Abstract
The mammalian brain ventricles are lined with ciliated ependymal cells. As yet little is known about the mechanisms by which neurotransmitters regulate cilia beat frequency (CBF).
Application of 5-HT to ependymal cells in cultured rat brainstem slices caused CBF to increase. 5-HT had an EC50 of 30 μM and at 100 μM attained a near-maximal CBF increase of 52.7 ± 4.1 % (mean ± s.d.) (n= 8).
Bathing slices in Ca2+-free solution markedly reduced the 5-HT-mediated increase in CBF. Fluorescence measurements revealed that 5-HT caused a marked transient elevation in cytosolic Ca2+ ([Ca2+]c) that then slowly decreased to a plateau level. Analysis showed that the [Ca2+]c transient was due to release of Ca2+ from inositol 1,4,5-trisphosphate (IP3)-sensitive stores; the plateau was probably due to extracellular Ca2+ influx through Ca2+ release-activated Ca2+ (CRAC) channels.
Application of ATP caused a sustained decrease in CBF. ATP had an EC50 of about 50 μM and 100 μM ATP resulted in a maximal 57.5 ± 6.5 % (n= 12) decrease in CBF. The ATP-induced decrease in CBF was unaffected by lowering extracellular [Ca2+], and no changes in [Ca2+]c were observed. Exposure of ependymal cells to forskolin caused a decrease in CBF. Ciliated ependymal cells loaded with caged cAMP exhibited a 54.3 ± 7.5 % (n= 9) decrease in CBF following uncaging. These results suggest that ATP reduces CBF by a Ca2+-independent cAMP-mediated pathway.
Application of 5-HT and adenosine-5′-O-3-thiotriphosphate (ATP-γ-S) to acutely isolated ciliated ependymal cells resulted in CBF responses similar to those of ependymal cells in cultured slices suggesting that these neurotransmitters act directly on these cells.
The opposite response of ciliated ependymal cells to 5-HT and ATP provides a novel mechanism for their active involvement in central nervous system signalling.
Cerebrospinal fluid (CSF) fills the ventricles and subarachnoid spaces enveloping the central nervous system (CNS) and has been thought to serve as a transport medium for disseminating global CNS signals (Nicholson, 1999). Ependymal cells form a single-layered, ciliated cellular interface that lines the ventricular surface of vertebrate brain and the central canal of the spinal cord (Del Bigio, 1995). Putative functions for these ciliated ependymal cells include: acting as neural stem cells (Johansson et al. 1999), moving cellular debris in the direction of bulk CSF flow (Cathcart & Worthington, 1964), and locally mixing CSF thereby minimizing the unstirred CSF layer over the ependyma and optimizing the dispersion of neural messengers in the CSF (Roth et al. 1985). Local mixing induced by ependymal cilia beating can influence the exchange between CSF and brain, thereby potentially regulating communication within the CNS. Unlike ciliated cells of the reproductive and respiratory epithelia, which have been extensively investigated, the functions and regulatory mechanisms of mammalian brain ependymal ciliated cells still remain poorly understood (Del Bigio, 1995). While there has been much speculation regarding the regulatory mechanisms involved in controlling the cilia beat frequency (CBF) of ependymal cells, the specific pathways involved in such regulation have not been elucidated.
The present experiments investigated the control of ciliary activity in ependymal cells by serotonin (5-HT) and ATP. Here we present the first direct evidence on mechanisms that control cilia activity in mammalian brain ependymal cells. The results show that ciliated ependymal cells have two unique and independent signalling pathways with opposing effects. One pathway that stimulates CBF is mediated by the activation of 5-HT receptors, resulting in IP3-mediated release of Ca2+ from intracellular Ca2+ stores. This initial elevation of cytosolic Ca2+ causes a prolonged increase of intracellular Ca2+ due to the opening of Ca2+ release-activated Ca2+ (CRAC) channels on the plasma membrane. Ca2+ influx through CRAC channels contributes to most of the observed increase in CBF. The second pathway that reduces CBF is activated by ATP. The inhibitory effect is independent of intracellular Ca2+ change and may involve the activation of purinergic receptors and is transduced by intracellular adenosine 3′,5′-cyclic monophosphate (cAMP).
METHODS
Ciliated ependymal cells of the 4th ventricle were studied either in brain slices grown for 1-3 days in culture or in a smaller number of experiments as acutely isolated ependymal cells. In both cases Sprague-Dawley rat pups (8-10 days old) were anaesthetized with either a ketamine-xylazine mixture (200 mg kg−1 and 14 mg kg−1, i.m., respectively) or 30 % halothane in air and then decapitated. The medulla was isolated and sliced in 300 μm-thick sections while being submerged in ice-cold Ringer solution containing (mM): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 11 glucose, and 2.5 CaCl2, bubbled with 95 % O2-5 % CO2. For studies of ependymal cells in slices we transferred these acutely prepared slices to a keratinocyte serum-free medium (Life Technologies, Gaithersburg, MD, USA) with 10 % fetal calf serum for 1-3 days. The University of Washington Animal Care and Use Committee approved all procedures.
For the preparation of isolated cells, slices were initially incubated in Hanks’ solution containing 10 units ml−1 protease (type XIV, Sigma, MO, USA) for 20 min at 37°C. The ciliated ependymal layer was then carefully removed from the brainstem slice and rinsed with Hanks’ solution. Gentle shear force through a hypodermic needle was used to break the ependymal layer into individual isolated ciliated cells. Aliquots of the cell suspension were then allowed to attach to the floor of polylysine-coated glass chambers and mounted on the stage of a microscope for observation.
All test substances were added to a static bath solution that contained the cultured slices or acutely isolated cells. Measurements of ciliary beating and cytosolic Ca2+ were carried out at 37°C, while measurements of electrical properties were performed at room temperature, approximately 22°C.
Measurement of ciliary beating
The ciliary activity measuring system is based on a previously described method, that has been modified to measure on-line ciliary beating of single ciliated cells (Ben-Shimol et al. 1991; Sanderson & Dirksen, 1995; Nguyen, 1996). The apparatus consists of a Nikon Diaphot inverted microscope with a ×100, 1.4 NA oil immersion objective and a DC illumination source. Images were captured by a thermoelectrically cooled, low dark noise (1.3 photoelectrons s−1 pixel−1 at -36°C) digital camera with a 336 × 243 CCD matrix, a 16-bit pixel resolution, and a readout rate of 105 pixel s−1 (Spectra Source Model 400, Westlake Village, CA, USA). The camera was mounted in the photoport of the microscope using a ×20 relay lens yielding a 10 pixel μm−1 resolution. To increase the sampling rate, we avoided capturing the whole image. Instead, single line scans were sampled at a rate of 256 scans s−1 and an on-line fast Fourier transform of the line samples gave the CBF. Each scan sampled an area 0.2 μm × 30 μm across the optical field of the ciliated ependymal cells. The CBF from this digital photography method was calibrated against the manual counting of ciliary beating in images captured with fast cinematography (128 pictures s−1). The CBF obtained by the two techniques yielded the same beat frequency over the range of 0-28 Hz (Nguyen, 1996).
Measurement of cytosolic [Ca2+]
Intact ependymal cells in cultured slices were equilibrated for 30 min in Hanks’ solution at 37°C, containing 5 μM rhod-2-acetoxymethyl ester (Molecular Probes, Eugene, OR, USA) from a stock solution in dimethyl sulfoxide (DMSO). The final concentration of DMSO in the Hanks’ solution was 0.1 %. Rhod-2 has a high Ca2+ affinity (Kd= 570 nM, in the absence of Mg2+), allowing the measurement of a low range of [Ca2+]c. The Kd of rhod-2 was calculated from direct measurements performed on cells treated with the Ca2+-ionophore A23187 following procedures described elsewhere (Kao, 1994; Nguyen et al. 1998). After dye loading, the cells were washed and equilibrated in Hanks’ solution for 30 min. Ciliated cells were also loaded with 500 ng ml−1 of the fluorescent dye DiOC6(3) (Molecular Probes) for 5-8 min and then washed in Hanks’ solution. This lipophylic, cationic dye has been used extensively to identify intracellular organelles including the ER of a variety of cells (Terasaki et al. 1984). Since the excitation wavelength of DiOC6(3) and the Ca2+ probes are different (484 and 525 nm, respectively), the double-labelling strategy allows selection of image planes and line scans without photobleaching the Ca2+ probes. After a selected ciliated cell was identified and focused using the DiOC6(3) label, the filter cube was changed to excite (546 ± 10 nm) and collect the emission of rhod-2 while the cells were stimulated with either ATP or 5-HT. The optical sectioning technique and its calibration have been described elsewhere (Monck et al. 1992; Nguyen et al. 1998).
Measurement of electrical properties of ependymal cells
Electrical measurements were performed on visually identified 4th ventricle ependymal cells in acutely prepared brainstem slices as described above from neonatal rats (8-10 days old). Immediately after slicing, the slices were incubated for 1 h at 37°C. During whole-cell recordings slices were perfused by a Ringer solution containing (mM): 119 NaCl, 26.2 NaHCO3, 1 NaH2PO4, 2.5 KCl, 11 glucose, 2.5 CaCl2, and 1.3 MgSO4. Using near-infrared DIC optics, ependymal cells were identified based on their location and morphology. On most occasions cells were filled with the fixable fluorescent dye Alexa 488 (200 mM, Molecular Probes), allowing visualization of ependymal cells during and after recordings. Whole-cell patch clamp recordings were performed at room temperature using an Axoclamp-2B amplifier and pCLAMP 7.0 software (Axon Instruments). Patch electrodes (resistance 6-8 MΩ) were filled with (mM): 145 KCl, 10 Hepes, 1 EGTA, 2 MgCl2, 2 ATP-Mg, 0.2 GTP-Tris (pH 7.2).
5-HT immunocytochemistry
Sprague-Dawley rats (7-14 days old) were anaesthetized by injection (i.m.) of a ketamine-xylazine mixture (200 mg kg−1 and 14 mg kg−1, respectively). Following decapitation, the brainstem was removed and transverse brainstem slices (300-1000 μm) were cut with a vibratome in a cold Ringer solution containing (mM): 119 NaCl, 26.2 NaHCO3, 1 NaH2PO4, 2.5 KCl, 11 glucose, 2.5 CaCl2, and 1.3 MgSO4. Tissue slices were then fixed in 4 % paraformaldehyde in 0.1 M sodium phosphate at 4°C overnight. After fixation, the tissue was washed in phosphate-buffered saline solution (PBS) and placed in 30 % sucrose in PBS overnight at 4°C. Following cryoprotection, slices were resectioned (50-70 μm thick) using a sliding microtome.
For immunocytochemistry, free-floating tissue sections were incubated in PBS containing 0.2 % Triton X-100 and 10 % donkey serum (Vector Laboratories) for 1 h. Tissues were then incubated overnight in tryptophan hydroxylase antibody (Sigma T0678, 1:400 or 1:500) in PBS containing 0.2 % Triton X-100 and 10 % donkey serum. After washing, tissue was then incubated in CY3 donkey anti-mouse secondary antibody (Jackson ImmunoResearch Inc, 1:600) for 90 min in PBS containing Triton X-100 and donkey serum. Tissue was washed and mounted onto slides using VectaShield (Vector Laboratories). Negative control assays were performed by omitting the primary antibody. Tissue was imaged using a BioRad confocal microscope.
RESULTS
Localization of 5-HT-containing processes in the rat brainstem ependymal cell layer
We used immunocytochemistry to demonstrate that 5-HT-containing nerve fibres and processes are present in the ependymal cell layer of the 4th ventricle and central canal in rats of the same age as used in our functional studies (see below). To do this we used an antibody against tryptophan hydroxylase, a key enzyme in the biosynthetic pathway for 5-HT, to show that the 4th ventricle ependymal cell layer has nerve fibres and varicosities that contain this enzyme (Fig. 1A, n= 5 rats). Figure 1B shows in the same section of the medulla the presence of 5-HT-containing neurons in the nearby midline nucleus raphe obscurus. Since raphe neurons are an important source of the brain’s 5-HT (Cooper et al. 1996) these results serve as a positive control for our findings in the ependymal cell layer. Further, we observed individual 5-HT-containing axons projecting into the ependymal cell layer that arose from single 5-HT-containing neurons (data not shown). Our results are in agreement with previous studies that reported the presence of a 5-HT-containing nerve plexus extending over most of the rat brain ependymal cell layer (Chan-Paly, 1976; Voutsinos et al. 1994). While distinct classical synaptic associations between 5-HT-containing nerve fibres and ependymal cells have been reported to be absent, it is nevertheless widely believed that ependymal cells are the targets of this 5-HT-containing nerve plexus (Lorez & Richards, 1982; Voutsinos et al. 1994; Dinopoulos & Dori, 1995).
Figure 1. 5-HT immunolabelling in transverse rat brainstem slices.

A, tryptophan hydroxylase-positive processes are present at the ependymal cell layer. Arrow points to the basal side of the ependymal cell layer where there are both 5-HT-containing nerve fibres and varicosities. Tissue section is from the medulla rostral to the obex where the 4th ventricle is clearly evident. This picture is derived from successive z-series images of the tissue section. B, tryptophan hydroxylase-positive neurons of the nucleus raphe obscurus are present in the same tissue section as in A. This picture is derived from single z-image of the tissue section. Calibration bars: 50 μm in A and 100 μm in B.
Response to 5-HT
The average basal CBF of the population of intact ciliated ependymal cells exposed to 5-HT was 19 ± 5 Hz (mean ±s.d.) (n= 8). The basal CBF remained fairly stable in single cells, with variability usually not larger than ±5 %. Exposure to 5-HT caused a sustained increase of CBF (Fig. 2A). The 5-HT dose-response data were fitted to a Hill equation which gave an EC50 of 30 μM and a slope factor of 1.7 (Fig. 2B). At a 5-HT concentration of 100 μM CBF attained a near-maximal response increase of 52.7 ± 4.1 % (n= 8) in CBF (Fig. 2B). The 5-HT response was completely blocked by the 5-HT receptor antagonist mianserin (5 μM, n= 6, data not shown). Mianserin has a high affinity for members of the 5-HT2 family of receptors (Zifa & Fillion, 1992). The blockade by mianserin shows that the observed CBF increase was a 5-HT receptor-mediated response.
Figure 2. Effect of 5-HT on ciliary beating of rat ependymal cells.
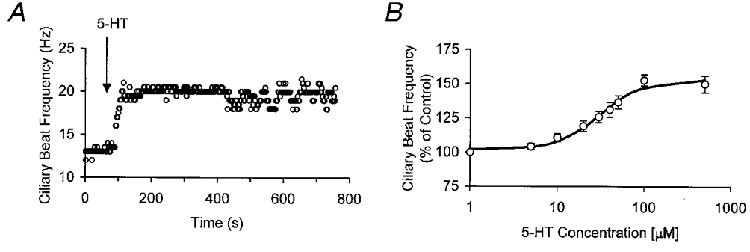
A, CBF response of an ependymal cell to 5-HT (50 μM) in normal Hanks’ solution. B, dose-response of CBF to varying 5-HT concentrations (n= 8). CBF increases with increasing 5-HT concentration with an EC50 of 30 μM and a near-maximal response at 100 μM. Data points are means ±s.d. and were fitted to a Hill equation.
Role of Ca2+ in the 5-HT response
The increase in CBF induced by 50 μM 5-HT was markedly reduced from 36.6 ± 4.7 % to 5.6 ± 2.3 % (n= 12) when the experiments were conducted in an EGTA-buffered Ca2+-free Hanks’ solution (Fig. 3A). While this markedly attenuated response in Ca2+-free solution indicates that the majority of the 5-HT-mediated CBF increase was dependent on extracellular [Ca2+], the small residual response observed in Ca2+-free solution suggests that the release from intracellular Ca2+ stores may be involved in this signalling pathway.
Figure 3. Role of Ca2+ in the 5-HT-mediated increase of ependymal cell CBF.

A, CBF response of an ependymal cell to 5-HT (50 μM) in EGTA-buffered Ca2+-free Hanks’ solution. The mean increase in CBF was markedly reduced to 5.6 ± 2.3 % in Ca2+-free medium (n= 12). B, effect of 5-HT (50 μM) in Hanks’ solution on [Ca2+]c. C, effect of 5-HT (50 μM) in EGTA-buffered Ca2+-free Hanks’ solution on [Ca2+]c.
In ependymal cells loaded with the Ca2+-sensitive dye rhod-2, bath application of 5-HT (50 μM) caused a marked transient elevation in cytosolic [Ca2+] ([Ca2+]c) that then slowly decreased to a plateau which was higher than the baseline [Ca2+]c level (Fig. 3B). However, in EGTA-buffered Ca2+-free Hanks’ solution, 5-HT (50 μM) also induced the characteristic spike in [Ca2+]c, but [Ca2+]c quickly returned to baseline values without exhibiting the plateau phase response (Fig. 3C). The presence of the initial [Ca2+]c spike observed in the absence of extracellular Ca2+ suggests that this initial transient increase in [Ca2+]c was due to the release of Ca2+ from intracellular stores. Thus, Ca2+ release from intracellular stores is probably responsible for the small residual increase in CBF observed in Ca2+-free Hanks’ solution.
A similar biphasic time course of [Ca2+]c, spike followed by plateau phase, has been observed in non-excitable KNRK-PAR-2 epithelial cell lines (rat kidney epithelial cells expressing human proteinase-activated receptor-2) stimulated with trypsin (Böhm et al. 1996). In that system the initial [Ca2+]c spike was shown to be due to release of Ca2+ from intracellular Ca2+ stores, while the subsequent [Ca2+]c plateau phase was shown to result from extracellular Ca2+ influx through Ca2+ release-activated Ca2+ (CRAC) channels (Böhm et al. 1996). Thus Ca2+ released from intracellular Ca2+ stores induced the opening of CRAC channels on the plasma membrane, leading to the longer-lasting plateau phase of [Ca2+]c increase.
To investigate whether this mechanism accounts for the biphasic [Ca2+]c response observed in ciliated ependymal cells, we first determined whether or not voltage-gated Ca2+ channels were present in these cells. These channels are found in many excitable cells. Using both current-clamp (n= 6) and voltage-clamp methods (n= 6) we studied the current-voltage properties of ependymal cells. Most patch-clamp electrodes were dye-filled in order to visualize the recorded ependymal cells. Figure 4A shows an Alexa-dye-filled ependymal cell whose lightly stained cilia (marked by an asterisk) are seen extending into the 4th ventricle. The ependymal cells have short basal processes (marked by an arrow); these have been observed previously in other mammalian ependymal cells (Perez-Martin et al. 2000). In current clamp we found that these cells lacked spontaneous electrical events, such as spontaneous synaptic potentials and action potentials and they were not excitable (data not shown). The resting membrane potential was −70 ± 10 mV (mean ±s.d., n= 6). The current-voltage relationships were found to be linear (6 cells studied in current clamp and 6 cells in voltage clamp) (Fig. 4B). In voltage clamp (n= 6) we observed an absence of any inward current when the membrane potential was stepped from a holding potential of −70 or −80 mV to −10 and 0 mV, respectively (Fig. 4C). These properties are characteristic of non-excitable cells that do not possess voltage-gated Ca2+ channels. Based on the slope of the steady-state current-voltage relationship obtained in voltage clamp (Fig. 4B), we computed the mean input resistance to be 82.5 ± 34.2 MΩ (mean ±s.d., n= 6).
Figure 4. Electrical properties of ependymal cells.

A, photomicrograph of a Alexa-dye-filled ependymal cell that was recorded from in voltage-clamp mode. Lightly stained cilia are seen (*) extending into the 4th ventricle. Arrow points to a short basal process of the filled cell. Calibration bar is 40 μm. B, the steady-state current-voltage relationship is linear. C, whole-cell current recordings from two different holding potentials shows an absence of any inward currents. Membrane current responses to 10 mV incrementing steps from a −70 mV holding potential (left-hand panel) and from a −80 mV potential (right-hand panel). All data shown are from the same ependymal cell.
Next, to test whether the initial [Ca2+]c transient was due to release of Ca2+ from IP3-sensitive Ca2+ stores, we exposed ependymal cells (n= 6) to xestospongin (20 μM), a membrane-permeable IP3 receptor antagonist (Gafni et al. 1997). Addition of xestospongin resulted in complete blockade of the effect of 5-HT on both CBF (Fig. 5A) and [Ca2+]c (Fig. 5B). Plasma membrane CRAC channels have been shown to be sensitive to both SKF-96365 and CdCl2 (Böhm et al. 1996; Parekh & Penner, 1997). To test the involvement of CRAC channels, we studied 5-HT (50 μM) stimulation of ependymal cells exposed to either SKF-96365 (50 μM, Tocris) or CdCl2 (500 μM), although SKF-96365 also blocks other ion channels (Franzius et al. 1994). These experiments resulted in a response very similar to that observed in cells stimulated with 5-HT in Ca2+-free Hanks’ solution (Fig. 3C), i.e. their response consisted of a large transient increase in [Ca2+]c without the subsequent plateau phase of [Ca2+]c increase observed in control experiments. Correspondingly, the CBF increase induced by 50 μM 5-HT was also markedly reduced to 5.8 ± 1.7 % (n= 5) and 4.2 ± 2.1 % (n= 7) in the presence of SKF-96365 and CdCl2, respectively (data not shown).
Figure 5. Xestospongin blockade of IP3 receptors abolishes the effect of 5-HT on CBF and [Ca2+]c.

A, effect of xestospongin, a membrane-permeable IP3 receptor antagonist, on the 5-HT-mediated effect on CBF. Xestospongin (20 μM) completely abolished the effect of 5-HT (50 μM) on CBF. B, xestospongin (20 μM) completely abolished the effect of 5-HT on [Ca2+]c.
Response to ATP
ATP has been demonstrated to function as an important neurotransmitter in the CNS. Released ATP can either interact with ATP receptors present in the CNS or participate in extracellular ecto-protein phosphorylation that may be involved in critical neural signalling pathways, such as long-term potentiation (LTP) (White, 1984; Edwards & Gibb, 1993; Wieraszko, 1996; Robertson & Edwards, 1998; Queiroz et al. 1999). In the mammalian respiratory and reproductive system, and in frog oesophagi, ATP has been shown to induce an increase in CBF that is mediated by an increase in [Ca2+]c (Nelson & Wright, 1974; Villalon et al. 1989; Korngreen & Priel, 1996; Evans & Sanderson, 1999). Therefore, we investigated the effect of ATP on intact ciliated brain ependymal cells whose mean basal CBF was 22 ± 6 Hz (n= 12). It is important to note that the mean CBF of this population was not significantly different from that of the population exposed to 5-HT (see above). Exposure of intact ciliated brain ependymal cells to ATP in Hanks’ solution resulted in a consistent and sustained decrease in CBF (Fig. 6A). The dose-response curve to ATP showed an EC50 of about 50 μM and a maximal response at 100 μM ATP resulting in a 57.5 ± 6.5 % (n= 12) decrease in CBF (Fig. 6B). In the presence of ATPase (0.1 units), ATP (100 μM) was unable to induce a decrease in CBF (n= 6) (Fig. 6C). However, ATP-γ-S (100 μM), a non-hydrolysable ATP analogue, was still able to decrease CBF in the presence of ATPase (n= 6) (Fig. 6C). Hence, the ATP-induced inhibition of ciliary beating does not involve the hydrolysis of ATP and most probably is a purinergic receptor-mediated response.
Figure 6. Effect of ATP on ciliary beating and [Ca2+]c of ependymal cells.

A, CBF response of an ependymal cell to ATP (100 μM) in normal Hanks’ solution. B, dose-response of CBF to varying concentrations of exogenously applied ATP (n= 12). CBF decreases with ATP concentration, with an EC50 of approximately 50 μM, and attains a maximal decrease at a concentration of 100 μM. Error bars are s.d.s. C, effect on CBF of ATP (100 μM) and ATP-γ-S (100 μM), a non-hydrolysable ATP analogue, in the presence of 0.1 units ATPase (n= 6). In the presence of ATPase, ATP does not cause a decrease in CBF; in contrast, application of ATP-γ-S results in a decrease in CBF. D, measurement of ependymal cell [Ca2+]c exposed to ATP (100 μM) in Hanks’ solution. No changes in [Ca2+]c were observed with ATP application.
The ATP-induced decrease in CBF was unaffected by lowering extracellular [Ca2+] to below 200 nM in the EGTA-buffered Hanks’ solution (n= 9) (data not shown). Further, we did not observe any changes in [Ca2+]c following ATP stimulation of ependymal cells (n= 9) (Fig. 6D). These findings suggest that ATP-mediated inhibition of CBF in ependymal cells is independent of changes in [Ca2+]c.
In mammalian respiratory tract ciliated cells, cAMP has been shown to modulate CBF by a pathway that is independent of [Ca2+]c (Sanderson et al. 1992). Exposure of ependymal cells to forskolin (100 μM), an adenylate cyclase agonist (Seamon et al. 1981; Seamon & Daly, 1981; Insel et al. 1982), resulted in a 27 ± 3.4 % (n= 7) decrease in CBF (data not shown). This observation suggests the involvement of cAMP in ATP-induced CBF decrease. Ciliated ependymal cells loaded with 50 μM caged cAMP (4,5-dimethoxy-2-nitrobenzyl adenosine 3′,5′-cyclic monophosphate, Molecular Probes) exhibited a 54.3 ± 7.5 % (n= 9) decrease in CBF (Fig. 7) following 2-5 s of illumination with 240-270 nm wavelength light. However, the frequency of ciliary beating returned to baseline within 20 min of uncaging. This slow recovery to baseline may be due to the gradual degradation of the uncaged cAMP. When ependymal cells were exposed to the same light, but in the absence of caged cAMP, they did not show any changes in CBF (n= 8) (data not shown). The involvement of cAMP in ATP-induced CBF decrease is consistent with previous reports of high concentrations of adenylate cyclase and pertussis toxin-sensitive G proteins on rat ependymal cell cilia (De Camilli et al. 1986; Shinohara et al. 1998).
Figure 7. Effect of uncaging of cAMP on ciliary beating of ependymal cells.

Effect of uncaging cAMP with a 240-270 nm light flash on CBF in an ependymal cell loaded with 50 μM caged cAMP. Releasing cAMP results in a 54 ± 7.5 % (n= 9) decrease in CBF.
We also investigated the possible interactions of the 5-HT- and ATP-stimulated pathways. At maximal concentrations, 100 μM ATP was able to overcome the stimulatory effect of 100 μM 5-HT and caused a 52.8 ± 8.2 % (n= 6) decrease in CBF. Hence, cAMP decreases CBF either by a pathway independent of Ca2+ or by inhibition of a point downstream of the Ca2+ regulatory cascade.
Response of isolated ciliated ependymal cells to 5-HT and ATP
Lastly, we sought to provide evidence that 5-HT and ATP act directly on ependymal cells to increase and decrease CBF, respectively. To do this we acutely isolated ependymal cells (see Methods) and investigated the effects of application of 5-HT and ATP-γ-S on CBF. Exposure of isolated ciliated ependymal cells to 5-HT (50 μM) caused an increase in CBF (Fig. 8A). We observed that CBF increased, on average, by 51.6 ± 14.4 % (n= 3). Exposure of isolated ciliated ependymal cells to ATP-γ-S (100 μM) caused a decrease in CBF (Fig. 8B). We observed that CBF decreased, on average, by 58.1 ± 10.4 % (n= 4). Thus these results demonstrate that 5-HT and ATP have a direct effect on ciliated ependymal cells.
Figure 8. Effect of 5-HT and ATP-γ-S on ciliary beating of isolated rat ependymal cells.

A, application of 5-HT (50 μM) to an isolated ependymal cell resulted in an increase in CBF. B, application of ATP-γ-S (100 μM), a non-hydrolysable ATP analogue, caused a marked decreased in CBF.
DISCUSSION
To our knowledge, this study is the first report to document the effects of 5-HT and ATP on ciliary beating of mammalian brain ependymal cells and to identify two distinct, independent pathways with opposing effects on CBF. Figure 9 presents a working model for these pathways. In the first pathway 5-HT increases ciliary beating by activating a 5-HT receptor, probably a member of the 5-HT2 receptor family located on the ependymal cell plasma membrane, that triggers the generation of cytosolic IP3. The binding of IP3 to its receptor on IP3-sensitive Ca2+ stores results in release of Ca2+ to the cytosol. This initial rise in [Ca2+]c opens CRAC channels on the plasma membrane, leading to a more sustained Ca2+ influx from the extracellular environment. The sustained elevation in [Ca2+]c is responsible for most of the 5-HT-stimulated increase in CBF. In contrast in the second pathway, ATP binding, probably to a purinergic receptor, possibly activates adenylate cyclase that generates cAMP that then inhibits ciliary beating. cAMP works by either inhibiting a point downstream of the Ca2+ regulatory cascade or a pathway independent of Ca2+. However, the exact nature by which cAMP and [Ca2+]c ultimately change ciliary movement remains unclear.
Figure 9. A model of the signalling pathways involved in the effect of 5-HT and ATP on ciliary beating of ependymal cells.

Exposure to 5-HT results in the intracellular production of IP3, which releases Ca2+ from intracellular Ca2+ stores. The released Ca2+ mediates the opening of CRAC channels that results in the influx of Ca2+ from the extracellular environment. The increase in [Ca2+]c ultimately increases CBF by an as yet unidentified mechanism. In contrast, ATP activates the production of intracellular cAMP, which inhibits CBF by a still to be determined mechanism.
While it is conceivable that the results observed in our slice culture experiments may involve not only ependymal cells but other cell types, we think this is unlikely. The fact that we observed the same effects of 5-HT and ATP on CBF in acutely isolated ciliated ependymal cells supports the model proposed in Fig. 9.
The 5-HT-mediated increase in CBF may have important functional implications. 5-HT is released into the CSF from the ependymal surface (Ternaux et al. 1977). Stimulation of the brainstem raphe nuclei, the location of the cell bodies that are the source of the 5-HT-containing nerve plexus of the ependymal layer (Chan-Paly, 1976), causes increased release of 5-HT into the CSF (Sheard & Zolovick, 1971). Thus the 5-HT-mediated increase in CBF may provide a mechanism for the wider distribution of this important neurotransmitter, whereby the CSF may be suited as a corridor for distributing neuronal messengers. This is analogous in the endocrine system to utilizing blood to distribute hormones. Finally, it is of interest that patients with immotile cilia syndrome (primary ciliary dyskinesia) have a number of neurological deficits including headache, depression and schizophrenia (Afzelius & Mossberg, 1995), all of which have been in part associated with alterations in the brain’s serotonergic system (Johnson et al. 1998; Peroutka, 1998).
Although the specific roles of ciliated ependymal cells in the CNS remain to be elucidated (Cathcart & Worthington, 1964; Roth et al. 1985; Del Bigio, 1995), here we have shown that these ciliated ependymal cells responded to 5-HT and ATP with opposing effects. By increasing their CBF, ciliated ependymal cells can preferentially disperse or transmit certain neural messengers into the bulk CSF and substantially spread these molecules to other regions of CNS, thereby initiating a more widely distributed signal. Conversely, by reducing the CBF, ciliated ependymal cells can also selectively retain or localize the dispersion of certain messengers in CSF, generating a short-range influence. These results provide compelling evidence that ependymal cells can play an active role in neural messenger exchange at the CSF-brain interface.
Acknowledgments
We thank Dr D. A. Bayliss, University of Virginia, for his advice on the 5-HT immunocytochemistry, and J. Steed and P. Huynh for technical assistance. This work was supported by grants from the NSF, NIH, DOE and Mellon Foundation. T. Nguyen was supported by training grants from the MSTP program and Internal Medicine residency program at University of Washington School of Medicine. J. A. O’Brien was supported by an NIH training grant. W.-C. Chin was supported by a fellowship from the Centre for Nanotechnology at the University of Washington.
References
- Afzelius BA, Mossberg B. Immotile-cilia syndrome (primary ciliary dyskinesia), including Kartagener syndrome. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 1995. pp. 3943–3954. [Google Scholar]
- Ben-Shimol Y, Dinstein I, Meisels A, Priel Z. Ciliary motion features from digitized video photography. Journal of Computer-Assisted Microscopy. 1991;3:103–116. [Google Scholar]
- Böhm SK, Khitin LM, Grady EF, Aponte G, Payan DG, Bunnett NW. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. Journal of Biological Chemistry. 1996;271:22003–22016. doi: 10.1074/jbc.271.36.22003. [DOI] [PubMed] [Google Scholar]
- Cathcart RS, Worthington WC. Ciliary movement in the rat cerebral ventricles: clearing action and directions of currents. Journal of Neuropathology and Experimental Neurology. 1964;23:609–618. doi: 10.1097/00005072-196410000-00002. [DOI] [PubMed] [Google Scholar]
- Chan-Paly V. Serotonin axons in the supra- and subependymal plexuses and in the leptomeninges; their roles in local alterations of cerebrospinal fluid and vasomotor activity. Brain Research. 1976;102:103–130. doi: 10.1016/0006-8993(76)90578-3. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 7. New York: Oxford University Press; 1996. [Google Scholar]
- De Camilli P, Moretti M, Donini SD, Walter U, Lohmann SM. Heterogeneous distribution of the cAMP receptor protein RII in the nervous system: evidence for its intracellular accumulation on microtubules, microtubule-organizing centers, and in the area of the Golgi complex. Journal of Cell Biology. 1986;103:189–203. doi: 10.1083/jcb.103.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. 1995;14:1–13. doi: 10.1002/glia.440140102. [DOI] [PubMed] [Google Scholar]
- Dinopoulos A, Dori I. The development of the serotonergic fiber network of the lateral ventricles of the rat brain: a light and electron microscopic immunocytochemical analysis. Experimental Neurology. 1995;133:73–84. doi: 10.1006/exnr.1995.1009. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ. ATP - a fast neurotransmitter. Federation of European Biochemical Societies. 1993;325:86–89. doi: 10.1016/0014-5793(93)81419-z. [DOI] [PubMed] [Google Scholar]
- Evans JH, Sanderson MJ. Intracellular calcium oscillations regulate ciliary beat frequency of airway epithelial cells. Cell Calcium. 1999;26:103–110. doi: 10.1054/ceca.1999.0060. [DOI] [PubMed] [Google Scholar]
- Franzius D, Hoth M, Penner R. Non-specific effects of calcium entry antagonists in mast cells. Pflügers Archiv. 1994;428:433–438. doi: 10.1007/BF00374562. [DOI] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Insel PA, Stengel D, Ferry N, Hanoune J. Regulation of adenylate cyclase of human platelet membranes by forskolin. Journal of Biological Chemistry. 1982;257:7485–7490. [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Johnson KW, Phebus LA, Cohen ML. Serotonin in migraine: theories, animal models and emerging therapies. Progress in Drug Research. 1998;51:219–244. doi: 10.1007/978-3-0348-8845-5_6. [DOI] [PubMed] [Google Scholar]
- Kao JPY. Practical aspects of measuring [Ca2+] with fluorescent indicators. In: Nuccitelli R, editor. Methods in Cell Biology. San Diego: Academic Press; 1994. pp. 155–181. [DOI] [PubMed] [Google Scholar]
- Korngreen A, Priel Z. Purinergic stimulation of rabbit ciliated airway epithelia: control by multiple calcium sources. The Journal of Physiology. 1996;497:53–66. doi: 10.1113/jphysiol.1996.sp021749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorez HP, Richards JG. Supra-ependymal serotoninergic nerves in mammalian brain: morphological, pharmacological and functional studies. Brain Research Bulletin. 1982;9:727–741. doi: 10.1016/0361-9230(82)90179-4. [DOI] [PubMed] [Google Scholar]
- Monck JR, Oberhauser AF, Keating TJ, Fernandez JM. Thin-section ratiometric Ca2+ images obtained by optical sectioning of fura-2 loaded mast cells. Journal of Cell Biology. 1992;116:745–759. doi: 10.1083/jcb.116.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DJ, Wright EM. The distribution, activity, and function of the cilia in the frog brain. The Journal of Physiology. 1974;243:63–78. doi: 10.1113/jphysiol.1974.sp010742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. Seattle, USA: University of Washington; 1996. Dynamics of cytosolic and compartmentalized Ca2+ during signal transduction. PhD Thesis. [Google Scholar]
- Nguyen T, Chin W-C, Verdugo P. Role of Ca2+/K+ ion exchange in intracellular storage and release of Ca2+ Nature. 1998;395:908–912. doi: 10.1038/27686. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Signals that go with the flow. Trends in Neurosciences. 1999;22:143–145. doi: 10.1016/s0166-2236(98)01388-5. [DOI] [PubMed] [Google Scholar]
- Parekh AB, Penner R. Store depletion and calcium influx. Physiological Reviews. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Grondona JM, Cifuentes M, Perez-Figares JM, Jimenez JA, Fernandez-Llebrez P. Ependymal explants from the lateral ventricle of the adult bovine brain: a model system for morphological and functional studies of the ependyma. Cell Tissue Research. 2000;300:11–19. doi: 10.1007/s004410000190. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ. Serotonin receptor variants in disease: new therapeutic opportunities? Annals of the New York Academy of Sciences. 1998;861:16–25. doi: 10.1111/j.1749-6632.1998.tb10168.x. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Meyer DK, Meyer A, Starke K, von Kügelgen I. A study of the mechanism of the release of ATP from rat cortical astroglial cells evoked by activation of glutamate receptors. Neuroscience. 1999;91:1171–1181. doi: 10.1016/s0306-4522(98)00644-7. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Edwards FA. ATP and glutamate are released from separate neurones in the rat medial bavenula nucleus: frequency dependence and adenosine-mediated inhibition of release. The Journal of Physiology. 1998;508:691–701. doi: 10.1111/j.1469-7793.1998.691bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth Y, Kimhi Y, Edery H, Aharonson E, Priel Z. Ciliary motility in brain ventricular system and trachea of hamsters. Brain Research. 1985;330:291–297. doi: 10.1016/0006-8993(85)90688-2. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ, Dirksen ER. Quantification of ciliary beat frequency and metachrony by high-speed digital video. Methods in Cell Biology. 1995;47:289–297. doi: 10.1016/s0091-679x(08)60822-5. [DOI] [PubMed] [Google Scholar]
- Sanderson MJ, Lansley AB, Dirksen ER. Regulation of ciliary beat frequency in respiratory tract cells. Chest. 1992;101:69S–71S. doi: 10.1378/chest.101.3_supplement.69s. [DOI] [PubMed] [Google Scholar]
- Seamon KB, Daly JW. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. Journal of Biological Chemistry. 1981;256:9799–9801. [PubMed] [Google Scholar]
- Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proceedings of the National Academy of Sciences of the USA. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard MH, Zolovick AJ. Serotonin: release in cat brain and cerebrospinal fluid on stimulation of midbrain raphé. Brain Research. 1971;26:455–458. [PubMed] [Google Scholar]
- Shinohara H, Asano T, Kato K, Kameshima T, Semba R. Localization of a G protein Gi2 in the cilia of rat ependyma, oviduct and trachea. European Journal of Neuroscience. 1998;10:699–707. doi: 10.1046/j.1460-9568.1998.00088.x. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Song J, Wong JR, Weiss MJ, Chen LB. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell. 1984;38:101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- Ternaux JP, Hery F, Hamon M, Bourgoin S, Glowinski J. 5-HT release from ependymal surface of the caudate nucleus in ‘encéphale isolé’ cats. Brain Research. 1977;132:575–579. doi: 10.1016/0006-8993(77)90207-4. [DOI] [PubMed] [Google Scholar]
- Villalon M, Hinds TR, Verdugo P. Stimulus-response coupling in mammalian ciliated cells. Demonstration of two mechanisms of control for cytosolic Ca2+ Biophysical Journal. 1989;56:1255–1258. doi: 10.1016/S0006-3495(89)82772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutsinos B, Chouaf L, Mertens P, Ruiz-Flandes P, Joubert Y, Belin MF, Didier-Bazes M. Tropism of serotonergic neurons towards glial targets in the rat ependyma. Neuroscience. 1994;59:663–672. doi: 10.1016/0306-4522(94)90185-6. [DOI] [PubMed] [Google Scholar]
- White TD. Characteristics of neuronal release of ATP. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1984;8:487–493. doi: 10.1016/0278-5846(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Wieraszko A. Extracellular ATP as a neurotransmitter: its role in synaptic plasticity in the hippocampus. Acta Neurobiologiae Experimentalis. 1996;56:637–648. doi: 10.55782/ane-1996-1168. [DOI] [PubMed] [Google Scholar]
- Zifa E, Fillion G. 5-Hydroxytryptamine receptors. Pharmacological Reviews. 1992;44:401–458. [PubMed] [Google Scholar]


