Abstract
We hypothesized that either the recruitment of additional muscle motor units and/or the progressive recruitment of less efficient fast-twitch muscle fibres was the predominant contributor to the additional oxygen 1uptake (V˙O2) observed during heavy exercise. Using surface electromyographic (EMG) techniques, we compared the V˙O2 response with the integrated EMG (iEMG) and mean power frequency (MPF) response of the vastus lateralis with the V˙O2 response during repeated bouts of moderate (below the lactate threshold, <LT) and heavy (above the lactate threshold, >LT) intensity cycle ergometer exercise.
Seven male subjects (age 29 ± 7 years, mean ±s.d.) performed three transitions to a work rate (WR) corresponding to 90 % LT and two transitions to a work rate that would elicit a V˙O2 corresponding to 50 % of the difference between peak V˙O2 and the LT (i.e. Δ50 %, >LT1 and >LT2).
The V˙O2 slow component was significantly reduced by prior heavy intensity exercise (>LT1, 410 ± 196 ml min−1; >LT2, 230 ± 191 ml min−1). The time constant (τ), amplitude (A) and gain (ΔV˙O2/ΔWR) of the primary V˙O2 response (phase II) were not affected by prior heavy exercise when a three-component, exponential model was used to describe the V˙O2 response.
Integrated EMG and MPF remained relatively constant and at the same level throughout both >LT1 and >LT2 exercise and therefore were not associated with the V˙O2 slow component.
These data are consistent with the view that the increased O2 cost (i.e. V˙O2 slow component) associated with performing heavy exercise is coupled with a progressive increase in ATP requirements of the already recruited motor units rather than to changes in the recruitment pattern of slow versus fast-twitch motor units. Further, the lack of speeding of the kinetics of the primary V˙O2 component with prior heavy exercise, thought to represent the initial muscle V˙O2 response, are inconsistent with O2 delivery being the limiting factor in V˙O2 kinetics during heavy exercise.
For exercise intensities that engender a sustained lactic acidosis (i.e. above the lactate threshold, LT), an additional increase in pulmonary O2 uptake (V˙O2) of delayed onset leads to a V˙O2 which is higher than predicted from the V˙O2-work rate relationship for exercise performed in the sub-LT domain (Whipp & Mahler, 1980; Roston et al. 1987; Henson et al. 1989; Paterson & Whipp, 1991; Barstow et al. 1993). The physiological mechanism(s) underlying the V˙O2 slow component (V˙O2 SC) have yet to be clearly established. However, identifying the process(es) that increase the O2 cost of performing heavy work would contribute significantly to our understanding of muscle energetics and the limitations to exercise tolerance in the heavy intensity exercise domain.
Since Poole et al. (1994) demonstrated that the excess increase in pulmonary V˙O2 observed during the slow component could, for the most part, be accounted for by an increase in leg V˙O2, considerable interest has been given to a mechanistic link between muscle fibre type and the V˙O2 SC. Studies using isolated muscle preparations have shown that fast-twitch (i.e. type II) fibres are less efficient than slow-twitch (i.e. type I) fibres (Crow & Kushmerick, 1982; Kushmerick et al. 1992) and, therefore, would utilize more O2 in order to regenerate the same amount of high-energy phosphates. Indeed, Barstow et al. (1996) recently demonstrated that the amplitude of the V˙O2 SC was positively correlated with the percentage of type II fibres. Thus, from a muscle energetics perspective, muscle fibre type distribution may be an important mechanism underlying the V˙O2 SC. However, muscle fibre type distribution alone, without knowledge as to the pattern of recruitment of the available motor units, may not predict the magnitude (or relative contribution) of the V˙O2 SC during heavy exercise.
Evidence supporting the contention that the V˙O2 SC may be associated with changes in muscle recruitment patterns has been reported by Shinohara & Moritani (1992), who found a significant correlation between the increase in V˙O2 and the increase in integrated electromyography (iEMG) during heavy constant load. However, in their study the increase in iEMG began at ∼240 s, which is well beyond the time course that is typically associated with the onset of the V˙O2 SC (∼90-180 s) (Roston et al. 1987; Casaburi et al. 1987; Paterson & Whipp, 1991; Barstow & Molé, 1991; Barstow et al. 1993; Scheuermann et al. 1998; Koga et al. 1999). In addition, iEMG indicates the overall recruitment of motor units but does not provide any information about the specific recruitment pattern of type I and II fibres. However, this information may be inferred from the frequency content (i.e. power density spectrum, PDS) of the EMG signal (Tesch et al. 1983; Gerdle et al. 1991; Kupa et al. 1995).
Recent studies have shown that when bouts of heavy constant load exercise are repeatedly performed, the V˙O2 SC becomes significantly reduced during the subsequent bout of exercise (Gerbino et al. 1996; MacDonald et al. 1997; Bohnert et al. 1998). It has been suggested that during successive bouts of heavy exercise, muscle perfusion and/or O2 off-loading at the muscle may be improved, resulting in faster V˙O2 kinetics during the on-transient and, as a consequence, a lower V˙O2 SC (Gerbino et al. 1996; MacDonald et al. 1997). The recruitment pattern of type I and type II muscle fibres during repeated bouts of heavy exercise has not been examined. If the amplitude of the V˙O2 SC is associated with the recruitment of the less efficient type II fibres (Barstow et al. 1996), then one could speculate that the recruitment of type II fibres during repeated bouts of heavy exercise may be altered. In addition, it is not clear from these studies (Gerbino et al. 1996; MacDonald et al. 1997; Bohnert et al. 1998) if the reported speeding of V˙O2 kinetics is the result of a speeding of the kinetics of the primary fast component (i.e. phase II), which reflects the rate of O2 utilization at the level of the muscle (Grassi et al. 1996), or rather reflects a smaller relative contribution of the V˙O2 slow component to the overall kinetics.
Thus, the purpose of this study was to test the hypotheses that (a) the V˙O2 SC observed during heavy exercise (and its reduction with repeated bouts of heavy exercise) is temporally and quantitatively correlated with either a change in neuromuscular activity, as evidenced by the iEMG, and/or a change in muscle fibre type recruitment, as implied by changes in the power density spectrum, and (b) any speeding of V˙O2 kinetics during a subsequent bout of heavy exercise is due to a reduction in the relative contribution of the V˙O2 SC to the overall kinetic response.
METHODS
Subjects
Seven healthy males (age 29 ± 7 years, mean ±s.d.) participated in this study. The exercise protocol and all possible risks and benefits associated with participation in the study were explained to each subject. Each subject provided written informed consent prior to participating in the study. The experimental protocol was approved by the Institutional Review Board for Research Involving Human Subjects at Kansas State University and is accordance with the guidelines set forth by the Declaration of Helsinki.
Materials and protocol
Subjects reported to the Human Exercise Physiology Laboratory at Kansas State University on three separate days within a 2-3 week period. Each subject was instructed to consume only a light meal prior to arriving at the laboratory and to abstain from vigorous exercise for ≥12 h prior to exercise testing. Exercise testing was performed at approximately the same time of day for each subject. The seat and handlebar position on the cycle ergometer were recorded on the first visit and replicated on subsequent exercise tests.
Preliminary exercise testing of each subject was performed to both familiarize the subjects with the exercise protocol and for the determination of the estimated lactate threshold (LT) and peak V˙O2 (V˙O2,peak). All exercise testing was performed on an electronically braked cycle ergometer (Corival 400, Lode, The Netherlands). The exercise test consisted of 4 min of unloaded cycling followed by a progressively increasing work rate (i.e. a ramp function at 20-30 W min−1) to volitional fatigue. The highest V˙O2 averaged over a 10 s interval was taken as V˙O2,peak. The estimated LT was determined by visual inspection from gas exchange indices using the V-slope, ventilatory equivalents and end-tidal gas tensions (Wasserman et al. 1973; Beaver et al. 1986). From the results of the ramp test, a work rate that would elicit a V˙O2 corresponding to approximately half-way between the LT and V˙O2,peak (Δ50 %= LT + ((V˙O2,peak - LT)0.50)) was determined.
On each of two subsequent visits to the laboratory, subjects were asked to perform a step increase in work rate from a baseline of unloaded cycling to both a moderate (90 % LT, <LT) and a heavy (Δ50 %), constant load work rate. For <LT exercise, each step transition was 6 min in duration with 6 min of unloaded cycling between each exercise transition. For >LT exercise, each step transition was 8 min in duration with 8 min of unloaded cycling between each transition. During each visit to the laboratory, the subject performed three transitions to the moderate work rate and two transitions to the heavy work rate, resulting in six repetitions for the moderate intensity exercise and two repetitions over the 2 days for each of the initial (>LT1) and subsequent (>LT2) heavy intensity exercise bouts.
Pulmonary gas exchange (V˙O2 and V˙CO2) and minute expired ventilation (V˙e) were measured breath by breath using a metabolic measurement system (MedGraphics CardiO2, Medical Graphics Corp., St Pauls, MN, USA). The system was calibrated prior to each exercise test according to the manufacturer’s instructions. The O2 and CO2 analysers were calibrated using gases of known concentrations. The volume signal was calibrated with a syringe of known volume (3.0 l). Dynamic validation of the breath-by-breath measurement system was performed on each day of testing using a gas exchange simulator (GESV, Medical Graphics Corp.) (Huszczuk et al. 1990). Over the course of the present study, the coefficient of variation (CV) for O2 was 1.9, 1.8 and 2.3 % for low, moderate and high simulated metabolic rates, respectively. Heart rate was monitored using an electrocardiogram with the electrodes placed in a modified V5 configuration.
Surface electromyography (EMG) was obtained from the vastus lateralis using bipolar, silver-silver chloride electrode (15 mm diameter sample area) with a fixed inter-electrode spacing of 30 mm (Norotrode, Noromed Inc., Seattle, WA, USA) during each exercise trial. Surface stimulation (Electronic Muscle-Neuromuscular Stimulator, Model EMS-2A, Med Labs Inc., Goleta, CA, USA) was used to detect motor points located near the electrode site in order to avoid placing the recording electrodes on or astride the motor point. Once the electrode site was determined, the skin was shaved and cleaned with alcohol. The reference electrode was placed over the head of the fibula. Inter-electrode resistance was measured (Electrode Impedance Meter, Model EZM-5, Grass Medical Instruments, Quincy, MA, USA) and considered acceptable if < 10 kΩ. To facilitate further recordings from the same site during subsequent visits, electrode placement and motor point were marked on the skin surface and recorded using the superior patella border as a landmark.
Surface EMG was amplified (differential amplifier, Model 12A14 DC/AC Amplifier, Grass Medical Instruments), and passed through a frequency window of 3-3000 Hz (Neurodata Acquisition System, Model 12, Grass Medical Instruments). The EMG signal was sampled at a rate of 2000 Hz using commercially available software (RCA Electronics, Inc., Santa Barbara, CA, USA) and stored on computer disk for later analysis.
Data analysis
The breath-by-breath data for each step transition in work rate were linearly interpolated at 1 s intervals, time aligned to the onset of exercise, and ensemble averaged to provide a single on- and off-transient for <LT, >LT1 and >LT2 exercise for each subject. The time course of O2 following the onset of exercise and during recovery were described for each subject and exercise intensity using a model that provides an estimate of the baseline (BSL), amplitudes (A0, A1, and A2), time delays (TD1 and TD2) and time constants (τ0, τ1 and τ2) (Fig. 1). The first exponential term started at the onset of exercise (i.e. no time delay), whereas the exponential terms describing the primary fast component (phase II) and the V˙O2 SC (phase III) kinetics began after independent time delays. For <LT exercise, the parameter estimates for the on-transient were determined as a function of time (t) using a two-component exponential model:
| (1) |
Figure 1. Parameters of the exponential models used to describe V˙O2 kinetics from moderate (below the lactate threshold, <LT) and heavy intensity (>LT) exercise.
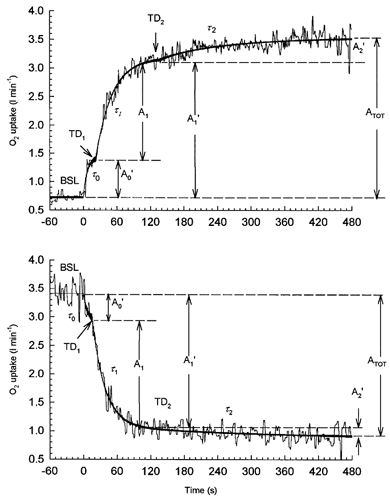
Upper panel, during the on-transition; lower panel, during recovery. Parameters correspond to those in eqns (1) and (2) (exercise) and (3) and (4) (recovery).
For >LT exercise, the parameters for the on-transient were determined using a three-component exponential model:
| (2) |
Recovery kinetics for <LT exercise were determined using a two-component model:
| (3) |
where V˙O2,EE is end-exercise V˙O2 (i.e. last 30 s of exercise). For recovery from >LT exercise, TD1 and TD2 were allowed to fit the V˙O2 response independently and were not constrained to begin simultaneously at the onset of phase II:
| (4) |
The exponential term describing phase I kinetics for both the on- and off-transients was truncated at the onset of phase II (i.e. at TD1) and the relevant amplitude of phase I (A0′) calculated according to the equation:
| (5) |
Thus, the first exponential term is descriptive up to the value of TD1. The physiologically relevant increase in V˙O2, that is, the amplitude of phase I and phase II (A1′) was determined as the sum of Ao′+A1 (Fig. 1). The amplitude of the slow component (A2′) at the end of exercise was determined as:
| (6) |
where ED is exercise duration. A mean response time (MRT), similar to the ‘effective time constant’ reported by Gerbino et al. (1996), was determined using a single exponential and time delay after removing the initial 20 s of the on-transient. The model parameters were determined by least-squares non-linear regression, in which the convergence criteria were satisfied by minimizing the sum of squared error.
Off-line processing of the EMG signal was performed using a computer program developed in our laboratory. The raw EMG signal was passed through a bandpass filter of 20-450 Hz and all signals between 59 and 61 Hz were removed. For each burst of muscle activity (i.e. once per pedal revolution) the integral of the rectified EMG signal (iEMG, μV s) and mean power frequency (MPF, Hz) were determined for each subject. For each exercise trial, the iEMG was normalized to the end-exercise value (15 s mean), which was assigned the value of 100 %. For each subject, the normalized iEMG and MPF values obtained on the two test days were averaged, resulting in a single response for each exercise condition for each subject. The iEMG and MPF values were then averaged over 15 s intervals from the onset to the end of exercise.
Statistical analysis
The V˙O2 kinetic parameter estimates were analysed using a repeated-measures ANOVA design with <LT versus >LT (>LT1, >LT2) exercise and on- versus off-transient as the main effects. The iEMG and MPF measures were analysed for <LT versus >LT (>LT1, >LT2) and time effects using a repeated-measures ANOVA design. A significant F ratio was further analysed using Student-Newman-Keuls post hoc analysis. Significance was declared when P < 0.05. All values are reported as the group mean of the individual subject responses ±s.d.
RESULTS
Subjects
The physical characteristics and peak V˙O2 values for the ramp exercise test are presented in Table 1. The group mean V˙O2,peak was 46.2 ± 9.6 ml kg−1 min−1. The V˙O2 at the estimated LT was 1835 ± 469 ml min−1, which corresponded to 49 ± 6 %V˙O2,peak. The mean V˙O2 achieved during Δ50 % exercise was ∼87 %V˙O2,peak for >LT1 and >LT2. The mean work rate for the Δ50 % exercise was 227 ± 44 W.
Table 1.
Subjects’ physical characteristics
| Subject | Age (years) | Height (cm) | Mass(kg) | V˙O2,peak (ml kg−1 min−1) | Δ50% WR (W) |
|---|---|---|---|---|---|
| 1 | 45 | 173 | 68 | 41.3 | 210 |
| 2 | 25 | 188 | 93 | 43.0 | 226 |
| 3 | 31 | 180 | 95 | 37.4 | 215 |
| 4 | 26 | 180 | 74 | 36.0 | 145 |
| 5 | 27 | 178 | 69 | 61.1 | 266 |
| 6 | 23 | 178 | 93 | 47.4 | 249 |
| 7 | 26 | 178 | 79 | 56.9 | 275 |
| Mean ± s.d | 29 ± 7 | 179 ± 4 | 82 ± 12 | 46.2 ± 9.6 | 227 ± 44 |
V˙O2,peak was determined during a preliminary ramp (20–30 W min−1) test.
o2 uptake kinetics
The response of an individual subject to a step increase in work rate corresponding to 90 % LT and Δ50 % are presented in Figs 2 and 3, respectively. V˙O2 kinetics (τ1) of the primary fast component during the on- and off-transition were similar (P > 0.05) for <LT exercise (Table 2). For exercise transitions above the LT, τ1 during recovery was similar to τ1 during exercise onset (Tables 3 and 4). Furthermore, τ1 of the primary fast component for the onset and recovery from <LT exercise were similar to the on- and off-transients for exercise above the LT (>LT1, >LT2). The kinetics (τ1) of the primary fast component during >LT2 was similar to that for >LT1 (Fig. 6). Prior heavy exercise (>LT1) was associated with a speeding (P < 0.05) of the MRT in >LT2 (Table 3).
Figure 2. On-transient responses to a moderate increase in work rate intensity.
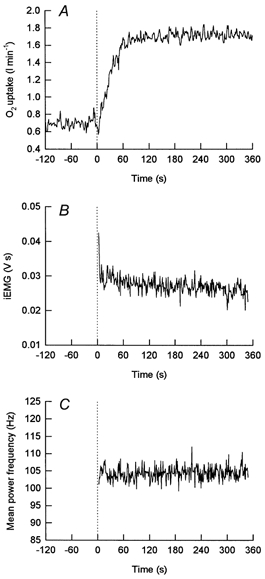
An example of the on-transient responses for O2 uptake (A), integrated EMG (B) and mean power frequency (C) for an individual subject to a step increase in work rate of moderate intensity below the lactate threshold. The group mean O2 response for a step increase in work rate to <LT and during repeated bouts of exercise above the LT (>LT1, >LT2) are presented in Figs 4 and 5, respectively.
Figure 3. On-transient responses during repeated bouts of heavy intensity exercise.
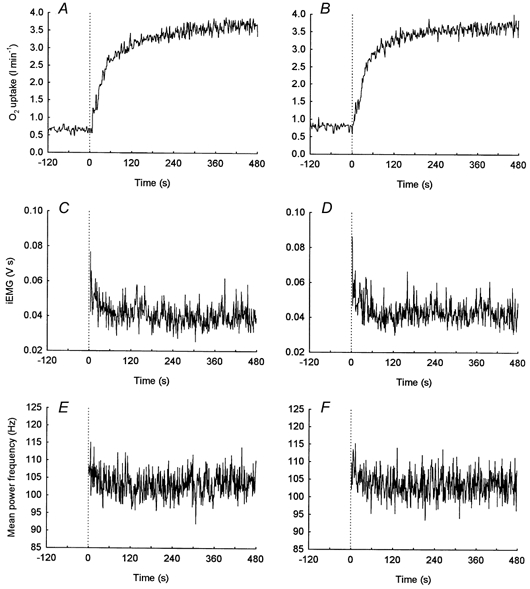
An example of the on-transient responses for O2 uptake (A and B), integrated EMG (C and D) and mean power frequency (E and F) for an individual subject during repeated bouts of constant load, heavy intensity exercise above the lactate threshold. The initial bout of heavy intensity exercise (>LT1) is shown in the left panels while the subsequent bout (>LT2) is presented in the right panels.
Table 2.
Parameter estimates for O2 response during the on-and off-transient to a step increase in work rate of moderate intensity (<LT)
| Parameter | On-transition | Off-transition |
|---|---|---|
| BSL (ml min−1) | 642 ± 96 | 1655 ± 393 |
| A0′(ml min−1) | 338 ± 113 | 263 ± 129 |
| TD1 (s) | 27.5 ± 8.4 | 20.1 ± 6.7 |
| A1′ (ml min-1) | 1010 ± 377 | 1001 ± 372 |
| τ1 (s) | 20.4 ± 10.5 | 25.6 ± 5.5 |
| Gain (ml min−1 W−1) | 9.0 ± 0.8 | 8.9 ± 0.8 |
Gain =A1′/ΔWR.
Table 3.
Parameter estimates for O2 response during the on-transient of repeated bouts of heavy intensity exercise
| Parameter | >LT1 | >LT2 |
|---|---|---|
| BSL (ml min−1) | 640 ± 121 | 751 ± 144* |
| A0′ (ml min−1) | 548 ± 264 | 568 ± 190 |
| TD1 (s) | 17.3 ± 5.5 | 18.0 ± 4.9 |
| A1′ (ml min−1) | 2191 ± 440 | 2256 ± 504 |
| τ1 (s) | 28.2 ± 13.1 | 27.3 ± 14.2 |
| TD2 (s) | 124.4 ± 39.2 | 171.2 ± 86.4 |
| A2′ (ml min−1) | 410 ± 196 | 230 ± 191* |
| ATOT (ml min−1) | 2602 ± 571 | 2485 ± 548* |
| V˙O2,EE (ml min−1) | 3241 ± 596 | 3237 ± 581 |
| Gain (ml min−1 W−1) | 9.6 ± 0.3 | 9.9 ± 0.8 |
| MRT (s) | 70.6 ± 23.8 | 53.1 ± 15.2* |
V˙O2,EE, end exercise V˙O2= BSL +A1′+A2′.
Significantly different from >LT1 (P < 0.05).
Table 4.
Parameter estimates for O2 response during the off-transient of repeated bouts of heavy intensity exercise
| Parameter | >LT1 | >LT2 |
|---|---|---|
| BSL (ml min−1) | 3225 ± 602 | 3307 ± 617 |
| A0′ (ml min−1) | 608 ± 288 | 634 ± 241 |
| TD1 (s) | 21.1 ± 3.4 | 20.8 ± 4.4 |
| A1′ (ml min−1) | 2334 ± 503 | 2353 ± 536 |
| τ1 (s) | 29.5 ± 7.0 | 27.2 ± 8.0 |
| TD2 (s) | 146.8 ± 85.7 | 132.4 ± 72.7 |
| A2′ (ml min−1) | 140 ± 85 | 230 ± 149 |
| ATOT (ml min−1) | 2473 ± 569 | 2582 ± 622 |
| V˙O2,ER (ml min−1) | 752 ± 136 | 724 ± 130 |
| Gain (ml min−1 W−1) | 10.3 ± 0.5 | 10.3 ± 0.5 |
VO2,ER
Figure 6. Amplitude and time constant for the primary fast component during repeated bouts of heavy excerise.
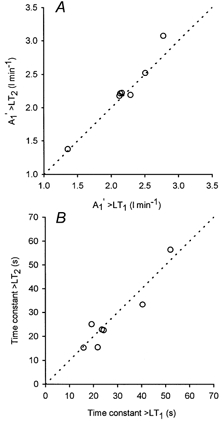
The relationship between the amplitudes (A1′; panel A), and the time constants (τ1; panel B) of the primary fast component during the initial heavy intensity exercise bout (>LT1) and the subsequent heavy intensity exercise bout (>LT2). The dotted line is the line of identity.
For <LT exercise, the amplitude of the primary fast component, expressed either in absolute terms (A1′) or as the gain (A1′/ΔWR), was similar during the on- and off-transients. As expected, A1′ was higher (P < 0.05) for exercise above the LT (>LT1, >LT2) compared to <LT, consistent with the higher WR. However, the gain for exercise above the LT (>LT1, >LT2) during both the on- and off-transients was similar to that observed for <LT exercise. Prior heavy intensity exercise (>LT1) was associated with an elevated (P < 0.05) baseline V˙O2 prior to the onset of >LT2 exercise, but the end-exercise V˙O2 was similar for >LT1 and >LT2 in spite of a lower (P < 0.05) total amplitude (ATOT=A1′+A2′) for >LT2. Since A1′ (and the gain) were similar during the on-transient of >LT1 and >LT2, the lower ATOT seen in >LT2 was the result of a lower A2′ compared to that of >LT1. The amplitudes A1′ and ATOT during recovery from exercise above the LT (>LT1, >LT2) were similar to the onset of exercise; further, no difference in either A1′ or ATOT was observed between >LT1 and >LT2 (Fig. 6).
Electromyographic responses
The burst-by-burst iEMG and MPF responses of an individual subject to a step increase in work rate to <LT are presented in Fig. 2, and to repeated step increases in work rate above the LT (>LT1, >LT2) in Fig. 3. The group mean iEMG and MPF responses for a step increase in work rate to <LT and during the repeated bouts of exercise above the LT (>LT1, >LT2) are presented in Fig. 4 and 5, respectively.
Figure 4. Group mean responses to a moderate step increase in work rate.
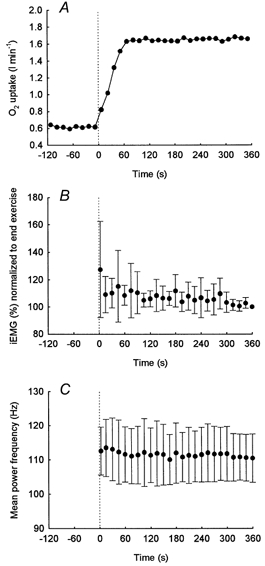
The group mean response for O2 uptake (A), integrated EMG (B) and mean power frequency (C) during a step increase in work rate of moderate intensity below the lactate threshold.
Figure 5. Group mean responses to repeated bouts of heavy intensity exercise.
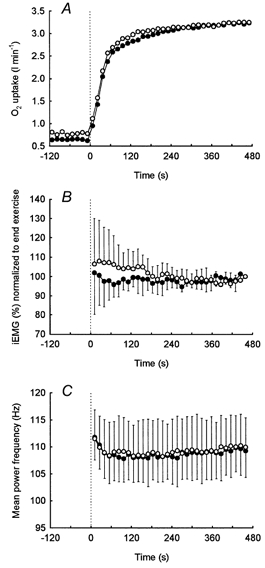
The group mean responses to the on-transient for O2 uptake (A), integrated EMG, normalized to end-exercise values (B), and mean power frequency (C) during repeated bouts of constant load, heavy intensity exercise above the lactate threshold. The initial exercise bout (>LT1) is represented by the filled circles (•) while the subsequent exercise bout (>LT2) is represented by the open circles (○). When the differences in baseline O2 uptake are accounted for, the kinetics of the primary exponential responses were similar for >LT1 and >LT2 (see Table 3).
Although care was taken to place the EMG electrodes on the same site for each test day, significant day-to-day differences in the surface EMG signal may have occurred due to small variations in electrode placement. Thus, for each test day we compared the raw iEMG and MPF values averaged over the last 60 s of exercise during exercise below and above the LT (i.e. <LT vs. >LT1 and >LT2). No day-to-day difference was observed for either iEMG or MPF between tests. Therefore, the values across days were averaged to provide a single value for each condition. iEMG was lower (P < 0.05) during <LT (440 ± 146 μV s) than above LT exercise (>LT1, 917 ± 435 μV s; >LT2, 896 ± 421 μV s), consistent with the WR. However, no difference in MPF was observed between <LT (111.3 ± 7.3 Hz) and either >LT1 (107.8 ± 5.2 Hz) or >LT2 (111.5 ± 7.4 Hz); this observation was consistent for both test days. Thus, prior heavy intensity warm-up exercise was not associated with changes in either iEMG or MPF as indicated by the similar values for >LT1 and >LT2.
In spite of the appearance of the V˙O2 SC during the on-transients of >LT1 and >LT2, no corresponding changes in either the iEMG or MPF were observed. Indeed, after the first 2 min of exercise, iEMG and MPF remained unchanged (i.e. the slope was not different from 0, P > 0.05) for the remainder of the exercise duration during <LT and above LT exercise (>LT1, >LT2).
DISCUSSION
Determining the mechanism underlying the V˙O2 SC observed during heavy, constant work rate exercise is fundamental to our understanding of muscle energetics in this domain of exercise intensity. The present study tested the hypothesis that the appearance of the V˙O2 SC would be associated with an increase in recruited muscle mass as indicated by an increase in iEMG and/or an increased reliance on type II muscle fibres (suggested by an increase in MPF) in order to maintain the required power output. In contrast to our first hypothesis, the results of the present study suggest that the V˙O2 SC during the first 8 min of exercise is not associated with either an increase in motor unit recruitment or a change in the proportion of type I and type II muscle fibres recruited. This was evidenced by a temporal and spatial independence of the onset and magnitude of the V˙O2 SC with the unchanging iEMG and MPF for both >LT1 and >LT2. Consistent with our second hypothesis, the overall apparent speeding of V˙O2 kinetics during >LT2 (reduced MRT) may be attributed to a smaller contribution of the V˙O2 SC to the overall change in V˙O2 since both the time constant and A1′ of the primary fast component were not altered by performing prior heavy intensity exercise. In addition, the similar τ1 and gain (A1′/ΔWR) for the primary fast component for exercise below and above the LT is consistent with V˙O2 kinetics being determined in young, healthy individuals by a similar mechanism for <LT and >LT exercise.
V˙o2 slow component during >LT1 and >LT2 exercise
The V˙O2 SC during the inital bout of heavy intensity cycle exercise was not accompanied by a change in either the iEMG or MPF. This observation is in contrast to an earlier report (Shinohara & Moritani, 1992) which showed a weak association between the V˙O2 SC and an increase in iEMG during heavy intensity, constant load cycling exercise. While this correlation was determined for V˙O2 and iEMG after 240 s of exercise, there is good evidence that the appearance of the V˙O2 SC occurs within 120-180 s of the onset of heavy exercise (Roston et al. 1987; Casaburi et al. 1987; Paterson & Whipp, 1991; Barstow & Molé, 1991; Barstow et al. 1993; Scheuermann et al. 1998; Koga et al. 1999). In the present study, the V˙O2 SC became evident on average 120 s after the onset of exercise during >LT1 but without corresponding changes in either the iEMG or the MPF. Likewise, no association between the onset of the V˙O2 SC and either iEMG or MPF was observed during >LT2. Thus, the lack of an association between the V˙O2 SC and either iEMG or MPF during >LT1 and >LT2 is inconsistent with the hypothesis that the V˙O2 SC is a consequence of the recruitment of additional motor units, or a change in the distribution of fast and slow motor units.
A significant finding of the present study was that while iEMG was higher during >LT exercise compared to <LT exercise, implying an increase in motor unit recruitment when progressing from moderate to heavy intensity exercise, the MPF was unchanged. Together, these results suggest that the increased power output and associated iEMG may have been accomplished without a discernible change in the distribution of fibre types recruited for <LT exercise (Tesch et al. 1983; Gerdle et al. 1991; Kupa et al. 1995). This observation is consistent with previous studies (Gollnick et al. 1974; Sargeant & Rademaker, 1996) demonstrating that exercise intensities corresponding to V˙O2,peak during incremental exercise require only a fraction of the maximal force the muscle group can generate, thereby precluding the necessity to recruit type II fibres for short-term exercise in this range of force development. In addition, these researchers confirmed that, based on glycogen depletion patterns, type II fibres were primarily recruited either during exercise associated with work rates above that required to elicit V˙O2,peak, or after sufficient time such that a significant proportion of type I fibres had become depleted of their glycogen stores (Gollnick et al. 1974). Thus, it is likely that the similar and unchanging MPF observed during exercise below and above the LT in the present study reflects the recruitment primarily of type I fibres, with only a small contribution of type II fibres, and that the proportion of active type I and type II fibres did not measurably change during the exercise duration utilized in this study (8 min) suggesting that any motor units that dropped out due to fatigue were replaced by motor units with similar EMG characteristics.
The amplitude of the V˙O2 SC was significantly reduced during the second bout of repeated exercise above the LT, in agreement with previous studies (Gerbino et al. 1996; MacDonald et al. 1997). In spite of a significant reduction in A2′ during >LT2 exercise, neither the iEMG nor MPF was different from the prior warm-up bout of heavy exercise. As stated above, these findings are inconsistent with the currently held view that the V˙O2 SC develops consequent to the progressive recruitment of the less efficient type II fibres (Shinohara & Moritani, 1992; Poole et al. 1994; Gaesser & Poole, 1996; Barstow et al. 1996) such that any reduction in V˙O2 SC, as observed here for >LT2 exercise, would be associated with fewer type II fibres being recruited (Koga et al. 1997). It may be argued that a decrease in iEMG may not be expected if type I fibres drop out as they fatigue and as a consequence a progressive recruitment of type II fibres occurs in order to maintain force development. However, if type II fibres are recruited coincident with or at some time after TD2, a shift to a higher MPF beyond TD2 would be expected to occur since type II fibres add higher frequency content than type I fibres to the power density spectrum (Tesch et al. 1983; Gerdle et al. 1991, 2000; Kupa et al. 1995). This was not the case and therefore the reduced amplitude of the V˙O2 SC during >LT2 exercise does not appear to be related to the recruitment of fewer type II fibres during the second bout of heavy exercise.
In apparent contrast to the present findings are the observations of Yoshida & Watari (1993), who described the splitting of the inorganic phosphate (Pi) peak of the NMR phosphorus spectrum into high-pH and low-pH peaks during repeated 2 min bouts of very heavy knee extension exercise. Associating the high pH peak with slow-twitch fibres, and the low pH with type II fibres (see also Mizuno et al. 1994), these authors concluded that the high-pH Pi metabolic pool was recruited first, while recruitment of the low-pH metabolic pool was more evident in subsequent bouts of exercise. One possible explanation for the apparent differences in recruitment patterns of their work with the present study is that the exercise intensity in their study, while not described in relative terms (i.e. %V˙O2,peak or MVC), was probably very heavy, as muscle pH fell to 6.2 during the first exercise bout. This intensity of muscle tension development would probably recruit type II motor units along with type I. In contrast, as noted above, the muscle tension developed during the cycle exercise at Δ50 % in the present study probably represented less than 20 % of MVC (Löllgen et al. 1980), and so would not be associated with obligatory recruitment of type II motor units.
Given the widely held view (as reviewed in Poole et al. 1994) that a progressive recruitment of type II muscle fibres may account for the V˙O2 SC, this dissociation of the V˙O2 SC from either the recruitment of additional motor units and/or a progressive recruitment of type II muscle fibres in the present study was somewhat surprising. The hypothesis of progressive recruitment is based on apparent differences in the energetics of contraction between type I and type II fibres. That is, the O2 cost associated with performing work might be expected to vary depending on the proportion of type I and type II fibres contributing to force production. Indeed, the metabolic cost for performing a brief tetanus (< 12 s) is ∼3-5 times higher for muscles composed primarily of type II rather than type I muscle fibres in the mouse (Crow & Kushmerick, 1982). In vitro measurements of heat production indicate that the energy turnover rate is significantly greater in type II than type I fibres (Gibbs & Gibson, 1972; Wendt & Gibbs, 1973). Finally, results from studies of isolated mitochondria indicate that type II fibres have a lower (∼18 %) P:O ratio than type I fibres (Willis & Jackman, 1994). Consistent with these studies, results for humans performing cycling exercise (Coyle et al. 1991, 1992; Horowitz et al. 1994), positive leg extension work following active pre-stretch (Aura & Komi, 1987), and running on a treadmill (Bosco et al. 1987) have all shown that subjects with predominantly type I muscle fibres exhibit greater mechanical efficiency (as a lower ΔV˙O2/ΔWR). Nonetheless, the literature relating mechanical efficiency to muscle fibre type distribution is far from unequivocal. For example, in isolated muscles performing isotonic contractions, the mechanical efficiency of type I fibres has been shown to be greater than (Barclay, 1994), similar to (Gibbs & Gibson, 1972; Wendt & Gibbs, 1973; Barclay et al. 1993), or less than (Heglund & Cavagna, 1987) that for type II fibres. Evidence in humans performing cycle exercise suggests that mechanical efficiency may (Medbo, 1990) or may not (Barstow et al. 1996, 2000) be independent of muscle fibre type distribution. Thus, while the argument may be widely accepted that the V˙O2 SC reflects the progressive recruitment of less efficient type II fibres, there is at present no consensus in the literature to conclusively support this hypothesis. The results of the present study suggest that alternative explanations need to be considered.
Sustained exercise <LT reflects steady-state conditions wherein ATP requirement and O2 remain relatively constant. However, given the physiological demands of performing >LT exercise on processes such as ion transport mechanisms (i.e. Ca2+- and Na+-K+-ATPases), acid-base regulation and energy substrate availability, it may be reasonable to suggest that the V˙O2 SC reflects either a progressive uncoupling of the mitochondrial P:O ratio or a progressive increase in ATP requirement (see Poole et al. 1994 for review). However, for either of these mechanisms to be plausible, they must be able to account for both the lower V˙O2 SC observed during >LT2 and the similar kinetic responses observed for the primary fast component during >LT1 and >LT2 (see below).
It has been suggested, based upon the reported difference in the P:O ratio between type I and type II muscle fibres, that the V˙O2 SC is associated with a lower metabolic efficiency of type II muscle fibres (Willis & Jackman, 1994). However, this hypothesis does not adequately explain the results of the present study since there did not appear to be any additional recruitment of type II muscle fibres. Alternatively, the reduced amplitude of the V˙O2 SC during >LT2 may reflect tighter metabolic coupling (i.e. an increased P:O ratio) for already active muscle fibres. However, a recent study (Tonkonogi et al. 1999) of isolated mitochondria, obtained from the vastus lateralis of humans performing repeated bouts of fatiguing exercise (130 % of V˙O2,peak), has shown that the O2 cost per ATP resynthesized was constant. The P:O ratio was similar at exhaustion for each exercise bout, including the longest exercise bout (∼3.4 min) which would have been of sufficient duration for a V˙O2 SC to develop. From these results it seems reasonable to infer that the P:O ratio remained constant under the conditions of the present study, and thus, the reduced V˙O2 SC observed for >LT2 does not appear to be associated with a change in efficiency which would reduce the O2 requirement.
During muscular contractions, the greatest demand for ATP may be attributed directly to the contractile elements or actomyosin (myo-ATPase). However, it has been shown that the energy requirement of non-actomyosin ATPases (predominantly the Ca2+-ATPase involved in Ca2+ sequestering) may approach 50 % of the total requirement (Bergström & Hultman, 1988). Thus, although speculative, the V˙O2 SC may reflect a progressive increase in ATP turnover rates, possibly associated with ion pumping, necessary to sustain exercise at this intensity. At present, there does not appear to be any evidence either in support of, or that would refute, the possibility that the V˙O2 SC is coupled to an increasing energy requirement of the Ca2+-ATPase (and other ATPases involved in maintaining intracellular homeostasis) during heavy exercise. Alternatively, the ATP requirement during heavy exercise may increase due to a reduction in the amount of free energy released from the splitting of phosphocreatine (Woledge, 1998). Although these mechanisms may account for the V˙O2 SC during exercise above the LT, the extent to which any or all of these mechanisms may explain the reduction in A2′ during >LT2 exercise is not readily apparent.
V˙o2 kinetics with prior heavy intensity exercise
Previous studies (Gerbino et al. 1996; MacDonald et al. 1997; Bohnert et al. 1998) have demonstrated that the overall time course of V˙O2 kinetics is speeded when a bout of heavy constant load exercise is preceded by a bout of heavy intensity warm-up exercise. However, in these studies only the overall time course of the V˙O2 response was considered and was described as either an effective time constant (Gerbino et al. 1996; Bohnert et al. 1998) or the calculated mean response time (MacDonald et al. 1997). In the present study, the MRT (or effective time constant) was speeded in >LT2 exercise by performing a prior bout of heavy intensity exercise, consistent with the previous studies. However, the more complex model utilized in the present study used to characterize the V˙O2 response applied independent exponential terms to the initial cardiodynamic phase, the primary fast component and, if present, the V˙O2 SC. This is an important distinction since much of our understanding of muscle energetics in the non-steady-state of exercise has relied, in part, on the premise that the τ for phase II kinetics measured at the mouth reflect the τ for O2 utilization at the level of the exercising muscle (Whipp & Mahler, 1980; Grassi et al. 1996). Nonetheless, the extent to which either O2 delivery or mitochondrial utilization of O2 may limit O2 kinetics during phase II remains a matter of debate (for review Tschakovsky & Hughson, 1999).
Gerbino et al. (1996) attributed the faster V˙O2 kinetics observed during the second of two repeated bouts of heavy intensity exercise to improved muscle perfusion, concluding that O2 delivery limited V˙O2 kinetics in the initial bout of heavy exercise. In the present study, A1′, gain and τ1 were similar for the on- and off-transitions during both >LT1 and >LT2 exercise. Furthermore, the gain and τ1 for >LT exercise were not different from <LT exercise. These findings are consistent with the argument that V˙O2 kinetics during the primary fast component rate are independent of O2 delivery, but rather are determined by the rate at which the active muscles utilize O2. The ‘apparent’ speeding of V˙O2 kinetics reported for repeated bouts of heavy intensity exercise seen in this study and in others (Gerbino et al. 1996; MacDonald et al. 1997; Bohnert et al. 1998) may therefore simply reflect the reduction in relative contribution of the V˙O2 SC to a mono-exponential description of the response as implied by the observations of Gerbino et al. (1996) of a reduction in the rise in V˙O2 between minutes 3 and 6 during similar heavy intensity exercise.
The similar gain for the fast component (A1′) between the first and second bouts of heavy exercise also suggests that, at the onset of the increased force requirements represented by the increased work rate, the motor centres in the brain organize the recruitment of a similar population of metabolically active motor units in order to accomplish the task. This leads to a quantitative rise in V˙O2 towards an initial steady state as A1′, irrespective of the exercise history of the exercising limbs. If true, then these data argue against the final V˙O2 response, which includes the V˙O2 SC, as being the initial projected V˙O2 for the exercise task. Rather, in this scheme, the V˙O2 SC represents metabolic processes which ‘add on’ to the initial metabolic response. This interpretation is supported by our findings in this study of stability in both the iEMG and the MPF between >LT1 and >LT2 exercise and in previous work by the observation of a linear rise in A1′ for exercise below and above the LT (Barstow & Molé, 1991; Barstow et al. 1993).
Limitations
Our conclusions are based on measurements of EMG activity from the vastus lateralis only. However, cycling exercise is a complex motor skill requiring the involvement of several muscle groups (Ericson et al. 1985) and thus the activation pattern of the vastus lateralis may not reflect the recruitment pattern of all muscle groups. There is evidence that the vastus lateralis and the vastus medialis are the most active muscles recruited during cycling exercise (Ericson et al. 1985) and therefore, may reflect the recruitment pattern of most muscle groups involved in the activity. Nonetheless, the simultaneous determination of recruitment patterns for all of the major muscle groups involved in cycling exercise are needed to clarify this potentially confounding use of surface EMG of a single muscle during multi-muscle, multi-joint activity.
Acknowledgments
The authors wish to thank the subjects for their participation in this study. The technical assistance of Mr Timothy Benson is greatly appreciated. B.W.S. was supported by a Medical Research Council of Canada Post Doctoral Fellowship. Funding for this project was provided in part by the National Heart, Lung and Blood Institute Grant HL46769 to T.J.B.
References
- Aura O, Komi P. Effects of muscle fiber distribution on the mechanical efficiency of human locomotion. International Journal of Sports Medicine. 1987;8(suppl.):30–37. doi: 10.1055/s-2008-1025701. [DOI] [PubMed] [Google Scholar]
- Barclay CJ. Efficiency of fast- and slow-twitch muscles of the mouse performing cyclic contractions. Journal of Experimental Biology. 1994;193:65–78. doi: 10.1242/jeb.193.1.65. [DOI] [PubMed] [Google Scholar]
- Barclay CJ, Constable JK, Gibbs CL. Energetics of fast- and slow-twitch muscles of the mouse. Journal of Physiology. 1993;472:61–80. doi: 10.1113/jphysiol.1993.sp019937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstow TJ, Casaburi R, Wasserman K. O2 uptake kinetics and the O2 deficit as related to exercise intensity and blood lactate. Journal of Applied Physiology. 1993;75:755–762. doi: 10.1152/jappl.1993.75.2.755. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. Journal of Applied Physiology. 1996;81:1642–1650. doi: 10.1152/jappl.1996.81.4.1642. [DOI] [PubMed] [Google Scholar]
- Barstow TJ, Jones AM, Nguyen PH, Casaburi R. Influence of muscle fibre type and fitness on the oxygen uptake/work rate slope during incremental exercise. Experimental Physiology. 2000;85:109–116. [PubMed] [Google Scholar]
- Barstow TJ, Molé PA. Linear and nonlinear characteristics of oxygen uptake kinetics during heavy exercise. Journal of Applied Physiology. 1991;71:2099–2106. doi: 10.1152/jappl.1991.71.6.2099. [DOI] [PubMed] [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. Journal of Applied Physiology. 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- Bergström M, Hultman E. Energy cost and fatigue during intermittent electrical stimulation of human skeletal muscle. Journal of Applied Physiology. 1988;65:1500–1505. doi: 10.1152/jappl.1988.65.4.1500. [DOI] [PubMed] [Google Scholar]
- Bohnert B, Ward SA, Whipp BJ. Effects of prior arm exercise on pulmonary gas exchange kinetics during high-intensity leg exercise in humans. Experimental Physiology. 1998;83:557–570. doi: 10.1113/expphysiol.1998.sp004138. [DOI] [PubMed] [Google Scholar]
- Bosco C, Montanari G, Ribacchi R, Giovenali P, Latteri F, Iachelli G, Faina M, Colli R, Dal Monte A, La Rosa M, Cortili G, Saibene F. Relationship between the efficiency of muscular work during jumping and the energetics of running. European Journal of Applied Physiology. 1987;56:138–143. doi: 10.1007/BF00640636. [DOI] [PubMed] [Google Scholar]
- Casaburi R, Storer TW, Ben-Dov I, Wasserman K. Effect of endurance training on possible determinants of O2 during heavy exercise. Journal of Applied Physiology. 1987;62:199–207. doi: 10.1152/jappl.1987.62.1.199. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Feltner ME, Kautz SA, Hamilton MT, Montain SJ, Baylor AM, Abraham LD, Petrek GW. Physiological and biomechanical factors associated with elite endurance cycling performance. Medicine and Science in Sports and Exercise. 1991;23:93–107. [PubMed] [Google Scholar]
- Coyle EF, Sidossis LS, Horowitz JF, Beltz J. Cycling efficiency is related to the percentage of type I muscle fibers. Medicine and Science in Sports and Exercise. 1992;24:782–788. [PubMed] [Google Scholar]
- Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. Journal of General Physiology. 1982;79:147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson MO, Nisell R, Arborelius UP, Ekholm J. Muscular activity during ergometer cycling. Scandinavian Journal of Rehabilitation Medicine. 1985;17:53–61. [PubMed] [Google Scholar]
- Gaesser GA, Poole DC. The slow component of oxygen uptake kinetics in humans. Exercise and Sport Sciences Reviews. 1996;24:35–70. [PubMed] [Google Scholar]
- Gerbino A, Ward SA, Whipp BJ. Effects of prior exercise on pulmonary gas-exchange kinetics during high intensity exercise in humans. Journal of Applied Physiology. 1996;80:99–107. doi: 10.1152/jappl.1996.80.1.99. [DOI] [PubMed] [Google Scholar]
- Gerdle B, Henriksson-Larsén K, Lorentzon R, Wretling ML. Dependence of the mean power frequency of the electromyogram on muscle force and fibre type. Acta Physiologica Scandinavica. 1991;142:457–465. doi: 10.1111/j.1748-1716.1991.tb09180.x. [DOI] [PubMed] [Google Scholar]
- Gerdle B, Karlsson S, Crenshaw AG, Elert J, Fridén J. The influences of muscle fibre proportions and areas upon EMG during maximal dynamic knee extensions. European Journal of Applied Physiology. 2000;81:2–10. doi: 10.1007/PL00013792. [DOI] [PubMed] [Google Scholar]
- Gibbs CL, Gibson WR. Energy production of rat soleus muscle. American Journal of Physiology. 1972;223:864–871. doi: 10.1152/ajplegacy.1972.223.4.864. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle after exercise of varying intensity and at varying pedalling rates. Journal of Physiology. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. Journal of Applied Physiology. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- Heglund NC, Cavagna GA. Mechanical work, oxygen consumption, and efficiency in isolated frog and rat muscle. American Journal of Physiology. 1987;253:C22–29. doi: 10.1152/ajpcell.1987.253.1.C22. [DOI] [PubMed] [Google Scholar]
- Henson LC, Poole DC, Whipp BJ. Fitness as a determinant of oxygen uptake response to constant-load exercise. European Journal of Applied Physiology. 1989;59:21–28. doi: 10.1007/BF02396575. [DOI] [PubMed] [Google Scholar]
- Horowitz JF, Sidossis LS, Coyle EF. High efficiency of type I fibers improves performance. International Journal of Sports Medicine. 1994;15:152–157. doi: 10.1055/s-2007-1021038. [DOI] [PubMed] [Google Scholar]
- Huszczuk A, Whipp BJ, Wasserman K. A respiratory gas exchange simulator for routine calibration in metabolic studies. European Respiratory Journal. 1990;3:465–468. [PubMed] [Google Scholar]
- Koga S, Shiojiri T, Kondo N, Barstow TJ. Effect of increased muscle temperature on oxygen uptake kinetics during exercise. Journal of Applied Physiology. 1997;83:1333–1338. doi: 10.1152/jappl.1997.83.4.1333. [DOI] [PubMed] [Google Scholar]
- Koga S, Shiojiri T, Shibasaki M, Kondo N, Fukuba Y, Barstow TJ. Kinetics of oxygen uptake during supine and upright heavy exercise. Journal of Applied Physiology. 1999;87:253–260. doi: 10.1152/jappl.1999.87.1.253. [DOI] [PubMed] [Google Scholar]
- Kupa EJ, Roy SH, Kandarian SC, De Luca CJ. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. Journal of Applied Physiology. 1995;79:23–32. doi: 10.1152/jappl.1995.79.1.23. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Meyer RA, Brown TR. Regulation of oxygen uptake consumption in fast- and slow-twitch muscle. American Journal of Physiology. 1992;263:C598–606. doi: 10.1152/ajpcell.1992.263.3.C598. [DOI] [PubMed] [Google Scholar]
- Löllgen H, Graham T, Sjogaard G. Muscle metabolites, force, and perceived exertion bicycling at varying pedal rates. Medicine and Science in Sports and Exercise. 1980;12:345–351. [PubMed] [Google Scholar]
- MacDonald MJ, Pedersen PK, Hughson RL. Acceleration of O2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. Journal of Applied Physiology. 1997;83:1318–1325. doi: 10.1152/jappl.1997.83.4.1318. [DOI] [PubMed] [Google Scholar]
- Medbo JI. Type I and type II fibres work with the same mechanical efficiency during bicycling. In: Maréchal G, Carraro U, editors. Muscle and Motility: Proceedings of XIXth European Conference in Brussels. Andover, Hampshire, UK: Intercept Ltd; 1990. pp. 303–308. [Google Scholar]
- Mizuno M, Secher NH, Quistorff B. 31P-NMR spectroscopy, rsEMG, and histochemical fiber types of human wrist flexor muscles. Journal of Applied Physiology. 1994;76:531–538. doi: 10.1152/jappl.1994.76.2.531. [DOI] [PubMed] [Google Scholar]
- Paterson DH, Whipp BJ. Asymmetries of oxygen uptake transients at the onset and offset of heavy exercise in humans. Journal of Physiology. 1991;443:575–586. doi: 10.1113/jphysiol.1991.sp018852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Barstow TJ, Gaesser GA, Willis WT, Whipp BJ. O2 slow component: physiological and functional significance. Medicine and Science in Sports and Exercise. 1994;26:1354–1358. [PubMed] [Google Scholar]
- Roston WL, Whipp BJ, Davis JA, Cunningham DA, Effros RM, Wasserman K. Oxygen uptake kinetics and lactate concentration during exercise in humans. American Review of Respiratory Disease. 1987;135:1080–1084. doi: 10.1164/arrd.1987.135.5.1080. [DOI] [PubMed] [Google Scholar]
- Sargeant AJ, Rademaker ACHJ. Human muscle fibre types and mechanical efficiency during cycling. In: Steinacker JM, Ward SA, editors. The Physiology and Pathophysiology of Exercise Tolerance. New York: Plenum Press; 1996. pp. 247–251. [Google Scholar]
- Scheuermann BW, Kowalchuk JM, Paterson DH, Cunningham DA. Oxygen uptake kinetics following acute acetazolamide administration during moderate and heavy exercise. Journal of Applied Physiology. 1998;85:1384–1393. doi: 10.1152/jappl.1998.85.4.1384. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Moritani T. Increase in neuromuscular activity and oxygen uptake during heavy exercise. Annals of Physiology and Anthropology. 1992;11:257–262. [PubMed] [Google Scholar]
- Tesch PA, Komi PV, Jacobs I, Karlsson J, Viitasalo JT. Influence of lactate accumulation of EMG frequency spectrum during repeated concentric contractions. Acta Physiologica Scandinavica. 1983;119:61–67. doi: 10.1111/j.1748-1716.1983.tb07306.x. [DOI] [PubMed] [Google Scholar]
- Tonkonogi M, Walsh B, Tiivel T, Saks V, Sahlin K. Mitochondrial function in human skeletal muscle is not impaired by high intensity exercise. Pflügers Archives. 1999;437:562–568. doi: 10.1007/s004240050818. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. Journal of Applied Physiology. 1999;86:1101–1113. doi: 10.1152/jappl.1999.86.4.1101. [DOI] [PubMed] [Google Scholar]
- Wasserman K, Whipp BJ, Koyal SN, Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. Journal of Applied Physiology. 1973;35:236–243. doi: 10.1152/jappl.1973.35.2.236. [DOI] [PubMed] [Google Scholar]
- Wendt IR, Gibbs CL. Energy production of rat extensor digitorum longus muscle. American Journal of Physiology. 1973;224:1081–1086. doi: 10.1152/ajplegacy.1973.224.5.1081. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Mahler M. Dynamics of pulmonary gas exchange during exercise. In: West JB, editor. Pulmonary Gas Exchange, Organism and Environment. Vol. 2. New York: Academic Press Inc.; 1980. pp. 33–96. [Google Scholar]
- Willis WT, Jackman MR. Mitochondrial function during heavy exercise. Medicine and Science in Sports and Exercise. 1994;26:1347–1354. [PubMed] [Google Scholar]
- Woledge RC. Possible effects of fatigue on muscle efficiency. Acta Physiologica Scandinavica. 1998;162:267–273. doi: 10.1046/j.1365-201X.1998.0294e.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Watari H. Changes in intracellular pH during repeated exercise. European Journal of Applied Physiology. 1993;67:274–278. doi: 10.1007/BF00864228. [DOI] [PubMed] [Google Scholar]


