Abstract
Hyperosmotic solutions cause markedly enhanced spontaneous quantal release of neurotransmitter from many nerve terminals. The mechanism of this enhancement is unknown. We have investigated this phenomenon at the frog neuromuscular junction with the aim of determining the degree to which it resembles the modulation of release by stretch, which has been shown to be mediated by mechanical tension on integrins.
The hypertonicity enhancement, like the stretch effect, does not require Ca2+ influx or release from internal stores, although internal release may contribute to the effect.
The hypertonicity effect is sharply reduced (but not eliminated) by peptides containing the RGD sequence, which compete with native ligands for integrin bonds.
There is co-variance in the magnitude of the stretch and osmotic effects; that is, individual terminals exhibiting a large stretch effect also show strong enhancement by hypertonicity, and vice versa. The stretch and osmotic enhancements also can partially occlude each other.
There remain some clear-cut differences between osmotic and stretch forms of modulation: the larger range of enhancement by hypertonic solutions, the relative lack of effect of osmolarity on evoked release, and the reported higher temperature sensitivity of osmotic enhancement. Nevertheless, our data strongly implicate integrins in a significant fraction of the osmotic enhancement, possibly acting via the same mechanism as stretch modulation.
Hypertonic solutions cause a marked increase in spontaneous quantal neurotransmitter release at many synapses (see for example, Fatt & Katz, 1952; Hubbard et al. 1968; Kita & Van der Kloot, 1977; Niles & Smith, 1982; Bourque & Renaud, 1984; Doherty et al. 1986; Brosius et al. 1992; Yu & Miller, 1995; Rosenmund & Stevens, 1996; Mochida et al. 1998). At frog neuromuscular junctions, 25-100 mosmol l−1 hypertonicity produces a reversible, many-fold increase in miniature endplate potential (mEPP) frequency that develops in 5-10 min to reach a broad peak, and then declines approximately exponentially over a period of 10-20 min to settle at an elevated plateau (Kita & Van der Kloot, 1977; Tanabe & Kijima, 1988; Van der Kloot & Molg–, 1994). Effects on evoked release are smaller and less consistent. Furshpan (1956) and Hubbard et al. (1968) reported no effect of moderate changes in osmolarity, while Barton et al. (1983) reported an initial increase in EPP quantal content that was proportional to the change in mEPP frequency, but of smaller magnitude. Large increases in osmolarity suppress evoked release at frog and rat neuromuscular junctions (Thesleff, 1959; Hubbard et al. 1968; Kita & Van der Kloot, 1977), possibly due to depletion of releasable quanta. Similarly, at the autapses formed on hippocampal cells in culture, Rosenmund & Stevens (1996) showed a transient potentiation of evoked release followed by depression, paralleling changes in spontaneous release. The depression was interpreted as a reflection of depletion, although the Ca2+ currents associated with evoked release also were reduced, as has also been reported for Ca2+ currents associated with hormone secretion from anterior pituitary cells (Matzner et al. 1996).
The mechanism of enhancement of spontaneous quantal release is not known. It is not dependent on Ca2+ entry (Furshpan, 1956; Hubbard et al. 1968; Blioch et al. 1968; Quastel et al. 1971; Kita & Van der Kloot, 1977), but there is support from work on the frog neuromuscular junction (Shimoni et al. 1977) and avian ciliary ganglion (Brosius et al. 1992) for the hypothesis that hyperosmolarity causes release of Ca2+ from internal stores. Conversely, in other systems there is convincing evidence that the hypertonicity effect is not dependent on elevated [Ca2+]i. In both hippocampal (Rosenmund & Stevens, 1996) and superior cervical ganglion cell cultures (Mochida et al. 1998), loading the cell with BAPTA and/or interfering with internal Ca2+ sequestering mechanisms, in a zero-Ca2+ Ringer solution, did not suppress the hypertonicity effect. The total increment in quantal release due to application of highly hypertonic solution has been used to determine the size of the ‘readily releasable pool’ of quanta (Stevens & Tsujimoto, 1995; Rosenmund & Stevens, 1996), which appears to correspond to the similarly named pool in the Drosophila neuromuscular junction, where use of the shibire mutant led to the identification of the ‘immediately releasable’, ‘readily releasable’ and ‘reserve’ pools (Delgado et al. 2000).
We became interested in the enhancement of release by hyperosmotic solutions while studying another form of modulation of release from frog motor nerve terminals, also first noted by Fatt & Katz (1952) - the enhancement of release by muscle stretch. This is a robust modulation, amounting to approximately a 10% increase in both spontaneous and evoked release for each 1% stretch within the physiological range (Hutter & Trautwein, 1956; Turkanis, 1973; Chen & Grinnell, 1995, 1997). This modulation, which is independent of changes in intraterminal [Ca2+]i, is mediated by mechanical stress on integrins which somehow link ligands in the extracellular matrix (ECM) to active zone structures in the terminal, regulating the probability of release (Chen & Grinnell, 1995, 1997). Since a change in osmolarity would be expected to cause shrinking or swelling of the terminal, and potentially a change in stress on molecules connecting the presynaptic and postsynaptic membranes (Robbins & Polak, 1989), this form of mechanical stress could explain at least part of the hypertonicity-stimulated release. In this paper, we present evidence that this is the case.
METHODS
Male and female Rana pipiens, ranging from 4 to 7 cm in size, were anaesthetized with 0.1% tricaine methane sulfonate (Sigma), doubly pithed and laid on a bed of ice. Both cutaneous pectoris (CP) muscles were dissected out with approximately 3-5 mm of the nerve attached and immediately bathed in normal frog Ringer (NFR) solution (composition, mm: 116 NaCl, 2 KCl, 1.8 CaCl2, 1 NaHCO3, 5 Hepes acid buffer, 1 MgCl2, 3 glucose and NaOH to adjust pH to 7.4 ± 0.1). In experiments testing the effect of elevated K+, Na+ was reduced proportionately to maintain the same osmolarity. Once the dissection was completed, the preparation was maintained at 4°C until use - typically 20-40 min later.
During all electrophysiological recordings, the preparation was pinned down on a thin layer of Sylgard in a Petri dish (1.5-3 ml volume) and maintained at 21 ± 1°C except where specified otherwise, with Peltier bimetallic elements underlying the metal plate holding the preparation chamber. Resting muscle length was taken to be 2.25 μm per sarcomere, the length at which the muscle was pinned when only osmotic effects were being studied. In most experiments where muscle length was changed, the muscle was stretched or relaxed until sarcomere spacing was the desired value and pinned at that length. Typically the effects of stretch were determined with a stretch of 15-20% above the original muscle length, i.e. to a sarcomere spacing of 2.6-2.7 μm. Since different fibres differed in sarcomere spacing by a few per cent, it was not feasible to specify length more accurately than this. In a few experiments, ‘floating’ microelectrodes were used to record continuously from individual fibres before, during and after stretch. Muscle stretch was imposed by a computer-controlled mechanical apparatus described in detail elsewhere (Chen & Grinnell, 1997).
Recordings from identified fibres were made with glass microelectrodes (Garner Glass Co., KG-33) pulled on a Narashige horizontal puller. The tips were then bent at about a 45 deg angle to permit penetration at an angle approximately perpendicular to the fibre surface at x200 using an Olympus x20 water immersion objective. Electrodes were filled with 0.6 m potassium acetate solution containing 5 mm KCl (D'Alonzo & Grinnell, 1985) and had tip resistances of 60-90 MΩ.
Baseline recordings of mEPPs and/or endplate potentials (EPPs) were obtained in NFR solution from a series of 10-20 identified surface fibres, which could be recognized using structural landmarks and studied repeatedly. Experimental changes in muscle length, external Ca2+ concentration, or Ringer solution tonicity were then made and recordings taken again from the same junctions. Changes in bathing solution were done in one of two ways. (1) In cases of change in Ca2+ concentration all of the previous solution was removed and exchanged with at least two bath volumes of the new solution. Bath changes took no more than 1-2 min, but were followed by 15-20 min to allow adjustment to the new solution before recordings were made again. (2) Changes in osmolarity were made by perfusion of the preparation with several bath volumes of NFR solution containing 25, 50, 75, or 100 mm sucrose at a flow rate of 1.4 ml min−1 (Minipuls 2 pump, Gilson). Most of our experiments involved elevations of only 25-50 mosmol l−1, which caused changes in mEPP frequency similar to those caused by stretch in the physiological range. This is much less elevation in osmolarity than has been used in many other studies, but more likely to be relevant to junctional physiology than elevations of 100-500 mosmol l−1. Preparations were allowed 30-45 min to stabilize after changes in bathing solution before recordings were made. The time course of the hyperosmotic enhancement was obtained by recording from single fibres during the perfusion of the hyperosmotic solution into the bathing chamber. In some cases 25 μmμ-conotoxin (Bachem), a selective blocker of muscle Na+ channels (Cruz et al. 1985), was applied for 10-20 min to prevent twitching (Robitaille & Charlton, 1992). In all experiments where only mEPPs were recorded, 1 μg ml−1 of neostigmine (Sigma) was added to enhance mEPP amplitudes. Records were saved on a Pentium processor-based computer using pCLAMP (v. 5.6, Axon Instruments). Means are given ±s.e.m. and significance was judged by Student's t test.
‘Zero-Ca2+ Ringer solution’ had the same composition as NFR solution but lacked CaCl2 and contained 2 mm Mg2+ and 1 mm EGTA (Sigma). In some experiments, preparations were loaded with 25 μm of the acetoxymethyl ester (AM) form of BAPTA (Molecular Probes) for 1-1.5 h at 4°C, in the presence of 10 μl ml−1 tetrakis(2-pyridylmethyl)ethylenediamine (TPEN, Molecular Probes) before baseline recordings were taken. To disable the native Ca2+ buffering by the endoplasmic reticulum, 20 μm thapsigargin (Gibco), prepared by dilution from a stock solution of 5 mm in dimethyl sulphoxide (DMSO), was added to the Ringer solution (Thastrup et al. 1989). To test the possible role of integrin binding in the response to hyperosmotic solutions, preparations were pre-treated for 1.5 h in a low divalent (LD) Ringer solution containing 50 μm Ca2+ and 50 μm Mg2+ (composition, mm: 116 NaCl, 1 NaHCO3, 2 KCl, 10 EDTA, 9.9 CaCl2, 0.175 MgCl2, 5 Hepes sodium salt, 5 Hepes acid) to weaken integrin-ligand bonds, which are divalent dependent in the frog junction (Chen & Grinnell, 1995, 1997), as in many other preparations (Gailit & Ruoslahti, 1988; Kirchhofer et al. 1991; Orlando & Cheresh, 1991). Experimental solutions contained 0.2 mm of the hexapeptide GRGDSP, while controls had NFR solution or the inactive peptide GRGESP. These low divalent solutions were applied for 90 min prior to testing the effects of changes in osmolarity. These were the same conditions used in earlier studies to test the effects of GRGDSP on the stretch enhancement of release (Chen & Grinnell, 1995, 1997).
RESULTS
Magnitude and kinetics of the hyperosmotic enhancement
Our results were consistent with earlier studies in showing that exposure to hypertonic Ringer solution caused a transient large increase in spontaneous quantal release that declined to a plateau level well above the original release frequency within 20-30 min. The mEPP frequency of control junctions in the present experiments showed a mean sustained increase of 2.45 ± 0.07-fold (n = 94) with addition of 25 mm sucrose (Fig. 1, columns 1 and 2) and a 5.74 ± 0.06-fold (n = 76) increase with addition of 50 mm sucrose. At these levels of osmolarity, the size distribution of mEPPs is the same as that in NFR solution, and all are effectively blocked by d-tubocurine chloride (data not shown).
Figure 1. Lack of Ca2+ dependence of hypertonicity response.
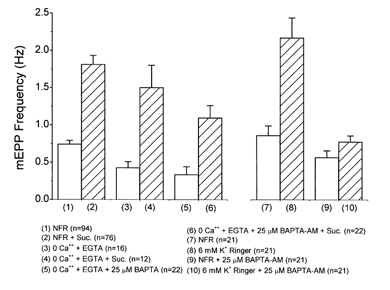
Addition of 25 mm sucrose to NFR solution caused a 2.45 ± 0.07-fold (n = 76) increase in the mEPP frequency (bars 1 and 2). Bathing preparations in a zero-Ca2+, EGTA-buffered Ringer solution, which eliminates Ca2+ influx, did not reduce the enhancement of mEPP frequency by addition of 25 mm sucrose. MEPP frequency increased by 3.52 ± 0.20 times (n = 12) (bars 3 and 4). Intracellular buffering with 25 μm BAPTA AM, coupled with the removal of extracellular Ca2+, also failed to eliminate osmotic enhancement of mEPP frequency. Addition of 25 mm sucrose caused a 3.86 ± 0.12-fold increase (n = 32) (bars 5 and 6). Hypertonicity caused a significant effect at the P < 0.01 level in all cases. Bars 7-10 show that loading of preparations with 25 μm BAPTA AM was effective in buffering intraterminal Ca2+. Preparations with and without BAPTA loading were slightly depolarized by elevation of K+ in the Ringer solution to 6 mm, increasing Ca2+ influx. Junctions without BAPTA showed an elevation in mEPP frequency of 2.27 ± 0.22-fold (n = 23). The same treatment of BAPTA-loaded preparations produced much smaller changes. The resting mEPP frequency after BAPTA loading was slightly reduced relative to the NFR solution condition. Addition of 6 mm K+ caused a much reduced increase (bars 9 and 10).
The effect of hyperosmolarity on EPP quantal content was much less dramatic. Of 22 junctions studied in three muscles, changing from NFR solution to NFR + 50 mm sucrose caused no significant change (< 5%) in seven junctions, an elevation in EPP amplitude in six (mean +70 ± 16%), and a decrease in nine (mean -23 ± 4%). The mean effect was a 9.6 ± 9.4%(n = 22) increase. Because the effect of hyperosmotic solutions on EPP quantal content was not consistent in direction or magnitude, we focused our research on the modulation of spontaneous release (mEPP frequency).
Ca2+ independence of hypertonicity enhancement of release
We have confirmed the findings that hyperosmotic solutions enhance mEPP frequency even in the absence of external Ca2+ (Blioch et al. 1968; Kita & Van der Kloot, 1977; Shimoni et al. 1977). After 1.5 h in a zero-Ca2+ Ringer solution containing 1 mm EGTA, the addition of 25 mosmol l−1 sucrose caused a 3.5 ± 0.20-fold increase in mEPP frequency, a larger effect than the mean 2.45 ± 0.07-fold increase produced by 25 mosmol l−1 sucrose in NFR solution (Fig. 1, columns 1-4). To test whether hyperosmotic enhancement was dependent on an elevation in [Ca2+]i due to release of Ca2+ from internal stores, we treated preparations for 1.5 h in an EGTA-buffered zero-Ca2+ Ringer solution containing 25 μm BAPTA AM. The combination of zero-Ca2+ external Ringer solution and internal Ca2+ buffering reduced the resting mEPP frequency by about 50%, but there was still a strong hyperosmotic enhancement (Fig. 1, columns 5 and 6). Independent experiments showed that the BAPTA loading of terminals was effective, since the same procedures strongly suppressed the increase in mEPP frequency produced by depolarization with 6 mm K+ in NFR solution (Fig. 1, columns 7-10). Without the extrinsic buffer, depolarization by 6 mm K+ caused a 127 ± 22% increase. In BAPTA-loaded terminals, the effect of was an increase of only 48 ± 12%.
It is possible that Ca2+ could be released from a compartment immediately adjacent to the Ca2+-sensing molecules responsible for triggering release, and that BAPTA is not able to buffer it before it has had its effect. To test this possibility, we treated preparations with 20 μm thapsigargin, an inhibitor of Ca2+-ATPase, which disables the Ca2+ buffering capabilities of the endoplasmic reticulum, one of the principal cytoplasmic Ca2+ sequestering/releasing compartments (Thastrup et al. 1989). Thapsigargin caused a transient increase in mEPP frequency as Ca2+ was released from internal compartments; then the frequency dropped back to a baseline level usually somewhat below that in NFR solution. In junctions treated for 1 h or more with thapsigargin, the hypertonicity enhancement was still present. The addition of 50 mm sucrose enhanced mEPP frequency by a factor of 5.74 ± 0.06 without thapsigargin treatment, and by a factor of 7.17 ± 0.08 in thapsigargin-treated junctions (Fig. 2, columns 1-4). Similarly, in a zero-Ca2+ Ringer solution and after thapsigargin treatment, 50 mm sucrose induced a 3.2-fold increase in mEPP frequency (Fig. 2, columns 5-6). This increase was less than that without thapsigargin (5.74 ± 0.06-fold increase), suggesting that Ca2+ from internal stores can contribute to the response to hypertonicity. There is still a strong response, however, even with [Ca2+]i release suppressed. We conclude, therefore, that the effect of hypertonicity in enhancing spontaneous release is not dependent on Ca2+ influx, and that a large fraction of the modulation persists when [Ca2+]i release from internal stores close to active zones has been blocked.
Figure 2. Effects of thapsigargin, an irreversible inhibitor of endoplasmic reticulum Ca2+-ATPase, on osmotic enhancement of release by Ringer solution containing 50 mm sucrose.
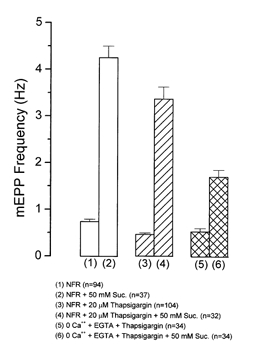
In NFR solution the baseline frequency (0.74 ± 0.05 Hz, n = 94) increased to 4.25 ± 0.25 Hz, (n = 37), a 5.74 ± 0.06-fold change (bars 1 and 2). Other preparations were treated for 1 h with 20 μm thapsigargin, during which the mEPP frequency increased sharply but then returned to slightly below the original level. Addition of 50 mm sucrose then increased the frequency from 0.47 ± 0.03 Hz (n = 104) to 3.37 ± 0.26 Hz (n = 32), a 7.17 ± 0.08-fold increase (bars 3 and 4). Similarly, in 0 Ca2+, EGTA-buffered Ringer solution, thapsigargin-treated terminals still showed a prominent sucrose effect (bars 5 and 6). The effect of hypertonicity was significant at P < 0.01 in all cases.
Role of integrins in the hyperosmolarity enhancement of release
Many integrins bind to a specific, characteristic sequence of three amino acids in ligands: arginine, glycine and aspartic acid (RGD) (Albeda & Buck, 1990; Reichardt & Tomaselli, 1991; Hynes, 1992). Addition of an extrinsic peptide containing the RGD sequence can interfere with the native binding. In many preparations, the hexapeptide GRGDSP (hereafter referred to as RGD) has proved particularly potent in interfering with integrin binding to extracellular matrix (ECM) or substrate molecules (Pierschbacher & Ruoslahti, 1987). A closely related hexapeptide, GRGESP (hereafter RGE), is a good inactive control. RGD reduces sharply the enhancement of spontaneous and evoked release by muscle stretch (Chen & Grinnell, 1995, 1997). Because integrin-ligand binding is divalent cation dependent, the interference can be increased if the preparation is pre-treated with a low divalent (LD) Ringer solution containing 0.2 mm RGD. Under these conditions, 0.2 mm RGD inhibits the stretch enhancement by about 60% (Chen & Grinnell, 1995, 1997). RGD does not have a significant effect on resting mEPP frequency (Chen & Grinnell, 1997).
Figure 3 shows the effect of 0.2 mm RGD on enhancement of spontaneous release measured 30 min or more after addition of 25-100 mm sucrose to LD Ringer solution. The hypertonicity-induced enhancement was reduced by about 40%. The blocking effect of RGD was significant at or exceeding the P < 0.01 level at all sucrose concentrations. RGE had no effect. Thus integrins appear to play a role in the modulation of release by hyperosmolarity.
Figure 3. Effect of 0.2 mm RGD on the enhancement of mEPP frequency by addition of 25, 50, 75 and 100 mm sucrose.
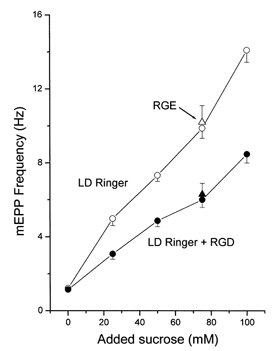
Control and experimental preparations were pretreated for 90 min in low-divalent (LD) Ringer solution. At all sucrose concentrations there was a significant (P < 0.01) inhibition of the hyperosmotic enhancement with the peptide (•) compared to the LD Ringer solution controls (•). Also plotted are control values showing the effect of 75 mm sucrose on mEPP frequency in the presence of the RGE peptide (▵, n = 30) and paired junctions in RGD (▴, n = 28).
In earlier studies of modulation of release by muscle stretch (Chen & Grinnell, 1997), it was found that the effect of RGD could be greatly magnified by bathing the preparation in zero-Ca2+ Ringer solution containing 0.5 mm Mn2+. Indeed, after such treatment, RGD almost completely blocked stretch enhancement of release. Hence we examined the effect of this treatment on the hypertonicity enhancement of release. Zero-Ca2+ Ringer solution slightly reduced the resting mEPP frequency (Fig. 1, column 3) and destabilized integrin bonds. However, addition of 0.5 mm Mn2+, itself a Ca2+ channel blocker, restored the resting mEPP frequency to approximately normal levels and allowed a robust enhancement by 25 mm hypertonicity (Fig. 4), probably because it takes the place of Ca2+ in stabilizing integrin bonds. However, Mn2+-stabilized integrin bonds are extremely sensitive to RGD, which displaces native ligands (see Discussion). When 0.2 mm RGD was added to muscles treated with zero-Ca2+ Ringer solution plus 0.5 mm Mn2+, the effect of an added 25 mm sucrose was largely eliminated (Fig. 4).
Figure 4. Effects of RGD on Mn2+-treated junctions.
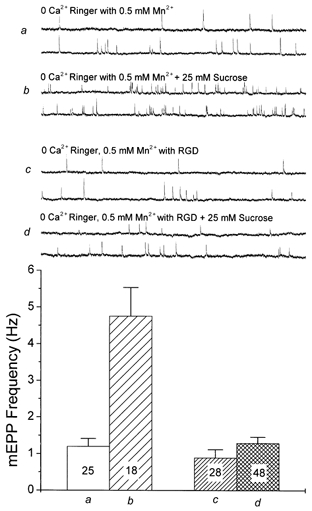
In muscles treated with zero-Ca2+ Ringer and 0.5 mm Mn2+, the resting mEPP frequency was close to normal (single traces and summary bar graph a), and addition of 25 mm sucrose caused an approximately 4-fold increase in frequency (b). Preparations treated similarly but in the presence of 0.2 mm RGD showed a slightly depressed resting mEPP frequency (c) and a severely reduced enhancement of release by 25 mm sucrose (d).
Co-variance of stretch and hyperosmolarity effects on spontaneous release
Different junctions exhibit great variability in the magnitude of the hyperosmotic enhancement, just as the magnitude of the stretch effect is highly variable (Chen & Grinnell, 1997). The explanation for this variability is not clear. However, it can be used to test the hypothesis that the mechanisms of hyperosmotic and stretch enhancement overlap to a significant degree. If they do, one would expect that junctions showing a large hyperosmotic enhancement would also be sensitive to stretch, while those that were insensitive to one treatment would be insensitive to the other. Figure 5 shows the data for 59 junctions from five preparations in which the effects of 15% stretch in NFR solution were compared with the effect of adding 25 mm sucrose to the Ringer solution at rest length in the same junctions. These experiments were done at 12-14°C to maintain the preparation in good condition throughout more than twice the usual experimental period. Cooling reduced the resting mEPP frequency, but did not affect the response to stretch or hypertonicity. Each treatment, by itself, caused a comparable increase in spontaneous release. Although there was a good deal of scatter in the relative effectiveness of stretch and hypertonicity, this figure shows a clear tendency for a large stretch effect to be correlated with a large hypertonicity effect.
Figure 5. Covariance of effects of 15-20% stretch and 25 mm hypertonicity on mEPP frequency.
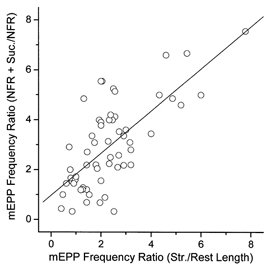
Data are shown for 59 identified junctions in 5 experiments with preparations held at 12-14° C to help keep fibres healthy through both manipulations. The correlation coefficient was 0.71.
Occlusion of stretch and hyperosmolarity enhancements
If stretch and hypertonicity enhance release by the same or related mechanisms, they might be expected to be additive at low levels of modulation and at least partially occlude each other at more extreme levels. If, for example, hyperosmotic solutions cause a shrinkage of terminals, enlarging the synaptic cleft (Robbins & Polak, 1989), this might exert stress on the same integrin bonds that mediate the stretch effect, partially occluding the effect of stretch itself. To test this hypothesis, parallel sets of experiments were done. In one of these, after recording baseline mEPP frequency at rest length in NFR solution, mEPP frequency was measured in the same identified fibres after an approximately 15% stretch, and again after a further treatment with 25 mm hypertonic Ringer solution. In the second set of experiments, the muscle was treated first with the hypertonic solution and then stretched. Only identified junctions in fibres that maintained a good resting potential throughout were used for data analysis. In the first set of experiments (Fig. 6, a), the resting mEPP frequency was a mean of 1.45 ± 0.25 Hz, which increased by approximately 2.7 times to 3.95 ± 0.46 Hz with stretch, and to 4.39 ± 0.4 Hz with subsequent hypertonicity (n = 26 fibres in two muscles). The hypertonic solution added a relatively small increment, a mean increase of < 0.5 Hz. This is a much smaller increase both absolutely as a percentage of the baseline frequency than is normally seen with 25 mm hypertonicity. Indeed, several junctions that had experienced a large increase in mEPP frequency with stretch actually showed a decrease with addition of sucrose.
Figure 6. Partial mutual occlusion of effects of stretch and hypertonicity on mEPP frequency.
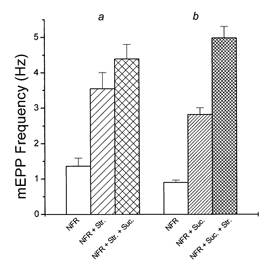
a, data from 23 identified fibres in two muscles studied first in NFR solution, then stretched by approximately 15%, and finally exposed to 25 mm sucrose in NFR solution. b, data from 25 identified fibres in the two muscles paired with those above, tested first in NFR solution, then in 25 mm hypertonic solution, and finally with stretch. Occlusion was particularly evident when stretch preceded hypertonicity.
In the second series of measurements (Fig. 6, b), the same hypertonicity caused the mEPP frequency to increase from a baseline of 0.9 ± 0.07 to 2.81 ± 0.2, an increase of > 3-fold. In this case, subsequent stretch increased the frequency to 4.98 ± 0.32 (n = 25 junctions in two muscles), a substantial addition to the effect of hypertonicity, but a smaller effect, as a percentage of the frequency before stretch, than was usually seen with stretch alone. In a larger set of seven experiments, comparing only junctions with rest frequencies between 0.25 and 1.5 Hz (where the populations overlapped extensively), the effect of stretch was a 4.13 ± 0.58-fold (n = 26) increase in NFR, and a 2.79 ± 0.24-fold (n = 17) increase in NFR solution plus 25 mm sucrose (P < 0.05). Thus both stretch and hyperosmolarity are capable of partially occluding the other.
DISCUSSION
Ca2+ independence of osmotic enhancement of spontaneous release
Our data show that the hypertonicity-induced increase in mEPP frequency does not depend upon the presence of external Ca2+, nor is it affected by loading of terminals with BAPTA, in agreement with findings in hippocampal (Rosenmund & Stevens, 1996) and superior cervical ganglion neurons (Mochida et al. 1998). We conclude, therefore, that it is not dependent on Ca2+ influx or on a general elevation of intraterminal Ca2+. We cannot exclude the possibility that part of the hypertonicity response is mediated by internal release of Ca2+ from compartments so close to release sites that BAPTA cannot fully block its effect. The response, while still quite large, was significantly reduced in a series of measurements where Ca2+ binding by internal compartments had been blocked by thapsigargin. The enhancement of release by stretch has been shown to require a basal level of intraterminal Ca2+ but to be independent of an elevation in [Ca2+]i, either from outside or by internal release from local compartments (Chen & Grinnell, 1995, 1997). A partial dependence on Ca2+ release from local internal stores might help explain the larger magnitude of the hypertonicity response relative to stretch, especially at high levels of hyperosmolarity. It might also help account for the findings of Shimoni et al. (1977) that the response to hypertonicity decreased in a reversed transmembrane Ca2+ gradient.
Involvement of integrins in the hyperosmolarity effect
Perhaps the most convincing evidence that a similar mechanism is involved in both forms of modulation is the observation that the peptide GRGDSP, which interferes with integrin binding to native ligands, sharply reduced the effect of hypertonicity in a low divalent Ringer solution (Fig. 3). The reduction in mEPP frequency (approximately 40%) was less than that observed with the stretch effect (approximately 60%), but still highly significant. More dramatically, in zero-Ca2+ Ringer solution containing 0.5 mm Mn2+, the osmotic enhancement of release by 25 mosmol l−1 sucrose was reduced about 90% by RGD (Fig. 4). This magnification of the effect of RGD by Mn2+, which was also clear in the case of stretch enhancement (Chen & Grinnell, 1997), is consistent with the observation by several groups that Mn2+ appears to sharply increase the affinity of integrin for short peptides containing the RGD sequence, which therefore are better able to displace the native ligand (Gailit & Ruoslahti, 1988; Kirchhofer et al. 1991; Grinnell & Backman, 1991). It has already been shown that the stretch enhancement is a mechanical modulation dependent on integrin binding, presumably to a molecule in the extracellular matrix (ECM) (Chen & Grinnell, 1997). Although integrins could in principle be operating in different ways, the reduction in effect seen with block of their binding is consistent with the hypothesis that at least part of the osmotic modulation involves tension on the same integrins that are involved in the stretch enhancement of release. It should be emphasized, however, that the integrin-blocking peptides did not totally block osmotic enhancement of release, even in a Mn2+-containing solution.
A role for integrins in modulation of release has not been reported at synapses other than the frog neuromuscular junction. However, similar experiments in larval Drosophila neuromuscular junctions reveal that integrins are involved in osmotic enhancement of spontaneous release in that preparation as well (K. Suzuki, A. D. Grinnell and Y. Kidokoro, unpublished observations). Moreover, a Drosophila integrin mutant, volado, has been shown to have defective olfactory memory (Grotewiel et al. 1998). Integrins have been demonstrated both pre- and postsynaptically at rat neuromuscular junctions where they appear to play a number of important developmental roles (Martin et al. 1996; Martin & Sanes, 1997; Nishimura et al. 1998; Wong et al. 1999). Integrins have been implicated in the stabilization of long-term potentiation in the mammalian hippocampus (Bahr et al. 1997), and the widespread and regionally differentiated distribution of different integrins subunits in the brain suggests that they may play a variety of roles in CNS function (Pinkstaff et al. 1999).
Co-variance and mutual occlusion of the effects of hypertonicity and stretch
Our experiments showed that junctions that were particularly sensitive to stretch tended to be particularly sensitive to hypertonicity as well. This does not mean that stretch and hyperosmolarity enhance spontaneous release via the same mechanism, but is consistent with that possibility. If similar mechanisms are involved, one might also expect the effects of stretch and hyperosmolarity to occlude each other to some extent. This prediction was at least partially borne out (Fig. 6). Although the effects of stretch and hypertonicity were clearly additive, some junctions, especially those that had undergone a large enhancement by one manipulation, showed a reduced or opposite effect of the other. It must be noted, however, that this occlusion is almost certainly not the result of the modulation reaching a saturation level, since high levels of hypertonicity, in particular, can produce much larger changes in release probability than occurred in our experiments.
Differences between the stretch and hyperosmolarity forms of modulation of release
Osmotic and stretch modulation are similar in being reduced by peptides that block integrin-ligand binding, in not being dependent on Ca2+ influx or release of Ca2+ from internal stores, and in exhibiting co-variance and partial occlusion. It is necessary also, however, to emphasize that there are differences between the two phenomena and to assess the significance of these differences, as follows.
(1) The absolute magnitude of enhancement of mEPP frequency achievable by changes in osmolarity far exceeds the enhancement that can be obtained by stretch. Mean mEPP frequency can be increased by more than 30-fold by large changes in the osmolarity of the Ringer solution (Blioch et al. 1968; Doherty et al. 1986). Stretch enhancement, in contrast, seldom exceeds a mean of 3- to 4-fold (Hutter & Trautwein, 1956; Turkanis, 1973; Chen & Grinnell, 1995, 1997). Either osmotic enhancement involves additional mechanisms or terminal shrinkage in a hypertonic medium is a much more efficient way of exerting tension on the mechanical pathway modulating release than is muscle stretch. Neither possibility can be ruled out.
(2) The two forms of modulation exhibit very different time courses. The osmotic enhancement develops over minutes, shows a prominent phasic component, and usually decays only slowly to a plateau level. Stretch modulation, in contrast, has a negligible delay and no phasic component and changes linearly with length (Chen & Grinnell, 1997). Nevertheless, shrinkage of a terminal due to osmotic loss of water might be an extremely effective way of exerting tension on bonds between integrins in the presynaptic membrane and their natural ligands in the ECM; and the time course of changes in tension might be very complicated as structures change shape and size due to changes in volume. We do not consider differences in time course to be compelling evidence for different mechanisms.
(3) The Q10 of osmotic enhancement has been reported by others to be about 2 in mammals (Hubbard et al. 1968) and up to 7 in frogs (Kita et al. 1982). The Q10 of the stretch enhancement is close to 1 (Chen & Grinnell, 1997). This difference clearly suggests a difference in mechanism, or in factors coupling stretch and osmotic changes to the same mechanism. We did not study the temperature coefficient systematically. It should be noted, however, that in experiments done at 12-14°C (Fig. 5), the mean enhancement of release by 25 mosmol l−1 was 3.04 ± 0.23-fold (n = 56), fully equivalent to the enhancement at 21°C (2.45 ± 0.07-fold, n = 76). Hence for moderate increases in osmolarity, the Q10 may not be large.
(4) Stretch increases the probability of evoked release in parallel with that of spontaneous release, and to a similar extent (Chen & Grinnell, 1997). In contrast, levels of hyperosmolarity up to 100 mosmol l−1 have been found to have no effect (Furshpan, 1956; Hubbard et al. 1968) or to increase evoked release only moderately (Barton et al. 1983; Tanabe & Kijima, 1988), while larger changes in osmolarity decreased evoked release (Thesleff, 1959; Hubbard et al. 1968; Kita & Van der Kloot, 1977; Rosenmund & Stevens, 1996). Although large increases in osmolarity might be depleting the pool of readily releasable vesicles, it seems unlikely that the moderate increase in frequency produced in our experiments could be causing depletion or explain the relative lack of effect on EPP amplitude of adding 50 mm sucrose. These differences in effect on mEPP frequency and evoked release imply, at the very least, an osmotically driven mechanism of suppression of evoked, but not spontaneous, release.
In summary, our data provide evidence that some fraction of the enhancement of spontaneous release by hypertonic solutions involves integrins, possibly acting via a mechanism common to stretch enhancement. However, there remain some major differences, the most interesting of which are the differences in effect on evoked release and the possible differences in Q10. Both stretch and hypertonicity modulation of release by-pass the Ca2+-triggering step in release. What other aspects of the docking and fusion processes are involved is not clear at this point. At mammalian neuromuscular junctions, hypertonicity is no longer effective after cleavage of SNAP-25 or synaptobrevin by clostridial toxins (Dreyer et al. 1987; Gansel et al. 1987), and at Drosophila neuromuscular junctions, the hypertonicity effect is eliminated in the absence of synaptobrevin, syntaxin, or Unc-13 (Aravamudan et al. 1999). Similarly, in hippocampal cell cultures, cleavage of syntaxin, synaptobrevin, or SNAP-25 eliminates the response to hypertonicity (Capogna et al. 1997). Thus the hypertonicity response, while bypassing Ca2+ triggering, does require much of the molecular apparatus thought to be involved in vesicle docking and fusion. An understanding of the mechanism(s) of integrin-mediated modulation of release is likely to be helpful in understanding molecular events involved in vesicle fusion.
The mechanical modulation of release responsible for the stretch and hypertonicity responses might conceivably also play a role in the long unexplained suppression of evoked and spontaneous release from frog neuromuscular junctions (Parmentier et al. 1981; Ashford et al. 1982) and of secretion from chromaffin cells and mast cells (Heinemann et al. 1987) by moderate levels of hydrostatic pressure. If increased hydrostatic pressure can compress some synaptic structures more than others (e.g. moving pre- and postsynaptic structures closer together), it could change the tension on mechanical links that are involved in regulating release.
Acknowledgments
We thank Drs B. Yazejian and O. Sand for helpful comments on the manuscript. This work was supported by grant from NASA NAG-3964 (to A.D.G.).
References
- Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB Journal. 1990;11:2868–2880. [PubMed] [Google Scholar]
- Aravamudan B, Fergestad T, Davis WS, Roedesch CK, Broadie K. Drosophila Unc-13 is essential for synaptic transmission. Nature Neuroscience. 1999;2:965–971. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- Ashford MLJ, Macdonald AG, Wann KT. The effects of hydrostatic pressure on the spontaneous release of transmitter at the frog neuromuscular junction. Journal of Physiology. 1982;333:531–543. doi: 10.1113/jphysiol.1982.sp014467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr BA, Staubli U, Xiao P, Chun D, Ji ZX, Esteban ET, Lynch G. Arg-Gly-Asp-Ser-selective adhesion and the stabilization of long-term potentiation: pharmacological studies and the characterization of a candidate matrix receptor. Journal of Neuroscience. 1997;17:1320–1329. doi: 10.1523/JNEUROSCI.17-04-01320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton SB, Cohen IS, Van der Kloot W. The calcium dependence of spontaneous and evoked quantal release at the frog neuromuscular junction. Journal of Physiology. 1983;337:735–751. doi: 10.1113/jphysiol.1983.sp014652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blioch ZL, Glagoleva IM, Liberman EA, Nenashev VA. A study of the mechanism of quantal transmitter release at a chemical synapse. Journal of Physiology. 1968;199:11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Activity patterns and osmosensitivity of rat supraoptic neurones in perfused hypothalamic explants. Journal of Physiology. 1984;349:631–642. doi: 10.1113/jphysiol.1984.sp015178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius DC, Hackett JT, Tuttle JB. Ca2+-independent and Ca2+-dependent stimulation of quantal neurosecretion in avian ciliary ganglion neurons. Journal of Neurophysiology. 1992;68:1229–1234. doi: 10.1152/jn.1992.68.4.1229. [DOI] [PubMed] [Google Scholar]
- Capogna M, McKinney RA, O'Connor V, Ghwiler BH, Thompson SM. Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. Journal of Neuroscience. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BM, Grinnell AD. Integrins and modulation of transmitter release from motor nerve terminals by stretch. Science. 1995;269:1578–1580. doi: 10.1126/science.7667637. [DOI] [PubMed] [Google Scholar]
- Chen BM, Grinnell AD. Kinetics, Ca2+ dependence, and biophysical properties of integrin-mediated mechanical modulation of transmitter release from frog motor nerve terminals. Journal of Neuroscience. 1997;17:904–916. doi: 10.1523/JNEUROSCI.17-03-00904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz LJ, Gray WR, Olivera BM, Zeikus RD, Kerr L, Yoshikami D, Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. Journal of Biological Chemistry. 1985;260:9280–9288. [PubMed] [Google Scholar]
- D'Alonzo AJ, Grinnell AD. Profiles of evoked transmitter release along the length of frog motor nerve terminals. Journal of Physiology. 1985;359:235–258. doi: 10.1113/jphysiol.1985.sp015583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rate of mobilization and recycling at the larval neuromuscular junction of a temperature-sensitive Drosophila mutant, shibire. Neuron. 2000 doi: 10.1016/s0896-6273(00)00165-3. in the Press. [DOI] [PubMed] [Google Scholar]
- Doherty P, Hawgood BJ, Smith IC. Changes in miniature end-plate potentials due to moderate hypertonicity at the frog neuromuscular junction. Journal of Physiology. 1986;376:1–11. doi: 10.1113/jphysiol.1986.sp016138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F, Rosenberg F, Becker C, Bigalke H, Penner R. Differential effects of various secretagogues on quantal transmitter release from mouse motor nerve terminals treated with botulinum A and tetanus toxin. Naunyn-Schmiedeberg's Archives Pharmacology. 1987;335:1–7. doi: 10.1007/BF00165027. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. Journal of Physiology. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Furshpan EJ. The effects of osmotic pressure changes on the spontaneous activity of motor nerve endings. Journal of Physiology. 1956;134:689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailit J, Ruoslahti E. Regulation of the fibronectin receptor affinity by divalent cations. Journal of Biological Chemistry. 1988;263:12927–12933. [PubMed] [Google Scholar]
- Gansel M, Penner R, Dreyer F. Distinct sites of action of clostridial neurotoxins revealed by double-poisoning of mouse motor nerve terminal. Pflügers Archiv. 1987;409:533–539. doi: 10.1007/BF00583812. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Backman R. Role of integrin receptors in manganese-dependent BHK cell spreading on albumin-coated substrata. Experimental Cell Research. 1991;195:218–223. doi: 10.1016/0014-4827(91)90520-5. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Heinemann SH, Stühmer W, Conti F. Single AChR-channel currents recorded at high hydrostatic pressures. Proceedings of the National Academy of Sciences of the USA. 1987;84:3229–3233. doi: 10.1073/pnas.84.10.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard JI, Jones SF, Landau EM. An examination of the effects of osmotic pressure changes upon transmitter release from mammalian motor nerve terminals. Journal of Physiology. 1968;197:639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter OF, Trautwein W. Neuromuscular facilitation by stretch of motor nerve-endings. Journal of Physiology. 1956;133:610–625. doi: 10.1113/jphysiol.1956.sp005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kirchhofer D, Grzesiak J, Pierschbacher MD. Calcium as a potential physiological regulator of integrin-mediated cell adhesion. Journal of Biological Chemistry. 1991;266:4471–4477. [PubMed] [Google Scholar]
- Kita H, Narita K, Van der Kloot W. The relation between tonicity and impulse-evoked transmitter release in the frog. Journal of Physiology. 1982;25:213–222. doi: 10.1113/jphysiol.1982.sp014146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Van der Kloot W. Time course and magnitude of effects of changes in tonicity on acetylcholine release at frog neuromuscular junction. Journal of Neurophysiology. 1977;40:212–224. doi: 10.1152/jn.1977.40.2.212. [DOI] [PubMed] [Google Scholar]
- Martin PT, Kaufman SJ, Kramer RH, Sanes JR. Synaptic integrins in developing, adult, and mutant muscle: selective association of α1, α7A, and α7B integrins with the neuromuscular junction. Developmental Biology. 1996;174:125–139. doi: 10.1006/dbio.1996.0057. [DOI] [PubMed] [Google Scholar]
- Martin PT, Sanes JR. Integrins mediate adhesion to agrin and modulate agrin signaling. Developmental Biology. 1997;124:3909–3917. doi: 10.1242/dev.124.19.3909. [DOI] [PubMed] [Google Scholar]
- Matzner O, Shlomo B-T, Nussinovitch I. Hyperosmotic regulation of voltage-gated calcium currents in rat anterior pituitary cells. Journal of Neurophysiology. 1996;75:1894–1900. doi: 10.1152/jn.1996.75.5.1894. [DOI] [PubMed] [Google Scholar]
- Mochida S, Yokayama CT, Kim DK, Itoh K, Catterall WA. Evidence for a voltage-dependent enhancement of neurotransmitter release mediated via the synaptic protein interaction site of N-type Ca2+ channels. Proceedings of the National Academy of Sciences of the USA. 1998;95:14523–14528. doi: 10.1073/pnas.95.24.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles WD, Smith DO. Effects of hypertonic solutions on quantal transmitter release at the crayfish neuromuscular junction. Journal of Physiology. 1982;329:185–202. doi: 10.1113/jphysiol.1982.sp014297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura SL, Boylen KP, Einheber S, Milner TA, Ramos DM, Pytela R. Synaptic and glial localization of the integrin αvβ8 in mouse and rat brain. Brain Research. 1998;791:271–282. doi: 10.1016/s0006-8993(98)00118-8. [DOI] [PubMed] [Google Scholar]
- Orlando RA, Cheresh DA. Arginine-glycine-aspartic acid binding leading to molecular stabilization between integrin α v β 3 and its ligand. Journal of Biological Chemistry. 1991;266:19543–19550. [PubMed] [Google Scholar]
- Parmentier JL, Shrivastav BB, Bennet PB. Hydrostatic pressure reduces synaptic efficiency by inhibiting transmitter release. Undersea Biomedical Research. 1981;8:175–183. [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. Journal of Biological Chemistry. 1987;36:17294–17298. [PubMed] [Google Scholar]
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. Journal of Neuroscience. 1999;19:1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel DMJ, Hackett JT, Cooke JD. Calcium: is it required for transmitter secretion? Science. 1971;172:1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- Reichardt LF, Tomaselli K. Extracellular matrix molecules and their receptors: Functions in neural development. Annual Reviews of Neuroscience. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N, Polak J. Filopodia, lamellipodia and retractions at mouse neuromuscular junctions. Journal of Neurocytology. 1989;17:545–561. doi: 10.1007/BF01189809. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. Journal of Neuroscience. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Alnaes E, Rahamimoff R. Is hyperosmotic neurosecretion from motor nerve endings a calcium-dependent process? Nature. 1977;267:170–172. doi: 10.1038/267170a0. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill a pool. Proceedings of the National Academy of Sciences of the USA. 1995;92:846–849. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe N, Kijima H. Transmitter release at frog end-plate loaded with a Ca2+-chelator, BAPTA: hypertonicity and erythrosin B augment the release independently of internal Ca2+ Neuroscience Letters. 1988;92:52–57. doi: 10.1016/0304-3940(88)90741-0. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drbak BK, Bjerrum PJ, Christensen SB, Hanley MR. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents and Actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Thesleff S. Motor end-plate ‘desensitization’ by repetitive nerve stimuli. Journal of Physiology. 1959;148:659–664. doi: 10.1113/jphysiol.1959.sp006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkanis SA. Effects of muscle stretch on transmitter release at endplates of rat diaphragm and frog sartorius muscle. Journal of Physiology. 1973;230:391–403. doi: 10.1113/jphysiol.1973.sp010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W, Molgo J. Quantal acetylcholine release at the vertebrate neuromuscular junction. Physiological Reviews. 1994;74:899–991. doi: 10.1152/physrev.1994.74.4.899. [DOI] [PubMed] [Google Scholar]
- Wong KC, Meyer T, Harding DI, Dick JR, Vrbova G, Greensmith L. Integrins at the neuromuscular junction are important for motoneuron survival. European Journal of Neuroscience. 1999;11:3287–3292. doi: 10.1046/j.1460-9568.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- Yu W, Miller RF. Hyperosmotic activation of transmitter release from presynaptic terminals onto retinal ganglion cells. Journal of Neuroscience. 1995;62:159–168. doi: 10.1016/0165-0270(95)00071-2. [DOI] [PubMed] [Google Scholar]


