Abstract
Body temperature has a circadian rhythm, and in women with ovulatory cycles, also a menstrual rhythm. Body temperature and sleep are believed to be closely coupled, but the influence on their relationship of gender, menstrual cycle phase and female reproductive hormones is unresolved.
We investigated sleep and 24 h rectal temperatures in eight women with normal menstrual cycles in their mid-follicular and mid-luteal phases, and in eight young women taking a steady dose of oral progestin and ethinyl oestradiol (hormonal contraceptive), and compared their sleep and body temperatures with that of eight young men, sleeping in identical conditions. All subjects maintained their habitual daytime schedules.
Rectal temperatures were elevated throughout 24 h in the luteal phase compared with the follicular phase in the naturally cycling women, consistent with a raised thermoregulatory set-point. Rectal temperatures in the women taking hormonal contraceptives were similar to those of the naturally cycling women in the luteal phase.
Gender influenced body temperature: the naturally cycling women and the women taking hormonal contraceptives attained their nocturnal minimum body temperatures earlier than the men, and the naturally cycling women had blunted nocturnal body temperature drops compared with the men.
Sleep architecture was essentially unaffected by either menstrual cycle phase or gender. The women taking hormonal contraceptives had less slow wave sleep (SWS), however, than the naturally cycling women.
Gender, menstrual cycle phase and hormonal contraceptives significantly influenced body temperature, but had only minor consequences for sleep, in the young men and women in our study.
Human body temperature has a circadian rhythm with a 0.8-1°C oscillation between a diurnal maximum and a nocturnal minimum (Moore-Ede et al. 1982; Cagnacci et al. 1996). This circadian rhythm is regulated by an endogenous pacemaker localised in the hypothalamic suprachiasmatic nuclei (Moore, 1999) and is also modulated by exogenous influences such as postural changes (Krauchi et al. 1997), physical activity (Gander et al. 1985), ambient temperature (see Heller et al. 1996 for review), meals (Driver et al. 1999b), and sleep (Barrett et al. 1993; Murphy & Campbell, 1997). In women with ovulatory menstrual cycles, the circadian rhythm is superimposed on a menstrual-associated rhythm. Daily average body temperature increases by approximately 0.4°C in the luteal phase compared with the pre-ovulatory follicular phase (Cagnacci et al. 1996; Driver et al. 1996). Whether menstrual events also affect the circadian rhythm is less clear. Progesterone apparently blunts the nocturnal decline in body temperature, reducing the amplitude (Lee, 1988; Kattapong et al. 1995; Cagnacci et al. 1996; Parry et al. 1997), and perhaps also delaying the phase (Cagnacci et al. 1996) of the circadian rhythm in the luteal phase compared with the follicular phase. One would therefore expect the circadian profile of body temperature in women to differ from that of men. Indeed, some studies have shown that young women with ovulatory cycles have phase-advanced circadian temperature rhythms compared with men (Lee, 1988; Baehr et al. 1999), but others have found no gender differences in circadian phase (Winget et al. 1977; Kattapong et al. 1995). The amplitude of the temperature rhythm may be reduced, compared with that of men, throughout the cycle (Rogacz et al. 1988) or only in the luteal phase (Kattapong et al. 1995).
Apart from its possible effect on circadian phase and amplitude, gender has more subtle effects on sleep architecture (Dijk et al. 1989; Driver & Baker, 1998). Although sleep architecture in healthy young men and women appears to be grossly similar, computerised analysis of the sleep electroencephalograph (EEG) has revealed that women have higher power densities (μV2 Hz−1) than men (Dijk et al. 1989; Armitage, 1995; Mourtazaev et al. 1995), which may be due to anatomical differences (Dijk et al. 1989) or to different physiological mechanisms (Ehlers & Kupfer, 1997). Subtle differences in sleep at different phases of the menstrual cycle also have been reported: in the luteal phase, rapid eye movement (REM) sleep is reduced (Driver et al. 1996; Baker et al. 1999) and stage 2 non-REM sleep and activity in the spindle frequency range (12-15 Hz) are increased (Driver et al. 1996). Apart from its hyperthermic properties, progesterone has hypnotic properties, and increases non-REM sleep when administered to young men (Friess et al. 1997). So the variations in sleep through the menstrual cycle may be in response to actions of progesterone or its analogues in the central nervous system (Driver et al. 1996). The sleep of women taking hormonal contraceptives, therefore, may also be influenced by the synthetic progestins, but Lee et al. (1990) did not find any differences in sleep, except for a shorter REM sleep onset latency in three women taking hormonal contraceptives, compared with ovulating women. On the other hand, menstrual-related variation in sleep may not result directly from hormonal changes, but may follow thermoregulatory variation (Deboer, 1998).
Sleep and body temperature are apparently closely coupled; sleep onset evokes a decrease of core body temperature (Barrett et al. 1993) and conversely a rapid decline in core body temperature associated with peripheral heat loss increases the likelihood of sleep initiation and facilitates entry into the deeper stages of sleep (Murphy & Campbell, 1997; Krauchi et al. 1999). Also, the peak in sleep propensity coincides with the nocturnal minimum of body temperature (Lack & Lushington, 1996). However, the investigations that have established an apparent relationship between body temperature and sleep-wake processes have rarely considered gender or menstrual cycle effects (Driver et al. 1999a).
To avoid the confounding effects of menstrual cycle phase, researchers in the field of human thermoregulation often prefer as subjects women who are taking hormonal contraceptives. These women are presumed to be free of the day-to-day variations in body temperature characteristic of ovulatory cycles. However, the synthetic oestrogens and progestins contained in hormonal contraceptives interfere with thermoregulation. Body temperature is elevated consistently over 24 h in women taking hormonal contraceptives, to the same extent as it is in ovulating women in the luteal phase (Kattapong et al. 1995; Wright & Badia, 1999). Rogers & Baker (1997) found that women taking such contraceptives had elevated rectal temperatures and sweating thresholds, both at rest and during exercise. We recently found that women taking hormonal contraceptives had significantly blunted nocturnal body temperature drops compared with young men (Baker et al. 1998). Women who are taking synthetic steroids, therefore, do not have normal thermoregulation and their circadian rhythms may not be the same as those of naturally cycling women.
A proper understanding of the relationship between circadian temperature rhythms and sleep architecture requires that the complications resulting from the menstrual-related hormones be confronted. We therefore investigated sleep and 24 h rectal temperatures in women with normal menstrual cycles, and in young women whose menstrual cycles were regulated artificially by exogenous hormones, and compared their sleep and temperature with that of age-matched young men sleeping in identical conditions. Also, we allowed sleep and temperature to be influenced by both endogenous and exogenous cues, by asking the subjects to pursue their habitual daytime activities. We used recently developed miniature thermometric data loggers to monitor rectal temperatures throughout sleep and daily activity (Fuller et al. 1999).
METHODS
Nine healthy young men, eight young women who were taking hormonal contraceptives and twelve naturally cycling women without any menstrual-associated complaints, were recruited from a university student population and consented to participate in our study. Ethical clearance was obtained from the Committee for Research on Human Subjects of the University of the Witwatersrand (Clearance no. M980503), which adheres to the principles of the Declaration of Helsinki. All the subjects completed questionnaires and were interviewed to ensure that they had regular sleep-wake schedules, were non-smokers, and showed no indication of sleep or medical disorders. The 30-item version of the General Health Questionnaire, which correlates well with psychiatric interview (Goldberg et al. 1976), was used to screen the volunteers for psychological distress. All the volunteers scored less than 12 out of 30, indicating normal psychological status. The naturally cycling women were asked specifically about any mood changes that occurred during their menstrual cycles and also completed a screening questionnaire for dysmenorrhoea (Andersch & Milsom, 1982). None reported evidence of premenstrual syndrome (Mortola, 1996) or dysmenorrhoea (Andersch & Milsom, 1982).
Women selected for inclusion in the group taking hormonal contraceptives had been taking monophasic hormonal contraceptives (21 active pills containing a fixed dosage of oestradiol and synthetic progestin; 7 placebo pills) for at least 3 months before the study. Six women were taking hormonal preparations containing 0.03 mg ethinyl-oestradiol and 0.15 mg of either desogestrel (Donmed Paharma) or levonorgestrel (Akromed). One woman was taking 0.02 mg ethinyl-oestradiol with 0.15 mg desorgestrel (Donmed), and one woman had 0.035 mg ethinyl-oestradiol with 2.0 mg cyproterone acetate (Schering). The women were only investigated while taking the active hormone pills, which all of them habitually took in the evening. The naturally cycling women had not taken any hormonal contraceptives for at least 6 months before our study.
During a month-long screening period, and for the duration of the study, the naturally cycling women completed a calendar of premenstrual experiences (Mortola, 1996), which confirmed that none suffered from premenstrual syndrome. They also recorded the dates of their menses. They measured their oral temperature every morning before getting out of bed, using a digital thermometer (Soar M.E., Nagoya, Japan), and used a commercially available self-testing kit which detects the presence of luteinising hormone (LH) in urine (ClearPlan One Step, Unipath, Bedford, UK) to confirm ovulation. During our study, eleven of the twelve women had regular, ovulatory menstrual cycles, as assessed by a mid-cycle surge of LH and a post-ovulatory increase in temperature. We recorded data from those eleven women only.
Recordings were made over a 3 month period during the late winter and spring months, when climatic conditions in the Southern hemisphere are relatively mild and dry. All the subjects were requested to maintain their customary weekday bedtime schedules, even on the weekend, for at least 1 week before a study night. On study nights, the subjects followed an identical night-time protocol in which they maintained their habitual schedules but slept in the controlled environment of our laboratory. The first night in the sleep laboratory served as an adaptation night, which allowed the subjects to familiarise themselves with the new environment and recording equipment. Between one and three nights later, the women taking oral contraceptives and the men returned to the sleep laboratory for their recording night. The naturally cycling women came to the laboratory on two occasions during one menstrual cycle: once during the mid-follicular phase (7-10 days after the onset of menstrual flow) and once during the mid-luteal phase (either the fifth or sixth night after the LH surge). The naturally cycling women had only one adaptation night, between one and three nights before their first recording night, in either the follicular or luteal phase. Since seven of the naturally cycling women had started the screening month in the luteal phase of their menstrual cycle, their first recording night was 1 month later, also in the luteal phase, whereas the remaining four women had their first recording night in the follicular phase. Two of the women had to return to the sleep laboratory for a repeat recording of one of their phases in the following month, as a result of incomplete data collection.
On study days, the subjects were allowed to pursue their usual daytime activities, such as attending lectures, but they refrained from drinking caffeinated or alcoholic beverages, and did not participate in any strenuous exercise, for 8 h before the start of the sleep recordings. We asked the subjects not to shower or bath in the evening of their recording nights, but we did not prohibit them from showering in the morning. We also did not regulate food intake or posture during the study. For the sleep recordings all the subjects wore light sleeping attire and slept under an eiderdown quilt, each in a separate bedroom, where the ambient temperature was maintained between 22 and 24°C. Lights-out and lights-on times were self-selected, based on the customary weekday bedtime schedules for each individual. Lights were turned off between 22.00 and 24.00 h and were turned on between 7 and 8 h later.
Standard polysomnographic electroencephalographic, electro-oculographic and electromyographic recordings were made on a digital electroencephalograph (Medelec DG 20, Vickers Medical, Surrey, UK) at a virtual recording speed of 15 mm s−1. Twenty-second epochs were scored according to modified standard criteria (Rechtschaffen & Kales, 1968) by two scorers blind to the identity of the subject or menstrual phase. If there were two sleep records for a particular individual, both records were scored by the same scorer. All the records were cross-checked by a third scorer. Total time spent in bed refers to the time from lights-out to lights-on. Total sleep time (TST) is reported only for the first 7 h after lights-out, as the time spent asleep minus in-bed wakefulness during that period. Sleep efficiency was calculated as a percentage of TST during the first 7 h after lights-out for each subject. Sleep onset latency (SOL) was taken as the time from lights-out to the appearance of the first of at least three consecutive epochs (60 s) of stage 2 sleep. The time between sleep onset and the first indication of any REM sleep was the REM sleep onset latency (ROL). The latency to slow wave sleep (SWS) was the time from sleep onset to the first of at least three consecutive epochs of stage 3 sleep.
Rectal temperatures were recorded every minute for at least 24 h, starting in the evening of the recording night, using indwelling rectal thermistors connected to miniature temperature loggers, custom-modified to a narrow temperature range (Stowaway XT1, Onset Computer Corporation, Pocasset, MA, USA). The thermistors were encased in a polythene sheath and inserted into the rectum to a depth of approximately 100 mm. Subjects recorded in a daily diary the times when they removed the probe for bathing or using the toilet, and the missing temperatures were calculated by linear interpolation. Overnight ambient dry-bulb temperature in the laboratory was recorded every 30 min by a thermocouple array connected to a fixed data logger (MC Systems, Cape Town, South Africa). All thermistors and thermocouples were calibrated, by water immersion, against a quartz thermometer (Quat 100, Heraeus, Hanau, Germany), to an accuracy of 0.1°C. The loggers maintained accuracy even in the face of changing environmental temperatures (Fuller et al. 1999).
Before going to bed, the subjects completed a questionnaire describing the events of that day and indicated their evening anxiety on a 100 mm visual-analog scale (VAS), anchored from ‘terribly agitated’ to ‘utterly calm and peaceful’. After each recording night, the subjects assessed the preceding night's sleep quality on a 100 mm VAS with anchor points of ‘worst possible’ and ‘best ever’ sleep. Morning vigilance was rated on a VAS with anchor points of ‘feeling awfully sleepy and lack-lustre’ and ‘feeling marvellously alert and energetic’.
A 5 ml blood sample was taken from all the women and from four of the men between 07.00 and 08.00 h, after the recording nights. The serum was frozen for later determination of oestradiol and progesterone, using solid-phase radioimmunoassay (Coat-A-Count Progesterone and Oestradiol, Diagnostic Products Corporation, Los Angeles, CA, USA).
We excluded one of the men and two of the naturally cycling women from the final analysis owing to incomplete temperature or sleep data. We also excluded one of the naturally cycling women from analysis because she did not show an increase in serum progesterone or body temperature in the latter period of her cycle, though she had done so in the screening period. Data from the remaining eight subjects in each group were used in the final analysis. The physical characteristics of the subjects are given in Table 1. There were no significant differences in age or body mass indices between the three study groups.
Table 1.
Physical characteristics of the subjects included in the analysis
| Women | Men | ||
|---|---|---|---|
| Naturally cycling | Contraceptives | ||
| Age (years) | 22 (4) | 21 (1) | 22 (1) |
| Mass (kg) | 66 (8) | 59 (11) | 66 (7) |
| Height (m) | 1·65 (0·08) | 1·66 (0·08) | 1·75 (0·07) |
| Body mass index (kg m−2) | 24 (2) | 21 (3) | 22 (2) |
| Menstrual cycle (days) | 30 (5) | — | — |
| n | 8 | 8 | 8 |
Values are means (s.d.).
Rectal temperature data were smoothed by a 15 min moving average of the 1 min recordings. We then calculated mean 24 h, mean 7 h in-bed and raw-minimum temperatures. We also determined from visual inspection of the smoothed temperature curves, the time of the raw-minimum temperature for each individual. We fitted 24 and 12 h sinusoidal curves to individual temperature curves using complex demodulation (described in Elsemore et al. 1995), after the data had been normalised by subtracting the mean of the time series from each data point. The fitted curves explained 80 ± 9 % (mean ±s.d.; n = 32) of the variance for the entire cohort of subjects. The time of occurrence of the fitted-minimum temperature was obtained from the fitted curve. Amplitude of the fitted temperature curve was estimated as half of the range between the fitted-maximum and fitted-minimum temperatures. We also measured the extent of the nocturnal drop in body temperature for each subject using an index utilised frequently by thermal physiologists, namely the thermal response index (TRI). In our case, the TRI was the time integral (°C h), over 7 h, of the change in rectal temperature from that temperature recorded at lights-out (i.e. the area between the actual curve of rectal temperature vs. time and a line drawn through the lights-out temperature).
We evaluated sleep over the first 7 h of the recording nights because all subjects were in bed for at least 7 h. The subjective VAS measurements were normalised before statistical analysis through the arcsin transform. We investigated differences in temperature, and subjective and objective sleep measures between the follicular and luteal phases in the naturally cycling women using a paired t test. A one-way analysis of variance (ANOVA) was used to evaluate differences between the men, women taking hormonal contraceptives and the naturally cycling women in the luteal phase, followed by Tukey's post hoc test when appropriate.
RESULTS
The naturally cycling women had significantly higher serum progesterone and oestrogen concentrations during the luteal phase than during the follicular phase, consistent with ovulatory cycles (Table 2). The women who were taking hormonal contraceptives had progesterone and oestrogen concentrations that did not differ significantly from follicular phase concentrations in naturally cycling women. The serum hormone concentrations in the women taking contraceptives reflect only endogenous progesterone and oestrogen since the assays that we used did not detect synthetic hormones. The four men from whom we took blood samples had significantly higher progesterone and lower oestrogen concentrations than the naturally cycling women in their follicular phase, but their hormone profile was much closer to that of the women in the follicular phase than the women in the luteal phase, and closest to the profile of women taking oral contraceptives.
Table 2.
Serum hormone concentrations in eight naturally cycling women in their follicular and luteal phases, in eight women taking hormonal contraceptives, and four men
| Women | Men | |||
|---|---|---|---|---|
| Follicular | Luteal | Contraceptives | ||
| Oestrogen (pmol l−1) | 179 (46) | 520* (310) | 111 (96) | 128 (35) |
| Progestin (nmol l−1) (as progesterone) | 3 (1) | 19* (13) | 3 (1) | 7 (1) |
Values are means (s.d.).
Significantly different from follicular phase, paired ttest, P < 0·01
Figure 1 shows the average smoothed rectal temperature curves of the subjects for 1 h before lights-out and 23 h afterwards. All groups showed a drop in temperature after lights-out and a marked rise in temperature after lights-on, associated with the rising phase of body temperature, increased morning activity and showering. Mean 24-hour, 7-hour in-bed, and raw-minimum temperatures were elevated, and to the same extent, in the women taking hormonal contraceptives and in the women in the luteal phase, compared with the women in the follicular phase and with the men (Table 3).
Figure 1. Mean rectal temperatures for 1 h before lights-out and 23 h afterwards in eight men, eight women taking hormonal contraceptives, and eight naturally cycling women in the mid-follicular and mid-luteal phases of their menstrual cycles.
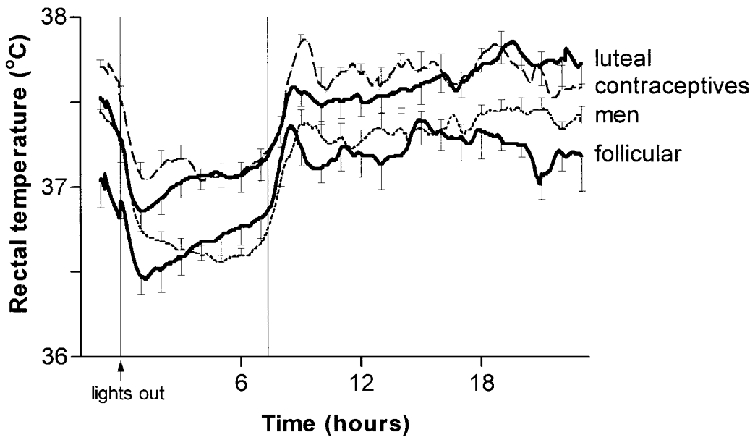
Bars denote s.e.m.; vertical lines indicate average time in bed.
Table 3.
Rectal temperature variables and statistical comparisons of eight young men, eight women taking hormonal contraceptives, and eight naturally cycling women in the mid-follicular and mid-luteal phases of the menstrual cycle
| Variable | Women | Men | Statistical comparisons | |||
|---|---|---|---|---|---|---|
| Follicular | Luteal | Contraceptives | ANOVA§ | Paired t test Follicular vs. luteal | ||
| Mean 24 h | 37·0 (0·3) | 37·4* (0·2) | 37·5 (0·2) | 37·1†‡ (0·1) | F2,21= 11·7 | t(7) = 4·0 |
| temperature (°C) | P = 0·0004 | P = 0·005 | ||||
| Raw-minimum | 36·4 (0·3) | 36·8* (0·3) | 37·0 (0·2) | 36·5†‡ (0·1) | F2,21= 8·6 | t(7) = 2·5 |
| temperature (°C) | P = 0·002 | P = 0·04 | ||||
| Mean 7 h in-bed | 36·7 (0·3) | 37·0*(0·3) | 37·1(0·2) | 36·7†‡ (0·1) | F2,21= 9·6 | t(7) = 2·9 |
| temperature (°C) | P = 0·001 | P = 0·02 | ||||
| Lights-out | 36·9(0·2) | 37·2(0·4) | 37·5(0·3) | 37·2(0·3) | F2,21= 2·4 | t(7) = 2·3 |
| temperature (°C) | P = 0·1 | P = 0·06 | ||||
| 7 h in-bed thermal | −1·9 (1·4) | −1·5 (1·3) | −2·8 (1·1) | −4·2‡(1·1) | F2,21= 11·1 | t(7) = 0·7 |
| response index (°C h) | P = 0·0005 | P = 0·5 | ||||
| Amplitude of fitted | 0·42(0·1) | 0·47(0·2) | 0·49(0·1) | 0·39(0·1) | F2·21= 1·2 | t(7) = 0·7 |
| temperature curve | P = 0·3 | P = 0·5 | ||||
| Time of lights-out | 23·38 h | 23·33 h | 23·02 h | 23·23 h | F2,21= 1·6 | t(7) = 0·6 |
| (38 min) | (41 min) | (36 min) | (26 min) | P = 0·22 | P = 0·6 | |
| Time of fitted-minimum | 02·31 h | 02·47 h | 03·20 h | 04·06 h‡ | F2,21= 3·4 | t(7) = 0·5 |
| temperature | (105 min) | (85 min) | (35 min) | (46 min) | P = 0·04 | P = 0·6 |
| Time of raw-minimum | 01·42 h | 00·48 h | 01·44 h | 03·41 h†‡ | F2,21= 7·8 | t(7) = 1·7 |
| temperature | (103 min) | (68 min) | (101 min) | (87 min) | P = 0·002 | P = 0·1 |
Values are means (s.d.).
Significantly different from follicular phase values, paired t test
Significantly different from values for women taking contraceptives, Tukey post hoc test, P < 0.05
Significantly different from luteal phase values, Tukey post hoc test, P < 0.05
Men vs. women taking hormonal contraceptives vs. naturally cycling women in the luteal phase
Also evident in Fig 1 is an earlier attainment of the raw-minimum body temperature in the naturally cycling women, compared with the men. Indeed, despite going to bed at a similar time (Table 3), the naturally cycling women reached their raw-minimum temperatures 124 ± 74 min (mean ±s.d.) after lights-out in the follicular phase and 74 ± 32 min after lights-out in the luteal phase, which was significantly earlier than the men at 258 ± 77 min after lights-out (ANOVA, F2,21= 11.6, P = 0.0004; Tukey's P = 0.0006). Consequently, the clock time of the raw-minimum body temperature was earlier in the naturally cycling women than in the men (Table 3). The women taking hormonal contraceptives also had significantly earlier raw-minimum temperatures than the men (Table 3), but took longer (215 ± 111 min; Tukey's P = 0.006) than the naturally cycling women in the luteal phase to reach their raw-minimum temperature after lights-out.
The clock time of the fitted-minimum body temperature was also significantly earlier in the naturally cycling women than in the men, even though there was a greater discrepancy between the times of the raw- and fitted-minimum temperatures in the women compared with the men. Whereas the clock times of the raw-temperature minimum and the fitted-minimum were similar in five of the eight men, the time of the fitted-minimum was delayed by at least 1 h compared with the raw-minimum in nine of the sixteen women, which was particularly evident in the luteal phase for all of the eight naturally cycling women.
There was no significant difference in the 7 h thermal response index or in circadian amplitude between the follicular phase and the luteal phase in the naturally cycling women. The average luteal-follicular difference in rectal temperature was the same during the 12 h day-period (09.00-21.00 h) as it was during the night (21.00-09.00 h) (0.40 ± 0.2 vs. 0.37 ± 0.3°C) in the naturally cycling women. The circadian amplitude also did not differ between the men, women taking hormonal contraceptives, and the naturally cycling women in the luteal phase (Table 3). Showering and activity influenced the daytime body temperatures of our subjects, so that there was no clear diurnal peak in the temperature curves for the majority of our subjects, which may have affected our estimate of circadian amplitude of the fitted curves. However, the thermal response index during the 7 h sleep period when conditions were constant was less negative in the naturally cycling women, indicating that they had significantly blunted nocturnal drops in body temperature, compared with the men (Table 3). The women taking hormonal contraceptives had nocturnal drops intermediate between those of naturally cycling women and men.
Before going to bed, levels of anxiety were the same in the naturally cycling women in their follicular (69 ± 25 mm) and luteal (63 ± 23 mm) phases (paired t test, t (7) = 0.9, P = 0.4), and were the same as those of the women taking hormonal contraceptives (47 ± 17 mm) and of the men (61 ± 26 mm) (ANOVA, F2,21= 1.2, P = 0.3). Subjective ratings of sleep quality were the same in the follicular (69 ± 15 mm) and luteal (60 ± 14 mm) phases in the naturally cycling women (paired t test, t (7) = 1.0, P = 0.3) and did not differ from the ratings of the women taking hormonal contraceptives (62 ± 18 mm) or the men (65 ± 13 mm) (ANOVA, F2,21= 0.3, P = 0.7). Subjective ratings of morning vigilance were the same in the follicular (46 ± 15 mm) and luteal (43 ± 13 mm) phases (paired t test, t (7) = 0.6, P = 0.5) in the naturally cycling women. Morning vigilance in the naturally cycling women, however, tended to be lower than in the women taking hormonal contraceptives (59 ± 21 mm) and in the men (63 ± 13 mm) (ANOVA, F2,21= 3.3, P = 0.06), but did not reach significance, possibly because of a lack of power due to the small sample sizes of the groups.
Total time spent in bed was the same in the naturally cycling women in their follicular (435 ± 11 min) and luteal (447 ± 19 min) phases (t (7) = 1.4, P = 0.2), which was the same as in the men (431 ± 26 min) and in the women taking hormonal contraceptives (449 ± 17 min) (ANOVA, F2,21= 1.7, P = 0.2). Selected sleep variables for the first 7 h of sleep after lights-out as derived from the polysomnograms, are shown in Table 4. Despite the significant differences in body temperature between the groups, their sleep architecture was remarkably similar, although the naturally cycling women in the luteal phase had more SWS compared with the women taking hormonal contraceptives. There were no significant gender differences in sleep composition, although the naturally cycling women tended to have more SWS than the men. The only significant difference in sleep associated with menstrual phase was that SWS occurred sooner in the luteal phase. The women showed a non-significant trend, however, for more REM sleep in the follicular phase compared with the luteal phase (paired t test, P = 0.07; Table 4). Although none of the subjects showed any signs of depression, according to their scores on the General Health Questionnaire (Goldberg et al. 1976), REM sleep onset latencies were relatively short in all the study groups, particularly in the contraceptive group.
Table 4.
Selected sleep variables during the first 7 h of sleep after lights-out and statistical comparisons in eight men, eight women taking hormonal contraceptives, and eight naturally cycling women in the mid-follicular and mid-luteal phases of their menstrual cycles
| Variable | Women | Men | Statistical comparisons | |||
|---|---|---|---|---|---|---|
| Follicular | Luteal | Contraceptives | ANOVA§ | Paired t test Follicular vs. luteal | ||
| Total sleep time (min) | 398(7) | 395(14) | 387(20) | 384(30)‡ | F2,21= 0·5 | t(7) = 0·7 |
| P = 0·6 | P = 0·5 | |||||
| Sleep efficiency (%) | 95(2) | 94(4) | 92(5) | 91(7) | F2,21= 1·1 | t(7) = 0·1 |
| P = 0·3 | P = 0·9 | |||||
| Sleep onset latency (min) | 11(3) | 11(6) | 19(11) | 15(8) | F2,21= 1·8 | t(7) = 0·31 |
| P = 0·2 | P = 0·8 | |||||
| Latency to stage 3 sleep (min) | 14(6) | 9*(2) | 12(4) | 10(2) | F2,21= 2·3 | t(7) = 2·8 |
| P = 0·1 | P = 0·03 | |||||
| Latency to REM sleep (min) | 70(12) | 80(16) | 62(23) | 76(23) | F2,21= 1·7 | t(7) = 1·3 |
| P = 0·2 | P = 0·2 | |||||
| Stage 2 sleep (%) | 42(6) | 45(6) | 45(3) | 42(4) | F2,21= 0·9 | t(7) = 1·7 |
| P = 0·4 | P = 0·13 | |||||
| Slow wave sleep (%) | 25(6) | 26†(6) | 18(4) | 22(6) | F2,21= 3·6 | t(7) = 0·1 |
| P = 0·045 | P = 0·9 | |||||
| REM sleep (%) | 22(3) | 19(3) | 21(3) | 19(6) | F2,21= 1·0 | t(7) = 2·2 |
| P = 0·38 | P = 0·07 | |||||
Values are means (s.d.).
Significantly different from follicular phase values, paired t test
Significantly different from values for women taking contraceptives, Tukey post hoc test, P = 0.05
Large s.d. because one man spent more than 1 h awake in the second half of the night
Men vs. women taking hormonal contraceptives vs. naturally cycling women in the luteal phase.
DISCUSSION
We recorded rectal temperatures continuously for 24 h, and sleep architecture for one night in young men, women taking synthetic oestradiol and progestin in hormonal contraceptives, and in naturally cycling women in both the mid-follicular and mid-luteal phases of their menstrual cycles. All the subjects slept under identical laboratory conditions, while maintaining their habitual daily schedules. The continuous recordings of rectal temperature were facilitated by the availability of unobtrusive, accurate, miniature thermometric data loggers.
The naturally cycling women had similarly shaped circadian body temperature curves in both the follicular and luteal phases, but the entire curve was raised by approximately 0.4°C in the luteal phase compared with the follicular phase, consistent with an upward shift in the thermoregulatory set point. Exogenous oestrogen and progestin influenced the circadian rhythm of body temperature: the women taking hormonal contraceptives had body temperatures similar to those of the naturally cycling women in the luteal phase throughout the 24 h recording period. Body temperature was also significantly affected by gender. The naturally cycling women in the follicular phase and the men had similar average 24 h and minimum temperatures, but the men had a greater nocturnal drop in body temperature, probably due to their having a higher temperature at lights-out. The men also had a minimum temperature that occurred later, compared with the naturally cycling women in both phases of the menstrual cycle.
Despite the hormonal and temperature changes, sleep macrostructure was relatively stable during the menstrual cycle in the naturally cycling women. They had significantly more SWS, however, than the women taking hormonal contraceptives. Although the naturally cycling women were inclined to have more SWS than the men, gender did not significantly affect sleep architecture. We therefore have found that menstrual cycle phase and hormonal contraceptives significantly affected body temperature but had only minor consequences for sleep composition in our subjects.
Body temperature and sleep architecture may well be correlated in the absence of external zeitgebers. An individual's circadian body temperature rhythm needs to be unmasked from exogenous influences to obtain the endogenous body temperature rhythm (Minors & Waterhouse, 1988). However, we chose to compare normal circadian body temperature rhythms, including both endogenous and exogenous components, in young men and women maintaining their habitual schedules. We did not restrict the subjects’ routine except for limiting their exercise during the recording period, and instructing them to maintain their customary bedtime schedule for at least 1 week before the recording night. Although the extent of our analysis of the endogenous circadian temperature rhythm may have been limited because our subjects were exposed to exogenous influences during the day, our study has the advantage of being relevant to actual physiological circumstances. As the sample size of each study group was small, we tried to eliminate variation between individuals, by including only women who were taking monophasic oral contraceptives in the contraceptive group, and by assessing day of ovulation and measuring serum progesterone and oestrogen in the cycling women to ensure that they were appropriately classified according to menstrual phase. We also recorded sleep and nocturnal body temperature in a controlled laboratory environment and avoided any seasonal variation in the circadian body temperature rhythm (Honma et al. 1992), by conducting our study over a 3 month period when climatic conditions varied little. Finally, we screened the subjects for psychological distress, and the women were screened for premenstrual syndrome and dysmenorrhoea, all of which may influence body temperature and sleep (Parry et al. 1997; Driver & Baker, 1998; Baker et al. 1999).
We did not find a blunted nocturnal drop in body temperature in the luteal phase compared with the follicular phase that has been reported by others (Rogacz et al. 1988; Kattapong et al. 1995; Cagnacci et al. 1996, 1997), which may be attributed to differences in study protocols. In some previous studies, women have been restricted to bedrest (Cagnacci et al. 1997), or to a constant environment (Rogacz et al. 1988; Cagnacci et al. 1996), where activity, and sleep and meal times are regulated. In another study of women who maintained their normal routines, Kattapong et al. (1995) found a blunted circadian amplitude of the temperature rhythm in the luteal phase compared with the follicular phase. Unfortunately though, they did not confirm menstrual cycle phase with blood progesterone and oestrogen measurements. Furthermore, their study design entailed comparisons of body temperature between different women in the follicular and luteal phases of the menstrual cycle, which is more susceptible to individual variation than a repeated-measures design. Our finding of a uniform upward shift in the circadian body temperature curve in the luteal phase compared with the follicular phase in the same women is consistent with the hypothesis of a raised thermoregulatory set-point in the luteal phase, around which body temperature is regulated, as occurs with fever (Cannon & Dinarello, 1985).
Although body temperature was raised in the luteal phase, the time of the raw-minimum body temperature was the same in both menstrual cycle phases, which is in agreement with the findings of some authors (Kattapong et al. 1995; Parry et al. 1997; Wright & Badia, 1999). Cagnacci et al. (1996), however, reported a phase delay in the body temperature minimum in the luteal phase compared with the follicular phase. Also, the times of the raw-minimum body temperature in the follicular (01.42 h) and luteal (00.48 h) phases in our naturally cycling women were earlier than those reported by Cagnacci et al. (1996) for women of the same age in the follicular (03.31 h) and luteal (05.09 h) phases. Following a fixed (Cagnacci et al. 1996) as opposed to a habitual schedule, seasonal effects on the body temperature rhythm (Honma et al. 1992), small sample sizes, and using different methods for determining the minimum (Kattapong et al. 1995) may have contributed to differences between studies in the estimated time of minimum body temperature.
Our finding that women taking monophasic hormonal contraceptives have body temperatures that are similar to those of women in the luteal phase confirms the findings of others (Kattapong et al. 1995; Wright & Badia, 1999). Although the mechanisms of action are unresolved, oestrogen and progesterone both affect thermoregulation (Rogers & Baker, 1997). The raised circadian body temperature curve in the women taking hormonal contraceptives, therefore, could be mediated by synthetic steroids increasing the hypothalamic thermoregulatory set-point temperature, as occurs during the natural luteal phase (Cannon & Dinarello, 1985). Alternatively, the raised body temperatures could be due to the relative absence in these women of endogenous oestrogen: oestrogen has a temperature-lowering effect (Rogers & Baker, 1997).
In our study, not only was body temperature influenced by menstrual cycle phase and hormonal contraceptives, but also by gender. The nocturnal drop in body temperature was blunted in the naturally cycling women, regardless of menstrual cycle phase, compared with the men, an observation that has been reported previously (Rogacz et al. 1988). Also, our finding that the women attained their minimum body temperatures earlier than the men supports that of others. Baehr et al. (1999) reported that a group of 71 young women had an earlier minimum body temperature by approximately 30 min compared with men, although menstrual cycle phase was not documented. Lee (1988) found that the body temperature acrophase occurred at approximately 15.30 h, regardless of menstrual cycle phase or hormonal contraceptive use, which is 1.5-2 h earlier than that documented previously for young men (17.18 h ± 33 min (±s.e.m.); Vitiello et al. 1986). We found a similar advance of approximately 2 h in the time of the temperature nadir in the women compared with the men. In contrast, Kattapong et al. (1995) did not find any differences in circadian phase between men, naturally cycling women and women taking hormonal contraceptives, but nocturnal conditions, when the minimum body temperature was attained, were not controlled in their study.
Body temperature was very different between our study groups but sleep was not. The naturally cycling women had similar sleep composition in the follicular and luteal phases of their menstrual cycles, apart from a marginally earlier onset to SWS in the luteal phase, which may have been mediated by progesterone. Progesterone administration to young men significantly decreased their latency to SWS (Friess et al. 1997), but others who have investigated the influence on sleep of the menstrual cycle did not find any change in the latency to SWS during the menstrual cycle (see Driver & Baker, 1998). Rather, investigators have reported variable effects on sleep of the menstrual cycle, including decreased SWS (Moldofsky et al. 1995), decreased REM sleep (Baker et al. 1999), or increased stage 2 sleep and a trend for decreased REM sleep (Driver et al. 1996), which we also found as a trend in this study, in the luteal phase compared with the follicular phase. Subtle effects of the menstrual cycle may become apparent with larger sample sizes and with more sensitive analysis of the sleep EEG. For example, activity in the upper spindle frequency band is increased in the luteal phase compared with the follicular phase (Driver et al. 1996).
Sleep was altered in the women taking hormonal contraceptives, in that they had less SWS compared with the naturally cycling women. Ho (1972) also reported that three women taking hormonal contraceptives had less SWS than three ovulating women in the luteal phase. Progesterone administration in male rats (Lancel et al. 1996) and young men (Friess et al. 1997) reduces slow wave activity in the EEG. Progestin in the oral contraceptive, therefore, may have mediated the decrease in SWS in the women taking hormonal contraceptives in our study. The rise in progesterone in the natural luteal phase, however, did not decrease SWS compared with the follicular phase. Exogenous progestin administered over a long period of time may influence sleep differently from endogenous progesterone. The acute and chronic effects on sleep of exogenous hormonal contraceptives have not been adequately explored and require further study.
We did not find any significant gender differences either in subjective assessments of sleep or in sleep composition, although the naturally cycling women were inclined to have more SWS than the young men. Williams et al. (1974) also did not find any gender differences in sleep, but Mourtazaev et al. (1995) found a significant increase in SWS in young women compared with men. Further studies in larger groups are needed to resolve gender effects on sleep macrostructure. Interestingly, computerised EEG analysis shows an increased amount of slow wave activity in women compared with men (Dijk et al. 1989; Armitage, 1995). As with the menstrual cycle, gender therefore may influence sleep EEG activity, but without significantly affecting overall sleep structure.
In conclusion, we have shown that naturally cycling women and women taking hormonal contraceptives have different 24 h body temperature curves from young men maintaining their habitual schedules. Also, sleep macrostructure is influenced by hormonal contraceptives compared with the normal menstrual cycle, and by menstrual cycle phase, to a limited extent.
Acknowledgments
We thank the subjects for their dedicated participation, Dr T. Elsemore for allowing us to use the complex demodulation program, and the University of the Witwatersrand Research Committee for funding.
References
- Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. American Journal of Obstetrics and Gynecology. 1982;144:655–660. doi: 10.1016/0002-9378(82)90433-1. [DOI] [PubMed] [Google Scholar]
- Armitage R. The distribution of EEG frequencies in REM and NREM sleep stages in healthy young adults. Sleep. 1995;18:334–341. doi: 10.1093/sleep/18.5.334. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in morningness-eveningness and the phase and amplitude of the circadian temperature rhythm. Sleep. 1999;22(suppl.):S164. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Baker FC, Driver HS, Rogers G, Paiker J, Mitchell D. High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. American Journal of Physiology. 1999;277:E1013–1021. doi: 10.1152/ajpendo.1999.277.6.E1013. [DOI] [PubMed] [Google Scholar]
- Baker FC, Selsick H, Driver HS, Taylor SR, Mitchell D. Different nocturnal body temperatures and sleep with forced-air warming in men and in women taking hormonal contraceptives. Journal of Sleep Research. 1998;7:175–181. doi: 10.1046/j.1365-2869.1998.00114.x. [DOI] [PubMed] [Google Scholar]
- Barrett J, Lack L, Morris M. The sleep-evoked decrease of body temperature. Sleep. 1993;16:93–99. [PubMed] [Google Scholar]
- Cagnacci A, Soldani R, Laughlin GA, Yen SSC. Modification of circadian body temperature rhythm during the luteal phase: role of melatonin. Journal of Applied Physiology. 1996;80:25–29. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Volpe A, Paoletti AM, Melis GB. Regulation of the 24-hour rhythm of body temperature in menstrual cycles with spontaneous and gonadotropin-induced ovulation. Fertility and Sterility. 1997;68:421–425. doi: 10.1016/s0015-0282(97)00242-2. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Dinarello CA. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985;227:1247–1249. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- Deboer T. Brain temperature-dependent changes in the electroencephalogram power spectrum of humans and animals. Journal of Sleep Research. 1998;7:254–262. doi: 10.1046/j.1365-2869.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Beersma DGM, Bloem GM. Gender differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep. 1989;12:500–507. doi: 10.1093/sleep/12.6.500. [DOI] [PubMed] [Google Scholar]
- Driver HS, Baker FC. Menstrual factors in sleep. Sleep Medicine Reviews. 1998;2:213–229. doi: 10.1016/s1087-0792(98)90009-0. [DOI] [PubMed] [Google Scholar]
- Driver H, Cachon J, Dableh L, Cushing M, Baker F, Cote K, Wolfson A. Letter to the Editor: ‘Gender representation in sleep research’. Journal of Sleep Research. 1999a;8:157–159. doi: 10.1046/j.1365-2869.1999.00147.x. [DOI] [PubMed] [Google Scholar]
- Driver HS, Dijk D-J, Werth E, Biedermann K, Borbely A. Menstrual cycle effects on sleep EEG in young healthy women. Journal of Clinical Endocrinology and Metabolism. 1996;81:728–735. doi: 10.1210/jcem.81.2.8636295. [DOI] [PubMed] [Google Scholar]
- Driver HS, Shulman I, Baker FC, Buffenstein R. An altered energy content of the evening meal alters nocturnal body temperature but not sleep. Physiology and Behaviour. 1999b;68:17–23. doi: 10.1016/s0031-9384(99)00145-6. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kupfer DJ. Slow-wave sleep: do young adult men and women age differently. Journal of Sleep Research. 1997;6:211–215. doi: 10.1046/j.1365-2869.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- Elsemore TF, Hegge FW, Naitoh P, Kelly T, Ryman D. WinCD: Windows software for complex demodulation. Chronobiology International. 1995;12:248–256. [Google Scholar]
- Friess E, Tagaya H, Trachsel L, Holsboer F, Rupprecht R. Progesterone-induced changes in sleep in male subjects. American Journal of Physiology. 1997;272:E885–891. doi: 10.1152/ajpendo.1997.272.5.E885. [DOI] [PubMed] [Google Scholar]
- Fuller A, Oosthuyse T, Maloney SK, Mitchell D. Evaluation of miniature data loggers for body temperature measurement during sporting activities. European Journal of Applied Physiology. 1999;79:341–346. doi: 10.1007/s004210050518. [DOI] [PubMed] [Google Scholar]
- Gander PH, Graeber RC, Connell LJ. Masking of the circadian rhythms of heart rate and core temperature by the rest-activity rhythm in man. Sleep Research. 1985;14:298. doi: 10.1177/074873048600100203. [DOI] [PubMed] [Google Scholar]
- Goldberg DP, Rickels K, Downing R, Hesbacher P. A comparison of two psychiatric screening tests. British Journal of Psychiatry. 1976;129:61–67. doi: 10.1192/bjp.129.1.61. [DOI] [PubMed] [Google Scholar]
- Heller HC, Edgar DM, Grahn DA, Glotzbach SF. Sleep, thermoregulation, and circadian rhythms. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology: Environmental Physiology. Oxford: Oxford University Press; 1996. pp. 1361–1374. [Google Scholar]
- Ho MA. Sex hormones and the sleep of women. Sleep Research. 1972;1:184. [Google Scholar]
- Honma K-I, Honma S, Kohsaka M, Fukuda N. Seasonal variation in the human circadian rhythm: dissociation between sleep and temperature rhythm. American Journal of Physiology. 1992;262:R885–891. doi: 10.1152/ajpregu.1992.262.5.R885. [DOI] [PubMed] [Google Scholar]
- Kattapong K, Fogg L, Eastman C. Effect of gender, menstrual cycle phase and hormonal contraceptive use on circadian body temperature rhythms. Chronobiology International. 1995;12:257–266. [Google Scholar]
- Krauchi K, Cajochen C, Werth E, Wirz-Justice A. Warm feet promote the rapid onset of sleep. Nature. 1999;401:36–37. doi: 10.1038/43366. [DOI] [PubMed] [Google Scholar]
- Krauchi K, Cajochen C, Wirz-Justice A. A relationship between heat loss and sleepiness: effects of postural change and melatonin administration. Journal of Applied Physiology. 1997;83:134–139. doi: 10.1152/jappl.1997.83.1.134. [DOI] [PubMed] [Google Scholar]
- Lack LC, Lushington K. The rhythms of human sleep propensity and core body temperature. Journal of Sleep Research. 1996;5:1–11. doi: 10.1046/j.1365-2869.1996.00005.x. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Holsboer F, Rupprecht R. Progesterone-induced changes in sleep comparable to those of agonistic GABAA receptor modulators. American Journal of Physiology. 1996;271:E763–772. doi: 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- Lee KA. Circadian temperature rhythms in relation to menstrual cycle phase. Journal of Biological Rhythms. 1988;3:255–263. [Google Scholar]
- Lee KA, Shaver JF, Giblin EC, Woods WF. Sleep patterns related to menstrual cycle phase and premenstrual affective symptoms. Sleep. 1990;13:403–439. [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM. Effects upon circadian rhythmicity of an alteration to the sleep-wake cycle: problems of assessment resulting from measurement in the presence of sleep and analysis in terms of a single shifted component. Journal of Biological Rhythms. 1988;3:23–40. doi: 10.1177/074873048800300102. [DOI] [PubMed] [Google Scholar]
- Moldofsky H, Lue FA, Shahal B, Jiang C-G, Gorczynski RM. Diurnal sleep/wake-related immune functions during the menstrual cycle of healthy young women. Journal of Sleep Research. 1995;4:150–159. doi: 10.1111/j.1365-2869.1995.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Moore RY. A clock for the ages. Science. 1999;284:2102–2103. doi: 10.1126/science.284.5423.2102. [DOI] [PubMed] [Google Scholar]
- Moore-Ede MC, Sulzman FM, Fuller CA. The Clocks that Time Us. Cambridge MA USA: Harvard University Press; 1982. p. 27p. [Google Scholar]
- Mortola J. Premenstrual syndrome. Trends in Endocrinology and Metabolism. 1996;7:184–189. doi: 10.1016/1043-2760(96)00060-4. [DOI] [PubMed] [Google Scholar]
- Mourtazaev MS, Kemp B, Zwinderman AH, Kamphuisen HAC. Age and gender affect different characteristics of slow waves in the sleep EEG. Sleep. 1995;18:557–564. doi: 10.1093/sleep/18.7.557. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Campbell SS. Nighttime drop in body temperature: A physiological trigger for sleep onset. Sleep. 1997;20:505–511. doi: 10.1093/sleep/20.7.505. [DOI] [PubMed] [Google Scholar]
- Parry BL, LeVeau B, Mostofi N, Naham HC, Loving R, Clopton P, Gillin JC. Temperature circadian rhythms during the menstrual cycle and sleep deprivation in premenstrual dysphoric disorder and normal comparison subjects. Journal of Biological Rhythms. 1997;12:34–46. doi: 10.1177/074873049701200106. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington DC USA: US Public Health Service (NIH publication no. 204), US Government Printing Office; 1968. [Google Scholar]
- Rogacz S, Duffy JF, Ronda JM, Czeisler CA. The increase in body temperature during the luteal phase of the menstrual cycle is only observed during the subjective night and is independent of sleep. Sleep Research. 1988;17:395. [Google Scholar]
- Rogers SM, Baker MA. Thermoregulation during exercise in women who are taking hormonal contraceptives. European Journal of Applied Physiology. 1997;75:34–38. doi: 10.1007/s004210050123. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, Smallwood RG, Avery DH, Pascualy RA, Martin DC, Prinz PN. Circadian temperature rhythms in young adult and aged men. Aging. 1986;7:97–100. doi: 10.1016/0197-4580(86)90146-6. [DOI] [PubMed] [Google Scholar]
- Williams RL, Karacan I, Hursch CJ. Electroencephalography (EEG) of Human Sleep: Clinical Applications. New York: John Wiley & Sons; 1974. [Google Scholar]
- Winget CM, DeRoshia CW, Vernikos-Danellis J, Rosenblatt WS, Hetherington NW. Comparison of circadian rhythms in male and female humans. Waking and Sleep. 1977;1:359–363. [Google Scholar]
- Wright KP, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behavioural Brain Research. 1999;103:185–194. doi: 10.1016/s0166-4328(99)00042-x. [DOI] [PubMed] [Google Scholar]


