Abstract
Intracortical inhibition in the human motor cortex has been previously demonstrated using paired-pulse transcranial magnetic stimulation (TMS) protocols at short intervals (1-6 ms; short interval intracortical inhibition, SICI) with a subthreshold conditioning pulse preceding a suprathreshold test pulse, and at long intervals (50-200 ms; long interval intracortical inhibition, LICI) with suprathreshold conditioning and test pulses.
We investigated whether different circuits mediate these inhibitory phenomena and how they interact. In nine healthy volunteers, we applied TMS to the motor cortex and recorded motor evoked potentials from the first dorsal interosseous muscle.
With increasing test pulse strength, LICI decreases but SICI tends to increase. There was no correlation between the degree of SICI and LICI.
We tested the interactions between SICI and LICI. SICI was reduced or eliminated in the presence of LICI. Loss of SICI was seen even with a conditioning stimulus too weak to induce significant LICI.
Our findings demonstrate that different cell populations mediate SICI and LICI. The results are consistent with the hypothesis that LICI inhibits SICI through presynaptic GABAB receptors. Testing of SICI in the presence of LICI may be a non-invasive way of evaluating inhibitory interactions in the human motor cortex.
Cortical inhibitory systems play a crucial role in modulating cortical output. Changes in cortical inhibition occur in many neurological and psychiatric disorders and may mediate cortical plasticity. Cortical output depends on the balance between excitatory and inhibitory systems. The inhibitory systems of human motor cortex can be evaluated non-invasively by transcranial magnetic stimulation (TMS) (Triggs et al. 1992; Hallett, 1995). A widely used protocol (Kujirai et al. 1993) involves a weak, subthreshold conditioning stimulus followed by a suprathreshold test stimulus. The test responses are inhibited at interstimulus intervals (ISIs) of 1-6 ms and are facilitated at ISIs of 8-30 ms. We will refer to these phenomena as short ISI cortical inhibition (SICI) and intracortical facilitation (ICF). Evidence that SICI occurs in the cortex includes the reduction of descending corticospinal waves (Nakamura et al. 1997; Di Lazzaro et al. 1998) and an anodal transcranial electrical stimulation (TES) test pulse, which directly activates corticospinal axons (Rothwell, 1997; Burke et al. 2000), is not inhibited by a TMS conditioning pulse (Kujirai et al. 1993).
SICI is reduced prior to voluntary movement in the intended agonist but not in the antagonist muscle (Reynolds & Ashby, 1999). Changes in SICI could serve to focus the subsequent excitatory drive to produce the intended movement (Floeter & Rothwell, 1999). Alteration in SICI and ICF may mediate cortical plasticity (Chen et al. 1998a; Ziemann et al. 1998b). Abnormalities of SICI and ICF have also been reported in some neurological and psychiatric disorders such as Parkinson's disease (Ridding et al. 1995a), dystonia (Ridding et al. 1995b) and Tourette's syndrome (Ziemann et al. 1997).
Another form of inhibition induced by TMS is from a suprathreshold conditioning pulse applied 50-200 ms prior to the test pulse (Valls-Soléet al. 1992; Wassermann et al. 1996). We will refer to this inhibition as long interstimulus interval intracortical inhibition (LICI). As for SICI, LICI at ISIs of more than 50 ms occurs in the cortex due to the absence of any change in spinal excitability (Fuhr et al. 1991), the failure to suppress the response to double TES (Inghilleri et al. 1993), and marked reduction in the corticospinal waves evoked by TMS (Nakamura et al. 1997; Chen et al. 1999). LICI has been shown to be abnormal in some neurological conditions, including stroke (Classen et al. 1997), dystonia (Chen et al. 1997), and Parkinson's disease (Priori et al. 1994).
Although both SICI and LICI are due to reduced cortical excitability, it is not known whether the same population of neurons mediates the two forms of cortical inhibition. Pharmacological studies suggest that the two forms of inhibition can be differentially modulated (Ziemann et al. 1996a; Werhahn et al. 1999). It has been hypothesized that SICI is primarily mediated by GABAA receptors (Hanajima et al. 1998), while LICI is mediated by GABAB receptors (Roick et al. 1993; Siebner et al. 1998; Werhahn et al. 1999). However, the evidence supporting activity at different inhibitory receptor subtypes does not resolve the question of whether the cell populations responsible for SICI and LICI are themselves distinct.
In the present study, we tested the hypothesis that different neuronal circuits mediate SICI and LICI and evaluated their interactions. We first determined the effects of different test stimulus intensities on SICI and LICI. A different pattern of response would support the existence of two distinct inhibitory circuits. We then examined the interaction of SICI and LICI in a triple-stimulation protocol that allows us to measure SICI in the presence of LICI. By testing different parameters of this interaction, we were able to differentiate between different models of intracortical connectivity.
METHODS
Subjects
We studied nine healthy volunteers (6 men, 3 women, mean age 40 years, range 25-47 years) except in Expt 5 in which six subjects participated. All subjects gave their written informed consent and the protocol was approved by the University Health Network Research Ethics Board in accordance with the Declaration of Helsinki on the use of human subjects in experiments.
EMG recording
Surface EMG was recorded from the right first dorsal interosseous (FDI) muscle with disposable disc electrodes in a tendon-belly arrangement. The subject maintained relaxation throughout the experiment and the EMG was monitored on a computer screen and via speakers at high gain. The signal was amplified (Intronix Technologies Corporation Model 2024F, Bolton, Ontario, Canada), filtered (band pass 2 Hz to 5 kHz), digitized at 5 kHz (Micro 1401, Cambridge Electronics Design, Cambridge, UK) and stored in a laboratory computer for off-line analysis.
Transcranial magnetic stimulation
TMS was performed with a 7 cm figure-of-eight coil and four Magstim 200 stimulators (The Magstim Company, Dyfed, UK) connected via three Bistim modules in a ‘pyramid’ set-up. The output of each of the two pairs of Magstim 200 stimulators was connected to one Bistim module. The output from the two Bistim modules was directed to a third Bistim module which was connected to the TMS coil. This set-up allowed us to deliver up to four pulses of different stimulus intensities through the same coil at very short interstimulus intervals. The power attenuation of the pyramid system is about 15%, similar to a single Bistim system. This is because the Bistim modules set the Magstim 200 stimulators to a lower voltage for technical reasons and the lower voltage remains the same regardless of the number of Bistim modules connected (personal communication, Dr R. Jalinous, Magstim Company).
The coil was placed at the optimal position for eliciting motor-evoked potentials (MEPs) from the FDI muscle. The optimal position was marked on the scalp to ensure identical placement of the coil throughout the experiment. The handle of the coil pointed backwards and was perpendicular to the presumed direction of the central sulcus, about 30 deg to the midsagittal line. The direction of the induced current was from posterior to anterior and was optimal to activate the motor cortex transynaptically (Werhahn et al. 1994; Kaneko et al. 1996).
Study design
To simplify the discussion of the results, we will define a consistent terminology for the various experimental configurations. Each trial consisted of a test pulse which could be preceded by one or two conditioning pulses. The conditioning pulses could occur 2, 10 or 100 ms prior to the test pulse. We labelled the pulses as ‘Test’, ‘CS2’, ‘CS10’, and ‘CS100’. CS2 was chosen because it consistently led to SICI (Kujirai et al. 1993; Chen et al. 1998b) and largely avoided the phenomenon of I-wave facilitation (Ziemann et al. 1998c; Chen & Garg, 2000) which may obscure SICI (Awiszus et al. 1999). CS10 was chosen because it consistently gave rise to ICF (Kujirai et al. 1993; Ridding et al. 1995c). CS100 was used to elicit LICI because at this interstimulus interval epidural recordings of corticospinal waves demonstrated reduced cortical excitability (Nakamura et al. 1997; Chen et al. 1999) and there was no change in spinal excitability (Fuhr et al. 1991).
Since the intensities of the test and CS100 stimuli were adjusted to achieve specific MEP amplitudes for each subject, we labelled the strength of the pulses accordingly. Thus, the stimulus intensity of ‘1 mV’ indicates the minimum stimulator setting (determined to the nearest 1% of the maximum stimulator power output) necessary to produce peak-to-peak MEP amplitudes of ≥ 1 mV in at least 5 out of 10 trials; ‘0.2 mV’ and ‘4 mV’ are defined similarly. The conditioning stimulus intensities were expressed as a percentage of the motor threshold (MT). MT was determined at rest and was the minimum stimulator output that produced MEPs of ≥ 50 μV in at least 5 out of 10 trials. A stimulator setting of 80% of MT is given as 0.8MT. Inhibition and facilitation were expressed as the ratio of the conditioned MEP amplitudes to the mean unconditioned MEP amplitude. For example, the ratio of the MEP amplitude of the response to the CS2-test stimulus pair to that of the response to the test stimulus alone gives SICI, the ratio of the MEP amplitudes in response to the CS10-test stimulus pair and the test stimulus alone gives ICF, and the ratio of the MEP amplitudes in response to the CS100-test stimulus pair and the test stimulus alone gives LICI.
Experiment 1: effects of test stimulus intensity on SICI, ICF and LICI
We examined whether changes in the test stimulus intensity had different effects on SICI, ICF and LICI. The intensities of the subthreshold CS2 (to elicit SICI) and CS10 (to elicit ICF) pulses were 0.8MT and the intensity of the suprathreshold CS100 pulse (to elicit LICI) was ‘1 mV’. Each run consisted of 10 trials each of a test stimulus alone, a CS2-test stimulus pair, a CS10-test stimulus pair and a CS100-test stimulus pair delivered in random order. Three test stimulus intensities of 0.2, 1 and 4 mV were studied in separate runs.
Experiment 2: effects of LICI on SICI and ICF
In this experiment, we investigated whether SICI and ICF were altered by LICI (induced by a CS100 pulse). Ten conditions were tested and are listed in Table 1 as conditions 2A-2J. Each run consisted of 10 trials of each of the 10 conditions delivered in a random order (100 trials). The baseline SICI, ICF and LICI for a 1 mV test MEP were determined from conditions 2A-2D. Because SICI and ICF may be affected by the size of the test response and the preceding CS100 pulse inhibits the test response, the strength of the test pulse was increased to compensate for this effect in conditions 2H-2J. The strength of the test pulse was adjusted to produce 1 mV MEPs in the presence of the earlier CS100 pulse (with CS100 at an intensity of 1 mV) and this test pulse is referred to as ‘1 mVCS100’. SICI and ICF in the presence of a preceding CS100 pulse were studied using three pulses in conditions 2I and 2J, respectively. Since the test intensity may also influence SICI and ICF, we also measured SICI and ICF with the increased test strength (1 mVCS100) in conditions 2F and 2G. Thus, we designed the experiment to compare SICI and ICF in the presence of the CS100 pulse (2I/2H and 2J/2H) to SICI and ICF in the absence of the CS100 pulse matched for test MEP amplitude (2B/2A and 2C/2A) and matched for test stimulus intensity (2F/2E and 2G/2E).
Table 1.
Stimulus conditions used in Expts 1–5
| Condition | Stimulus intensity |
|||
|---|---|---|---|---|
| CS100 | CS10 | CS2 | Test | |
| 2A | — | — | — | 1 mV |
| 2B | — | — | 0.8MT | 1 mV |
| 2C | — | 0.8MT | — | 1 mV |
| 2D | 1 mV | — | — | 1 mV |
| 2E | — | — | — | 1 mVCS100 |
| 2F | — | — | 0.8MT | 1 mVCS100 |
| 2G | — | 0.8MT | — | 1 mVCS100 |
| 2H | 1 mV | — | — | 1 mVCS100 |
| 2I | 1 mV | — | 0.8MT | 1 mVCS100 |
| 2J | 1 mV | 0.8MT | — | 1 mVCS100 |
| 3A | 1 mV | — | — | 1 mVCS100 |
| 3B | 1 mV | — | 0.8MTCS100 | 1 mVCS100 |
| 3C | 1 mV | 0.8MTCS100 | — | 1 mVCS100 |
| 4A | — | — | — | 1 mVCS2 |
| 4B | — | — | 0.8MT | 1 mVCS2 |
| 4C | — | 0.8MT | — | 1 mVCS2 |
| 4D | 1, 1.3 or 1.5MT | — | — | 1 mVCS2 |
| 4E | 1, 1.3 or 1.5MT | — | 0.8MT | 1 mVCS2 |
| 4F | 1, 1.3 or 1.5MT | 0.8MT | — | 1 mVCS2 |
| 4G | — | — | — | 1 mV |
| 4H | 1, 1.3 or 1.5MT | — | — | 1 mV |
CS100, conditioning stimulus delivered 100ms before test stimulus (CS80 at 1.1MT was used in Expt 5); CS10, conditioning stimulus delivered 10ms before test stimulus; CS2, conditioning stimulus delivered 2ms before test stimulus; Test, test stimulus. See Methods for definitions of test stimulus intensity.
Experiment 3: effects of stronger CS2 and CS10 pulses
Since the preceding CS100 pulse may also inhibit or reduce the effectiveness of the CS2 or CS10 pulses, we tested SICI and ICF with stronger CS2 and CS10 stimuli to compensate for the effects of CS100 (conditions 3A-3C, Table1). In this case, we determined the resting motor threshold in the presence of CS100 (Tergau et al. 1999). The intensities of CS2 and CS10 were adjusted to be 80% of the ‘conditioned’ motor threshold in the presence of CS100, and we labelled this intensity ‘0.8MTCS100’. As before, each run consisted of 10 trials of each of the conditions delivered in a random order.
Experiment 4: effects of variations in LICI intensity
While in the previous experiments the strength of the CS100 stimulus was held constant (1 mV), here we tested the effects of different strengths of the CS100 stimulus. We examined the effects of adding a CS2 pulse on LICI by comparing a test MEP generated by a single pulse to one generated by a CS2-test pulse combination matched for either test MEP amplitude or test stimulus intensity. We also studied the effects of different strengths of CS100 stimulus on LICI and on the subsequent SICI and ICF.
The CS100 stimulus was set at intensities of 110, 130, or 150% of the motor threshold (1.1MT, 1.3MT and 1.5MT). Table 1 shows the different stimulus conditions (4A-4H) used. Conditions 4G and 4H evaluated the effects of different intensities of the CS100 stimulus on LICI with the test stimulus adjusted to evoke MEPs of about 1 mV. In conditions 4A-4F the test pulse was increased to compensate for CS2 so that the MEP was approximately 1 mV in the presence of CS2 (indicated by ‘1 mVCS2’). Thus, LICI in the presence of CS2 (4E/4B) was matched for MEP amplitude (4H/4G) or test stimulus intensity (4D/4A) in the absence of CS2. Each run consisted of 10 trials of each condition in random order. Different CS100 stimulus intensities were tested in separate runs.
Experiment 5: effects of weak LICI within the silent period
With CS100 at 1.1MT, we found that the CS2 and test pulses were delivered around the end of the silent period (see below). Since the cortical neurons may have increased excitability at the end of the silent period, we performed an additional experiment to examine the effects of weak LICI within the silent period. Six subjects were studied. The conditions were identical to conditions 4A-4H (Table 1) except that the CS100 was changed to CS80 at 1.1MT.
Experiment 6: silent period duration
We measured the duration of the silent period following single stimuli at 1.1MT, 1.3MT and 1.5MT and at a strength of ‘1 mV’. The EMG passed through a leaky integrator and the EMG level was displayed on an oscilloscope. With visual and auditory feedback, the subjects maintained a constant background contraction of 20% of the maximum filtered EMG. Ten trials were obtained for each intensity tested.
Data analysis
The peak-to-peak MEP amplitude for each trial was measured off-line. The inhibition or facilitation for each trial was expressed as a ratio of the mean conditioned to unconditioned MEP amplitude for each subject. Ratios less than 1 indicate inhibition, and ratios greater than 1 indicate facilitation. The silent period for each trial was measured off-line from onset of the MEP to the resumption of voluntary EMG activity. Values are expressed as means ± standard error of the mean.
Statistical analysis
For Expt 1, the effects of test stimulus intensity on SICI, ICF and LICI were evaluated by analysis of variance (ANOVA). Correlation between SICI and LICI was tested by linear regression and Pearson's correlation coefficient. For Expts 2, 3 and 4, SICI, ICF and LICI with and without the CS100 stimulus were compared using Student's paired t test. For Expt 4, the effects of different levels of baseline MEPs and CS100 intensities on the extent of inhibition or facilitation induced by CS100 were analysed with ANOVA. The threshold for significance was set at P < 0.05.
RESULTS
Experiment 1
Figure 1 shows the change in SICI, LICI and ICF as the test stimulus strength was varied from 0.2 to 4 mV. For SICI, there was only slight inhibition for small test MEPs of about 0.2 mV and the inhibition increased with test MEPs of about 1 mV. There was little further change in SICI with test MEPs increased to about 4 mV. The effect of test stimulus strength on SICI did not reach significance (P = 0.06, repeated-measures ANOVA). ICF was similar for test MEP amplitudes of 0.2 and 1 mV, but appeared to be reduced at a test MEP amplitude of 4 mV. This may be due to a ‘ceiling effect’ for the large test MEP. The effect of the test stimulus intensity on ICF was not significant. In contrast, LICI decreased with higher test stimulus intensities (P = 0.004). When the test stimulus was 0.2 mV, the CS100 stimulus led to a marked inhibition of the test response (20 ± 3.5% (mean ±s.e.m.) of the test stimulus alone). With the test stimulus at 4 mV, the same CS100 stimulus only caused a slight inhibition (82 ± 16%). This effect was evident in all nine subjects studied.
Figure 1. Effects of different test stimulus intensities on cortical inhibition and facilitation.
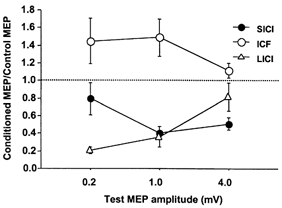
Data from 9 subjects. Inhibition and facilitation are shown as the ratio (mean ±s.e.m.) of the conditioned MEP amplitude to the unconditioned MEP amplitude. Ratios less than 1 indicate inhibition and ratios greater than 1 indicate facilitation. Test pulse strength (0.2, 1, or 4 mV) is specified in terms of the target MEP amplitude evoked by the test pulse alone. SICI tended to increase while LICI decreased with increasing test pulse intensity.
There was no correlation between the degree of SICI and LICI in the same subject for any test stimulus strength, or when all test stimulus strengths were combined (Pearson's correlation coefficient < 0.2, P > 0.3 in all cases).
Experiment 2
The average MEP amplitude for all subjects was 1.3 ± 0.23 mV (condition 2A in Table 1) for the 1 mV test stimulus alone and 2.6 ± 0.5 mV (condition 2E) for the 1 mVCS100 test stimulus alone. With CS100 at 1 mV intensity and the test stimulus at 1 mVCS100 (condition 2H), the MEP amplitude was 1.3 ± 0.3 mV. Thus, the MEP responses to the test stimulus in conditions 2A and 2H were matched.
The effect of combining CS100 with CS2 for a representative subject is shown in Fig. 2. Compared to the test pulse alone (Fig. 2A), a preceding CS2 (Fig. 2B) or CS100 (Fig. 2C) stimulus inhibited the response. However, in the presence of CS100, CS2 did not inhibit the test response (Fig. 2D) compared to the same pulse combination without the CS2 (Fig. 2C). Similar effects were seen in all subjects tested, and the results are summarized in Fig. 3. CS2 alone at strength 0.8MT followed by the test pulse caused a similar degree of SICI whether the test stimulus was set at 1 mV (inhibited to 41 ± 5% of baseline, condition 2B/2A shown as points above a in Fig. 3) or 1 mVCS100 (44 ± 7%, condition 2F/2E, points above b in Fig. 3). However, the addition of CS100 eliminated the SICI caused by CS2 (112 ± 29%, condition 2I/2H, points above c in Fig. 3). This change in SICI was significant whether compared to the test stimulus set at 1 mV (P = 0.0064, conditions 2I/2H vs. 2B/2A, matched for test MEP amplitude) or at 1 mVCS100 (P = 0.0022, conditions 2I/2H vs. 2F/2E, matched for test stimulus intensity). The CS100 stimulus did not significantly change ICF (Fig. 3, conditions 2J/2H compared to 2C/2A or 2G/2E).
Figure 2. The effect of a preceding CS100 conditioning pulse on SICI.
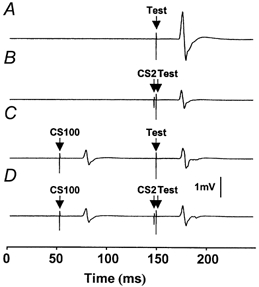
Traces show average MEPs for a single subject. Only trials with test stimulus intensity at 1 mVCS100 are shown. The CS100 stimulus (62% of maximum stimulator output) was adjusted to produce 1 mV MEPs, the CS2 stimulus (35% of maximum stimulator output) set at 80% of resting motor threshold and the test stimulus (83% of maximum stimulator output) was set to produce 1 mV MEPs in the presence of a CS100 stimulus (1 mVCS100). A, response to the test pulse alone (condition 2E). TMS was delivered at 150 ms. B, a preceding CS2 conditioning stimulus at 148 ms leads to inhibition of the test pulse compared to the baseline in A (condition 2F). C, a preceding CS100 conditioning stimulus at 50 ms also leads to inhibition of the test pulse (condition 2H). D, in the presence of a CS100 pulse, the CS2 pulse does not lead to a decrease in the MEP size compared to that shown in C (condition 2I).
Figure 3. Changes in SICI and ICF in the presence of a CS100 stimulus in all 9 subjects (mean ±s.e.m.).
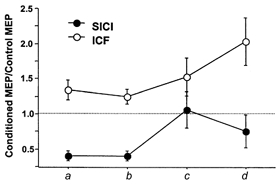
•, ICF; •, SICI. Inhibition and facilitation are shown as the ratio of the conditioned MEP amplitude to the unconditioned MEP amplitude. Ratios less than 1 indicate inhibition and ratios greater than 1 indicate facilitation. Points above a (conditions 2B/2A and 2C/2A) and b (conditions 2F/2E and 2G/2E) represent SICI and ICF without the CS100 stimulus. Points above a show that the test pulse evokes a 1 mV MEP and points above b that the test pulse evokes 1 mV MEP if preceded by a CS100 stimulus. Points above c (conditions 2I/2H and 2J/2H) and d (conditions 3B/3A and 3C/3A) represent SICI and ICF with the CS100 stimulus. The CS100 stimulus evokes a 1 mV MEP and the test MEP evokes a 1 mV MEP in the presence of a CS100 stimulus. Points above c show the CS2/10 was at 80% of resting motor threshold and points above d that the CS2/10 was at 80% of resting motor threshold in the presence of CS100. Points above a, c and d are matched for test MEP amplitude and points above b, c and d are matched for test stimulus intensity.
Experiment 3
The resting motor threshold (MT) for all subjects was 47 ± 2.4% of the maximum stimulator output. In the presence of the CS100 stimulus, the motor threshold (MTCS100) was increased to 65 ± 3.6% of the maximum stimulator output, so the CS2 stimulus (0.8MTCS100) was increased by an average of 18% to compensate for CS100.
The results for SICI and ICF are shown in the points above d of Fig. 3. The stronger CS2 stimulus partially restored SICI in the presence of CS100. The SICI for condition 3B/3A (CS100 at 1 mV, CS2 at 0.8MTCS100, Test at 1 mVCS100) was significantly stronger than condition 2I/2H (CS100 at 1 mV, CS2 at 0.8MT, Test at 1 mVCS100, points above c in Fig. 3, P = 0.038). However, there was still a significant reduction in SICI compared to CS2 at 0.8MT in the absence of LICI, whether matched for test MEP amplitude (condition 2B/2A, points above a in Fig. 3, P = 0.05) or matched for test stimulus strength (condition 2F/2E, points above b in Fig. 3, P = 0.015).
The results for ICF are shown in the top traces of Fig. 3. The stronger CS10 at 0.8MTCS100 (conditions 3C/3A, points above d) led to higher ICF compared to CS10 at 0.8MT without LICI, whether matched for test MEP amplitude (condition 2C/2A, points above a, P = 0.015) or matched for test stimulus strength (condition 2G/2E, points above b, P = 0.011).
Experiment 4
In conditions 4A-4F (Table 1), the test stimulus intensity was adjusted to achieve 1 mV in the presence of CS2, yielding an increased stimulus strength of 83 ± 4% of maximum stimulator output (‘1 mVCS2’). The test stimulus of 1 mVCS2 alone gave a MEP amplitude of 2.8 ± 0.26 mV (condition 4A, Table 1), and when preceded by the CS2 stimulus the MEP amplitude was 1.44 ± 0.5 mV (condition 4B).
We found that weak CS100 stimuli increased MEP amplitudes produced by the CS2-test stimulus combination. Figure 4 shows an example of one subject's averaged response. Figure 4A gives the response to a test MEP of strength 1 mVCS2 alone (condition 4A). Figure 4B shows inhibition when a CS2 pulse of strength 0.8MT preceded the test MEP (condition 4B). Figure 4C shows that there was no inhibition when a weak CS100 pulse of strength 1.1MT preceded the test MEP (condition 4D). Figure 4D shows that when a CS100 pulse at 1.1MT (which did not inhibit single pulse test MEP) was added before a CS2 pulse (condition 4E), there was MEP facilitation compared to condition 4B without the CS100 pulse in Fig. 4B. This is probably due to a reduction in the inhibitory effects of CS2.
Figure 4. Averaged EMG tracing from a representative subject in Expt 4.
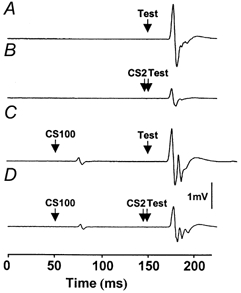
A, response to the test pulse alone (1 mVCS2, 58% of maximum stimulator output). B, addition of a CS2 pulse (0.8MT, 30% of maximum stimulator output) inhibited the test MEP. C, addition of a CS100 pulse at 1.1MT (48% of maximum stimulator output) did not inhibit the test pulse. D, addition of both CS100 (1.1MT) and CS2 pulses (0.8MT) reduced the inhibitory effect of CS2 and led to higher test MEP amplitude compared to those shown in B.
The effects of adding a CS2 pulse to the test pulse on LICI at varying CS100 intensities for all subjects are shown in Fig. 5A. ANOVA showed significant effects of both CS100 intensity (P = 0.0003) and the nature of the baseline MEP (Test at 1 mV, Test at 1 mVCS2 or CS2 at 0.8MT followed by Test at 1 mVCS2) (P < 0.0001) on LICI. With CS100 at 1.1MT, there was no significant LICI whether the test stimulus was set at 1 mV (conditions 4H/4G, Table 1) or at 1 mVCS2 (conditions 4D/4A). As expected, the LICI increased with higher strengths of CS100. The LICI was more prominent for the weaker test stimulus of 1 mV than the stronger test stimulus of 1 mVCS2, confirming the results of Expt 1. When CS2 at 0.8MT was added (test stimulus at 1 mVCS2, conditions 4E/4B, top trace in Fig. 5A), the effect of the CS100 pulse was markedly changed compared to baseline MEPs generated by single pulses when matched for MEP amplitude (1 mV, conditions 4H/4G) or test stimulus intensity (1 mVCS2, conditions 4D/4A). At an intensity of 1.1MT, the CS100 stimulus led to significant MEP facilitation (P = 0.01, paired t test) in the presence of CS2, although the same CS100 stimulus (1.1MT) had little effect on an unconditioned MEP of similar amplitude or an unconditioned MEP produced by the same stimulus intensity (Fig. 5A). This finding is consistent with the CS100 stimulus causing a reduction in SICI mediated by CS2. Addition of the CS100 stimulus at 1.3 and 1.5MT did not cause significant inhibition or facilitation of the response to a CS2-test pulse combination (Fig. 5A, top trace).
Figure 5. Effects of different CS100 stimulus strengths.
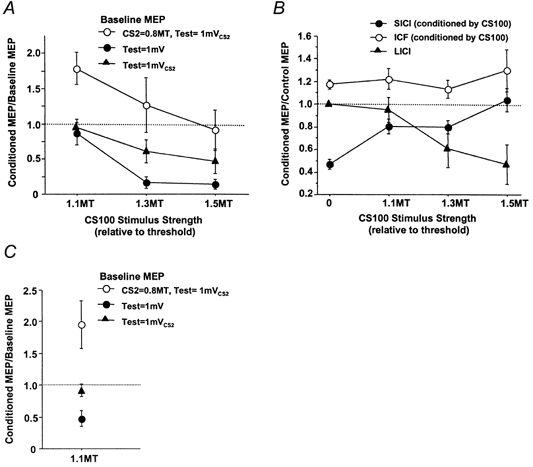
A, effects of different strengths of the CS100 stimulus depend on the nature of the baseline MEPs. Data from 9 subjects (mean ±s.e.m.). The MEP amplitudes conditioned by the CS100 stimulus are expressed as a ratio of the baseline MEP amplitudes. •, baseline MEPs with test stimulus intensity of 1 mV (conditions 4H/4G); ▴, baseline MEPs with test stimulus intensity of 1 mVCS2 (conditions 4D/4A); •, baseline MEPs generated by adding CS2 at 0.8MT to the test stimulus at 1 mVCS2 (conditions 4E/4B). • and • are matched for baseline MEP amplitude; • and ▴ are matched for test MEP intensity. The CS100 stimulus tended to cause facilitation of baseline MEPs produced by the CS2-test stimulus pair but inhibition of baseline MEPs produced by the test stimulus alone. B, the effect of different strengths of CS100 pulse on SICI and ICF. Data from 9 subjects. CS2 or CS10 was 0.8MT and the test pulse was 1 mVCS2. ‘0’ on the x-axis represents SICI, ICF and a test pulse alone without the CS100 stimulus. SICI without the CS100 stimulus was calculated from conditions 4B/4A and ICF from conditions 4C/4A. SICI with the CS100 stimulus was calculated from conditions 4E/4D and ICF from conditions 4F/4D. LICI was calculated from conditions 4D/4A. The CS100 pulse caused reduction of SICI at all stimulus intensities tested. C, the effects of CS80 stimulus at 1.1MT on different baseline MEPs. Data from 6 subjects. The symbols used are identical to A. The CS80 stimulus inhibited baseline MEPs produced by the test stimulus alone but facilitated the MEPs produced by the CS2-test stimulus pair.
Figure 5B shows the effect of different CS100 strengths on LICI, SICI and ICF. The SICI was reduced even for a CS100 strength of 1.1MT, which is insufficient by itself to produce significant LICI. The change in SICI was significant (paired t test) at all CS100 strengths compared to the SICI in the absence of the CS100 pulse (CS100 = 1.1MT, P = 0.0008; CS100 = 1.3MT, P = 0.0035; CS100 = 1.5MT, P = 0.0005). The change in ICF was not significant at any CS100 strength.
Experiment 5
CS80 at 1.1MT resulted in slight LICI when the test pulse was 1 mVCS2, with the MEP being reduced from 2.44 ± 0.3 mV (condition 4A) to 2.27 ± 0.35 mV (condition 4D). LICI was stronger when the test pulse was 1 mV, and the MEP was reduced from 1.39 ± 0.2 mV (condition 4G) to 0.61 ± 0.1 mV (condition 4H). This is consistent with the results of Expts 1 and 4. Despite the inhibitory effects of CS80 on single test pulses, CS80 resulted in significant facilitation (P = 0.04, paired t test) of the CS2-test (1 mVCS2) combination from 1.11 ± 0.1 mV (condition 4B) to 2.75 ± 0.4 mV (condition 4E). These results are illustrated in Fig. 5C.. The SICI, expressed as conditioned MEP/control MEP, was 54 ± 9% without CS80 (conditions 4B/4A) and was reduced to 91 ± 4% with CS80 (conditions 4E/4D).
Experiment 6
The duration of the silent period was 105 ± 7 ms for a stimulus at 1.1MT, 160 ± 8 ms for a stimulus at 1.3MT, 198 ± 8 ms for a stimulus at 1.5MT, and 174 ± 8 ms for a stimulus at ‘1 mV’. The 1 mV strength was equivalent to 137 ± 16% of the MT. Therefore, in Expts 2 and 3 with CS100 at 1 mV strength the test MEP (ISI of 100 ms) occurred well within the silent period. In Expt 4 the test MEP was expected near the end of the silent period for the CS100 intensity of 1.1MT and the test MEP occurred well within the silent period for CS100 intensities of 1.3 and 1.5MT. In Expt 5 the test MEP occurred within the silent period.
DISCUSSION
We attempted to answer two related questions. Do different cell populations mediate the two inhibitory phenomena? If so, what are their interactions?
Different cell populations mediate LICI and SICI
Changes in the strength of the test stimulus had markedly different effects on SICI and LICI (Fig. 1) and there was no correlation between the extent of SICI and LICI. This suggests that different cell populations mediate LICI and SICI. Since LICI was greatest for low intensity test stimuli, neurons with the lowest threshold are more sensitive to LICI than those with higher thresholds. Increasing the test stimulus intensity may recruit neurons that are less excitable or are spatially further away from the centre of activation by TMS. These neurons may be less susceptible to LICI.
Reduction of SICI by LICI
In Expt 2 (Fig. 2 and 3), we established that LICI could abolish SICI elicited by a subsequent CS2 stimulus. Thus, LICI does not potentiate nor is it additive to the effects of SICI, but reduces or even reverses SICI.
We used several controls to address the possibility that activation of different populations of cortical or spinal motoneurons may explain the results. In order to produce a similar degree of corticospinal activation with and without LICI, we matched the test MEP amplitude by increasing the test stimulus when preceded by the CS100 stimulus. Even with matched MEP amplitude, it could still be argued that LICI preferentially inhibited low threshold cortical motoneurons and therefore the CS100-1 mVcs100 test pulse combination (condition 2H) may predominately activate high threshold cortical motoneurons or produce direct activation of cortical output neurons. However, we demonstrated in Expt 1 that neurons activated at high intensities are equally or more susceptible to SICI than the first recruited cortical motoneurons (Fig. 1). This is confirmed by the similar extent of SICI with the test stimulus at 1 mV or 1 mVCS2 (points above a and b, Fig. 3). Therefore, activation of different populations of cortical motoneurons in the presence of LICI cannot explain our results. We also matched the test stimulus intensity and found virtually identical results (Fig. 3). In addition, we found no effect of LICI on ICF. This is consistent with previous observations that different circuits probably mediate SICI and ICF (Ziemann et al. 1996c; Chen et al. 1998b). If the changes in SICI were related to changes in the motoneuron pool, then similar effects would be expected for ICF.
We also investigated whether increasing the CS2 intensity to the same extent as the MT elevation induced by the CS100 stimulus can restore SICI in the presence of LICI. Similar to the results of Tergau et al. (1999), we found that MT was elevated during LICI. At the higher CS2 intensity, there is some restoration of SICI (Fig. 3). This is probably because the SICI circuits were more strongly activated. However, SICI was still significantly reduced compared to SICI without the CS100 stimulus, indicating that SICI was still inhibited by LICI (Fig. 3). The increase in ICF with the stronger CS10 is consistent with previous findings (Kujirai et al. 1993; Chen et al. 1998b) and may be related to activation of descending volleys from cortical output neurons.
The circuitry mediating interaction between LICI and SICI
How does LICI cause reduction of SICI? Several possible models of interactions between LICI, SICI and ICF are shown in Fig. 6. It is known that both SICI (Nakamura et al. 1997; Di Lazzaro et al. 1998; Hanajima et al. 1998) and LICI (Nakamura et al. 1997; Chen et al. 1999) produce MEP inhibition by reducing the late indirect (I) waves. ICF is less well studied but probably causes facilitation by increasing the late I waves (Nakamura et al. 1997). The first I wave was relatively unaffected suggesting that there is little direct inhibition of the pyramidal neurons. We therefore indicate in Fig. 6 that SICI, LICI and ICF act on neurons producing I waves.
Figure 6. Models to explain the experimental results.
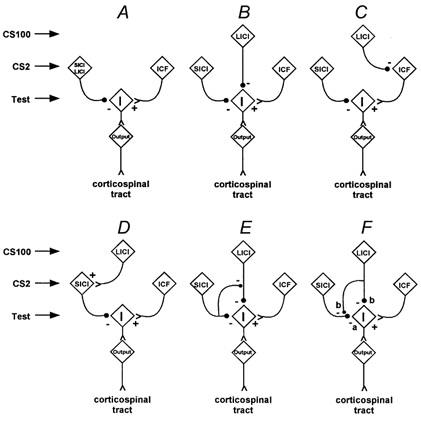
Each box in the figure schematically indicates the population of cells responsible for mediating ‘SICI’, ‘LICI’, ‘ICF’, or the response to the test stimulus alone. The box labeled ‘I’ indicates the source of descending I-waves, and ‘output’ indicates the corticospinal output cell populations. The diagram is for illustration, and the populations may be heterogeneous or include further internal circuitry. The filled circles represent inhibitory synapses, and the letters ‘a’ and ‘b’ indicate the hypothesized presence of primarily GABAA or GABAB receptors.
The first model (Fig. 6a) postulates that the same circuit mediates LICI and SICI. The second model (Fig. 6b) postulates that LICI and SICI are independent sources of inhibition. In the third model, LICI causes inhibition by reducing ICF (Fig. 6c). Another possibility, shown in Fig. 6D, is that LICI and SICI are mediated through a common pathway in which LICI activates the SICI circuit. The reduction of SICI by LICI might then be explained by a saturation effect, such that addition of the CS2 stimulus to the CS100 stimulus produces little or no further inhibition. The fifth model suggests that SICI inhibits LICI (Fig. 6E), causing an apparent reduction of SICI, since the test MEP would be larger than expected. The sixth possibility is that LICI inhibits the SICI circuit (Fig. 6F).
The first model (Fig.6A) can be excluded on the basis of the different responses of SICI and LICI to test stimulus strength shown in Expt 1. The second model (Fig. 6b) can be excluded because we demonstrated that LICI inhibits SICI and therefore the two inhibitory systems are not independent. The third model (Fig. 6c) can also be excluded since we found no significant inhibition of ICF by CS100 (Fig. 3). Two of our observations are not compatible with the schemes depicted in Fig. 6D and E. Firstly, reduction of SICI is evident at CS100 intensities that do not cause significant MEP inhibition (Figs 4 and 5). This finding makes a saturation effect (Fig. 6D) unlikely and cannot be explained as inhibition of LICI by SICI (Fig. 6E) since there was no LICI. Secondly, a weak CS100 at 1.1MT followed by CS2 and the test stimulus (condition 4E) resulted in a higher test MEP amplitude than the CS2-test stimulus pair alone (condition 4B) (Figs 4 and 5A and C). This facilitation cannot be explained by activation of the SICI inhibitory circuit by LICI (Fig. 6D) or inhibition of LICI by SICI (Fig.6E), but is consistent with inhibition of the SICI circuit by LICI (Fig. 6F).
Thus, our findings suggest that LICI reduces SICI by inhibiting the SICI circuit as well as by directly inhibiting the output neurons, as shown in Fig. 6F.. Our data do not allow us to decide whether LICI inhibits SICI by presynaptic or postsynaptic mechanisms. We favour presynaptic inhibition, as depicted in Fig. 6F, because of the similarity with known GABAA- and GABAB-mediated effects (see below). Moreover, the mechanisms mediating SICI inhibition and MEP inhibition are probably distinct, with different thresholds for activation, since a weak CS100 stimulus can cause strong SICI inhibition without significant MEP inhibition (Fig. 4). The pathway for SICI inhibition also appears to be different from that mediating the silent period. With CS100 stimuli at 1.1MT, SICI reduction was evident, but the duration of the silent period was close to the 100 ms ISI for the test pulse. While we believe that Fig. 6F provides the most parsimonious explanation of our results, we cannot exclude the possibility of more complex polysynaptic interactions that might lead to similar observations. Since some of our experiments are based on roughly linear neuronal interactions, the model may need to be revised if the interactions among cortical neurons are markedly non-linear.
Implications for studies of SICI
SICI has been reported to be abnormal in many neurological and psychiatric disorders and in settings of cortical plasticity. Our findings suggest that changes in SICI can be caused by alterations in LICI-mediated SICI inhibition, in addition to changes in the SICI circuit itself. For example, reduced SICI can be due to increased LICI-mediated inhibition of the SICI circuit. Further studies are needed to distinguish between the relative contributions of the various factors that affect SICI in different experimental settings.
Possible roles of GABAA and GABAB receptors
GABA is the most important inhibitory neurotransmitter in the brain and is distributed through all layers of the cortex (Hendry & Jones, 1981; Jones, 1993). One possible explanation for our results is that LICI acts primarily through GABAB receptors and inhibits SICI presynaptically, while SICI normally activates postsynaptic GABAA receptors as shown in Fig. 6F.. Based on the time course of inhibition (McCormick, 1989) and results of pharmacological intervention, several authors have supported a role for GABAA in SICI (Hanajima et al. 1998) and GABAB in LICI and the silent period (Ziemann et al. 1996a; Werhahn et al. 1999). Stimulation of the neocortex produced disynaptic fast and slow IPSPs of markedly different time course (Davies et al. 1990; Kang et al. 1994; Deisz, 1999a). The fast IPSP is mediated by GABAA receptors coupled to chloride channels and lasts approximately 20 ms. The slow IPSP is mediated by GABAB receptors which activate potassium channels and peaks around 150-200 ms (McCormick, 1989; Davies et al. 1990; Kang et al. 1994; Deisz, 1999a). The different time courses therefore correspond roughly to the different ISIs of SICI (1-6 ms) and LICI (50-150 ms). While GABAA receptors are primarily postsynaptic, GABAB receptors are both presynaptic and postsynaptic (Mott & Lewis, 1994).
Presynaptic GABAB receptors mediate inhibition of GABA release (Davies et al. 1990; Pitler & Alger, 1994; Deisz, 1999b). Supporting our finding that LICI reduces SICI, paired stimuli in the rat hippocampus (Davies et al. 1990; Pitler & Alger, 1994) and neocortex (Deisz, 1999b) caused a marked decrease of the GABAA-mediated fast IPSPs evoked by the second stimulus. This phenomenon, known as paired-pulse depression, was maximal at interstimulus intervals between 100 and 200 ms and was inhibited by a GABAB antagonist. Paired-pulse depression increased with higher conditioning stimulus intensity (Davies et al. 1990; Pitler & Alger, 1994), similar to the more pronounced inhibition of SICI with higher CS100 intensity that we observed (Fig. 5b). The pharmacological properties (Pitler & Alger, 1994; Deisz, 1999b) and the time course (Deisz, 1999b) of the presynaptic paired-pulse depression also differed from the postsynaptic GABAB-mediated IPSPs, with a faster decay for the postsynaptic GABAB-mediated IPSPs. This may account for the SICI inhibition produced by a weak CS100 stimulus (at 1.1MT) that did not produce MEP inhibition delivered towards the end of the silent period (Fig. 4).
Pharmacological studies showed that SICI may be mediated by GABAA, since SICI can be enhanced by drugs that enhance GABAA transmission (Ziemann et al. 1996a, b, 1998a). Conversely, the silent period may be mediated by GABAB. In a patient with dystonia, the GABAB agonist baclofen administered intrathecally caused dose-dependent prolongation of the silent period (Siebner et al. 1998). Since LICI and the silent period may be related phenomena (Wassermann et al. 1996), this suggests a role for GABAB receptors in mediating LICI. Further evidence comes from the use of tiagabine, which inhibits GABA reuptake and primarily affects the response at GABAB receptors (Thompson & Gahwiler, 1992). Tiagabine was shown to inhibit SICI but facilitate LICI and the silent period (Werhahn et al. 1999). The authors suggested that while facilitation of LICI was due to effects at postsynaptic GABAB-dependent IPSPs, the reduction in SICI was due to stimulation of presynaptic GABAB receptors with a secondary decrease in GABA release.
Our results are consistent with the hypothesis that the neurons that mediate LICI cause a reduction of SICI through activation of presynaptic GABAB receptors and a reduction of MEP amplitude through postsynaptic GABAB receptors on the cortical neurons. The evaluation of SICI in the presence of LICI may be a non-invasive way of testing presynaptic inhibition in the human motor cortex.
Acknowledgments
We thank Dr Peter Ashby for helpful discussions and for allowing us to use his equipment. This work was supported by the Medical Research Council of Canada (grant no. MT 15128), the Canada Foundation for Innovation and the University Health Network Krembril Family Chair in Neurology. R.C. is a Medical Research Council of Canada Scholar.
References
- Awiszus F, Feistner H, Urbach D, Bostock H. Characterisation of paired-pulse transcranial magnetic stimulation conditions yielding intracortical inhibition or I-wave facilitation using a threshold-hunting paradigm. Experimental Brain Research. 1999;129:317–324. doi: 10.1007/s002210050901. [DOI] [PubMed] [Google Scholar]
- Burke D, Bartley K, Woodforth IJ, Yakoubi A, Stephen JP. The effects of a volatile anaesthetic on the excitability of human corticospinal axons. Brain. 2000;123:992–1000. doi: 10.1093/brain/123.5.992. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG. Mechanisms of cortical reorganization in lower-limb amputees. Journal of Neuroscience. 1998a;18:3443–3450. doi: 10.1523/JNEUROSCI.18-09-03443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. Journal of Neurophysiology. 2000;83:1426–1434. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Experimental Brain Research. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. Journal of Neurophysiology. 1998b;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Chen R, Wassermann EM, Caños M, Hallett M. Impaired inhibition in writer's cramp during voluntary muscle activation. Neurology. 1997;49:1054–1059. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, Benecke R. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic stroke. Brain. 1997;120:605–619. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. Journal of Physiology. 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA. GABAB receptor-mediated effects in human and rat neocortical neurones in vitro. Neuropharmacology. 1999a;38:1755–1766. doi: 10.1016/s0028-3908(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Deisz RA. The GABAB receptor antagonist CGP 55845A reduces presynaptic GABAB actions in neocortical neurons of the rat in vitro. Neuroscience. 1999b;93:1241–1249. doi: 10.1016/s0306-4522(99)00203-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Maonezz P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates inhibitory circuits. Experimental Brain Research. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Floeter MK, Rothwell JC. Releasing the brakes before pressing the gas pedal. Neurology. 1999;53:664–665. doi: 10.1212/wnl.53.4.664. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalography and Clinical Neurophysiology. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: negative effects. In: Hallett M, Lüders H, Marsden CD, editors. Negative Motor Phenomena. Advances in Neurology. vol. 67. Philadelphia: Lippincott-Raven; 1995. pp. 107–113. [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. Journal of Physiology. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Sizes and distributions of intrinsic neurons incorporating tritiated GABA in monkey sensory-motor cortex. Journal of Neuroscience. 1981;1:390–408. doi: 10.1523/JNEUROSCI.01-04-00390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human motor cortex and cervicomedullary junction. Journal of Physiology. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Jones EG. GABAergic neurons and their role in cortical plasticity in primates. Cerebral Cortex. 1993;3:361–372. doi: 10.1093/cercor/3.5.361-a. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalography and Clinical Neurophysiology. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kang Y, Kaneko T, Ohishi H, Endo K, Araki T. Spatiotemporally differential inhibition of pyramidal cells in the cat motor cortex. Journal of Neurophysiology. 1994;71:280–293. doi: 10.1152/jn.1994.71.1.280. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. Journal of Physiology. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. GABA as an inhibitory neurotransmitter in human cerebral cortex. Journal of Neurophysiology. 1989;62:1018–1027. doi: 10.1152/jn.1989.62.5.1018. [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV. The pharmacology and function of central GABAB receptors. International Review of Neurobiology. 1994;36:97–223. doi: 10.1016/s0074-7742(08)60304-9. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. Journal of Physiology. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Differences between presynaptic and postsynaptic GABAB mechanisms in rat hippocampal pyramidal cells. Journal of Neurophysiology. 1994;72:2317–2327. doi: 10.1152/jn.1994.72.5.2317. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Inghilleri M, Accornero N, Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial magnetic brain stimulation in normal subjects, patients with Parkinson's disease and drug-induced parkinsonism. Brain. 1994;117:317–323. doi: 10.1093/brain/117.2.317. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Annals of Neurology. 1995a;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. Journal of Neurology, Neurosurgery and Psychiatry. 1995b;39:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. Journal of Physiology. 1995c;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roick H, von Giesen R, Benecke R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Experimental Brain Research. 1993;94:489–498. doi: 10.1007/BF00230207. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. Journal of Neuroscience Methods. 1997;74:113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle and Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tergau F, Wanschura V, Canelo M, Wischer S, Wassermann EM, Ziemann U, Paulus W. Complete suppression of voluntary motor drive during the silent period after transcranial magnetic stimulation. Experimental Brain Research. 1999;124:447–454. doi: 10.1007/s002210050640. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Effects of the GABA uptake inhibitor tiagabine on inhibitory synaptic potentials in rat hippocampal slice cultures. Journal of Neurophysiology. 1992;67:1698–1701. doi: 10.1152/jn.1992.67.6.1698. [DOI] [PubMed] [Google Scholar]
- Triggs WJ, Macdonell RA, Cros D, Chippa KH, Shahani BT, Day BJ. Motor inhibition and excitation are independent effects of magnetic cortical stimulation. Annals of Neurology. 1992;32:345–351. doi: 10.1002/ana.410320307. [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalography and Clinical Neurophysiology. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscle. Experimental Brain Research. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JKY, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalography and Clinical Neurophysiology. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. Journal of Physiology. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallet M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998a;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. Journal of Neuroscience. 1998b;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Annals of Neurology. 1996a;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Experimental Brain Research. 1996b;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette's disorder: evidence from transcranial magnetic stimulation. American Journal of Psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. Journal of Physiology. 1996c;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. Journal of Physiology. 1998c;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


