Abstract
The effects of joint immobilization on the contractile properties of human skeletal muscle were examined using the first dorsal interosseous (FDI) muscle.
The middle finger, index finger and thumb were immobilized for a period of 6 weeks, and the contractile properties of FDI were tested before immobilization, after 3 and 6 weeks of immobilization, and after a 6 week recovery period.
Twitch and tetanic contractions of FDI were evoked by per-cutaneous electrical stimulation. The peak twitch tension (Pt), contraction time (CT) and half-relaxation time (1/2RT) were measured from twitch contractions, while the stimulus frequency-force relationship was obtained from the tetanic contractions (2 s) evoked using various frequencies of stimulation (10-100 Hz). The fatigability of FDI was tested using Burke's fatigue protocol.
Pt was significantly increased after 6 weeks of immobilization (P < 0.05) but little alteration was observed in CT or 1/2RT. No change was noted in the FDI fatigue index throughout the immobilization period.
The stimulus frequency-force relationship was shifted to the left by immobilization, indicating that a larger percentage of maximal force was evoked by the lower rates of stimulation. Indeed, the tetanic force evoked by a stimulus frequency of 10 Hz was enhanced after immobilization (P < 0.05). On the other hand, the force evoked by frequencies above 50 Hz, including maximal tetanic tension, was decreased (P < 0.05). As a result, the twitch/tetanus ratio was increased (P < 0.01) after immobilization.
The changes induced by immobilization in the FDI twitch/tetanus ratio and the estimated maximal firing rate of FDI motoneurones showed a significant correlation (r = 0.80, P < 0.05).
It is suggested that the changes in the contractile properties of the FDI muscle seen after joint immobilization are causally linked to the changes in firing rate modulation of FDI motoneurones.
There is general agreement that the properties of skeletal muscle and those of the motoneuronal system correspond well with each other in many ways; for example, muscle units with a shorter contraction time are generally governed by motoneurones with a faster conduction velocity (Burke, 1967), a higher recruitment threshold (Henneman, 1957) and a shorter duration of after-hyperpolarization (Bakels & Kernell, 1993). Furthermore, the synaptic input to motoneurones is also known to correlate well with the properties of the motoneurones and muscle (Heckman, 1994).
Long-term immobilization of a joint is known to modify such properties of skeletal muscle as morphology (Tabary et al. 1972), the proportion of fast and slow muscle fibres (Tomanek & Lund, 1974) and contractility (Fischbach & Robbins, 1969; Duchateau & Hainaut, 1987). Several reports have also suggested that immobilization affects such features of the neural system controlling muscular force as the intrinsic properties of the motoneurones (Gallego et al. 1979a), the inputs to motoneurones from peripheral afferents (Gallego et al. 1979b; Manabe et al. 1989) and the descending system to motoneurones (Zanette et al. 1997). Furthermore, electromyographic (EMG) activity has also been reported to be influenced by joint immobilization (Fudema et al. 1961; Hnik et al. 1985), while Fischbach & Robbins (1969) found that aggregate EMG activity, as well as the pattern of EMG bursts, was modified after immobilization in association with changes in the contractile properties of skeletal muscle. Since EMG activity is regulated by modulating motoneuronal activity, it seemed reasonable to assume that motoneuronal activity during voluntary contractions in humans (Milner-Brown et al. 1973; Monster & Chan, 1977) would also be affected by immobilization. However, there is little evidence to indicate the existence of any correlation between joint immobilization-induced changes in motoneuronal activity and the associated changes in the contractile properties of the muscle.
In the preceding paper (Seki et al. 2001) on the effects of joint immobilization on the firing properties of human motoneurones during voluntary isometric contractions of the first dorsal interosseous (FDI), we suggested that there might be two kinds of adaptation to joint immobilization: a restriction of motoneurone firing to the lower rates and an enhancement of the voluntary force exerted at low firing rates. To examine how these changes in motoneuronal activity might correlate with any changes in the contractile properties of the FDI muscle, we investigated the effect of a 6 week joint immobilization on the contractile properties of FDI.
Preliminary results have been reported in abstract form (Seki et al. 1997).
METHODS
Experiments were performed on the left hand of seven right-handed male human subjects (21-22 years of age) after their informed consent had been obtained. All subjects were right-handed and had no known neural disorders. The experimental procedures were in accordance with the recommendations of the Declaration of Helsinki for Human Experimentation and were approved by our institutional ethics committee.
The procedure used for immobilization, the basic experimental protocols and the methodology used for the recording of muscle force are described in the accompanying paper (Seki et al. 2001). Briefly, FDI was immobilized at a shortened length for 6 weeks and eight experimental sessions were held, two before immobilization, two after 3 weeks of immobilization, two after 6 weeks of immobilization and two after 6 weeks of recovery. The two sessions in each pair were performed on separate days: motoneuronal firing frequency during voluntary contraction was measured on the first day and the contractile properties of the FDI were assessed on the second day. The sessions on the second day are the main concern of the present paper.
Muscle stimulation
To evoke twitch and tetanic contractions, electrical stimulation (3F45; NEC San-ei, Tokyo, Japan) was applied per-cutaneously over FDI. A silver-foil electrode (covered with gauze impregnated with conductive paste and saline) was used for stimulation. The skin over the FDI muscle was scrubbed with alcohol and the cathode (40 mm × 21 mm or 36 mm × 19 mm) was positioned over the muscle belly, with the anode (40 mm × 39 mm) between the proximal interphalangeal joint and metacarpophalangeal joint. The cathode was positioned so that clear abduction movement of the index finger was evoked by single-pulse stimulation at low voltage. Once the cathode was correctly positioned, the electrodes were taped to the skin. Throughout the experiment, skin temperature was kept at 35.5-36.5 °C with the aid of an infra-red lamp. After each session, the position of the electrodes was marked on the skin using a permanent marker as a reference for subsequent experiments.
Force recording
The devices for recording abduction and flexion forces are described in the accompanying paper (Seki et al. 2001). Briefly, the left arm was abducted to the horizontal plane and the forearm and hand were clamped to a supporting device using Velcro tapes. Flexion force and abduction force were measured separately by two force transducers. Both were positioned at the proximal interphalangeal joint of the index finger. In the pilot experiments, this position was moved slightly distally during the tetanic contractions evoked by stimulation at 30-100 Hz (see below) when palmar flexion of the fingers was simultaneously evoked in some subjects. To prevent these movements during tetanic contractions, the metacarpophalangeal joints of the index to little finger were held onto the supporting device by applying slight vertical pressure on them via a wooden block.
Experimental procedures
Once the stimulating electrodes were in position, the intensity of stimulation was adjusted by monitoring the twitch contraction. A single square-wave pulse (300 μs) was applied with an interval of approximately 2 s. The voltage was increased gradually and the stimulus voltage at which the twitch ceased to increase was determined. We used 1.2 times this voltage (290-350 V) as the intensity for the subsequent stimulation of FDI.
Twitch contraction
Once the stimulation intensity had been determined, 10 pulses were delivered at intervals of 30 s to evoke twitch contractions of FDI (Fig. 1A). During this period, the subject was asked not to move his hand voluntarily. The twitch contractions so obtained were averaged and peak twitch tension (Pt) and contraction time (CT) were measured from this averaged trace; these were taken as the force achieved and the time elapsed, respectively, when the twitch contraction had reached its maximal force (measured from the onset of the twitch contraction). The half-relaxation time (1/2RT) was also measured; this was taken as the time elapsed when the twitch contraction had reached half of its Pt (measured from the time needed for the twitch contraction to reach Pt).
Figure 1.
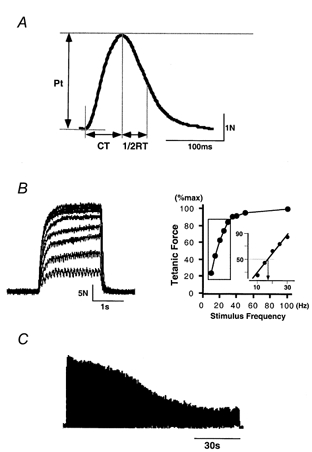
Methods used for evaluation of contractile and fatigue properties in FDI muscle
A, twitch contraction evoked by per-cutaneous muscle stimulation with a single square-wave pulse (300 μs). Ten traces were averaged. Pt, peak twitch tension; CT, contraction time; 1/2RT, half-relaxation time. B, left, superimposed tetanic contractions evoked by application of nine different stimulus frequencies (10, 15, 20, 25, 30, 35, 40, 50 and 100 Hz), each for 2 s. Right, stimulus frequency-force relationship obtained by plotting peak force achieved in nine tetanic contractions against stimulus frequency. Inset, a regression line was calculated for data from the steep region of this curve (indicated by the boxed area) and the stimulus frequency required to generate half the maximal tetanic force (arrow) was obtained from it. C, fatigability tested using electrical stimulation (a 40 Hz burst for 330 ms administered every second for 120 s). See Methods for details.
Tetanic contraction
Tetanic contractions were evoked using 2 s trains of single square-wave pulses (300 μs) at nine different stimulus frequencies (10, 15, 20, 25, 30, 35, 40, 50 and 100 Hz). Intervals of 2 min were interposed between stimulations with different frequencies. Values for maximal tetanic tension were obtained from the various tetanic contractions, and these values were plotted against the stimulus frequency to enable us to evaluate the stimulus frequency-force relationship (Fig. 1B). Then a regression line was fitted (see inset in Fig.1B) to data from the steep region of each curve (indicated by the boxed area). From the regression line, we obtained the stimulus frequency required to generate half the maximal tetanic force (Macefield et al. 1996).
Fatigue test
After a sufficient period for rest (usually 20-30 min), the fatigability of FDI was tested (Fig. 1C) using Burke's fatigue protocol (Burke et al. 1973). This entailed delivering a 40 Hz burst for 330 ms every second for 2 min. Peak tension was obtained from the tetanic contraction evoked by each stimulus burst by measuring the force achieved when the tetanic contraction had reached the maximal level (measured from the onset of each tetanic contraction). The fatigue index was obtained from the following equation: Fatigue index (%) = (Peak tension of the 120th tetanus/Peak tension of the first tetanus) × 100.
Data analysis
Mechanical signals stored on analog FM tape were analysed using the MacLab system (ADInstruments, Castle Hill, NSW, Australia) with a sampling frequency of 1 kHz, and values for the various parameters described above were obtained using this system. An analysis of variance (ANOVA), a Student's t test or a Wilcoxon test was used for the comparison of data.
RESULTS
This paper is based on data from the seven subjects who were also involved in the recording of motor unit activity in the preceding paper (Seki et al. 2001). All subjects volunteered for the recording sessions held before immobilization and after 3 and 6 weeks of immobilization. Four of them were also examined after a 6 week recovery period.
Twitch contraction
As shown in Fig. 2, twitch contractions of the FDI were affected by joint immobilization. In the subject whose data are shown in Fig. 2A, twitch tension was enhanced after immobilization and this was followed by a partial recovery towards the pre-immobilization level. The peak tension (Pt) values for the traces before, after 3 and 6 weeks of immobilization and recovery were 1.2, 2.7, 5.7 and 2.6 N, respectively, the Pt being enhanced by more than 4 times after a 6 week immobilization (by comparison with the control value). The Pt values for all subjects are presented as means ±s.d. in Fig. 2B and Table 1. In good correspondence with Fig. 2A, Pt was significantly increased after 6 weeks of immobilization (P < 0.05) and had returned to a value similar to the pre-immobilization value after the recovery period.
Figure 2.
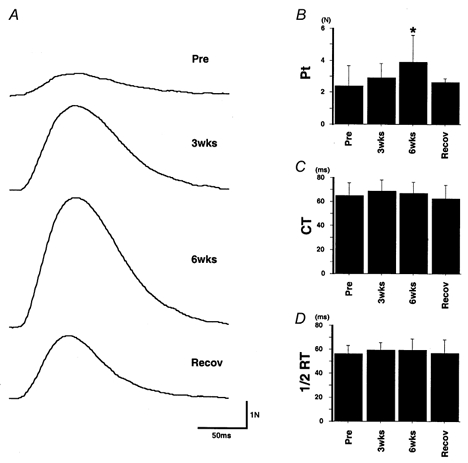
Effect of immobilization on twitch contraction
A, twitch contraction (10 traces averaged) in the pre-immobilization period, after 3 weeks (3wks) or 6 weeks (6wks) of immobilization, and after the recovery period (Recov). Each trace starts at the time the stimulus was applied. Typical examples from one subject. Pt was 1.2, 2.7, 5.7 and 2.6 N from top to bottom. B-D, mean ±s.d. of Pt (B), CT (C) and 1/2RT (D) values in all subjects. * Significantly different from pre-immobilization value (P < 0.05).
Table 1.
Effects of a 6 week immobilization on twitch and tetanic force
| Tetanus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Immobilization | Twitch | 10 Hz | 15 Hz | 20 Hz | 25 Hz | 30 Hz | 35 Hz | 40 Hz | 50 Hz | 100 Hz |
| Pre | 2.4 ± 1.3 | 6.9 ± 2.5 | 11.6 ± 3.1 | 15.9 ± 3.5 | 18.7 ± 3.7 | 20.9 ± 3.9 | 22.7 ± 3.7 | 23.6 ± 3.8 | 24.7 ± 4.0 | 25.5 ± 5.2 |
| 3 weeks | 2.9 ± 0.9 | 7.5 ± 1.7 | 12.0 ± 2.6 | 14.9 ± 2.8 | 16.7 ± 3.0 | 17.8 ± 3.2 | 18.5 ± 3.7 | 18.8 ± 3.7 | 19.5 ± 3.7* | 20.3 ± 4.2* |
| 6 weeks | 3.9 ± 1.7* | 9.1 ± 2.3* | 13.5 ± 2.6 | 15.7 ± 3.5 | 17.1 ± 3.6 | 18.3 ± 3.8 | 18.7 ± 4.2 | 19.2 ± 4.0 | 19.9 ± 4.2* | 20.2 ± 4.5 |
| Recovery | 2.6 ± 0.3 | 7.9 ± 2.3 | 12.7 ± 3.6 | 17.6 ± 5.8 | 20.3 ± 6.0 | 22.1 ± 7.2 | 22.6 ± 7.8 | 22.3 ± 7.4 | 22.9 ± 7.9 | 23.5 ± 8.8 |
Values are means ±s.d. in absolute terms (N).
Significantly different from pre-immobilization value (P < 0.05).
The effect of a 6 week immobilization on the time course of the twitch contraction is shown in Fig. 2C and D. Neither contraction time (CT) nor half-relaxation time (1/2RT) changed significantly throughout the immobilization period.
Tetanic contraction
Figure 3 shows examples of tetanic contractions and stimulus frequency-force relationships in one subject. Three features are clear in these plots. First, before immobilization (Fig. 3A) tetanic force increased as the stimulus frequency increased, at least up to 50 Hz. After 3 weeks of immobilization (Fig. 3B), and even more so after 6 weeks (Fig. 3C), increments in tetanic force were clear at the lower frequencies but were less pronounced at the higher frequencies. In fact, at 3 weeks the tetanic forces evoked by frequencies higher than 30 Hz were more similar to each other (Fig. 3B) than were the corresponding pre-immobilization traces (Fig. 3A). At 6 weeks, the tetanic forces evoked at 25 Hz or more were close to the maximal tetanic tension.
Figure 3.
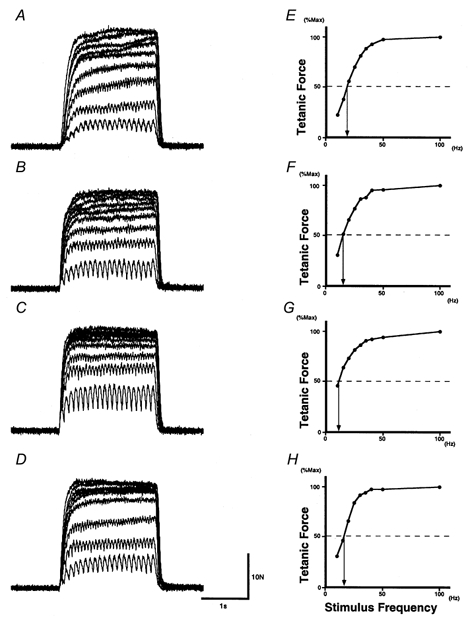
Effect of immobilization on tetanic contraction
Tetanic contractions evoked by stimuli of nine different frequencies (A-D) and the stimulus frequency-force relationships (E-H, where peak force is shown as a percentage of the maximal tetanic force) in one subject before (A and E), after 3 weeks (B and F) or 6 weeks (C and G) of immobilization, and after the recovery period (D and H). In A-D, nine tetanic contractions are superimposed. Note that the tetanic contraction evoked by the 10 Hz stimulation (bottom trace in each panel) showed larger peak force during immobilization, while that evoked by 100 Hz (top trace in each panel) was reduced during immobilization. In E-H, the arrow points to the stimulus frequency required to generate half the maximal tetanic force.
Second, in the tetanic contraction evoked by stimulation at 10 Hz (bottom trace in Fig. 3A–D), the peak tension in control (A; 5.6 N) was increased after both 3 weeks (B; 6.4 N) and 6 weeks (C; 10.4 N) of immobilization.
Third, in the subject whose data are shown in Fig. 3A–D, the tetanic tension evoked by stimulation at 100 Hz (maximal tetanic force) declined from the pre-immobilization value of 25.6 N to 20.9 N at 3 weeks and to 22.8 N at 6 weeks. This observation is consistent with those in previous reports (Simard et al. 1982; Witzmann et al. 1982, 1983; Davies et al. 1987; Duchateau & Hainaut, 1987) showing a reduction in maximal tetanic tension accompanying atrophy in immobilized muscles.
The stimulus frequency-force relationships for the data of Fig. 3A–D are shown in Fig. 3E–H, respectively, with peak tetanic force expressed as a percentage of the maximal tetanic force (evoked by 100 Hz). The characteristics of the relationship were affected by immobilization: after immobilization, a greater force could be evoked by stimulation at low frequencies, as clearly shown by the decrement in the stimulus frequency required to generate half the maximal tetanic force after immobilization (see arrows in Fig. 3E-H).
The changes in the stimulus frequency-force relationship induced by immobilization are summarized in Fig. 4. In Fig. 4A, the relationships obtained for individual subjects were averaged (standard deviations are not shown for visual clarity). As in Fig. 3, the relationship was shifted to the left by immobilization, which meant that a larger percentage of the maximal force was evoked by a given rate of stimulation (particularly at the lower frequencies: see Fig. 4A inset).
Figure 4.
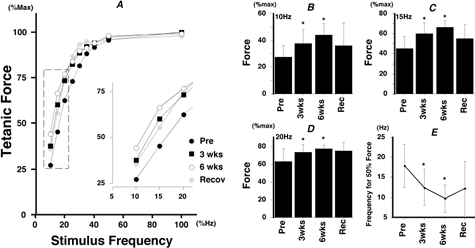
Effects of immobilization on the stimulus frequency-force relationship: normalized tetanic force
A, the stimulus frequency-force relationships were averaged for all subjects (using normalized force). The part of each curve at the lower frequency range (boxed region) is shown on an expanded scale in the inset. B-D, mean ±s.d. of the tetanic force evoked by 10, 15 and 20 Hz stimulation, respectively. E, mean ±s.d. of the stimulus frequency required to generate half the maximal tetanic force. * Significantly different from pre-immobilization value (P < 0.05).
These features are more clearly shown in Fig. 4B–D. These plots show mean tetanic force data (±s.d.) for 10 Hz (Fig. 4B), 15 Hz (Fig. 4C) and 20 Hz (Fig. 4D) stimulation. The tetanic force evoked at 10 Hz (Fig. 4B) was significantly increased after 3 weeks (P < 0.05) and 6 weeks (P < 0.05) of immobilization, with recovery to a value less than that seen at 3 weeks. Similar results were obtained at 15 Hz (Fig. 4C) and 20 Hz (Fig. 4D), although recovery was slight at these frequencies. Immobilization did not affect the tetanic force significantly when the stimulus frequency was more than 25 Hz. Mean data for the stimulus frequency required to generate half the maximal tetanic force are shown in Fig. 4E; this frequency was reduced (P < 0.05) after both 3 and 6 weeks of immobilization and showed partial recovery.
Since the tetanic force was expressed as a normalized value in Figs 3 and 4, the enhancement of the muscle force evoked by a given low frequency of stimulation observed might be the result of (1) an enhancement of some properties of the muscle related to its ability to generate tetanic force on stimulation at specific frequencies, (2) a reduction of the maximal tetanic force by immobilization or (3) points (1) and (2) occurring simultaneously. To clarify this issue, evoked force was expressed in absolute terms and plotted against the frequency of stimulation, as shown in Fig. 5A (which shows data from one subject). Two features stand out in this figure. First, the maximal tetanic force in control (29.0 N) had declined strongly after 3 weeks of immobilization (16.5 N) and also after 6 weeks (18.4 N). Second, in contrast to the effect on maximal tetanic force, the force evoked at 10 Hz in control (7.1 N) tended to be increased after both 3 weeks (7.7 N) and 6 weeks (9.3 N) of immobilization. This enhancement was seen only at frequencies less than 15 Hz, the force evoked at higher frequencies being decreased, just as the maximal tetanic force was.
Figure 5.
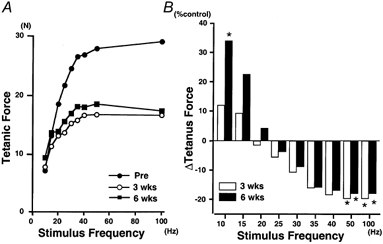
Effects of immobilization on the stimulus frequency-force relationship: absolute tetanic force
A, absolute (non-normalized) force plotted against stimulus frequency. Typical examples from one subject. Note that recovery was not tested in this subject. B, alteration induced by immobilization in tetanic force (absolute values) evoked at each stimulus frequency, shown as a percentage of the pre-immobilization value (% control). Open and filled bars represent the data for 3 and 6 weeks of immobilization, respectively. Each bar shows the mean from seven subjects (standard deviations are not shown for visual clarity: see Table 1). * Significantly different from pre-immobilization value (P < 0.05).
The effect of immobilization on the tetanic force is summarized in Fig. 5B and Table 1. Mean ±s.d. values in absolute terms are shown in Table 1 and changes as a percentage of the pre-immobilization level are shown in Fig. 5B. Two contrasting characteristics were noted with regard to the alteration in tetanic force. Firstly, the force evoked at 10 Hz was increased after immobilization (P < 0.05) and, secondly, the force evoked at 50 and 100 Hz was decreased after immobilization (P < 0.05). After the recovery period, both returned towards control values (Table 1). It is interesting to note that a stimulus frequency of around 20 Hz seems to be the border frequency between an enhancement (less than 20 Hz) and a decrement (more than 20 Hz) in tetanic force (Fig. 5B).
Twitch/tetanus ratio
The effect of immobilization on the twitch/tetanus ratio is shown in Fig. 6. The twitch/tetanus ratios for all subjects are plotted in Fig. 6A, using different symbols for the different subjects, and they are shown as means ±s.d. in Fig. 6B. In all subjects (Fig. 6A), the ratio was significantly increased after both 3 weeks (P < 0.05) and 6 weeks (P < 0.01) of immobilization and there was a return to near control values after the recovery period (Fig. 6B).
Figure 6.
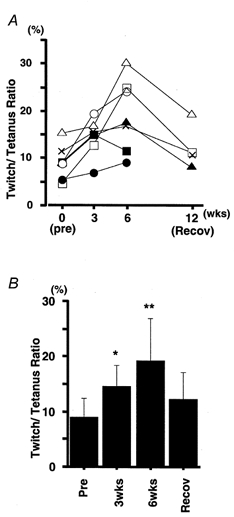
Changes in twitch/tetanus ratio
A, twitch/tetanus ratio (twitch force expressed as a percentage of maximal tetanic force) in individual subjects (each symbol represents a different subject). B, mean ±s.d. of values of twitch/tetanus ratio in seven subjects. Asterisks indicate a significant difference from pre-immobilization value (* P < 0.05, ** P < 0.01).
Fatigue index
The fatigue index was calculated to be 21.1 ± 6.5 % (pre-immobilization), 23.2 ± 10.2 % (3 weeks) and 22.61 ± 5.2 % (6 weeks) and no differences were found throughout the immobilization period. Fatigability of muscle has also been reported to be independent of immobilization in most previous studies (White & Davies, 1984; Davies et al. 1987; Miles et al. 1994) though not in all (Robinson et al. 1991).
DISCUSSION
In this paper, we found that a 6 week immobilization of the human first dorsal interosseous (FDI) causes (1) an enhancement of the force evoked at lower rates of stimulation as well as of twitch force, (2) a decrement in the force evoked at higher rates of stimulation, including the maximal tetanic force, and consequently (3) a displacement of the stimulus frequency-force relationship towards lower frequencies. We shall begin this discussion with the factors contributing to these alterations. Then, we shall discuss the possible link between these changes and the alterations in motoneuronal activity reported in the preceding paper (Seki et al. 2001).
Decrement in maximal tetanic force
Several studies have reported that a reduction in tetanic force is induced after immobilization in the rat (Simard et al. 1982; Witzmann et al. 1982, 1983) and in humans (Davis et al. 1987; Duchateau & Hainaut, 1987). These reductions in maximal tetanic force are thought to occur as a consequence of muscular atrophy (Booth, 1982). Indeed, in the hindlimb muscles of the rat Witzmann et al. (1982) found that muscle weight, as well as maximal tetanic tension, was decreased by immobilization. In humans, it has been shown that the force induced during a maximal voluntary contraction (MVC) is reduced by immobilization together with the muscle cross-sectional area (White & Davies, 1984; Miles et al. 1994), the circumference of the limb (around the immobilized muscle) (MacDougall et al. 1977) or the mean fibre area (MacDougall et al. 1980). However, as shown in the preceding paper (Seki et al. 2001), a large part of the reduction in MVC force could be induced by neural adaptation and consequently it is likely that the reduction in MVC force shown in previous studies did not simply reflect adaptation by the muscle.
To distinguish muscle change from neural change (Duchateau & Hainnaut, 1987), we recorded the maximal tetanic force evoked by means of electrical stimulation and found that it was reduced after immobilization (Table 1), as was the muscle volume (Seki et al. 2001). Hence, it is now clear that the reduction in maximal tetanic force could conceivably occur as a consequence of muscular atrophy in the human finger muscle.
Enhancement of twitch force
The twitch force was enhanced significantly after immobilization (Fig. 2). In view of the muscle atrophy, this result was unexpected. However, several previous studies have also shown an enhancement of twitch force after reduced use in humans (Davies et al. 1987; Fuglevand et al. 1995) or experimental animals (Witzmann et al. 1982; Robinson et al. 1991). The most likely explanation for this enhancement may be an alteration in the excitation-contraction coupling mechanism in the FDI muscle. Kim et al. (1982) showed that the re-uptake of Ca2+ by the sarcoplasmic reticulum occurred more slowly during immobilization. This would reflect a slower dissociation of calcium from the myofibrillar protein (Briggs et al. 1977), which in turn would enhance cross-bridge formation.
In human hand muscles, an enhancement or non-reduction of twitch force after immobilization seems to be a fairly common phenomenon since most reports in the literature did not find a reduction in twitch force. In these reports, it is interesting to note that while immobilization had little effect on the twitch force of adductor pollicis (ADP) (Sale et al. 1982; Duchateau & Hainaut, 1987), it enhanced that of FDI (Fuglevand et al. 1995), as in Fig. 2B. This difference in the adaptation to immobilization shown by the two muscles might be ascribed to their different fibre-type profiles. ADP is predominantly occupied by slow-twitch fibres, while FDI is known to possess equal proportions of slow- and fast-twitch fibres (Johnson et al. 1973). Interestingly, Robinson et al. (1991) found an enhancement of twitch force specifically in fast twitch motor units in the tibialis posterior muscle of the cat, while Witzmann et al. (1982) found an enhancement of twitch force in vastus lateralis (predominantly fast) but not in soleus (nearly all slow). Hence, it may be that the twitch enhancement after immobilization found in FDI reflects a preferential twitch enhancement in fast-twitch motor units.
Twitch/tetanus ratio
As a consequence of the reduction in maximal tetanic force and the enhancement of twitch tension, we found a significant increase in the twitch/tetanus ratio (Fig. 6). This ratio is known to be higher in slow muscles (Simard et al. 1982) because of their lower fusion frequency (which is ascribed to their slower contraction speed). On this basis, one might suppose that the enhancement of the twitch/tetanus ratio seen in FDI might reflect a reduction in the contraction speed of this muscle as a result of immobilization. However, we found no significant change in CT (Fig. 2C) or 1/2RT (Fig. 2D) or in the fatigue index of FDI. Therefore, we could not conclude that a change in contraction speed had any great effect on the twitch/tetanus ratio during immobilization. Rather, as already discussed, the reduction in muscle mass and alterations in muscle membrane properties, which may affect twitch force more than contraction time, might be the major factors inducing an increase in the twitch/tetanus ratio.
Changes in stimulus frequency-force relationships
As shown in Figs 3 and 4, the stimulus frequency-force relationship was shifted to the left by immobilization, indicating that a larger percentage of the maximal force was evoked at the lower (10-20 Hz) rates of stimulation. Furthermore, this shift was shown to involve an enhancement or reduction (Table 1, Fig. 5B) in the absolute tetanic force when it was evoked by stimulation at lower (10 Hz) or higher (50 and 100 Hz) frequencies, respectively. It is likely that these changes were induced by a mechanism similar to the one that induced the reductions in twitch and maximal tetanic force. In the lower frequency range, where the twitches are largely unfused, the evoked tetanic contraction might be more affected by the enhanced twitch tension caused by alterations in membrane properties, i.e. excitation-contraction coupling (Kim et al. 1982; Dulhunty & Gage, 1985). On the other hand, in the higher frequency range, where the twitches are largely fused, the evoked contraction might be more influenced by the muscle atrophy caused by immobilization.
Petit & Gioux (1993) found a shift in the force-frequency relationship, like that observed in the present study, in the motor units of hindlimb muscles in the cat. Interestingly, they found this change only in fast motor units, not in slow units. In contrast, maximal tetanic force was reduced in all types of motor unit. These observations seem to support our speculation that the twitch enhancement due to immobilization occurs mainly in fast muscle units in FDI. If this is indeed the case, the shift in the stimulus frequency-force relationship may be preferentially due to changes affecting fast motor units.
Correlation between changes in muscle and those in motoneuronal characteristics
In this pair of papers, we have shown that the force-frequency relationships for both voluntary and electrically elicited contractions were modified after immobilization for 6 weeks. These results are summarized in Fig. 7. During voluntary contraction (Fig. 7A), the slope of the voluntary force-mean firing rate (MFR) relationship was decreased by immobilization (data from Fig. 5A in Seki et al. 2001), while during electrically evoked contractions (Fig. 7B), the stimulus frequency-force relationship (note that the axes have been changed to the same orientation as in Fig. 7A) was shifted downwards. In essence, both relationships are being shifted in the same direction (see arrows in Fig. 7A and B): namely, a lower frequency of motoneuronal firing (Fig. 7A) or of electrical stimulation (Fig. 7B) was needed to obtain a given force (as a percentage of maximal force). As already discussed, the relationship shown in Fig. 7A was apparently influenced by changes in neural activity (Seki et al. 2001), while that shown in Fig. 7B was influenced by alterations in the twitch and tetanic properties of the muscle. Although the mechanisms underlying these changes in the two systems have already been discussed independently, we now need to consider whether there might be some link between the changes in the two systems.
Figure 7.
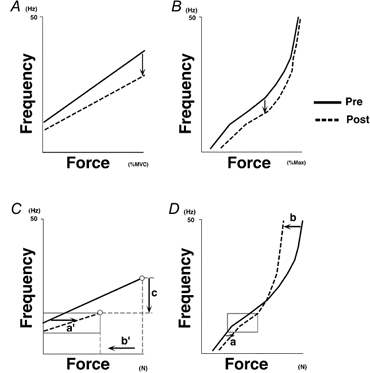
Possible link between the immobilization-induced changes in the voluntary force-MFR relationship and those induced in the stimulus frequency-force relationship
A and C are derived from Figs 5A and 8D in the preceding paper (Seki et al. 2001). B and D were prepared from Fig. 4A and Table 1 in this paper. Note that mean twitch force in all subjects (Table 1) is extrapolated to B and D (taken as being evoked by 1 Hz). In each plot, continuous and dashed lines represent data from pre- and post-immobilization (6 weeks), respectively. The orientation of the axes was changed in B and D to allow easier comparison with A and C. A and B, after immobilization, both the voluntary force-MFR relationship (A) and the stimulus frequency-force relationship (B, electrically evoked contractions) were shifted downwards. These shifts (shown by arrows) are well matched (in the sense that after immobilization, a lower frequency is associated with a given force in each plot). C and D, possible factors underlying the shifts shown in A and B. Forces shown in C and D are not normalized. Open circles in C indicate the MVC force and the maximal firing rate. Arrows labelled a, a’ and b, b’ indicate shifts induced by alterations in the contractile properties of the muscle: (1) enhancement of the force associated with a low rate of stimulation (a, a’) and (2) reduction in the force associated with a high rate of stimulation (b, b’). Boxed areas in C and D correspond to the range spanning minimal and maximal firing rates after immobilization in C. Note that within this range of frequencies, evoked force at a given stimulus frequency was always larger post-immobilization. Arrow c represents the decrement in motoneuronal firing rate associated with stronger voluntary efforts (e.g. MVC). See text for details.
To help us discuss this issue, Fig. 7C was prepared from one subject (Fig. 8D in the preceding paper, Seki et al. 2001) and Fig. 7D was prepared from Table 1 (using the values from pre- and 6 weeks of immobilization). In Fig. 7C and D, force (C: voluntary force, D: electrically evoked force) was not normalized (it is shown in N). Changes in the contractile properties (Fig. 7D) of the FDI muscle after immobilization can be summarized as follows: (1) an enhanced force is evoked at lower rates (twitch and 10 Hz) of stimulation (arrow labelled a in Fig. 7D) and (2) a reduced force is evoked at higher rates of stimulation (50 and 100 Hz: arrow labelled b in Fig. 7D).
Figure 8.
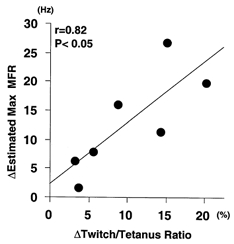
Correlation between immobilization-induced changes in motoneuronal firing rate and those induced in contractile properties of muscle
Abscissa, difference in twitch/tetanus ratio obtained by subtracting the pre-immobilization value from that obtained at 6 weeks of immobilization. Ordinate, change in estimated maximal MFR of motoneurones (see preceding paper) obtained by subtracting the value obtained at 6 weeks of immobilization from the pre-immobilization value. Results from seven subjects are shown. The regression line was significant (P < 0.05, r = 0.82, n = 7), suggesting that the change labelled c in Fig. 7 is well correlated with those labelled a and b. See text for details.
Similar changes can also be seen in Fig. 7C, in which the voluntary force evoked at lower motoneurone firing rates was enhanced (arrow labelled a’ which seems to correspond to a in D). Furthermore, within the range of minimal (intercept) and maximal firing rates after immobilization (boxed areas in Fig. 7C and D), the evoked force after immobilization was always higher than that evoked pre-immobilization. One exception to this consistency would be the time course of adaptation: while changes in the force at lower firing rates occurred largely in the initial 3 weeks (Fig. 9B in Seki et al. 2001), twitch force and force at 10 Hz electrical stimulation (Table 1) changed more in the second 3 weeks. This difference might suggest that other mechanisms that act to reduce the force at lower firing rates (e.g. changes in recruitment gain of FDI motoneurones) could be involved (see Seki et al. 2001). Besides the similarity between the change in force induced at low firing rates, including twitch force, part of the reduction in voluntary force during stronger efforts (b’ in Fig. 7C) seems to correspond to b in Fig. 7D. These ideas about correspondence are reasonable since the same force-generating properties of FDI muscle should be reflected in the force-frequency relationship, regardless of whether the force was evoked by electrical stimulation (Fig. 7D) or by voluntary activation (Fig. 7C). In contrast, the decrement in the firing rate associated with stronger voluntary efforts (c in Fig. 7C) may reflect a change in neural activity that is independent of any alterations in contractile properties (not present in Fig. 7D). Therefore, if c does have some correlation with alterations in contractile properties such as those shown by a and/or b, we may assume that neural and muscular systems change to some extent in a linked way. We examine this possibility below.
In Fig. 8, the abscissa shows the immobilization-induced change in twitch/tetanus ratio (which could be an appropriate index of the change in contractile properties shown in Fig. 7D) that occurred in the 6 week period of immobilization (equivalent to a/b in Fig. 7D). The ordinate indicates the change in the estimated maximal MFR resulting from immobilization (obtained by extrapolating the MVC force in the regression equations describing the voluntary force-MFR relationships in the various subjects (see Seki et al. 2001) and equivalent to c in Fig. 7C). As shown, we found a significant correlation between the two (r = 0.82, P < 0.05). On this basis, the neural and muscular adaptations to joint immobilization are very likely to be occurring in a linked way.
What might be the mechanism underlying the correlation between those changes in motoneuronal and muscular properties that represent adaptations to immobilization? It has been suggested that neurotropic substances could modify the characteristics of skeletal muscle and its motoneurones. For example, subcutaneous injection of ciliary neurotrophic factor (CNTF), which is known to be expressed in peripheral nerves, reduced denervation atrophy (Helgren et al. 1994). On the other hand, Suliman et al. (1999) reported that receptors for insulin-like growth factor I (IGF-I), a nerve- and muscle-derived neurotrophic factor, were increased after 4 weeks of hindlimb immobilization in the rat. Conceivably, the linked changes in the activity of FDI motoneurones and the contractile properties of the muscle might be partly induced by such trophic influences on skeletal muscle and motoneurone. However, it should be pointed out that the significance of neurotrophic influences on contractile properties is still unclear for several reasons (Gundersen, 1998).
Since the pioneering cross-reinnervation experiments of Buller et al. (1960), it has been well known that motoneurones have marked influences over the contractile properties of their muscle fibres; moreover, these influences are known to be related to motoneuronal activity (Salmons & Vrbova, 1969). Since the amount of activity in FDI motoneurones was changed by immobilization (Seki et al. 2001), the changes in the contractile properties demonstrated in this present paper may be secondary to this reduced input to FDI muscle. If reduced motoneuronal activity reflects changes in the intrinsic mechanism for motoneuronal spike generation, it is possible that the changes occurring in the intrinsic properties of the motoneurone induce the observed alterations in the muscle's contractile properties, or vice versa. However, no report seems to support the idea that a reduced firing rate is induced only by an alteration in the spike-generating properties of the motoneurone during immobilization.
Alternatively, changes in the synaptic input to the motoneurones might be a more reasonable mechanism to account for the reduced firing rate. For example, Liepert et al. (1995) showed that the area of the motor cortex activating the ankle flexor muscles was reduced after immobilization of the ankle joint in humans. Several studies have also suggested plastic changes in the synaptic input to motoneurones after peripheral nerve block (Brasil-Neto et al. 1993), disuse (Zanette et al. 1997) or motor nerve lesion (Sanes et al. 1990). Hence, the correlation between the adaptations in neural and muscular properties in FDI (Fig. 8) might suggest that the alteration in contractile properties seen after immobilization was triggered by reduced activity in the FDI motoneurones, which in turn was induced by alterations in their synaptic inputs.
We conclude from these results that after 6 weeks of immobilization, the neural and muscular adaptations (found in the firing rate modulation of motoneurones and in the contractile properties of the FDI muscle) occur in a correlated way, not independently. These results could be an example of the concept that, as a response to an environmental alteration, it is more likely that the various elements in the human motor system will show adaptation as a system than that adaptation will be shown by an individual element.
Acknowledgments
This research was supported by grants from the Japanese Ministry of Education, Science, Sports and Culture, the Meiji Foundation for Health Science and the Budo and Sports Science Institute of International Budo University.
References
- Bakels R, Kernell D. Matching between motoneurone and muscle unit properties in rat medial gastrocnemius. Journal of Physiology. 1993;463:307–324. doi: 10.1113/jphysiol.1993.sp019596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW. Effect of limb immobilization on skeletal muscle. Journal of Applied Physiology. 1982;52:1113–1118. doi: 10.1152/jappl.1982.52.5.1113. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R, Maccabee P, Cracco J, Hallett M, Cohen LG. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Briggs FN, Poland JL, Solaro RJ. Relative capabilities of sarcoplasmic reticulum in fast and slow mammalian skeletal muscles. Journal of Physiology. 1977;266:587–594. doi: 10.1113/jphysiol.1977.sp011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller AJ, Eccles JC, Eccles RM. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. Journal of Physiology. 1960;176:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE. Motor unit types of cat triceps surae muscle. Journal of Physiology. 1967;193:141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE. Physiological types and histochemical profiles in motor units of the cat gastrocnemius. Journal of Physiology. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CT, Rutherford IC, Thomas DO. Electrically evoked contractions of the triceps surae during and following 21 days of voluntary leg immobilization. European Journal of Applied Physiology and Occupational Physiology. 1987;56:306–312. doi: 10.1007/BF00690897. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Electrical and mechanical changes in immobilized human muscle. Journal of Applied Physiology. 1987;62:2168–2173. doi: 10.1152/jappl.1987.62.6.2168. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Gage PW. Excitation-contraction coupling and charge movement in denervated rat extensor digitorum longus and soleus muscles. Journal of Physiology. 1985;358:75–89. doi: 10.1113/jphysiol.1985.sp015541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach GD, Robbins N. Changes in contractile properties of disused soleus muscles. Journal of Physiology. 1969;201:305–320. doi: 10.1113/jphysiol.1969.sp008757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudema JJ, Fizzell JA, Nelson EM. Electromyography of experimentally immobilized skeletal muscles in cats. American Journal of Physiology. 1961;200:963–967. doi: 10.1152/ajplegacy.1961.200.5.963. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Bilodeau M, Enoka RM. Short-term immobilization has a minimal effect on the strength and fatigability of a human hand muscle. Journal of Applied Physiology. 1995;78:847–855. doi: 10.1152/jappl.1995.78.3.847. [DOI] [PubMed] [Google Scholar]
- Gallego R, Kuno M, Nunez R, Snider WD. Dependence of motoneurone properties on the length of immobilized muscle. Journal of Physiology. 1979a;291:179–189. doi: 10.1113/jphysiol.1979.sp012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R, Kuno M, Nunez R, Snider WD. Disuse enhances synaptic efficacy in spinal mononeurones. Journal of Physiology. 1979b;291:191–205. doi: 10.1113/jphysiol.1979.sp012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen K. Determination of muscle contractile properties: the importance of the nerve. Acta Physiologica Scandinavica. 1998;162:333–341. doi: 10.1046/j.1365-201X.1998.0336e.x. [DOI] [PubMed] [Google Scholar]
- Heckman CJ. Alterations in synaptic input to motoneurons during partial spinal cord injury. Medicine and Science in Sports and Exercise. 1994;26:1480–1490. [PubMed] [Google Scholar]
- Helgren ME, Squinto SP, Davis HL, Parry DJ, Boulton TG, Heck CS, Zhu Y, Yancopoulos GD, Lindsay RM, Distefano PS. Trophic effect of ciliary neurotrophic factor on denervated skeletal muscle. Cell. 1994;76:493–504. doi: 10.1016/0092-8674(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relations between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1346. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Hnik P, Vejsada R, Goldspink DF, Kasicki S, Krekule I. Quantitative evaluation of electromyogram activity in rat extensor and flexor muscles immobilized at different lengths. Experimental Neurology. 1985;88:515–528. doi: 10.1016/0014-4886(85)90067-6. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Polger J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. Journal of the Neurological Sciences. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kim DH, Witzmann FA, Fitts RH. Effect of disuse on sarcoplasmic reticulum in fast and slow skeletal muscle. American Journal of Physiology. 1982;243:C156–160. doi: 10.1152/ajpcell.1982.243.3.C156. [DOI] [PubMed] [Google Scholar]
- Liepert J, Tegenthoff M, Malin JP. Changes of cortical motor area size during immobilization. Electroencephalography and Clinical Neurophysiology. 1995;97:382–386. doi: 10.1016/0924-980x(95)00194-p. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Elder GC, Sale DG, Moroz JR, Sutton JR. Effects of strength training and immobilization on human muscle fibres. European Journal of Applied Physiology and Occupational Physiology. 1980;43:23–34. doi: 10.1007/BF00421352. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Ward GR, Sale DG, Sutton JR. Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. Journal of Applied Physiology. 1977;43:700–703. doi: 10.1152/jappl.1977.43.4.700. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Fuglevand AJ, Bigland-Ritchie B. Contractile properties of single motor units in human toe extensors assessed by intraneural motor axon stimulation. Journal of Neurophysiology. 1996;75:2509–2519. doi: 10.1152/jn.1996.75.6.2509. [DOI] [PubMed] [Google Scholar]
- Manabe T, Kaneko S, Kuno M. Disuse-induced enhancement of Ia synaptic transmission in spinal motoneurons of the rat. Journal of Neuroscience. 1989;9:2455–2461. doi: 10.1523/JNEUROSCI.09-07-02455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles MP, Clarkson PM, Bean M, Ambach K, Mulroy J, Vincent K. Muscle function at the wrist following 9 d of immobilization and suspension. Medicine and Science in Sports and Exercise. 1994;26:615–623. [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. Changes in firing rate of human motor units during linearly changing voluntary contractions. Journal of Physiology. 1973;230:371–390. doi: 10.1113/jphysiol.1973.sp010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. Journal of Neurophysiology. 1977;40:1432–1443. doi: 10.1152/jn.1977.40.6.1432. [DOI] [PubMed] [Google Scholar]
- Petit J, Gioux M. Properties of motor units after immobilization of cat peroneus longus muscle. Journal of Applied Physiology. 1993;74:1131–1139. doi: 10.1152/jappl.1993.74.3.1131. [DOI] [PubMed] [Google Scholar]
- Robinson GA, Enoka RM, Stuart DG. Immobilization-induced changes in motor unit force and fatigability in the cat. Muscle and Nerve. 1991;14:563–573. doi: 10.1002/mus.880140611. [DOI] [PubMed] [Google Scholar]
- Sale DG, McComas AJ, MacDougall JD, Upton AR. Neuromuscular adaptation in human thenar muscles following strength training and immobilization. Journal of Applied Physiology. 1982;53:419–424. doi: 10.1152/jappl.1982.53.2.419. [DOI] [PubMed] [Google Scholar]
- Salmons S, Vrbova G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. Journal of Physiology. 1969;201:535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Experimental Brain Research. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Seki K, Taniguchi Y, Narusawa M. XXXII International Congress of Physiological Sciences. P043.10. Russia: St Petersburg; 1997. Adaptation of motoneuronal activity to immobilization and to overloading of skeletal muscle in human subjects. [Google Scholar]
- Seki K, Taniguchi Y, Narusawa M. Effects of joint immobilization on firing rate modulation of human motor units. Journal of Physiology. 2001;530:507–519. doi: 10.1111/j.1469-7793.2001.0507k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard CP, Spector SA, Edgerton VR. Contractile properties of rat hind limb muscles immobilized at different lengths. Experimental Neurology. 1982;77:467–482. doi: 10.1016/0014-4886(82)90221-7. [DOI] [PubMed] [Google Scholar]
- Suliman IA, Lindgren JU, Gillberg PG, Elhassan AM, Monneron C, Adem A. Alteration of spinal cord IGF-I receptors and skeletal muscle IGF-I after hind-limb immobilization in the rat. NeuroReport. 1999;10:1195–1199. doi: 10.1097/00001756-199904260-00007. [DOI] [PubMed] [Google Scholar]
- Tabary JC, Tabary C, Tardieu C, Tardieu G, Goldspink G. Physiological and structural changes in the cat's soleus muscle due to immobilization at different lengths by plaster casts. Journal of Physiology. 1972;224:231–244. doi: 10.1113/jphysiol.1972.sp009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek RJ, Lund DD. Degeneration of different types of skeletal muscle fibres. II. Immobilization. Journal of Anatomy. 1974;118:531–541. [PMC free article] [PubMed] [Google Scholar]
- White MJ, Davies CT. The effects of immobilization, after lower leg fracture, on the contractile properties of human triceps surae. Clinical Science. 1984;66:277–282. doi: 10.1042/cs0660277. [DOI] [PubMed] [Google Scholar]
- Witzmann FA, Kim DH, Fitts RH. Hindlimb immobilization: length-tension and contractile properties of skeletal muscle. Journal of Applied Physiology. 1982;53:335–345. doi: 10.1152/jappl.1982.53.2.335. [DOI] [PubMed] [Google Scholar]
- Witzmann FA, Kim DH, Fitts RH. Effect of hindlimb immobilization on the fatigability of skeletal muscle. Journal of Applied Physiology. 1983;54:1242–1248. doi: 10.1152/jappl.1983.54.5.1242. [DOI] [PubMed] [Google Scholar]
- Zanette G, Tinazzi M, Bonato C, di Summa A, Manganotti P, Polo A, Fiaschi A. Reversible changes of motor cortical outputs following immobilization of the upper limb. Electroencephalography and Clinical Neurophysiology. 1997;105:269–279. doi: 10.1016/s0924-980x(97)00024-6. [DOI] [PubMed] [Google Scholar]


