Abstract
We investigated the effects of 6 weeks of immobilization on firing rate modulation in motor units in the first dorsal interosseous (FDI) of human volunteers. The middle finger, index finger and thumb were immobilized for a period of 6 weeks in a fibre-glass cast, which kept FDI in a shortened position.
During isometric contraction at 20, 40, 60 and 80 % of maximal voluntary contraction (MVC) (index finger abduction), motor unit action potentials were recorded from the FDI using a tungsten microelectrode, and the relationship between voluntary force and mean firing rate (MFR) was obtained by plotting the MFR of each motor unit action potential train as a function of voluntary force. Four recording sessions were held for each subject: before immobilization, after 3 and 6 weeks of immobilization, and after a 6 week recovery period.
As a result of immobilization, FDI volume (as measured by computerized tomography (CT) scanning) decreased, with an accompanying reduction in aggregate EMG activity per day (P < 0.01). The force measured during MVC also decreased (P < 0.05).
The slope of the relationship between voluntary force and MFR was significantly decreased after immobilization, as was the range of firing rate modulation (P < 0.01). Maximal MFR, estimated from the relationship between voluntary force and MFR, was decreased (P < 0.05).
MFR was also plotted against voluntary force without being normalized with respect to MVC, and the slope of the regression line was decreased (P < 0.05). Voluntary force when the MFR was 15 Hz was estimated from regression equations for the absolute force-MFR relationship, and it was increased after immobilization (P < 0.05).
These results suggest that firing rate modulation shows two different adaptations to joint immobilization: a restriction of motoneurone firing to the lower rates and an enhancement of the voluntary force exerted when the MFR is relatively low.
As a way of reducing the activity of the neuromuscular system, joint immobilization has been used in several studies on human subjects and experimental animals, and it is well known that joint immobilization induces several changes in such skeletal muscle properties as morphology (Tabary et al. 1972), fatigability (Robinson et al. 1991; Yue et al. 1997), muscle fibre type (Tomanek & Lund, 1974; Mayer et al. 1981; Booth, 1982) and contractility (Fischbach & Robbins, 1969; Duchateau & Hainaut, 1987).
In addition to those concentrating on muscle properties, some studies have focused on the alterations in the function of the neural system that occur in response to limb immobilization or chronic disuse. These include alterations in the intrinsic properties of the motoneurone (Gallego et al. 1979a), in the efficacy of synaptic transmission onto the motoneurone (Gallego et al. 1979b;Manabe et al. 1989) and in the cortical representation of immobilized muscle (Zanette et al. 1997). All have been reported to be altered by immobilization. Furthermore, the electromyographic (EMG) activity associated with daily behaviour has been reported to be altered by immobilization, with both the amount of EMG activity (Fudema et al. 1961; Fournier et al. 1983; Hnik et al. 1985) and its burst pattern (Fischbach & Robbins, 1969) being affected. From these data, it is reasonable to assume that motoneuronal activity during voluntary muscle contraction will be influenced by joint immobilization. However, the actual effect of prolonged joint immobilization on such activity is largely unknown.
During voluntary muscle contraction, muscle force is known to be regulated both by the recruitment of motoneurones (Henneman, 1957) and by the modulation of their firing rate (Monster & Chan, 1977). Our previous report (Seki & Narusawa, 1996), and an earlier one by Kukulka & Clamann (1981), suggested that firing rate modulation is the predominant mechanism controlling muscle force in a small muscle like the first dorsal interosseous (FDI), which controls the abduction and flexion force of the index finger (Brandell, 1970).
We therefore set out to examine the effect of prolonged immobilization of the finger joints on the firing rate modulation of FDI motoneurones by recording motor unit activity in the human FDI muscle during voluntary isometric contractions of various magnitudes (Seki & Narusawa, 1996).
If the firing properties of human motor units are indeed affected by joint immobilization, it would be worth examining the correlation between the firing properties and the contractile properties of immobilized muscle. Even though it is well known that the characteristics of individual motoneurones and their muscles correspond well in vertebrate neuromuscular systems (Burke, 1981; Kernell, 1992), little is known about any relationship between the alterations in muscular properties and the changes in motoneuronal activity evoked by prolonged joint immobilization. This issue will be examined in the accompanying paper (Seki et al. 2001), in which we also discuss the possible link between the alterations occurring in the neural and muscular systems during immobilization.
Preliminary results have been reported in abstract form (Seki et al. 1997).
METHODS
Experiments were performed on the left hand of nine male subjects (21-22 years of age) after their informed consent had been obtained. All subjects were right-handed and had no known neural disorders. Of these subjects, seven underwent joint immobilization, one simply provided a day-long recording of the FDI EMG and one acted as a control subject for CT scanning of FDI. The experimental procedures were in accordance with the recommendations of the Declaration of Helsinki for Human Experimentation and with the guidelines issued by our institutional ethics committee.
Immobilization procedure
In seven subjects, the left middle finger, index finger and thumb were immobilized for a period of 6 weeks within a fibre-glass cast (Delta-Lite 6822; Johnson & Johnson, TX, USA) (Fig. 1). The cast kept the metacarpophalangeal joint of the index finger flexed (by approximately 30-40 deg) and the proximal and distal interphalangeal joints slightly extended. The middle finger was immobilized in the same position as the index finger to prevent co-activity of FDI during the movement of the middle finger (Schieber, 1996). The thumb was adducted to make contact with the radial aspect of the index finger and all three fingers pressed lightly against each other within the cast. Some cotton-wool was placed between the fingers, especially between the proximal interphalangeal joints of the index and middle fingers, to avoid excessive pressure on the joints, which was the main source of the discomfort reported in the pilot study. With the hand in this position, the movements of the thumb and of the metacarpophalangeal and proximal interphalangeal joints of the index finger were virtually suppressed, and the movements of the wrist were also restricted to some extent. In our pilot study with three subjects, removing the cast on 1 day per week or once a day, even if only during bathing, greatly diminished the observed effects of immobilization. For this reason, subjects were not allowed to remove or change the cast themselves; this was done by one of the experimenters. On average, the cast was replaced weekly (except in the first week, in which it was replaced twice). Special care was taken to prevent finger movement while replacing the cast. To minimize the isometric contraction in FDI that may accompany arm movements, subjects were asked to make an effort not to use their left hand and arm in their daily life.
Figure 1.
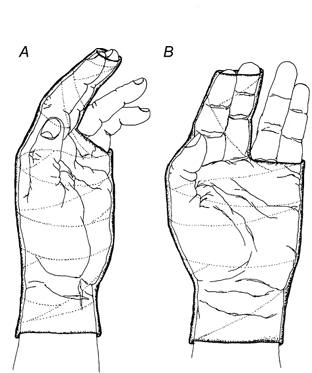
Immobilization of joints
The left middle finger, index finger and thumb were immobilized for a period of 6 weeks within a fibre-glass cast. The cast covered the left hand as shown, from a level about 1 cm proximal to the wrist to a level distal to the interphalangeal joints of the index and middle fingers and to the level of the metacarpophalangeal joint of the ring and little fingers. The thumb, which was completely enclosed within the cast, was adducted to make contact with the radial aspect of the index finger. The thumb and the first two fingers were pressed lightly against each other within the cast. A, radial view; B, palmar view.
Muscle volume
In four subjects, we evaluated the changes in the volume of the FDI induced by immobilization, using CT scanning (Hi-speed Advantage-RP; General Electronics). To try to ensure that the hand was in the same position for each measurement, a hand-supporting device was tailored for each subject using modelling clay. With the aid of this device, the subject's forearm was kept in the mid-position (half-way between pronation and supination) and all five fingers were placed exactly along a custom-fitted grip.
During the measurement, the subject lay prone within the scanning device. The arms were relaxed and positioned straight along the sides of the body with the left hand placed in the supporting device. Scanning was begun from a line drawn between the styloid process of the radius and ulna (using a permanent marker) and contiguous slices, each 1.5 mm thick, were collected (see below).
The cross-sectional area of the muscle in each slice was measured by tracing the boundary of the muscle and then calculating the traced area. The muscle volume was calculated by summing the slice volumes, which were determined by multiplying the size of the traced area by the slice thickness (1.5 mm). The base of the second metacarpal bone was selected as a landmark and this was then used as a reference for each subject. The same number of slices (27-34) from the reference was used to obtain the volume of FDI on each occasion in a given subject. All measurements of muscle volume were performed by the same investigator (K.S.)
Daily activity in FDI
A surface EMG was recorded from the FDI for 18.0-18.5 h per day on two successive days in one subject (for 18.5 h without immobilization and for 18.0 h with immobilization). For this purpose, a surface electrode (MDI-X10 active electrode; Neuromuscular Research Center, Boston, MA, USA) was attached to the skin over the muscle belly of FDI, with a reference electrode being attached around the wrist. The cast used for immobilization was placed in position (covering these electrodes) on the second day. The EMG signal was amplified (x1000), filtered (10-500 Hz) and stored on a portable personal computer (PC). A long lead between the amplifier and the PC allowed the subject to move around without needing to pay much attention to the recording system. Whenever the subject had to move outside the recording area, he was accompanied by one of the investigators, carrying the PC system. The recorded signal was rectified, and integrated EMG activity was calculated for each 10 min period. To eliminate the background noise level from the integrated value, the period without muscle activity was determined in a notional 10 min period, and the integrated values obtained during that period were taken as the noise level and subtracted from the integrated EMG.
Experimental sessions
An experiment consisted of eight experimental sessions, two before immobilization, two after 3 weeks of immobilization, two after 6 weeks of immobilization and two after a 6 week recovery period. Seven subjects were involved in sessions one to six and four of these subjects also took part in the last two sessions. Each session was begun just after the removal of the cast, and the cast was replaced immediately after the end of the session (except of course for the sessions before immobilization and in the recovery period). On average, each pair of sessions was performed with an inter-session interval of 1 day. On the first day of each pair of sessions, myoelectrical signals were recorded to allow measurement of the individual firing frequency of motor units during isometric contractions of various magnitudes. On the second day, the FDI's contractile properties were evaluated by means of per-cutaneous electrical stimulation. The measurements made on the first day are the main focus of the present paper, while those made on the second day are described in the accompanying paper (Seki et al. 2001).
Force recording
These experiments were conducted with the subject in a seated position. The left (non-dominant) arm was abducted to the horizontal plane so that the hand and the forearm were pronated and resting on a supporting device. The forearm and hand were clamped in the device at several points: (1) three belts were used to hold the wrist, the middle to little finger and the thumb and (2) a rigid support was used for the thumb and middle finger. The clamping unit and the location of the two transducers were adjustable to allow for individual variations in the hands of the various subjects. To measure the isometric abduction force exerted by the index finger, a force transducer (TU-CR5K; TEAC Instruments, Tokyo, Japan: capacity < 49.03 N) was fixed to the radial aspect of the proximal interphalangeal joint of this finger by means of a wooden fitting pad that held the angle between phalanges I and II at 1.5 rad. The FDI, which is responsible for about 93 % of the maximum achievable abduction force of the index finger, also has some role in index finger flexion (Brandell, 1970). For this reason, we also measured the flexion force of the index finger by fitting a force transducer (LM5KA; Kyowa Electronic Instruments, Tokyo, Japan: capacity < 49.03 N) to the palmar aspect of the proximal interphalangeal joint of this finger (again, using a wooden fitting pad). Signals from these two transducers were simultaneously displayed on oscilloscopes for visual feedback. Subjects were instructed not to exert a flexion force during the experiments proper, and were allowed several practice sessions to enable them to exert an abduction force without any flexion force.
Myoelectrical signal recording
The technique developed by Bigland-Ritchie and colleagues (Bellemare et al. 1983; Bigland-Ritchie et al. 1983) was employed to record single-unit potentials from FDI during voluntary isometric contraction. Potentials from single muscle fibres were recorded using a tungsten microelectrode (Frederick Haer & Co., MI, USA) which had an insulated tungsten shaft 8.5 cm long and 0.24 mm in diameter, with an exposed tip 5-25 μm in length. Electrode impedance was initially 2-12 MΩ; however, this decreased somewhat after several penetrations into muscles, with an accompanying reduction in spike amplitude and a poorer isolation of single-unit activity. For this reason, electrode impedance was repeatedly checked between trials and if it was found to have decreased, the electrode was replaced. Typically, two or three electrodes were used in each experiment. An insulated stainless-steel wire (diameter, 30 μm) inserted into the subcutaneous tissue around the active electrode was used as a reference electrode.
After the skin had been punctured with a hypodermic needle, subjects were asked to perform a gentle contraction. Subsequently, while monitoring the recorded signal with the aid of a loudspeaker, one experimenter inserted the microelectrode through the puncture hole until its tip lay just under the muscle surface. The electrode was inserted into the medial portion of FDI through the dorsal surface of the hand in the mid-line between the metacarpophalangeal joint and the carpometacarpal joint.
In the course of each trial, the tungsten electrode was slowly advanced under manual control so as to record spike trains from as many muscle fibres as possible. The electrode was then re-inserted at a different angle or at another location (typically 0.5-1 cm distal to the first one). The single-unit potentials were amplified (MS20; Oxford Instruments, Oxford, UK: frequency range, 100 Hz to 20 kHz), displayed on an oscilloscope and stored on magnetic tape.
The location of each penetration was marked on the skin by means of a permanent marker as a landmark for subsequent sessions and electrodes were inserted into approximately the same portions of muscle in each session.
Experimental protocol
First, the maximal voluntary contraction (MVC) was determined by taking the largest of several brief non-fatiguing maximal isometric efforts, with 3 min being allowed between contractions. To assess the degree of muscle activation during MVC, a twitch-interpolation technique (Belanger & McComas, 1981) was used. A supramaximal stimulus (350 V, 300 μs duration, x2 with a 10 ms interval) was delivered through a silver-foil electrode covered with gauze impregnated with a mixture of conductive paste and saline. The cathode (40 mm x 21 mm or 36 mm x 19 mm) was positioned over the muscle belly, with the anode (40 mm x 39 mm) over the distal tendon of the muscle. If no force increment was found during a maximal effort, the level of required contraction was then assigned a value as a percentage of this force (approximately 20, 40, 60 or 80 % MVC). In general, several trials were necessary to isolate abduction without flexion of the index finger and to achieve a sufficient level of effort.
After the MVC and target force levels had been determined, the stimulating electrodes were removed. After a rest period of more than 10 min, subjects were asked to maintain the pre-determined force levels as accurately as possible (with the aid of an oscilloscope display providing a visual feedback of the force they exerted and with the verbal encouragement of one experimenter). Each trial was terminated within 10 s and a rest period of more than 2 min was allowed between trials. Two to four sets of four trials each were performed in a given experiment. Each set consisted of contractions of 20, 40, 60 and 80 % of MVC, the four force levels being performed in a random order within each set. Once more than 20 spike trains had been recorded for each of the four force levels, the experiment was terminated (in most cases). Skin temperature was kept between 35.5 and 36.5 °C with the aid of an infra-red lamp.
Data analysis
The mechanical and myoelectrical signals were stored on analog FM tape and then transferred to a PC with a sampling rate of 10 kHz. Custom-made software was used to analyse these signals. Single-unit recordings from each trial were displayed on the screen of the PC and the positive peaks above a given amplitude were detected in each spike train. Then, inter-spike intervals were measured and the mean firing rate (MFR) in each spike train was calculated. In addition, the maximal spike amplitude in each train, the mean exerted force (% MVC) with s.d., and the number of spikes within each train were obtained. An analysis of variance, a Student's t test, or a Wilcoxon test were used for the comparison of results.
RESULTS
The present study is based on experiments performed on nine subjects. Of these, one was only involved in the chronic recording of the surface EMG and another volunteered only for the measurement of muscle volume as a control subject without immobilization. The firing frequency of FDI motor units during voluntary contraction was examined before, during and after the 6 week period of immobilization in the other seven subjects, and four out of the seven were also involved in the examination of the recovery from immobilization. Changes in FDI muscle volume were measured in four subjects.
Muscle volume
A reduction in muscle volume in FDI was clearly seen in those subjects who underwent immobilization (Fig. 2). The plots in Fig. 2 show results from three subjects with immobilization (A-C) and one without immobilization (D). After 3 weeks of immobilization, muscle volume was reduced to 94, 85 or 92 % of the pre-immobilization value (Fig. 2A, B and C, respectively) and after 6 weeks to 88 or 80 % (Fig. 2A and B) of the pre-immobilization value. These reductions were far larger than the test-to-test variance (less than 1 %, as shown in Fig. 2D). Recovery was examined in one subject (Fig. 2A); in this subject, the volume had returned to the pre-immobilization value (99 % of control) after a 6 week recovery period.
Figure 2.
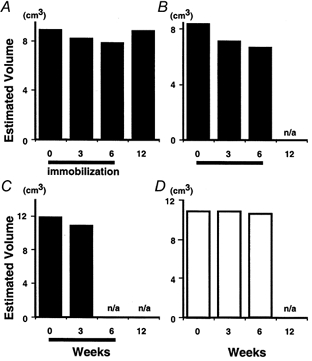
Effect of immobilization on muscle volume of first dorsal interosseous (FDI)
The effect of immobilization on the volume of FDI was evaluated by summing the slice volumes of 27-34 CT images, the volume being determined by multiplying the area in a slice by its thickness (1.5 mm). Each panel shows data from one subject. A-C, subjects each with a 6 week period of immobilization. A reduction in FDI volume can be seen after both 3 and 6 weeks in A and B, and after 3 weeks in C. Data were not available (n/a) at 12 weeks for B or at 6 or 12 weeks for C. Note that recovery from the effect of immobilization (to 99 % of control) was recorded in one subject (A) at 12 weeks. D, a subject without immobilization. Test-to-test variance was less than 1 %.
Chronic EMG recording
A chronic EMG was recorded in one subject for 18.0 h without immobilization (day 1) and for 18.5 h with immobilization (day 2). Figure 3A and B shows the integrated EMG during a day without (A) and a day with immobilization (B). On both days, the subject spent most of the daytime hours sitting in front of the display doing his normal deskwork. Without immobilization (Fig. 3A), the FDI was clearly more active during the daytime than during the time spent in bed (from t = 250 min to t = 700 min, approximately). A qualitatively similar pattern was seen during immobilization (Fig. 3B), but the integrated value of the EMG signal was far lower than on the day without immobilization. The mean integrated value obtained for the day with immobilization was significantly smaller than the control without immobilization (Fig. 3C: 0.23 ± 0.31 vs. 0.03 ± 0.03 mV min, P < 0.01). There was slight noise in the rough EMG and the above values are those obtained after subtracting the integrated noise level from the rough data. In actual fact, the integrated noise level (normalized to a notional 10 min period, as were the corrected data) showed a slight, but significant, difference between control and immobilization (0.26 ± 0.06 vs. 0.23 ± 0.06 mV min; P < 0.01). However, this difference can be ignored since it is very small (-10 %) compared to the huge immobilization-induced reduction in the noise-corrected integrated EMG (-88 %).
Figure 3.
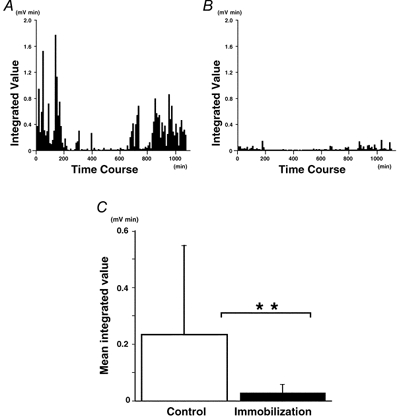
Effect of immobilization on daily activity of FDI
Chronic EMG recording was carried out for 18.0 h without immobilization (A) and for 18.5 h with immobilization (B). EMG signals were rectified and integrated every 10 min. Note that the amplitude of the integrated EMG was smaller during immobilization and that the activity was more marked in the daytime (< 250 min and > 700 min). C, mean values from integrated EMGs (**P < 0.01).
Maximal voluntary contraction
The force achieved during MVC was decreased after 6 weeks of immobilization (Table 1: 38.9 ± 9.1 vs. 32.5 ± 9.4 N, P < 0.05). A decrement in MVC force due to immobilization has also been demonstrated in some previous reports (MacDougall et al. 1977, 1980; White & Davies, 1984; Duchateau & Hainaut, 1987; Miles et al. 1994), but not in all (Fuglevand et al. 1995). The force had returned to the pre-immobilization value after the 6 week recovery period (39.1 ± 9.0 N).
Table 1.
Summary of the number of spike trains, action potential amplitude and MVC force
| No. of spike trains | ||||||||
|---|---|---|---|---|---|---|---|---|
| Immobilization | No. of subjects | MVC force (N) | 20% | 40% | 60% | 80% | Total | Spike amplitude (mV) |
| Pre | 7 | 38.9 ± 9.1 | 28.4 ± 10.1 | 25.0 ± 6.5 | 24.1 ± 9.7 | 20.5 ± 11.4 | 98.1 ± 27.4 | 20.4 ± 5.4 |
| 3 weeks | 7 | 34.1 ± 5.9 | 24.2 ± 7.8 | 23.8 ± 3.4 | 22.0 ± 5.6 | 21.2 ± 8.3 | 91.4 ± 18.6 | 16.2 ± 1.0 |
| 6 weeks | 7 | 32.5 ± 9.4* | 25.0 ± 5.7 | 25.2 ± 4.9 | 26.8 ± 6.9 | 26.0 ± 13.9 | 103.5 ± 23.5 | 19.3 ± 4.0 |
| Recovery | 4 | 39.1 ± 9.0 | 27.0 ± 4.3 | 26.5 ± 13.1 | 24.2 ± 4.7 | 22.5 ± 5.7 | 100.2 ± 10.4 | 16.8 ± 3.3 |
Values are means ±s.d. MVC, maximal voluntary contraction.
Significantly different from pre-immobilization value (P < 0.05).
Features of recorded spike trains
Figure 4A shows typical spike trains recorded from muscle fibres in FDI (pre-immobilization) during voluntary contractions at 80 % MVC. The characteristics of these spike trains were very similar to those in earlier reports (Bellemare et al. 1983; Bigland-Ritchie et al. 1983). For example, (i) each action potential had a large positive spike (mean 18.4 ± 9.6 mV, about 1-2 ms duration), (ii) the spike amplitude typically increased and then decreased as the electrode was advanced and (iii) each spike train exhibited a regular inter-spike interval (coefficient of variation (CV) = 11.3 ± 6.5 %). These characteristics suggested that each spike train originated from a single muscle fibre (Bellemare et al. 1983; Bigland-Ritchie et al. 1983). Only spike trains with more than four intervals were accepted for analysis, and the MFR within each train was calculated.
Figure 4.
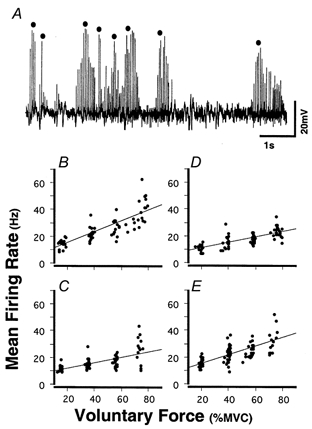
Representive spike trains recorded from FDI during voluntary contractions and relationships between voluntary force and mean firing rate (MFR) in one subject
A, during isometric contraction (80 % MVC), a tungsten electrode was slowly advanced from superficial to deeper portions of FDI. The MFR was obtained for each spike train (•) by measuring the inter-spike interval within each train. B-E, the voluntary force-MFR relationships in one subject pre-immobilization, after 3 and 6 weeks of immobilization, and in recovery, respectively. The MFR of each spike train was plotted against the mean voluntary force measured during the same spike train. Continuous line in each plot indicates the linear regression line (r = 0.82, 0.65, 0.76 and 0.72, respectively; P < 0.01).
In total, we analysed 2450 spike trains (pre-immobilization, 687; 3 weeks, 640; 6 weeks, 727; recovery, 401) from FDI in seven subjects (only four subjects for recovery). The essential features of the sampled spike trains are summarized in Table 1. In each experimental session, we recorded 91.4-103.5 spike trains from a given subject on average (20.5-28.4 spike trains per target force level). There was no difference in the number of spike trains among the various levels of target force or among the experimental sessions. As well as the interval between spikes, we measured the maximal amplitude of the spikes in each train: no difference was found in spike amplitude among the experimental sessions.
Mean firing rates in spike trains during voluntary contraction
To obtain the relationship between voluntary force and MFR (Seki & Narusawa, 1996), the MFR in each spike train (ordinate) was plotted against the mean voluntary force (abscissa) for the period in which each spike train was recorded. Voluntary force was normalized with respect to the MVC force to enable us to compare the relationships among subjects with different MVC values.
Figure 4B–E shows, for one subject, voluntary force-MFR plots for pre-immobilization, 3 and 6 weeks of immobilization, and recovery, respectively. Each of the linear relationships was significant (r = 0.65-0.82, P < 0.01). Note that the slope of the regression lines obtained after immobilization was less steep (Fig. 4C and D) than that obtained pre-immobilization (Fig. 4B). Voluntary force- MFR relationships drawn using pooled data obtained from seven subjects are shown in Fig. 5A. It was clear that the voluntary force-MFR relationships were less steep for the 3 and 6 week immobilization periods than for pre-immobilization and that there was a return to the pre-immobilization slope after the recovery period. The difference was relatively minor between week 3 and week 6. In each of these (pre-immobilization, 3 weeks, 6 weeks, recovery), the linear relationship between voluntary force (% MVC) and MFR (Hz) was significant (r = 0.59-0.72, P < 0.01), the relevant equations being as follows (MFR = Slope x Force + Intercept):
Figure 5.
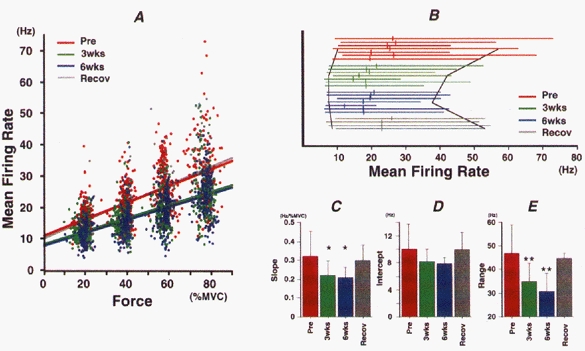
Effect of immobilization of FDI on the relationship between voluntary force and MFR
Red, green, blue and grey colours correspond to pre-immobilization, 3 and 6 weeks of immobilization and recovery, respectively. A, relationships between voluntary force and MFR before immobilization (Pre; n = 687), after 3 weeks (3wks; n = 640) or 6 weeks (6wks; n = 727) of immobilization and in recovery (Recov; n = 401). Data were pooled from seven subjects (four in recovery). Continuous line in each plot indicates the linear regression line (r = 0.59, 0.60, 0.63 and 0.72, respectively; P < 0.01). B, each horizontal line represents the range of MFR values in a given subject (plotted in the same order in each group). Small vertical bars across each line indicate the mean MFR value for that subject, and the black lines show the means of the maximal and minimal MFR values for all subjects. C-E, mean ±s.d. values for slope (C) and intercept (D) obtained from the force-MFR relationships for each subject, and for the range of MFR values (E). Asterisks indicate a significant difference from the pre-immobilization value (* P < 0.05; ** P < 0.01).
 |
The voluntary force-MFR relationship for the FDI in the pre-immobilization period was similar to that in our previous study (slope = 0.27, intercept = 10.46: Seki & Narusawa, 1996). On the other hand, even though the relationship became less steep after immobilization, it was still considerably steeper than that reported for the biceps brachii (slope = 0.13, intercept = 14.78: Seki & Narusawa, 1996).
The range of MFR values was calculated by subtracting the minimal from the maximal MFR values for each subject at each experimental stage, as summarized in Fig. 5B. The results obtained for all four target levels were pooled in this analysis. It is clear that the range covered by the MFR values narrowed after immobilization. The effects of 6 weeks of immobilization on the slope and intercept of the linear regression line and on the range of firing rates are shown in Fig. 5C–E, respectively. Significant decreases were found in slope (Fig. 5C; P < 0.05) and range (Fig. 5E; P < 0.01) after a 3 and 6 week immobilization but not in intercept (Fig. 5D). The maximal MFR in each subject was estimated by extrapolating from the MVC force using the regression equation, and the effect of immobilizaion on MFR was examined (Fig. 6). The estimated maximal MFR was decreased (P < 0.05) after both 3 and 6 weeks of immobilization.
Figure 6.
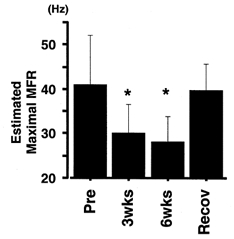
Effect of immobilization on maximal MFR of FDI
Maximal MFR (means ±s.d.) was estimated by extrapolating to MVC force using the regression equation relating voluntary force to MFR in each subject. * Significantly different from pre-immobilization value (P < 0.05).
It is clear from Fig. 5 that after immobilization, the slope of the voluntary force-MFR relationship was decreased and that the range of the MFR values was narrowed. The decrement in maximal MFR (Fig. 6) suggested that the changes in slope and range of firing rates are largely effected by the decrement in maximal discharge rate shown by the FDI motoneurone.
To examine further the underlying mechanism inducing the alteration in the voluntary force-MFR relationship, lines of the type obtained after immobilization are shown (for illustrative purposes) without force being normalized with respect to MVC (Fig. 7). In this schema, three possible voluntary force-MFR relationships after immobilization are drawn (grey lines, labelled a, b and c) relative to that obtained pre-immobilization (black line). Since no significant difference was found among the intercepts of the voluntary force-MFR relationships (Fig. 5D), all of these lines are assumed to have the same intercept (note that the intercept values are not affected by whether force is or is not normalized). In this hypothetical illustration, the voluntary force-MFR relationships are extended up to MVC and maximal MFR (open circles). Both showed a reduction after immobilization (Table 1 and Fig. 6). If the force was normalized with respect to MVC, lines a, b and c of Fig. 7 could be redrawn as one line (less steep than the pre-immobilization line but with the same intercept). In line a, the reduction in maximal firing rate could be the only factor to reduce the MVC, and the force-frequency relationship in the lower force range is not different from that obtained pre-immobilization. In this case, the slope of this relationship is unchanged. On the other hand, any change of slope in these plots suggests that factors other than a reduction in maximal firing rate (Fig. 6) should be taken into account when trying to explain the reduction in MVC, since the force-frequency relationship at a lower force level is altered. For example, an increment in the slope (line b) after immobilization suggests a deficit in the ability to exert force at sub-maximal MFR, since the force induced by a given MFR is reduced (filled circle on line b). In this case, MVC force would be reduced even if the maximal firing rate was assumed to be the same as the pre-immobilizaion value (see dashed part of line b). On the other hand, a decrement in the slope (line c) suggests an improvement in the ability to exert force at sub-maximal MFR, since the force induced by a given MFR is enhanced (filled circle on line c). In this case, MVC would be increased if the maximal firing rate was assumed to be the same as the pre-immobilization value (see dashed part of line c).
Figure 7.
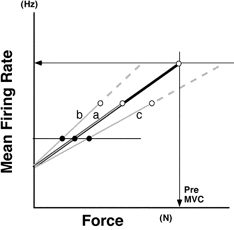
Schema showing illustrative voluntary force-MFR relationships plotted without force being normalized with respect to MVC
The voluntary force-MFR relationship after immobilization was drawn in three different ways for illustrative purposes (grey lines, labelled a-c) relative to the pre-immobilization line (black line). All of these lines are assumed to have the same intercept. The relationships are each extended up to MVC and maximal MFR (○), both of which show a reduction after immobilization (Table 1, Fig. 6). See text for further explanation.
In Fig. 8, the voluntary force-MFR relationships are shown for all subjects (A-G) with force expressed in absolute terms (N). The continuous and dashed lines represent the regression lines obtained pre- and post- (6 week) immobilization, respectively. In five subjects (Fig. 8A-E), the slope of the voluntary force-MFR relationship was decreased post-immobilization (as it was in line c in Fig. 7). One subject (Fig. 8F) showed no decrement in the slope, although the regression line in this subject shifted downwards (see also Fig. 8D and E) and the similar consequences to those described for line c of Fig. 7 would apply in this subject. One subject (Fig. 8G) showed a line similar to line a in Fig. 7. No subject showed a relationship like line b in Fig. 7. Therefore, since only changes producing regression lines like a and c were found in the subjects in this study, these results seemed to suggest an improvement in the ability to exert force at sub-maximal MFR as well as a reduction in the maximal firing rate of FDI motoneurones (Fig. 6) after immobilization.
Figure 8.
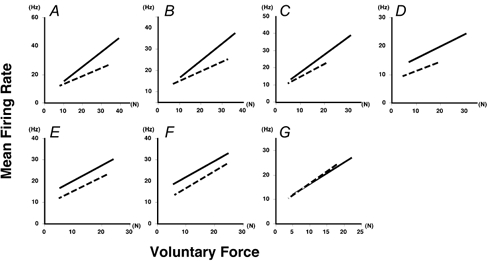
Relationships between absolute force and MFR
The voluntary force-MFR relationships are shown for all subjects (A-G) with force expressed in absolute terms (N). The continuous and dashed lines represent the regression lines for pre- and post-immobilization (6 weeks), respectively.
Figure 9 supports this idea. Figure 9A shows the effect of immobilization on the slope of the absolute force-MFR relationship. The slope was decreased after 6 weeks of immobilization (as in line c in Fig. 7), which suggested, again, an enhanced ability to produce sub-maximal force. We also examined the ability to exert force at sub-maximal MFR by estimating the voluntary force when the MFR was 15 Hz (Fig. 9B) using the regression equation relating absolute force and MFR. The estimated force was enhanced after both 3 and 6 weeks of immobilization (P < 0.05). From Figs 6–9, consequently, it is obvious that two important changes occur as a result of immobilization: force enhancement (a greater force for a given MFR) and a decrease in the maximal firing rate.
Figure 9.
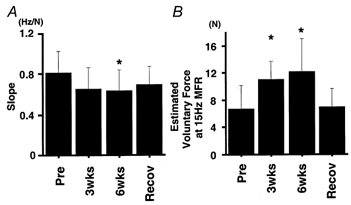
Reduction in the slope of the absolute force-MFR relationship and enhancement of voluntary force produced by a given MFR
A, mean ±s.d. values for the slope of the regression equation for absolute force-MFR relationship (see Figs 7 and 8). B, mean ±s.d. values for voluntary force when MFR was 15 Hz (as calculated from the regression equation relating absolute force and MFR). * Significantly different from the pre-immobilization value (P < 0.05).
DISCUSSION
From the experiments performed in this study, we conclude that a 6 week immobilization of FDI induced (1) a reduction in muscle volume (Fig. 2), (2) a decrease in the slope of the relationship between voluntary force and MFR (Fig. 5) and (3) a narrowing of the range of firing rates shown by FDI motor units (Fig. 5). Furthermore, our data suggest that after immobilization (1) the maximal firing rate of FDI motoneurones during voluntary contractions is decreased (Fig. 6) and (2) the force-generating capacity of the neuromuscular system at a sub-maximal frequency of activation is enhanced (Fig. 9). To date, the effects of joint immobilization on the firing properties of human motor units have been examined in only two studies (including this paper) and the conclusions drawn in this study are based on the firing rate of motor units in healthy volunteers, whereas the other report was concerned almost entirely with patients with fractures (Duchateau & Hainaut, 1990).
Change in muscle volume in FDI during immobilization
As shown in Fig. 2, the volume of FDI, as estimated from a series of CT images, was reduced to 80-92 % of control after immobilization, suggesting an atrophy of the immobilized muscle. This observation is consistent with previous reports on human subjects (MacDougall et al. 1977; White & Davies, 1984; Miles et al. 1994) or non-human mammals (Tabary et al. 1972). Indeed, muscle atrophy is known to be a general consequence of joint immobilization (Booth, 1982). Since the EMG activity in FDI was substantially reduced by immobilization (Fig. 3), the muscle atrophy may have been a consequence of the reduced activity (Fournier et al. 1983; Hnik et al. 1985). Two other groups (Duchateau & Hainaut, 1990; Fuglevand et al. 1995) have examined the effect of immobilization on muscle properties in human FDI. Duchateau & Hainaut (1990) found a decline in MVC force in FDI after immobilization for 6-8 weeks, a result similar to ours. In contrast, Fuglevand et al. (1995) found that 3-5 weeks of immobilization of the index finger and thumb caused little change in the strength of the FDI muscles. We can think of three possible explanations for this discrepancy. Firstly, in the study of Fuglevand et al. (1995) the thumb and the index finger were immobilized but the middle finger and wrist were not. In contrast, in the present study the movement of the middle finger was abolished and that of the wrist was restricted. It has been shown that flexion of the middle finger or wrist joint is concomitant with flexion of the index finger in non-human primates (Schieber, 1991). Since FDI is a synergist for the flexion of the index finger (Brandell, 1970), it is likely that part of FDI is recruited by the ‘distributed control system’ (Schieber, 1996) during immobilization, and muscle atrophy might be prevented to some extent by the myoelectrical activity and/or the mechanical stimulus (to the muscle) occurring during the resulting movements. It is difficult to compare the amount of EMG activity during immobilization between the two studies because the methods used to quantify the EMG signals were different, although we can say that a reduction in the EMG signal was found in each study (95 % in Fuglevand et al. 1995; 88 % in this study). It may still be a reasonable assumption that immobilization of the middle finger was causally related to the muscle atrophy in FDI seen in our study. Our observations seem to be supported by the findings of Duchateau & Hainaut (1990) who reported a decline in MVC force in FDI after immobilization achieved using an immobilization method similar to ours.
Secondly, the subjects in the study by Fuglevand et al. (1995) were allowed to remove the splint at least once a day for bathing. In our pilot study with three subjects, we used a cast like that shown in Fig. 1 but with a slit for removal, and we allowed the subjects to remove it for bathing. Interestingly, when that design was used little change was found in MVC force after 3-6 weeks of immobilization. Consequently, as mentioned in Methods, subjects in the study proper were not allowed to remove the cast by themselves; instead, an experimenter removed it and washed their hand, once a week on average. From these observations, it would appear that even brief activity and/or passive stretch (Brownson & Loughna, 1996) of FDI during the immobilization period may prevent or impede the development of muscle atrophy.
Thirdly, the duration of immobilization was somewhat shorter in the study by Fuglevand et al. (1995) (3 weeks in most subjects) than in the present study (6 weeks). As shown in Fig. 2A and B and Table 1, both the estimated volume of FDI and its MVC force decreased progressively throughout the immobilization period. Duchateau & Hainaut (1990) also found a decrement in MVC in FDI after 6-8 weeks of immobilization. Conceivably, it might take longer in FDI than in other human muscles (Davies et al. 1987; Miles et al. 1994) for joint immobilization to induce a reduction in muscle force or volume.
Mechanism producing the alteration in the relationship between voluntary force and MFR
After a 6 week immobilization of FDI, we found a reduction in the slope of the relationship between voluntary force and MFR, together with a narrowing of the range of motoneuronal firing rates (Fig. 5) during submaximal voluntary contractions. As shown in Table 1, no difference was found in the number of recorded spike trains at a given force level (either at a given immobilization stage or among the various stages). Furthermore, we did our utmost to insert the electrode from the same points and at similar angles and directions so as to compile the spike train from a similar portion of FDI each time in a given subject. Consequently, we think it unlikely that our observations were affected by sampling bias.
As depicted in Fig. 7, we can envisage three types of voluntary force-MFR relationship after immobilization (lines a-c), and we can hypothesize that three mechanisms might underlie the changes: a reduction in the maximal firing rate of motoneurones (Fig. 6 and lines a-c in Fig. 7), an enhanced ability to induce submaximal force (Fig. 7, line c) and a deficit in the force produced at sub-maximal MFR (Fig. 7, line b). The results shown in Figs 6, 8 and 9 suggest, firstly, that the estimated maximal MFR was significantly decreased by immobilization (Fig. 6). This could be one of the main reasons for the reduction in the MVC force of the FDI (Table 1), together with the change found in the voluntary force-MFR relationship (Fig. 5).
Secondly, we found that after immobilization in six (Fig. 8A-F) out of the seven subjects the absolute force-MFR relationship showed changes similar to those illustrated by line c in Fig. 7; that is, the slope of the absolute force-MFR relationship was decreased (P < 0.05) (Fig. 9A), and the voluntary force when the MFR was 15 Hz was enhanced (P < 0.05) (Fig. 9B). This was somewhat surprising since both the muscle volume and the MVC force were reduced by immobilization. In view of such a loss of the ability to exert strong force, we might have expected a deficit in the ability of FDI to exert force at sub-maximal MFR after immobilization (like line b in Fig. 7). However, this was not the case, and most subjects showed an enhancement, rather than a deficit, in the ability to induce force at a sub-maximal MFR. Therefore, it could be concluded that the force-generating capacity of the neuromuscular system at a sub-maximal frequency of activation is enhanced by immobilization, and this could be the second factor responsible for the change found inthe voluntary force-MFR relationship (Fig. 5).
Factors enhancing the voluntary force at lower firing rates
An interesting feature of our findings was that after immobilization there was an enhancement of the isometric force induced by a lower MFR (Fig. 9B). Several possible factors might be causally involved in this phenomenon.
It is well known that the organization of muscle fibre types can be altered by limb immobilization (Booth, 1982). Since recruitment of motoneurones under normal conditions is known to occur in order of ‘size’ (Henneman, 1957), the recruitment characteristics of FDI motoneurones would be changed if the normal distribution of motoneurone properties, not only of muscle fibre types, was altered by immobilization. In this case, the observed enhancement of voluntary force at sub-maximal MFR might be attributed to altered recruitment properties in the FDI motoneurone pool, such that more motor units are active when a sub-maximal voluntary force is produced. Two earlier studies addressed this issue. Mayer et al. (1981), who investigated the effect of limb immobilization on the motor unit population of the cat gastrocnemius muscle, found no change in the distribution of motor unit types. In a similar experiment, Robinson et al. (1991) found no significant difference in cat tibialis posterior. Therefore, even though no information is available as to the effects of immobilization on the organization of the motoneuronal pool in the human subject, it would seem unlikely that the enhancement of sub-maximal force is induced by a change in motoneuronal organization.
It has been proposed that the recruitment gain within a motoneuronal pool might be affected by various synaptic inputs (Kernell & Hultborn, 1990). If the recruitment gain in the FDI motoneurone pool was altered so as to compress the recruitment range, then more motoneurones could be recruited and more voluntary force could be expected at a given mean rate of motoneuronal discharge. For example, the efficacy of synaptic transmission from Ia afferents to the spinal motoneurone is known to be enhanced after a period of disuse (Gallego et al. 1979b;Manabe et al. 1989). These kinds of alterations in synaptic efficacy might modify the recruitment gain of the FDI motoneurone pool. However, to verify this possibility, more information would be required as to how immobilization affects the various kinds of synaptic input to motoneurones (see Henneman & Mendell, 1981).
A plausible explanation for the phenomenon under discussion could be an alteration in the relationship between activation frequency and evoked isometric force in the FDI muscle itself. In animal preparations, an alteration in the stimulus frequency-force relationship has been reported after joint immobilization in the hind-limb muscle of the cat (Petit & Gioux, 1993) and rat (Simard et al. 1982). In humans, we found a shift in the stimulus frequency-force relationship towards lower frequencies after joint immobilization and we concluded that these changes could be ascribed mainly to an enhancement of the twitch force, and to a decrement in maximal tetanic force (Seki et al. 2001). We shall discuss the possible factors contributing to these changes in the accompanying paper (Seki et al. 2001).
Factors restricting motoneuronal firing at higher rates
Repetitive firing of neurones is known to be limited by the after-hyperpolarization (AHP) that follows each action potential (Kernell, 1965; Schwindt & Crill, 1982). The duration of the AHP might be a limiting factor preventing FDI motoneurones from firing at higher rates, if it was extended by joint immobilization. However, Gallego et al. (1979a) found that the duration of the AHP in soleus motoneurones decreased significantly after 2 weeks of joint immobilization. It is known that the minimal rate of motoneuronal firing is largely determined by the duration of the AHP (Kernell, 1965). Even though the minimal firing rate of each motor unit was not measured in this study, the changes in the lowest frequency (within the force range used in this study) were less marked than those in the highest frequency (Fig. 5B). Hence, it seems unlikely that the reduction in the estimated maximal firing rate after immobilization was effected by a change in AHP duration.
Kernell (1992) suggested that the maximum firing rate of the motoneurone is dependent on the spike-generating properties of its membrane. Even though several intrinsic properties of the motoneuronal membrane (e.g. Hounsgaard et al. 1984; Kiehn & Eken, 1997; Gorassini et al. 1999) may affect repetitive firing, it is not known whether these properties are influenced by joint immobilization. Therefore, it is an open question as to whether and how limb immobilization might affect the intrinsic spike-generating properties of the motoneurone.
An alteration in the synaptic effects on FDI motoneurones seems a more probable explanation for the restriction of motoneurone firing at higher rates. For example, the response of the FDI muscle to transcranial magnetic stimulation has been reported to be affected by immobilization (Zanette et al. 1997). Moreover, Sale et al. (1982) suggested a reduction in the ‘central drive’ to upper limb motoneurones during immobilization in human subjects. These reports seem to support the notion that the descending command facilitating motoneuronal excitability is reduced by immobilization. Therefore, it is possible that the reduction in maximal firing rate may reflect this alteration. It has been shown that feedback from peripheral afferents could affect a motoneurone's ability to fire at higher frequencies (Bigland-Ritchie et al. 1986; Gandevia et al. 1990). If this feedback system is influenced by immobilization, this is likely to alter the motoneurone's ability to discharge at higher rates.
Time course of changes
By way of conclusion, it might be interesting to discuss the time course of the immobilization-induced changes in motoneuronal activity. We found that immobilization induced changes in MVC, muscle volume, the slope of the relationship between voluntary force and MFR, the range of firing rates, the estimated maximal MFR and the estimated tetanic force induced at 15 Hz MFR. In the present study, we noticed that the magnitude of the changes was much greater in weeks 1-3 than in weeks 4-6 of immobilization. For example, the percentage of the total change occurring in the first 3 weeks of immobilization was 94 % (slope), 67 % (firing rate range), 91 % (estimated maximal firing rate) and 78 % (tetanic force induced by 15 Hz MFR). The remainder (only 6-33 %) of the total change occurred in the second 3 weeks. These results suggest not only that a considerable proportion of the changes induced by immobilization takes place within 3 weeks, but also that the immobilization-induced alteration in motoneuronal firing rate modulation would begin much earlier than 3 weeks. It is interesting to note that motor unit activity in human muscles is reported to be noticeably altered within a few days of the beginning of strength training (Patten & Kamen, 1996). Furthermore, we found support for this notion in our preliminary data indicating a reduction in maximal firing rate of FDI motoneurones after 1 week of immobilization (Seki et al. 1999).
Acknowledgments
We thank Asaji Nitta for his help with CT scanning. This research was supported by grants from the Japanese Ministry of Education, Science, Sports and Culture, the Meiji Foundation for Health Science and the Budo and Sports Science Institute of International Budo University.
References
- Belanger AY, McComas AJ. Extent of motor unit activation during effort. Journal of Applied Physiology. 1981;51:1131–1135. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Bellemare F, Woods JJ, Johansson R, Bigland-Ritchie B. Motor-unit discharge rates in maximal voluntary contractions of three human muscles. Journal of Neurophysiology. 1983;50:1380–1392. doi: 10.1152/jn.1983.50.6.1380. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Dawson NJ, Johansson R, Lippold OCJ. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. Journal of Physiology. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OCJ, Smith S, Woods JJ. Changes in motoneurone firing rates during sustained maximal voluntary contractions. Journal of Physiology. 1983;340:335–346. doi: 10.1113/jphysiol.1983.sp014765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW. Effect of limb immobilization on skeletal muscle. Journal of Applied Physiology. 1982;52:1113–1118. doi: 10.1152/jappl.1982.52.5.1113. [DOI] [PubMed] [Google Scholar]
- Brandell BR. An electromyographic-cinematographic study of the muscles of the index finger. Archives of Physical Medicine and Rehabilitation. 1970;51:278–285. [PubMed] [Google Scholar]
- Brownson C, Loughna P. Alterations in the mRNA levels of two metabolic enzymes in rat skeletal muscle during stretch-induced hypertrophy and disuse atrophy. Pflügers Archiv. 1996;431:990–992. doi: 10.1007/s004240050097. [DOI] [PubMed] [Google Scholar]
- Burke R. Motor units: anatomy, physiology, and functional organization. In: Brooks VB, editor. Handbook of Physiology. Vol. 2. Bethesda MD USA: American Physiological Society; 1981. pp. 354–422. section 1, The Nervous System, Motor Control. [Google Scholar]
- Davies CT, Rutherford IC, Thomas DO. Electrically evoked contractions of the triceps surae during and following 21 days of voluntary leg immobilization. European Journal of Applied Physiology and Occupational Physiology. 1987;56:306–312. doi: 10.1007/BF00690897. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Electrical and mechanical changes in immobilized human muscle. Journal of Applied Physiology. 1987;62:2168–2173. doi: 10.1152/jappl.1987.62.6.2168. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. Journal of Physiology. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach GD, Robbins N. Changes in contractile properties of disused soleus muscles. Journal of Physiology. 1969;201:305–320. doi: 10.1113/jphysiol.1969.sp008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Roy RR, Perham H, Simard CP, Edgerton VR. Is limb immobilization a model of muscle disuse. Experimental Neurology. 1983;80:147–156. doi: 10.1016/0014-4886(83)90011-0. [DOI] [PubMed] [Google Scholar]
- Fudema JJ, Fizzell JA, Nelson EM. Electromyography of experimentally immobilized skeletal muscles in cats. American Journal of Physiology. 1961;200:963–967. doi: 10.1152/ajplegacy.1961.200.5.963. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Bilodeau M, Enoka RM. Short-term immobilization has a minimal effect on the strength and fatigability of a human hand muscle. Journal of Applied Physiology. 1995;78:847–855. doi: 10.1152/jappl.1995.78.3.847. [DOI] [PubMed] [Google Scholar]
- Gallego R, Kuno M, Nunez R, Snider WD. Dependence of motoneurone properties on the length of immobilized muscle. Journal of Physiology. 1979a;291:179–189. doi: 10.1113/jphysiol.1979.sp012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R, Kuno M, Nunez R, Snider WD. Disuse enhances synaptic efficacy in spinal mononeurones. Journal of Physiology. 1979b;291:191–205. doi: 10.1113/jphysiol.1979.sp012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Macefield G, Burke D, McKenzie DK. Voluntary activation of human motor axons in the absence of muscle afferent feedback. Brain. 1990;113:1563–1581. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. Journal of Neurophysiology. 1999;82:709–717. doi: 10.1152/jn.1999.82.2.709. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relations between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1346. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneuron pool and its inputs. In: Brooks VB, editor. Handbook of Physiology. Bethesda USA MD: American Physiological Society; 1981. pp. 423–507. section 1, The Nervous System, vol. 2, Motor Control. [Google Scholar]
- Hnik P, Vejsada R, Goldspink DF, Kasicki S, Krekule I. Quantitative evaluation of electromyogram activity in rat extensor and flexor muscles immobilized at different lengths. Experimental Neurology. 1985;88:515–528. doi: 10.1016/0014-4886(85)90067-6. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Experimental Brain Research. 1984;55:391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiologica Scandinavica. 1965;65:87–100. [Google Scholar]
- Kernell D. Organized variability in the neuromuscular system: a survey of task-related adaptations. Archives Italiennes de Biologie. 1992;130:19–66. [PubMed] [Google Scholar]
- Kernell D, Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurine pools. Brain Research. 1990;507:176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons. Journal of Neurophysiology. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Research. 1981;219:45–55. doi: 10.1016/0006-8993(81)90266-3. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Elder GC, Sale DG, Moroz JR, Sutton JR. Effects of strength training and immobilization on human muscle fibres. European Journal of Applied Physiology and Occupational Physiology. 1980;43:25–34. doi: 10.1007/BF00421352. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Ward GR, Sale DG, Sutton JR. Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. Journal of Applied Physiology. 1977;43:700–703. doi: 10.1152/jappl.1977.43.4.700. [DOI] [PubMed] [Google Scholar]
- Manabe T, Kaneko S, Kuno M. Disuse-induced enhancement of Ia synaptic transmission in spinal motoneurons of the rat. Journal of Neuroscience. 1989;9:2455–2461. doi: 10.1523/JNEUROSCI.09-07-02455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer RF, Burke RE, Toop J, Hodgson JA, Kanda K, Walmsley BW. The effect of long-term immobilization on the motor unit population of the cat medial gastrocnemius muscle. Neuroscience. 1981;6:725–739. doi: 10.1016/0306-4522(81)90156-1. [DOI] [PubMed] [Google Scholar]
- Miles MP, Clarkson PM, Bean M, Ambach K, Mulroy J, Vincent K. Muscle function at the wrist following 9 d of immobilization and suspension. Medicine and Science in Sports and Exercise. 1994;26:615–623. [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. Journal of Neurophysiology. 1977;40:1432–1443. doi: 10.1152/jn.1977.40.6.1432. [DOI] [PubMed] [Google Scholar]
- Patten C, Kamen G. Adaptation in human motor unit discharge behaviour to strength training. Society for Neuroscience Abstracts. 1996;22:130. [Google Scholar]
- Petit J, Gioux M. Properties of motor units after immobilization of cat peroneus longus muscle. Journal of Applied Physiology. 1993;74:1131–1139. doi: 10.1152/jappl.1993.74.3.1131. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Enoka RM, Stuart DG. Immobilization-induced changes in motor unit force and fatigability in the cat. Muscle and Nerve. 1991;14:563–573. doi: 10.1002/mus.880140611. [DOI] [PubMed] [Google Scholar]
- Sale DG, McComas AJ, MacDougall JD, Upton AR. Neuromuscular adaptation in human thenar muscles following strength training and immobilization. Journal of Applied Physiology. 1982;53:419–424. doi: 10.1152/jappl.1982.53.2.419. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Individuated finger movements of rhesus monkeys: a means of quantifying the independence of the digits. Journal of Neurophysiology. 1991;65:1381–1391. doi: 10.1152/jn.1991.65.6.1381. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Individuated finger movements. In: Wing A, Haggard P, Flanagan JR, editors. Hand and Brain. San Diego USA: Academic Press; 1996. pp. 81–98. [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. Journal of Neurophysiology. 1982;48:875–890. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- Seki K, Kizuka T, Yamada H. Immobilization of skeletal muscle induces a reduction in maximal firing rate of human motor unit. Society for Neuroscience Abstracts. 1999;25:1147. [Google Scholar]
- Seki K, Narusawa M. Firing rate modulation of human motor units in different muscles during isometric contraction with various forces. Brain Research. 1996;719:1–7. doi: 10.1016/0006-8993(95)01432-2. [DOI] [PubMed] [Google Scholar]
- Seki K, Taniguchi Y, Narusawa M. XXXII International Congress of Physiological Sciences. P043.10. Russia: St Petersburg; 1997. Adaptation of motoneuronal activity to immobilization and to overloading of skeletal muscle in human subjects. [Google Scholar]
- Seki K, Taniguchi Y, Narusawa M. Alterations in contractile properties of human skeletal muscle induced by joint immobilization. Journal of Physiology. 2001;530:521–532. doi: 10.1111/j.1469-7793.2001.0521k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard CP, Spector SA, Edgerton VR. Contractile properties of rat hind limb muscles immobilized at different lengths. Experimental Neurology. 1982;77:467–482. doi: 10.1016/0014-4886(82)90221-7. [DOI] [PubMed] [Google Scholar]
- Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. Journal of Physiology. 1972;224:231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek RJ, Lund DD. Degeneration of different types of skeletal muscle fibres. II. Immobilization. Journal of Anatomy. 1974;118:531–541. [PMC free article] [PubMed] [Google Scholar]
- White MJ, Davies CT. The effects of immobilization, after lower leg fracture, on the contractile properties of human triceps surae. Clinical Science. 1984;66:277–282. doi: 10.1042/cs0660277. [DOI] [PubMed] [Google Scholar]
- Yue GH, Bilodeau M, Hardy PA, Enoka RM. Task-dependent effect of limb immobilization on the fatigability of the elbow flexor muscles in humans. Experimental Physiology. 1997;82:567–592. doi: 10.1113/expphysiol.1997.sp004048. [DOI] [PubMed] [Google Scholar]
- Zanette G, Tinazzi M, Bonato C, Di Summa A, Manganotti P, Polo A, Fiaschi A. Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalography and Clinical Neurophysiology. 1997;105:269–279. doi: 10.1016/s0924-980x(97)00024-6. [DOI] [PubMed] [Google Scholar]


