosin cross-bridges shifts the thin filament from the blocked state through the closed state to the open state. Therefore, complete activation of the thin filament involves several factors including co-operative binding of myosin cross-bridges to actin and co-operative binding of Ca2+ to the thin filament regulatory protein troponin (reviewed by Moss, 1992). A model proposed by Campbell (1997) suggests that positive co-operativity is a dynamic component of force generation, i.e. at low Ca2+ levels the rate of activation is slowed (compared to the rate at high Ca2+ levels) by progressive co-operative recruitment of myosin cross-bridges into force-generating states. The effects of co-operative cross-bridge binding on the rate of force development would be minimized at high levels of Ca2+ since Ca2+ binding would initially recruit most of the available myosin cross-bridges so that few remained for co-operative binding. The Ca2+ dependence of the rate of force redevelopment (ktr) has been reported in skinned skeletal muscle fibres (Brenner & Eisenberg, 1986; Metzger et al. 1989) and both skinned (Wolff et al. 1995; Palmer & Kentish, 1998) and intact (Baker et al. 1998) myocardial preparations. However, the role of co-operative cross-bridge binding in the activation of myocardial force is not yet known.
In the present study, N-ethylmaleimide-modified myosin subfragment-1 (NEM-S1), a strong-binding, non-tension-generating derivative of myosin subfragment-1 (Swartz & Moss, 1992), was applied to chemically skinned myocardium to test the idea that the strong binding of myosin cross-bridges co-operatively activates force and the kinetics of force development. If co-operativity in cross-bridge binding to actin contributes to the rate of activation, saturating the co-operative mechanism with NEM-S1 should speed the rate of force redevelopment and reduce or eliminate its dependence on force and Ca2+ concentration.
METHODS
Experimental animals
Female Sprague-Dawley rats (≈3 months of age, weighing 200-224 g) were purchased from Harlan Sprague-Dawley (Madison, WI, USA). Rats were housed in groups of three per cage in temperature- and light-controlled quarters and were provided with food and water ad libitum. All rats were inspected daily for signs of distress or discomfort throughout the course of the project. Rats were anaesthetized by placing them in a glass bell jar containing room air and 3-4% methoxyflurane and were subsequently killed by a pneumothorax following the establishment of deep anaesthesia (confirmed by the loss of the pedal reflex and muscular tension of the limbs). Animal use was approved by the University of Wisconsin Animal Care Committee.
Experimental solutions
The compositions of relaxing solution used for the preparation of skinned myocardium and activating solutions used in mechanical measurements were as follows. Relaxing solution contained (mm): KCl, 100; imidazole, 20; MgATP, 4; EGTA, 2; and free Mg2+, 1; pH 7.0 at 22°C. Pre-activating solution contained (mm): KCl, 91.9; imidazole, 20; creatine phosphate, 14.5; MgATP, 4; free Mg2+, 1; and EGTA, 0.07; pH 7.0 at 15°C and an ionic strength of 180 mm. Activating solution contained (mm): KCl, 79.2; imidazole, 20; creatine phosphate, 14.5; EGTA, 7; MgATP, 4; and free Mg2+, 1; with free Ca2+ concentration ([Ca2+]free) ranging from 1 nm (i.e. pCa 9.0) to 32 μm (i.e. pCa 4.5); pH 7.0 at 15°C and an ionic strength of 180 mm. The compositions of solutions used in the flash photolysis experiments are listed in the legend of Fig. 6. A computer program (Fabiato, 1988) was used to calculate the final concentrations of each metal, ligand and metal-ligand complex using stability constants listed by Godt & Lindley (1982).
Figure 6. NEM-S1 markedly slows the rate of force relaxation.
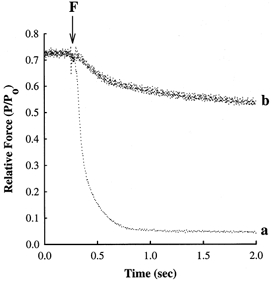
Myocardial preparations were bathed for 15 min in a pCa 9.0 solution in the absence or presence of 6 μm NEM-S1 and subsequently incubated for 2 min in loading solution containing (mm): Bes, 100; potassium propionate, 40.9; creatine phosphate, 15; dithiothreitol, 5; MgATP, 4; free Mg2+, 1; CaCl2, 0.12; and diazo-2, 0.25; pH 7.0 at 15°C. Force relaxation following flash photolysis of diazo-2 was recorded either in the absence (trace a) or in the presence of 6 μm NEM-S1 (trace b), where F refers to the time point at which the flash lamp was triggered. Each of the myocardial preparations generated ≈0.70 Po in the loading solution prior to photolysis of diazo-2.
Myocardial preparation
After the rats had been killed, the heart was rapidly excised, placed in a beaker of ice-cold relaxing solution and trimmed free of atria and great vessels. The left ventricle was cut into small pieces and homogenized in ice-cold relaxing solution for 4 s using a Polytron homogenizer to yield multicellular bundles with dimensions of 600-900 μm x 100-180 μm. The homogenate was centrifuged at 120 g for 1 min and resuspended in fresh relaxing solution. After the second spin, the pelleted myocardial preparations were chemically skinned by resuspending them for 30 min in fresh relaxing solution containing 1% Triton X-100. The preparations were sedimented by gravity, washed twice with fresh relaxing solution and stored on ice until use.
Preparation of NEM-S1
Myosin subfragment-1 (S1) was purified from rabbit fast-twitch skeletal muscle and modified with N-ethylmaleimide (NEM), as described by Swartz & Moss (1992). While NEM-S1 significantly increases actomyosin ATPase activity in the presence of Ca2+ (Williams et al. 1984; Greene et al. 1987), it exhibits little apparent intrinsic ATPase activity (Williams et al. 1984). NEM-S1 forms long-lasting complexes with actin in the presence of ATP and in the presence or absence of Ca2+ (Swartz & Moss, 1992). In this study, the concentration of NEM-S1 was estimated by light absorption at 280 nm (with light-scattering correction performed at 320 nm) using a mass absorptivity value of 0.75 and a molecular weight of 118 000 for S1. Before use, a stock solution of NEM-S1 was dialysed overnight against 20 mm imidazole and 1 mm DTT (pH 7.0). A working solution of NEM-S1 was prepared immediately before use by mixing equal volumes of NEM-S1 stock and a 2x stock of pCa 9.0 solution. The NEM-S1 concentration was adjusted to 6 μm by adding the appropriate amount of 1x pCa 9.0 solution. Prior to mechanical measurements, skinned myocardial preparations were incubated for 15 min at 15°C in pCa 9.0 solution containing 6 μm NEM-S1. Following 15 min of NEM-S1 treatment, skinned myocardium generated a significant amount of Ca2+-independent tension with the rate of force redevelopment (ktr) at pCa 9.0 similar to that measured during maximal activation in a solution of pCa 4.5. We observed no further increase in these values when skinned myocardium was treated with NEM-S1 for longer periods (i.e. > 15 min). Although NEM-S1 has little catalytic activity, it still generates phosphate at an appreciable rate (Swartz & Moss, 1992), which would influence both steady-state force and cross-bridge kinetics of skinned myocardium. Therefore, skinned preparations were initially incubated with NEM-S1 in a solution of pCa 9.0 to allow NEM-S1 binding to actin and were subsequently transferred to pre-activating solution for 1 min and then into activating solutions of varying pCa (i.e. pCa 6.6-4.5) without NEM-S1 for 15-45 s to measure steady-state force and the kinetics of cross-bridge interaction. After each mechanical measurement, the preparation was returned to the pCa 9.0 solution containing NEM-S1. Thus, the skinned myocardium was incubated in NEM-S1-free activating solutions for no more than 2 min, during which time negligible amounts of NEM-S1 would be lost since an extensive time (i.e. ≈1-2 h) is required to wash out NEM-S1 from the myocardial preparations (data not shown).
Experimental apparatus
For mechanical measurements, skinned myocardial preparations (dimensions, 600-800 μm x 100-180 μm) were mounted between a force transducer (model 400A; Aurora Scientific) and a DC torque motor (model 308B; Aurora Scientific) by placing each end (≈100 μm) into stainless steel 25 gauge troughs in an experimental apparatus (Moss, 1979) set on the stage of an inverted microscope (Zeiss). Each myocardial preparation was secured in place by first overlaying each end with a small segment (≈1 mm) of 3-0 monofilament suture, which in turn was subsequently tied in place by two loops of 10-0 monofilament. Light photomicrographs of a skinned myocardial preparation used in this study are shown in Fig. 1. Length and force signals were digitized at 1 kHz, using a 12-bit A/D converter (model AT-MIO-16F-5; National Instruments Corp.), and displayed and stored on a personal computer using customized software based on LabView Full Development System for Windows (version 5.01; National Instruments Corp.). Length changes were driven by computer-generated voltage commands delivered to the torque motor via a 12-bit D/A converter. All experiments were performed at 15°C with sarcomere length set at ≈2.30 μm in relaxing solution. During activation and relaxation, sarcomere length and cell dimensions were recorded on videotape using a video camera (model WV-BL730; Panasonic) and VHS recorder (model SVO-1420; Sony).
Figure 1. Photomicrographs of rat skinned myocardium.

Representative skinned myocardial preparation (dimensions: 430 μm w 174 μm) while relaxed (A, pCa 9.0) and during maximal activation (B, pCa 4.5). Sarcomere length in pCa 9.0 and 4.5 was 2.35 and 2.27 μm, respectively. Scale bar represents 50 μm.
Experimental measurements
Rate of tension redevelopment
The rate constant of force redevelopment (ktr) in skinned myocardium was assessed using a modification of the protocol described previously by Brenner & Eisenberg (1986). Measurement of ktr involves a mechanical slack-restretch manoeuvre to dissociate bound cross-bridges from actin in a steadily Ca2+-activated preparation. The rate of force redevelopment following the slack-restretch manoeuvre reflects the sum of the rate-limiting steps in the transition of myosin cross-bridges to and from (i.e. fapp and gapp, respectively) the strongly bound, force-generating state in steadily activated preparations (Brenner & Eisenberg, 1986). The myocardial preparation was transferred from relaxing solution to activating solutions of varying pCa (i.e. pCa 6.6-4.5) and allowed to develop steady force. The preparation was then rapidly (< 2 ms) slackened by 20% of its original length, resulting in an abrupt reduction in force to near zero and a brief period of unloaded shortening (≈20 ms). To further disrupt cross-bridges, the preparation was rapidly (< 2 ms) restretched to its initial length. A ktr-pCa relationship was obtained by first measuring ktr in a solution of pCa 4.5 and subsequently in a series of submaximal pCa solutions between pCa 6.6 and 5.6. To assess any decline in maximal ktr, the preparation was activated in a solution of pCa 4.5 at the end of each protocol. The reference maximal ktr value for successive submaximal activations was interpolated between the initial and final measurements of maximal ktr. Force redevelopment traces were fitted by a single exponential equation, Ft =Fo(1 - e-kt), where Ft is force at any time t after restretch, Fo is maximum force, and k is ktr. The ktr values reported here were determined without sarcomere length control and may therefore underestimate the true rate constant of tension redevelopment during maximal activation by 15-20% (Chase et al. 1994).
Force-pCa relationship
During measurements of ktr, the skinned myocardial preparation was exposed to solutions of varying pCa (pCa 6.6-4.5) and allowed to develop steady-state force. As a consequence of the slack-restretch manoeuvre, a force baseline was obtained. To obtain Ca2+-activated force for a given pCa, Ca2+-independent force measured in a solution of pCa 9.0 was subtracted from total force (i.e. Ca2+-activated force plus Ca2+-independent force). Force-pCa relationships were determined by (1) expressing submaximal Ca2+-activated force (P) at each pCa as a fraction of maximal Ca2+-activated force (Po) determined at pCa 4.5, i.e. P/Po; and (2) expressing submaximal total force (P*) at each pCa as a fraction of maximal total force (PTot) determined at pCa 4.5, i.e. P*/PTot. The apparent co-operativity of force development was inferred from the steepness of the force-pCa relationship at forces less than half-maximal, which was quantified using a Hill plot transformation of the force-pCa data (Strang et al. 1994). We focused on this region of the curve because the force-pCa relationship is biphasic, i.e. the curve is steeper at high pCa, indicating that most of the co-operative activation of the thin filament occurs at forces less than 0.50 Po (Moss, 1992). Force-pCa data were analysed by least-squares regression using the Hill equation: log(Prel/(1 - Prel)) =n2(log[Ca2+]+k), where Prel is force as a fraction of either Po or PTot, n2 is the Hill coefficient for forces below half-maximal, and k is the [Ca2+] required for half-maximal activation (pCa50). Using pCa50 values derived from the Hill equation, force-pCa relationships (Fig. 3A and B) were fitted using the equation Prel=[Ca2+]n/(kn+[Ca2+]n).
Figure 3. Effects of 6 μm NEM-S1 on force-pCa relationships in skinned myocardium.
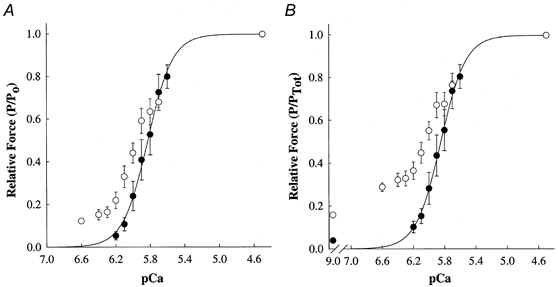
Force-pCa relationships were determined from skinned ventricular myocardium (n = 7) in the absence (0) and presence (1) of NEM-S1. Smooth lines were generated by fitting the mean data with the equation: Prel=[Ca2+]n/(kn+[Ca2+]n), where Prel is force as a fraction of maximal Ca2+-activated force (Po) or maximal total force (PTot), n is the Hill coefficient, and k is the [Ca2+] required for half-maximal activation (i.e. pCa50). Data points are means and the error bars are s.e.m. A, Ca2+-activated force-pCa relationship in the absence (pCa50= 5.85 ± 0.05, n2= 4.72 ± 0.38) and presence of NEM-S1 (pCa50= 5.95 ± 0.04, n2= 1.54 ± 0.07). B, total force-pCa relationship in the absence (pCa50= 5.86 ± 0.05, n2= 4.50 ± 0.69) and presence of NEM-S1 (pCa50= 6.09 ± 0.05, n2= 0.93 ± 0.22). Total force is the sum of Ca2+-independent and Ca2+-activated forces at each pCa.
Rate of force relaxation
The rate constant of force relaxation (krel) in skinned myocardium was determined following flash photolysis of diazo-2 (Molecular Probes) as described previously (Patel et al. 1996). When exposed to a flash of UV light diazo-2 rapidly (> 3000 s−1) chelates Ca2+ due to an increase in the Ca2+-binding affinity from 2.2 μm to 73 nm (Adams et al. 1989). Diazo-2 has been used previously to examine the rate of force relaxation of skinned myocardium (Palmer & Kentish, 1998; Fitzsimons et al. 1998). The rate of force relaxation (krel) is a complex process governed by several factors, including the rate of cross-bridge detachment and of Ca2+ dissociation from troponin C (TnC). Of these, the intrinsic rate of cross-bridge detachment most probably dominates the rate of force relaxation, since the off-rate (koff) of Ca2+ from TnC is approximately 350 s−1 (Dong et al. 1996), which is significantly faster than the rates of relaxation measured in the current study. All photolysis experiments were performed using the solutions listed in the legend of Fig. 6. The amount of force generated prior to flash photolysis of diazo-2 (P) was expressed as a fraction of maximal force (Po) determined at pCa 4.5, i.e. P/Po. The rate of force relaxation was determined using the following equation: krel= ln 2/t1/2, where t1/2 is the time taken for force to decrease by 50% of the initial value.
Statistics
All data are expressed as means ±s.e.m. Where appropriate, either Student's two-tailed t test for independent samples or Student's paired t test was used as a post hoc test of significance, with significance set at P < 0.05.
RESULTS
Influence of NEM-S1 on steady-state force
Previous experiments on skinned skeletal muscle fibres (Swartz & Moss, 1992) and cardiac myocytes (Fitzsimons & Moss, 1998) showed that 6 μm NEM-S1 elicited a steady increase in submaximal Ca2+-activated force, without reducing maximum Ca2+-activated force (Po). However, higher levels of NEM-S1 caused a progressive reduction in Po, most probably due to competitive inhibition of endogenous cross-bridge binding (Swartz & Moss, 1992). Therefore, in the present study of the activating effects of strong-binding myosin cross-bridges in myocardium, skinned preparations were treated with 6 μm NEM-S1. Control experiments showed no effects of unmodified S1 (i.e. no NEM modification) on steady force or cross-bridge kinetics because of the rapid cycling rate of unmodified exogenous S1 (data not shown).
The effects of 6 μm NEM-S1 on the maximum total tension (PTot), maximum Ca2+-activated tension (Po), Ca2+ sensitivity of tension and Hill coefficient (n2) in rat skinned myocardium are summarized in Table 1. Although 6 μm NEM-S1 did not alter total maximum tension, Ca2+-independent tension at pCa 9.0 was significantly increased and there was a concomitant reduction in maximal Ca2+-activated tension. In the absence of NEM-S1, Ca2+-independent tension accounted for ≈4% of total tension (4.4 ± 0.7%) in maximally activated preparations, which increased nearly 4-fold after NEM-S1 treatment (16.5 ± 1.4%) (Fig. 2). This large NEM-S1-induced increase in Ca2+-independent force (3.7 ± 0.8 vs. 0.8 ± 0.3 mN mm−2) is unique to myocardium since skinned skeletal muscle fibres treated with 6 μm or higher concentrations of NEM-S1 exhibited much smaller increases in Ca2+-independent tension (Swartz & Moss, 1992). The increase in Ca2+-independent force appeared to be the result of increased binding of endogenous cross-bridges due to NEM-S1-induced activation of the thin filament (as discussed below).
Table 1.
Steady-state mechanical measurements in skinned left ventricular myocardium in the absence and presence of 6 μM NEM-S1
| Group | PTot | Po | Prest | n2 | pCa50 | ΔpCa50 |
|---|---|---|---|---|---|---|
| (mN mm-2) | (mN mm-2) | (mN mm-2) | ||||
| Control (7) | 25.8 ± 1.8 | 24.7 ± 1.7 | 0.8 ± 0.3 | 4.72 ± 0.38 | 5.85 ± 0.05 | |
| 6 μM NEM-S1 (7) | 23.7 ± 2.1 | 19.7 ± 1.6* | 3.7 ± 0.8* | 1.54 ± 0.07* | 5.95 ± 0.04* | 0.11 ± 0.02 |
All values are expressed as means ± S.E.M., with the number of ventricular preparations given in parentheses. PTot, maximal total tension (maximal Ca2+-activated tension plus Ca2+-independent tension); Po, maximal Ca2+-activated tension; Prest, Ca2+-independent tension at pCa 9.0; n2, Hill coefficient for Ca2+-activated force below 0.50 Po; pCa50, pCa required for half-maximal activation; ΔpCa50, change in pCa50 following addition of 6 μM NEM-S1.
*Significantly different from control (P < 0.05).
Figure 2. NEM-S1 increases Ca2+-independent force.
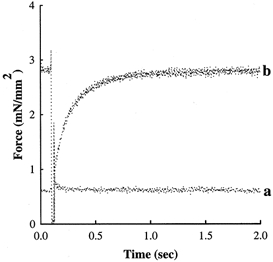
A mechanical release-restretch manoeuvre was used to measure the rate of force redevelopment in a solution of pCa 9.0 in the absence and presence of 6 μm NEM-S1. This skinned myocardial preparation generated Ca2+-independent force of 0.05 Po in the absence of NEM-S1 in pCa 9.0 (trace a), whereas Ca2+-independent force increased to 0.22 Po following NEM-S1 treatment (trace b).
NEM-S1 also increased submaximal Ca2+-activated force (Fig. 3a). Mean pCa50 increased significantly after NEM-S1 treatment (ΔpCa50= 0.11 ± 0.02, P < 0.05), indicating an increase in the Ca2+ sensitivity of force. These results are consistent with the idea that NEM-S1 promotes the formation of strongly bound force-generating cross-bridges at low levels of Ca2+. At progressively higher Ca2+ concentrations (i.e. pCa < 5.8), NEM-S1 had negligible effects on Ca2+-activated tension compared to control. Commensurate with the increase in force at low [Ca2+], NEM-S1 significantly reduced the steepness (n2) of the force-pCa relationship, in agreement with results reported previously in skinned ventricular myocytes (Fitzsimons & Moss, 1998) and skeletal muscle fibres (Swartz & Moss, 1992). Similar effects of NEM-S1 were evident in the total force-pCa relationships obtained from these same preparations (Fig. 3b). Prior to NEM-S1 treatment, mean pCa50 was 5.86 ± 0.05, which increased to 6.09 ± 0.05 in the presence of NEM-S1 (ΔpCa50= 0.23 ± 0.03, P < 0.05). The Hill coefficient for the total force-pCa data was markedly reduced in the presence of NEM-S1 (n2 was 0.93 ± 0.22 vs. 4.50 ± 0.69 in untreated myocardium; P < 0.05), which suggests that 6 μm NEM-S1 saturated the co-operativity evident in the activation of tension in the absence of NEM-S1.
Acceleration of cross-bridge kinetics by NEM-S1
Rate of force redevelopment
The rate constant of force redevelopment (ktr) following rapid release and restretch (Brenner & Eisenberg, 1986) has been used to estimate the rate of weak- to strong-binding cross-bridge transitions in muscle. We recorded force redevelopment in skinned myocardium in the absence and presence of NEM-S1 to determine whether the NEM-S1-induced potentiation of submaximal Ca2+-activated force was associated with an acceleration of the transition(s) to strongly bound cross-bridge states. Examples of the effects of NEM-S1 on the time course and rate of force development are shown in Fig. 4. In the absence of NEM-S1, maximum Ca2+-activated force (Po) developed in a solution of pCa 4.5 with an apparent rate constant (ktr) of 8.25 s−1, whereas force in a solution of pCa 5.8 developed to a steady level of 0.47 Po with a ktr of 2.00 s−1. However, addition of NEM-S1 increased the steady-state force at pCa 5.8 to 0.61 Po and increased ktr to a near-maximal value of 7.41 s−1.
Figure 4. NEM-S1 potentiates submaximal force and the rate of force redevelopment.
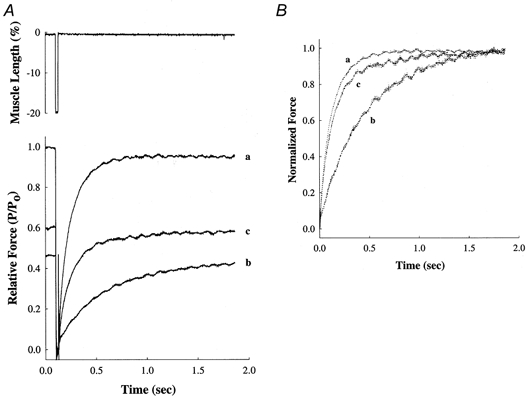
A, force records showing redevelopment of submaximal force developed by a skinned ventricular preparation at pCa 5.8 both in the absence (trace b) and in the presence of 6 μm NEM-S1 (trace c). Force in each case is scaled to the maximum force (Po) generated by the same preparation when exposed to a solution of pCa 4.5 (trace a). The upper panel shows the mechanical release-restretch manoeuvre used to measure ktr. B, to illustrate differences in the rate of force redevelopment, the force records in A are re-plotted with the peak force at each pCa scaled to 1.0.
A summary of the ktr and relative ktr values for several levels of Ca2+ activation is presented in Table 2, and cumulative ktr-pCa relationships obtained in the presence or absence of NEM-S1 are presented in Fig. 5A.. In the absence of NEM-S1, ktr varied with the level of Ca2+, increasing progressively from 1.11 ± 0.21 s−1 at pCa 6.0 to 9.28 ± 0.41 s−1 at pCa 4.5. The Ca2+ dependence of ktr in skinned myocardium observed here is consistent with the results of several earlier studies (Wolff et al. 1995; Palmer & Kentish, 1998; Baker et al. 1998). However, addition of NEM-S1 significantly reduced the Ca2+ dependence of ktr; that is, mean ktr was 9.38 ± 0.30 s−1 at pCa 6.6 as compared to 9.23 ± 0.27 s−1 at pCa 4.5 (Fig. 5a). Even at very low [Ca2+], i.e. pCa 9.0, the induction of Ca2+-independent force by NEM-S1 was accompanied by an increase in ktr to 9.42 ± 0.67 s−1.
Table 2.
Summary of ktr and Ca2+-activated force at maximal and submaximal concentrations of Ca2+ before and after treatment with 6 μM NEM-S1 in skinned left ventricular myocardium
| Control (7) | pCa 6.0 | pCa 5.8 | pCa 4.5 |
|---|---|---|---|
| P/Po | 0.24 ± 0.07 | 0.53 ± 0.09 | 1.00 ± 0.00 |
| ktr (s-1) | 1.11 ± 0.21 | 2.93 ± 0.37 | 9.28 ± 0.41 |
| Relative ktr | 0.10 ± 0.01 | 0.25 ± 0.03 | 1.00 ± 0.00 |
| 6 μM NEM-S1 (7) | pCa 6.4 | pCa 6.2 | pCa 5.9 | pCa 4.5 |
|---|---|---|---|---|
| P/Po | 0.15 ± 0.02 | 0.22 ± 0.04 | 0.59 ± 0.06 | 1.00 ± 0.00 |
| ktr (s-1) | 9.69 ± 0.44 | 8.91 ± 0.40* | 7.45 ± 0.57* | 9.23 ± 0.27 |
| Relative ktr | 1.02 ± 0.06 | 0.96 ± 0.03 | 0.88 ± 0.02 | 1.00 ± 0.00 |
All values are expressed as means ± S.E.M., with the number of ventricular preparations given in parentheses. P/Po, relative Ca2+-activated force; ktr, rate of tension redevelopment; relative ktr, relative rate of force redevelopment normalized to the maximal rate obtained at pCa 4.5 under the same conditions (i.e. either control or in the presence of NEM-S1).
*Significantly different from control for given level of force (P < 0.05).
Figure 5. NEM-S1 nearly eliminates the activation dependence of the rate of force redevelopment.
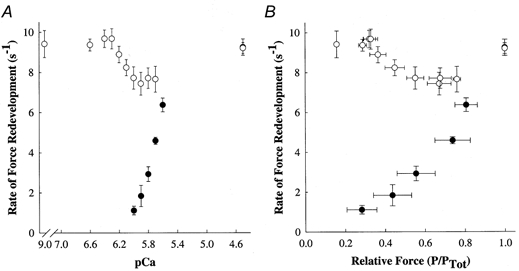
Force redevelopment following rapid release and restretch was measured in rat skinned myocardium (n = 7) in the absence (0) and presence (1) of NEM-S1. Data points are means and the error bars are the s.e.m. A, the Ca2+ dependence of the rate constant of force redevelopment, ktr. B, the rate constant of force redevelopment (ktr) as a function of force (as a fraction of total force) measured at each pCa. Total force is the sum of Ca2+-independent and Ca2+-activated forces at each pCa.
The rate constant of force redevelopment was also plotted vs. total steady-state isometric force at each pCa, in order to assess the variation of ktr with a variable that reflects the effects of both Ca2+ and strong-binding cross-bridges on thin filament activation (Fig. 5B). In the absence of NEM-S1, ktr increased as a function of increasing isometric force, gradually at low forces and more steeply at high forces, an observation that is consistent with previous studies in skeletal (Brenner, 1988; Metzger et al. 1989; Swartz & Moss, 1992) and cardiac muscles (Wolff et al. 1995; Palmer & Kentish, 1998; Baker et al. 1998). However, the relationship between ktr and steady-state isometric force was substantially altered by NEM-S1. At very low forces and at maximum force, ktr was similar to values obtained during maximal activation of the same preparation in the absence of NEM-S1. At intermediate forces, NEM-S1 increased ktr to values greater than control at similar relative forces but still less than the values obtained at both low and maximum forces. These data are consistent with the idea that NEM-S1 co-operatively promotes the binding of endogenous cross-bridges at low and intermediate levels of activation.
Rate of force relaxation (krel) following flash photolysis of diazo-2
To investigate the possible involvement of strong-binding cross-bridges in determining the rate of thin filament inactivation, the relaxation of steady-state force was recorded in the presence and absence of NEM-S1 following photolysis of the photolabile Ca2+ chelator diazo-2 (Fig. 6). The conditions used to determine the rate of relaxation were designed so that control preparations would relax by greater than 90% from pre-photolysis steady-state force. When incubated in loading solution containing 0.12 mm CaCl2 and 0.25 mm diazo-2, each preparation generated a pre-flash steady force of ≈0.70 Po. Following flash photolysis of diazo-2, there is an almost instantaneous decrease in intracellular [Ca2+] (Adams et al. 1989), such that relaxation, and hence the rate of thin filament inactivation, most probably reflects the rate of cross-bridge detachment (Palmer & Kentish, 1998). Although steady force decreased in each preparation following flash photolysis of diazo-2, the extent and rate of force relaxation were dramatically reduced by NEM-S1. In the absence of NEM-S1, steady-state force declined to near-zero active force with an apparent rate constant (krel) of 6.9 s−1, whereas force decreased by only 20% with a krel of ≈0.9 s−1 in the NEM-S1-treated preparation.
DISCUSSION
The main objective of the present study was to determine the role of strong-binding myosin cross-bridges in the activation of myocardial force. Our results show that application of NEM-S1 to skinned myocardium co-operatively promotes the formation of endogenous strongly bound force-generating cross-bridges, as manifested by: (1) significant increases in Ca2+-independent tension at pCa 9.0, (2) potentiation of submaximal Ca2+-activated tension and a corresponding increase in the Ca2+ sensitivity of force (i.e. a leftward shift in the force-pCa relationship), (3) significant reductions in the Ca2+ dependence of the rate of force redevelopment (ktr), and (4) marked slowing of the rate of relaxation (krel).
Effects of NEM-S1 on steady-state force
Our initial hypothesis was that an increase in the number of strong-binding cross-bridges would increase the co-operative activation of the thin filament, which would be manifested by increases in submaximal Ca2+-activated tension and in the Ca2+ sensitivity of tension. The approach to test this idea was to treat chemically skinned myocardium with NEM-S1, a strong-binding non-tension-generating derivative of myosin sub-fragment-1, as a means to increase the number of strongly bound bridges and thereby presumably promote the formation of endogenous strong-binding cross-bridges (Swartz & Moss, 1992). NEM-S1 elicited a marked increase in Ca2+-independent tension (Fig. 2 and Table 1), as measured in the virtual absence of Ca2+, i.e. pCa 9.0 ([Ca2+]free= 1 nm). The amount of Ca2+-independent tension observed in these experiments suggests that the cardiac-specific thin filament proteins confer greater activating effects of strongly bound cross-bridges in myocardium, since a similar concentration of NEM-S1 had little or no effect on Ca2+-independent tension in skeletal muscle fibres (Swartz & Moss, 1992). This result would imply that relatively few NEM-S1 heads are required to switch on the cardiac thin filament, in contrast to the case for the skeletal muscle thin filament. In addition, both submaximal tension (Fig. 3) and the [Ca2+] required for half-maximal activation (pCa50) (Table 1) were significantly increased in the presence of NEM-S1. These NEM-S1-induced increases in Ca2+-independent force, submaximal force and Ca2+ sensitivity of force could be due to NEM-S1-induced effects to either co-operatively promote the formation of endogenous strong-binding cross-bridges or increase the Ca2+-binding affinity of troponin C. However, the latter possibility seems unlikely since recent studies have suggested that force and not the number of cross-bridges per se leads to increases Ca2+ binding to TnC (Wang & Fuchs, 1994; Fuchs & Wang, 1996), and NEM-S1 does not generate force.
NEM-S1 eliminates the Ca2+ dependence of the rate of force development
In the absence of NEM-S1, the rate of force redevelopment (ktr) was highly variable with the level of activating Ca2+, increasing as Ca2+ was increased from submaximal to maximal concentrations (Fig. 5a). The Ca2+ dependence of ktr observed in the present study is consistent with previous results from both skeletal (Brenner, 1988; Metzger et al. 1989) and cardiac muscle preparations (Wolff et al. 1995; Palmer & Kentish, 1998; Baker et al. 1998). A recent model proposed by Campbell (1997) provides a plausible explanation for such a relationship by predicting that ktr should be slow at low levels of activation due to inter-molecular co-operation in the activation process. According to Campbell, cross-bridges exist in non-cycling and cycling pools, and the distribution between these pools is determined by activating effects on the thin filament due to Ca2+ and strong-binding of cross-bridges. At saturating levels of Ca2+, virtually all cross-bridges are part of the cycling pool so that the rate of force development is determined by the sum of the rate constants of cross-bridge attachment (f) and detachment (g), i.e. there is little effect of cross-bridge binding on thin filament activation. However, at low levels of Ca2+, only a small fraction of cross-bridges are initially recruited into the cycling pool by the binding of Ca2+ to the thin filament, leaving a large fraction in the non-cycling pool. In such a case, the first cross-bridges that bind would co-operatively recruit additional cross-bridges from the non-cycling pool, which would then lead to successive cycles of recruitment. Thus, the rate of force development would be slow at low [Ca2+] and would progressively increase as [Ca2+] is increased due to a progressive reduction in the size of the non-cycling pool.
In the context of Campbell's model (Campbell, 1997), application of NEM-S1 should increase the number of strongly bound cross-bridges and thereby accelerate ktr at low levels of Ca2+, which is the result we observed. The rates of force redevelopment increased to maximal or near-maximal levels in the presence of NEM-S1 at all levels of Ca2+ activation, significantly reducing the Ca2+ dependence of ktr (Fig. 5a). Even at very low [Ca2+]free (i.e. pCa 9.0), NEM-S1-treated myocardium exhibited maximal rates of force redevelopment (Fig. 2), similar to those observed during maximal Ca2+ activation. Furthermore, the relationship between ktr and steady-state isometric force prior to the release- restretch protocol, which is an index of the total activation of thin filaments by Ca2+ and strongly bound cross-bridges, was dramatically altered by NEM-S1 (Fig. 5b). In the presence of NEM-S1, ktr values at very low forces and at maximal force were similar to values obtained during maximal activation in the absence of NEM-S1. At intermediate levels of force, NEM-S1 increased ktr to values greater than control but not as great as those observed at both low and high relative forces.
The fact that NEM-S1 did not completely eliminate the activation dependence of ktr could have several explanations, which we have addressed in another study done on skinned skeletal muscle fibres (D. P. Fitzsimons, J. R. Patel, K. S. Campbell & R. L. Moss, manuscript in preparation). One possibility is that the amount of NEM-S1 we applied was insufficient to bind to all of the functional groups (1 troponin, 1 tropomyosin and associated actins) within the thin filament. While we cannot completely rule out this possibility, we still observed a minimum in ktr at intermediate levels of activation when NEM-S1 concentration was increased to 10 μm (data not shown). It is also possible that other mechanisms contribute to ktr, which seems likely in view of results on skeletal muscle fibres showing that the residual depression of ktr at intermediate levels of activation is eliminated by partial extraction of TnC from the thin filament. Such an intervention is thought to disrupt co-operation between adjacent functional groups within the thin filament (Moss et al. 1985), suggesting that NEM-S1 does not significantly facilitate such co-operation. We have discussed these mechanisms in detail in the above study (D. P. Fitzsimons, J. R. Patel, K. S. Campbell & R. L. Moss, manuscript in preparation), but whatever the cause of the lesser effects of NEM-S1 on ktr at intermediate levels of activation, the present results show clearly that strong-binding cross-bridges accelerate the kinetics of cross-bridge binding in myocardium.
Additional results from the present study further support the idea that activation of the myocardial thin filament is modulated by strong binding of myosin cross-bridges. Application of NEM-S1 to skinned myocardium reduced both the extent and rate of relaxation from a steady force of 0.70 Po (Fig. 6) and 0.4 Po (data not shown) following photolysis of diazo-2. By forming long-lasting complexes with actin, NEM-S1 appears to promote the attachment of endogenous cross-bridges to actin, thereby slowing the rate of relaxation. A co-operative mechanism similar to this has recently been proposed as an explanation for the slower rates of relaxation observed in mouse hearts expressing significant amounts of α-tropomyosin on a normal β-tropomyosin background (Wolska et al. 1999; Moss, 1999).
Acknowledgments
This study was supported by grants HL-54581 from the National Institutes of Health (R.L.M.) and 97-GB-90 from the American Heart Association, Wisconsin Affiliate (D.P.F.). The authors would like to thank Chad Warren for the preparation of NEM-S1.
References
- Adams SR, Kao JPY, Tsien RY. Biologically useful chelators that take up Ca2+ upon illumination. Journal of the American Chemical Society. 1989;111:7957–7968. [Google Scholar]
- Baker AJ, Figueredo VM, Keung EC, Camacho SA. Ca2+ regulates the kinetics of tension development in intact cardiac muscle. American Journal of Physiology. 1998;275:H744–750. doi: 10.1152/ajpheart.1998.275.3.H744. [DOI] [PubMed] [Google Scholar]
- Brenner B. Effects of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proceedings of the National Academy of Sciences of the USA. 1988;85:3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B, Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proceedings of the National Academy of Sciences of the USA. 1986;83:3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophysical Journal. 1997;72:254–262. doi: 10.1016/S0006-3495(97)78664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase PB, Martyn DA, Hannon JD. Isometric force redevelopment of skinned muscle fibers from rabbit with and without Ca2+ Biophysical Journal. 1994;67:1994–2001. doi: 10.1016/S0006-3495(94)80682-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong WJ, Rosenfeld SS, Wang CK, Gordon AM, Cheung HC. Kinetic studies of calcium binding to the regulatory site of troponin C from cardiac muscle. Journal of Biological Chemistry. 1996;271:688–694. doi: 10.1074/jbc.271.2.688. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free and free from specified total ionic concentrations in aqueous solutions containing multiple metals or ligands. Methods in Enzymology. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fitzsimons DP, Moss RL. Strong-binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circulation Research. 1998;83:602–607. doi: 10.1161/01.res.83.6.602. [DOI] [PubMed] [Google Scholar]
- Fitzsimons DP, Patel JR, Moss RL. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. Journal of Physiology. 1998;513:171–183. doi: 10.1111/j.1469-7793.1998.171by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F, Wang Y-P. Sarcomere length versus interfilament lattice spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. Journal of Molecular and Cellular Cardiology. 1996;28:1375–1383. doi: 10.1006/jmcc.1996.0129. [DOI] [PubMed] [Google Scholar]
- Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. Journal of General Physiology. 1982;80:279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene LE, Williams DL, Eisenberg E. Regulation of actomyosin ATPase activity by troponin-tropomyosin: effect of the binding of the myosin subfragment 1 (S-1)-ATP complex. Proceedings of the National Academy of Sciences of the USA. 1987;84:3102–3106. doi: 10.1073/pnas.84.10.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Collins JH, Robertson SP, Potter JD. A fluorescent probe study of Ca2+ binding to the Ca2+ specific sites of cardiac troponin and troponin C. Journal of Biological Chemistry. 1980;255:9635–9640. [PubMed] [Google Scholar]
- Lehrer SS. The regulatory switch of the muscle thin filament: Ca2+ or myosin heads. Journal of Muscle Research and Cell Motility. 1994;15:232–236. doi: 10.1007/BF00123476. [DOI] [PubMed] [Google Scholar]
- McKillop DFA, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophysical Journal. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Journal of General Physiology. 1989;93:855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RL. Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. Journal of Physiology. 1979;292:177–202. doi: 10.1113/jphysiol.1979.sp012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RL. Ca2+ regulation of mechanical properties of striated muscle. Circulation Research. 1992;70:865–884. doi: 10.1161/01.res.70.5.865. [DOI] [PubMed] [Google Scholar]
- Moss RL. Plasticity in the dynamics of myocardial contraction: Ca2+, crossbridge kinetics or molecular cooperation. Circulation Research. 1999;84:862–865. doi: 10.1161/01.res.84.7.862. [DOI] [PubMed] [Google Scholar]
- Moss RL, Giulian GG, Greaser ML. The effects of partial extraction of TnC upon the tension-pCa relation in mammalian skeletal muscle. Journal of General Physiology. 1985;86:585–600. doi: 10.1085/jgp.86.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Kentish JC. Roles of Ca2+ and crossbridge kinetics in determining the maximum rates of Ca2+ activation and relaxation in rat and guinea pig skinned trabeculae. Circulation Research. 1998;83:179–186. doi: 10.1161/01.res.83.2.179. [DOI] [PubMed] [Google Scholar]
- Patel JR, Diffee GM, Moss RL. Myosin regulatory light chain modulates the Ca2+ dependence of the kinetics of tension development in skeletal muscle fibers. Biophysical Journal. 1996;70:2333–2340. doi: 10.1016/S0006-3495(96)79799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaro RJ, Rarick HM. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Circulation Research. 1998;83:471–480. doi: 10.1161/01.res.83.5.471. [DOI] [PubMed] [Google Scholar]
- Strang KT, Sweitzer NK, Greaser ML, Moss RL. Beta-adrenergic receptor stimulation increases unloaded shortening velocity of skinned single ventricular myocytes from rats. Circulation Research. 1994;74:542–549. doi: 10.1161/01.res.74.3.542. [DOI] [PubMed] [Google Scholar]
- Swartz DR, Moss RL. Influence of a strong-binding myosin analogue on calcium-sensitive mechanical properties of skinned skeletal muscle fibers. Journal of Biological Chemistry. 1992;267:20497–20506. [PubMed] [Google Scholar]
- Tobacman LS. Thin filament-mediated regulation of cardiac contraction. Annual Review of Physiology. 1996;58:447–481. doi: 10.1146/annurev.ph.58.030196.002311. [DOI] [PubMed] [Google Scholar]
- Wang Y-P, Fuchs F. Length, force and Ca2+-troponin C affinity in cardiac and slow skeletal muscle. American Journal of Physiology. 1994;266:C1077–1082. doi: 10.1152/ajpcell.1994.266.4.C1077. [DOI] [PubMed] [Google Scholar]
- Williams DL, Greene LE, Eisenberg E. Comparison of effects of smooth and skeletal muscle tropomyosins on interactions of actin and myosin subfragment 1. Biochemistry. 1984;23:4150–4155. doi: 10.1021/bi00313a022. [DOI] [PubMed] [Google Scholar]
- Wolff MR, McDonald KS, Moss RL. Rate of tension development in cardiac muscle varies with level of activator calcium. Circulation Research. 1995;76:154–160. doi: 10.1161/01.res.76.1.154. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Keller RS, Evans CC, Palmiter KA, Philips RM, Muthuchamy M, Oehlenschlager J, Wieczorek DF, De Tombe PP, Solaro RJ. Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells expressing beta-tropomyosin. Circulation Research. 1999;84:745–751. doi: 10.1161/01.res.84.7.745. [DOI] [PubMed] [Google Scholar]


