Abstract
Neurophysiological evidence that afferent information from skin receptors is important for proprioception has been gathered mainly in experiments relating to the human hand and finger joints. To investigate if proprioceptive information is also provided by skin mechanoreceptor afferents from skin areas related to large joints of postural importance, microneurography recordings were obtained in humans from skin afferents in the lateral cutaneous femoral nerve to study their responses to knee joint movements.
Data were collected from 60 sequentially recorded afferents from slowly (n= 23) and fast (n= 6) adapting low-threshold mechanoreceptors, hair follicle receptors (n= 24), field receptors (n= 1) and C mechanoreceptors (n= 6). Fascicular recordings showed that the lateral cutaneous femoral nerve supplies extensive areas of the thigh: from 5–10 cm below the inguinal ligament down to below and lateral to the knee joint; accordingly, the afferents originated in receptors located in wide areas of the human thigh.
All afferents from fast and slowly adapting low-threshold mechanoreceptors, as well as C mechanoreceptors, responded to manually applied skin stretch. In contrast, the same stimulus elicited, at most, feeble responses in hair follicle receptors.
Qualitative and quantitative analyses of the responses of a subset of afferents revealed that in particular slowly adapting afferents effectively encode both static and dynamic aspects of passively imposed knee joint movements.
It was concluded that receptors in the hairy skin of humans can provide high-fidelity information about knee joint movements. A previously undefined type of slowly adapting receptor (SA III) seemed particularly suited for this task whereas this does not seem to be the case for either hair follicle receptors or C mechanoreceptors.
‘Kinaesthesia’ was introduced by Bastian in 1880 to describe the role of the motor cortex in eliciting motor behaviours that orchestrate specific and functionally appropriate somatosensory afferent patterns, whereas ‘proprioceptors’ was initially used by Sherrington for a set of somatosensory afferents that convey information about the position and movements of our body parts (Finger, 1994). Today ‘kinaesthesia’ and ‘proprioception’ are used practically synonymously to denote an organism’s ability to assess the configuration and movements of its body parts.
Muscle spindles have been regarded as the most important among proprioceptors as defined by Sherrington, since the early 1970s (McCloskey, 1978; Matthews, 1988). Twenty years ago, however, it was reported that afferents from the palmar skin of the human hand respond to finger movements (Hulliger et al. 1979). In recent years it has become increasingly apparent that mechanoreceptors in the hairy dorsal skin of the human hand may also play a role with respect to proprioception. It was reported in 1991 that a large majority of the mechanoreceptors on the back of the hand are activated by movements at nearby joints (Edin & Abbs, 1991). Moreover, the manner in which they respond suggests that they provide high-fidelity information about joint movements. Indeed, slowly adapting skin afferents from the dorsum of the hand display a static (Edin, 1992) and a dynamic (Grill & Hallett, 1995) position sensitivity for movements at the metacarpophalangeal (MCP) joints at least on a par with that reported for human muscle spindle afferents (Edin & Vallbo, 1990; Grill & Hallett, 1995). Finally, two independent studies suggest that humans use inputs from skin mechanoreceptors when they assess finger joint movements and postures. First, when a conflict is artificially created between the input pattern from skin mechanoreceptors and that of muscle spindle afferents, humans seem to primarily judge finger joint movements and postures on the basis of sensory cues from the skin (Edin & Johansson, 1995). Second, Collins & Prochazka reported in 1996 that not only does electrical stimulation of the back of the hand induce movement illusions in a fair proportion of subjects but so do various spatial patterns of skin stretch.
The neurophysiological evidence that afferent information from skin mechanoreceptors is important for proprioception has all been gathered in experiments relating to the human hand and finger joints. Given the unique functions of the human hand these results cannot be extrapolated to other body parts without caution, but they do warrant investigations of the proprioceptive capacity of skin afferents originating in skin areas other than the human hand. Microneurographic recordings were therefore obtained from the lateral cutaneous femoral nerve that innervates skin areas that are deformed by movements at the knee joint, i.e. a joint with functional properties that are quite different from those of the finger joints: it is one of the largest joints in the body, it is regularly subjected to large torque, and its control is of utmost importance for postural stability.
METHODS
Subjects
The experiments were performed on 16 subjects (15 females and 1 male, aged 17-35 years) and useful recordings were obtained from 11 of these. The experimental procedure was approved by the local Ethics Committee of the Faculty of Medicine and Odontology, Umeå University. All subjects gave their informed, written consent prior to the experiments in accordance with the Declaration of Helsinki. The subjects were seated in an almost supine position in an adjustable dentist chair with the left leg resting on a support on the side of the chair, i.e. the left leg was adducted by 20-30 deg and the knee joint was extended. The experimenter induced knee joint movements manually by grasping the lower leg with one hand and gently supporting the thigh on its medial dorsal aspect with the other hand. Movement amplitudes were gradually increased during single-unit recordings to a maximum 90 deg flexion. Likewise, the angular velocities were gradually increased during the recordings; the highest recorded velocity was 65 deg s−1 (Fig. 2D).
Figure 2. Knee joint movements and associated neural responses in SA III units.
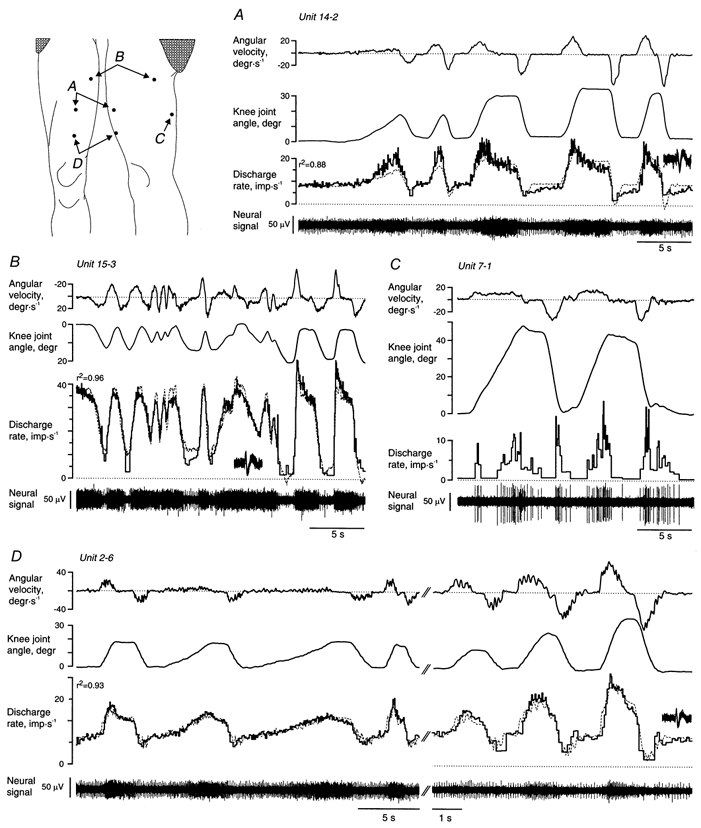
The instantaneous discharge rate was fitted by a linear combination of the angular velocity and the knee joint angle and shown with dotted lines superimposed on the recorded discharge rate (unit 7-1 in C responded to both flexion and extension). Insets show unitary spikes superimposed for the whole displayed time window. The scales of knee joint position and angular velocity were reversed for unit 15-3 (in B) to make it easier to compare them with the unit’s neural response that increased with knee extension rather than flexion. The knee joint angle was defined as zero when the knee was held in a fully extended but not hyperextended position. Note separate time scales in D.
Neurophysiological technique
Detailed descriptions of the microneurographic technique can be found elsewhere (Edin & Abbs, 1991). An uninsulated tungsten electrode (50 mm long, diameter 200 μm, distal 1 mm tapered to a 5-15 μm tip) was inserted transcutaneously 5 cm below and just medial to the left superior anterior iliac spine. The lateral cutaneous femoral nerve in most subjects evidently had split in numerous small branches at this level. To identify single nerve branches, the electrode was moved in small steps until paraesthesia could be elicited with stimulus currents less than 200 μA; it was never possible to evoke muscle twitches or ‘deep’ sensations. Using the uninsulated electrode as a landmark, a similar electrode but coated with lacquer to within 20-40 μm from the tip was then inserted intraneurally (impedance at 1 kHz was 300-800 kΩ measured in situ). The neural signals were amplified (× 10 000; bandwidth 470-5000 Hz) close to the recording site. The microneurographic technique used in this study is biased towards large-diameter axons, yet the functional properties among fast-conducting afferents are not related to their conduction velocity, i.e. to the axon diameter (Mackel, 1988). Relevant skin areas of the thigh were stimulated by stroking hand movements while the electrode was moved in minute steps until single unit activity was observed in the neural signal. Fascicular fields were defined as well-demarcated skin areas from which multi-unit activity could be evoked (Vallbo & Hagbarth, 1968; Hagbarth et al. 1970).
Slowly (SA) and fast adapting (FA) afferents were classified according to standard criteria (Knibestöl & Vallbo, 1970; Vallbo & Johansson, 1984). The threshold force required to evoke responses was determined using von Frey hairs (12.5 mg to 3.2 g) and the receptive field (RF) was defined by determining the skin area in which a von Frey hair of 4-5 times the threshold evoked a response at least 50 % of the time. Hair follicle afferents could not be activated by mechanical stimulation of the skin but only by manipulating individual hair shafts and accordingly their RFs were defined as these hairs. C mechanoreceptors were characterised by their slow conduction velocity (< 2 m s−1) as determined from the distance between the RF and the recording electrode and the delay following a rapidly applied von Frey hair stimulation or direct electrical stimulation of the RF via intracutaneous electrodes (see Fig. 3B). Electrical intracutaneous stimulation of skin afferents was obtained by delivering 200 μs pulses of 0.5-5 mA at most every 5 s through an unshielded tungsten electrode inserted into the superficial dermis immediately adjacent to the RF of the afferent.
Figure 3. Recordings from C mechanoreceptors.
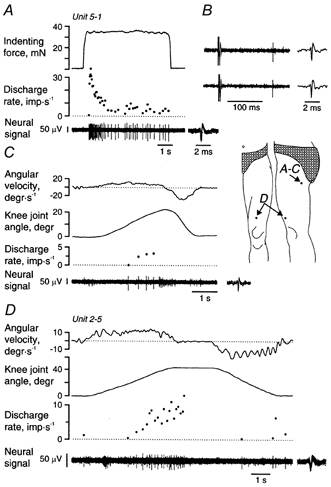
Recordings from two C mechanoreceptive afferents with receptive fields just proximal to the patella and on the lateral proximal thigh (schematic inset). As illustrated for unit 5-1, these receptors responded vigorously to mechanical stimulation but fatigued rapidly (A), and showed low conduction velocities (B). Feeble responses with long delays were observed in response to knee joint movements (C and D).
Recordings from two C mechanoreceptive afferents with receptive fields just proximal to the patella and on the lateral proximal thigh (schematic inset). As illustrated for unit 5-1, these receptors responded vigorously to mechanical stimulation but fatigued rapidly (A), and showed low conduction velocities (B). Feeble responses with long delays were observed in response to knee joint movements (C and D).
Data sampling and processing
The nerve signal was sampled digitally at 12.4 kHz. Individual nerve spikes were retrieved off-line under visual inspection by the use of a previously described algorithm (Edin et al. 1988). The knee joint angle was measured by recording the position of three reflectors on the lateral side of the leg by means of a CCD camera (100 Hz frame rate) and applying standard trigonometric functions off-line (Sandström et al. 1996). The angle of the knee joint when it was held in an extended but not hyperextended position was defined as zero degrees. The angular velocity was calculated by symmetrical numerical differentiation of the knee joint angle. For units that increased or decreased their discharge rate in a monotonous manner as a function of the knee joint angle, multiple linear regression was applied to model the instantaneous discharge rate (Fq) as a linear combination of the knee joint angle (α) and the angular velocity (ω) at the knee joint: Fq = k0 + k○α+ k2ω, where k0 corresponded to the spontaneous discharge rate, k1 the static position sensitivity and k2 the dynamic sensitivity. The recorded discharge rate was shifted within a ± 250 ms (± 25 samples) time window relative to the kinematic signals to find an optimal fit despite any transduction delay.
RESULTS
Recordings of mass activity in individual nerve fascicles demonstrated that the lateral cutaneous femoral nerve supplies remarkably extensive areas of the anterior and lateral aspects of the thigh (Fig. 1). The innervated area reached from 5-10 cm below the inguinal ligament down to below and lateral to the knee joint. In contrast to the nerves supplying the human hand (Hagbarth et al. 1970), tongue (Trulsson & Essick, 1997), and face (Nordin & Thomander, 1989), the fascicular organisation of the lateral cutaneous femoral nerve did not suggest that there were skin areas with particularly high receptor densities. Despite a considerable variability, however, all subjects had fascicles that innervated the lateral distal part of the thigh, i.e. the area typically involved in meralgia paraesthetica.
Figure 1. Fascicular and receptive fields.
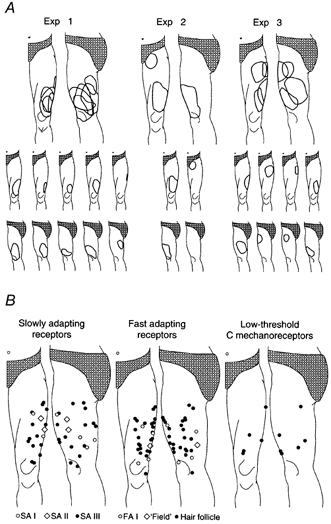
A, fascicular receptive fields mapped by light tactile stimuli while recording multi-unit neural activity in microneurographic recordings in three subjects. B, location of the centre of all receptive fields for slowly and fast adapting afferents and C mechanoreceptive afferents. There was no sign of increased receptor densities close to the knee joint.
Characteristics of slowly adapting afferents
The 23 SA afferents were of three types. Table 1 summarises the descriptions that follow.
Table 1.
Slowly adapting afferents in human hairy skin
| SA I | SA II | SA III | |
| RFs with multiple zones of high sensitivity | • | ○ | ○ |
| Small, sharply demarcated RFs | • | ○ | • |
| Omnidirectional strain sensitivity | • | ○ | • |
| High skin strain sensitivity | ○ | • | • |
| Regular interspike intervals | ○ | • | • |
○, absent; •, present.
Five SA afferents matched the description of SA type I afferents according to Iggo (Iggo & Muir, 1969): clearly demarcated RFs, sustained but irregular discharge in response to maintained skin indentation, and one or more particularly sensitive spots which are associated with dome-like structures in the skin. According to Iggo (Chambers et al. 1972), SA II afferents show a Gaussian distribution of their interspike intervals when the receptor is subjected to a constant indentation, they have RFs with obscure borders, and are markedly sensitive to skin stretch in particular directions (see also Knibestöl & Vallbo, 1970).
Of the 18 SA units that clearly did not belong to the SA I category, only two fitted Iggo’s description of SA type II. The remaining group of 16 afferents, tentatively called SA III, shared features with both SA I and SA II units. The so-called SA III units showed sharply demarcated minute RFs that typically could not be resolved beyond the tip of a von Frey hair (RF area < 1 mm2 for all but one unit; threshold to indentation ranged from 12.5 to 200 mg, median 100 mg). Similar to SA I units, the SA III units responded with high discharge rates (300-550 Hz) when the tip of a glass rod was moved across their RFs. In contrast to SA I units, however, the RFs of SA III units were never fractionated, i.e. they all showed a single highly sensitive zone in their RFs. Moreover, the SA III units were distinctly different from SA I units in their response to maintained skin indentation by showing a very regular discharge similar to SA II units (the experimental protocol did not include a formal test of interspike distributions, cf. Edin, 1992; Grill & Hallett, 1995). In contrast to the SA II proper, however, SA III afferents did not show any preferred direction of skin stretch, i.e. they showed omnidirectional strain sensitivity.
Responses of slowly adapting afferents to knee joint movements
All SA afferents responded to manually applied skin stretch. Knee joint movements evoked responses in all tested SA II and SA III units (2 SA II, 10 SA III) despite, in some cases, a considerable distance between the knee joint and the corresponding RF (cf. Fig. 1B). The single unit recording position was often lost as soon as manipulation of the subject’s knee commenced, yet recordings lasting more than just a few seconds were obtained from nine SA units (1 SA II and 8 SA III, Fig. 2). Knee joint position and angular velocity both influenced the discharge of these units. The observed movement-related responses could in all cases be predicted from the location of the afferents’ RFs and their increased discharge in response to manually applied skin stretch (Edin & Abbs, 1991). The RFs of units 14-2 and 2-6 (Fig. 2A and D), for instance, were both subjected to increased strain during knee flexion and their discharge accordingly increased during flexion movements. In contrast, the skin area of unit 15-3 (Fig. 2B) was stretched during knee extension while that of unit 7-1 (Fig. 2C) was stretched during both knee extension and flexion. The distance between the RFs and the knee joint poorly predicted movement sensitivity and this suggests significant differences in strain sensitivity among the SA units. Unit 15-3, for instance, was the unit furthest away from the knee joint and yet showed by far the highest static and dynamic position sensitivity (1.85 impulses s−1 deg −1 and 0.47 impulses s−1 (deg s−1)−1, respectively).
The dashed lines superimposed on the instantaneous discharge rates in Fig. 2A-C represent the discharge rates calculated from a multiple linear regression model that predicted the instantaneous discharge rate from the knee joint angle and angular velocity (i.e. the discharge rate was modelled as a weighted sum of the knee joint angle and the angular velocity). The median coefficient of determination (r2) for the two SA II and five SA III afferents subjected to this analysis was 0.88 (range 0.58-0.96). The median static position sensitivity for the five units with r2≥ 0.7 was 0.35 impulses s−1 deg−1 (range 0.33-1.85), the dynamic sensitivity was 0.20 impulses s−1 (deg s−1)−1 (range 0.16-0.47). The impact of the knee joint angle and the angular velocity on the discharge rate was similar (the median partial r2 for the knee joint angle and angular velocity was 0.65 and 0.60, respectively). The optimal regression fit was obtained if the discharge rate was shifted in time in relation to the knee joint angle and the angular velocity records. The median shift required to maximise the fit was, however, less than 20 ms indicating that the afferents were able to convey information about knee movements with just a small transduction delay.
Although the linear regression yielded good fits, this does not imply that the skin mechanoreceptors are ‘linear’ transducers of joint positions and velocities except for limited, albeit significant, movement and velocity ranges. Unit 14-2 (Fig. 2A), for instance, did not increase its discharge significantly when the knee joint was flexed beyond 15-20 deg. Similarly, although the linear model predicted the discharge rate in unit 2-6 remarkably well for flexion movements less than about 40 deg, this unit did not increase its discharge proportionally when the knee joint was flexed beyond 45-50 deg (not shown).
Only two SA I afferents were tested with knee joint movements. Unfortunately, their RFs were located 8 and 11 cm proximal to the lateral meniscus on the lateral aspect of the thigh in a skin area not much affected by knee joint movements (see Fig. 1B). Although they both readily responded to manually applied skin stretch only the unit furthest away from the knee joint responded in a characteristically irregular fashion during both joint extension and flexion. It was not possible to test either of these units with large movement amplitudes or high velocities.
Fast adapting afferents
The largest single group of afferents originated in hair follicles (24/60) and they were all fast adapting. Spikes could be evoked by manipulating single hair shafts (median 5, range 1-18) and yet the subjects often denied perceiving any stimulus (Hensel & Boman, 1960). More importantly, all hair follicle afferents were either definitely insensitive or showed feeble responses to skin stretch and none of 17 tested hair follicle receptor afferents responded to knee joint movements. FA I units, in contrast, all responded briskly to manually applied skin stretch. The three FA I units that were tested happened to be located on the anterior aspect of the thigh within 10 cm of the patella and they all responded to knee joint movements. One of these FA I units discharged 5-10 impulses during knee joint flexion only whereas the other two discharged briefly during both flexion and extension movements (Edin & Abbs, 1991).
C mechanoreceptive afferents
Recordings were obtained from six low-threshold C mechanoreceptive afferents (Fig. 3). They all showed low conduction velocities (< 2 m s−1; Fig. 3B), responded readily to indenting or slowly moving tactile stimuli or manually applied skin stretch with discharge rates of 50 Hz or more, but the receptors were markedly fatigable (Fig. 3A). In all respects they were thus similar to their counterparts in the human forearm (Vallbo et al. 1993) and facial skin (Nordin, 1990). The two C mechanoreceptive afferents tested for sensitivity to knee joint movements both increased their discharge with knee joint flexion as could be predicted from their responses to manually applied skin stretch and the skin stretch pattern observed during knee joint movements (Fig. 3C and D). Due to their slow conduction velocities these responses were, however, markedly delayed in relation to the actual movements.
DISCUSSION
The recordings showed that the skin of the human thigh contains an abundance of stretch-sensitive mechanoreceptors that may convey information about knee joint positions and movements. Indeed, with the exception of hair follicle receptors, all mechanoreceptors were capable of conveying proprioceptive information but to different degrees. The most important group with respect to their potential role for proprioception clearly is the slowly adapting receptors; the unknown receptor type corresponding to SA III units seems particularly suited for this task. FA afferents, on the other hand, presumably make a marginal contribution because they showed meagre responses in the tested velocity ranges and seemed to be few in numbers. Finally, it is difficult to see how the C mechanoreceptive afferents - even if they in reality may outnumber all low-threshold mechanoreceptors - may at all contribute to motor control considering the long delays by which their afferent signals reach the central nervous system.
Slowly adapting afferents
SA III afferents have not previously been described as such but they are not unique to the human thigh. The same type of afferents has been recorded from the radial nerve that innervates the back of the human hand (Edin & Abbs, 1991; Edin, 1992). Edin & Abbs (1991) subdivided SA units into type I and II based on multiple criteria but primarily on the distribution of interspike intervals during maintained skin indentations. Although they pointed out that it was ‘non-trivial’ to subdivide SA units into types I and II, the presence of a third type of SA unit was not recognised and the classification problem was considered immaterial to the main purpose of the study. In retrospect, some of the units causing classification problems fitted the description of SA III. The unit illustrated in Fig. 4 of Edin & Abbs (1991), for instance, represents an SA III unit as described in the present study. Edin (1992) in a sense evaded the classification problem by operationally subdividing SA units using a single criteria, namely the distribution of interspike intervals; Grill & Hallet (1995) seem to have taken the same approach but they did not describe the RFs of the SA units with sufficient detail to allow detailed comparisons. Vallbo et al. (1995) recorded afferents from the skin of the human forearm and studied their responses to moving tactile stimuli. They identified SA units type I and II on the basis of multiple criteria, including interspike interval variability. It is not clear if all these SA units fulfilled all criteria for SA type I and II, respectively, or if a subset might have resembled SA III units (see Table 1). Nevertheless, at least in previous studies of afferents originating in the dorsum of the human hand, some units reported as SA II units fit the description of SA III as given in the present study.
The RF characteristics of SA III units suggest that their histological counterpart is located superficially in the skin, presumably at the junction between the dermis and the epidermis, i.e. just like SA I units (Iggo & Muir, 1969). Moreover, the fact that they display an omnidirectional strain sensitivity, i.e. show a similar sensitivity to skin strain in all directions, suggests that they are symmetrical in planes perpendicular to the skin surface.
Regional differences
We still have limited information on the receptor populations in the hairy skin. Vallbo et al. (1995) pointed out that there seems to be regional differences between the back of the hand, the face and the forearm. They suggested that the proportion of afferents and the RF characteristics of the units recorded from the forearm are more representative of the hairy skin that covers the main parts of the body than either facial skin or the skin on the hand dorsum. When comparing the data in the present study with those of Vallbo et al. (1995), it can be concluded, however, that the proportion of hair follicle receptor afferents is higher in the thigh than in the forearm (26/54 vs. 12/55 of myelinated afferents, P= 0.03, Fisher’s exact probability test), that the proportion of SA I afferents is lower in the skin of the thigh than in the forearm (5/23 vs. 21/36 of the SA units, P= 0.06, Fisher’s exact probability test), and that FA I afferents can be recorded from nerves supplying the skin of the thigh but apparently not from the forearm. Thus, it seems as if each hairy skin area in humans has its own unique characteristics; this presumably reflects regional differences in functional roles.
There are large differences in the magnitude of the skin strains associated with angular movements of the knee and the MCP joints because of their different physical dimensions. Accordingly, skin afferents on the thigh showed a dynamic sensitivity almost an order of magnitude larger than reported for both SA afferents on the back of the hand (0.20 vs. 0.033 impulses s−1 (deg s−1)−1 in Grill & Hallett, 1995), and muscle spindle afferents in the long finger extensor muscles (0.027 impulses s−1 (deg s−1)−1 in Edin & Vallbo 1990; Grill & Hallett, 1995). In contrast, the median static sensitivity was close to a conservative indirect estimate of the corresponding sensitivity of SA receptors located 2-3 cm from the MCP joints (0.35 vs. 0.36-0.54 impulses s−1 deg−1 in Edin, 1992; cf. 0.4 impulses s−1 deg−1 in Grill & Hallett, 1995).
Hair follicle receptor afferents
That none of the hair follicle receptors responded to knee joint movements was unexpected. Appenteng et al. (1982) demonstrated that hair follicle receptors in rabbits show vigorous responses during chewing and concluded that these receptors may contribute the ‘perception of jaw movement, but not jaw position’. The most likely explanation for this discrepancy is the marked difference in the density of hair follicles between human and rabbit skin. While hair follicle receptors are exquisitely sensitive to minute movements of the hair shaft also in humans, skin deformations associated with joint movements may simply not be large enough to result in any mechanical interactions between nearby hairs and therefore do not result in activation of the hair follicle receptor. The experimental conditions in the present study did not allow the testing of unitary responses within the full range of physiological knee joint movements. It seems unlikely, however, that the hair follicle receptor afferents would respond to larger amplitude and higher velocities than those tested, given their poor responses to vigorously applied manual skin stretch that mimicked strain changes during very fast joint movements.
Skin afferents and proprioception
The hypothesis that skin mechanoreceptors provide information about skin strain patterns induced by various joint postures and that this information can be used by the CNS to determine joint postures and movements is supported by recent evidence obtained from studies of the human hand and fingers (Edin & Johansson, 1995; Collins & Prochazka, 1996). Although the present study provides strong evidence that cutaneous mechanoreceptors provide high-fidelity information about knee joints movements, it does not address the crucial question of whether or not the human central nervous system also takes advantage of this information. There are, however, clinical data that suggest that it does. Physiotherapists have for a long time claimed that taping a joint improves its ‘stability’. Yet taping is known to hardly make any mechanical contribution to the stability of large joints such as the knee and ankle. Joint stability, however, is not only a result of biomechanical constraints but also of the ability of an organism to appropriately control the muscles acting on the joint. As such, the ‘stabilising’ effect of tapes and braces may be due to altered somatosensory inflow from the skin. Joint movements are necessarily associated with changes in skin strain. When the tape immobilises certain skin areas, movements always cause larger strain than normal in other skin areas. Indeed, several reports claim that taping near the knee joint alone effectively improves a subject’s proprioceptive acuity, whether the subject is healthy (Perlau et al. 1995), has suffered cruciate ligament injuries (Jerosch & Prymka, 1996), or suffers from knee osteoarthrosis (Barrett et al. 1991).
Clark et al. (1979) blocked skin afferents from a ‘15 cm band’ around the knee joint and found that this had little or no effect on the subject’s ability to detect passive knee joint movements. They therefore concluded that skin afferents are relatively unimportant for proprioception. Their conclusion may be correct but it cannot be based on their findings alone because the anaesthesia they applied could only have blocked a small proportion of the skin afferents that respond to knee joint movements (see Fig. 1B).
Proprioceptors - unresolved issues
The present consensus regarding the central role of muscle spindles for proprioception is based mainly on the striking movement illusions that can be evoked in most subjects by muscle vibrations (Goodwin et al. 1972). Several studies prove beyond doubt that muscle spindles somehow are involved in proprioception and motor control (Goodwin et al. 1972; Craske, 1977; Roll & Vedel, 1982; Lackner, 1988; Inglis et al. 1991). It is, however, a complex task to interpret these experiments. For instance, vibrations applied to muscle tendons and bellies evoke phase-locked discharges in muscle spindle afferents at frequencies known to be most effective in eliciting movement illusions, i.e. 80-120 Hz (Roll & Vedel, 1982; Roll et al. 1989), but the discharge rates observed in human muscle spindles at movement velocities similar to illusory movements are about 10 Hz (Hulliger & Vallbo, 1979; Vallbo et al. 1981; Roll & Vedel, 1982; Gilhodes et al. 1986). Movement illusions evoked by muscle vibrations thus seem to require markedly abnormal muscle spindle inputs, i.e. synchronous activation at rates almost an order of magnitude higher than that which can be expected at the perceived movement velocities. Muscle spindles thus provide proprioceptive information but their normal role in providing such information during passive movements may have been exaggerated.
We know that sensory activity among different types of mechanoreceptors is integrated or ‘fused’ to generate the patterns of neural activity that correspond to ‘proprioception’; this has been demonstrated for proprioceptive sensations related to the interphalangeal joints of humans (Gandevia et al. 1983), but is also evident in proprioceptive tasks related to whole body movements (Jürgens et al. 1999). Yet the mechanisms by which such integration may occur are unknown. Moreover, it is largely unknown if and when specific afferent channels are particularly important for proprioception and motor control, respectively. Yet, skin mechanoreceptors, for instance, seem to be particularly important in the human hand (Edin & Johansson, 1995; Collins & Prochazka, 1996) and face (Gracco & Abbs, 1985; Johansson et al. 1988), mechanoreceptors in joint ligaments and capsules may be crucial in extreme, but not necessarily noxious, joint positions (Burke et al. 1988; Edin, 1990), and muscle spindles perhaps for gaze control (Roll et al. 1991). Finally, since sensory activity has to be interpreted in a context of actual motor behaviour, ‘proprioception’ requires integration not only of signals originating in various types of mechanoreceptors but also of centrally generated efferent activity. ‘Proprioception’ and ‘kinaesthesia’ has for technical and other reasons traditionally most often been investigated in a behavioural context where it is particularly unlikely that the central nervous mechanisms for motor control are engaged, i.e. in passive subjects (but see, for instance Cordo et al. 1995; Jürgens et al. 1999). It would be of great theoretical and clinical value to define differences in specific control requirements as well as useful sensory sources in different behavioural contexts, e.g. joint movements in various phases of locomotion, complex arm movements, swallowing and speech production.
Acknowledgments
I would like to thank Andreas Wennström for his technical support. This work was supported by grants from the Swedish Medical Research Council.
References
- Appenteng K, Lund JP, Seguin JJ. Behavior of cutaneous mechanoreceptors recorded in mandibular division of Gasserian ganglion of the rabbit during movements of lower jaw. Journal of Neurophysiology. 1982;47:151–166. doi: 10.1152/jn.1982.47.2.151. [DOI] [PubMed] [Google Scholar]
- Barrett DS, Cobb AG, Bentley G. Joint proprioception in normal, osteoarthritic and replaced knees. Journal of Bone and Joint Surgery. British Volume. 1991;73:53–56. doi: 10.1302/0301-620X.73B1.1991775. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, Macefield G. Responses to passive movement of receptors in joint, skin and muscle of the human hand. Journal of Physiology. 1988;402:347–361. doi: 10.1113/jphysiol.1988.sp017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MR, Andres KH, Duering M, Iggo A. The structure and function of slowly adapting type II mechanoreceptors in hairy skin. Quarterly Journal of Experimental Physiology. 1972;57:417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Horch KW, Bach SM, Larson GF. Contributions of cutaneous and joint receptors to static knee-position sense in man. Journal of Neurophysiology. 1979;42:877–888. doi: 10.1152/jn.1979.42.3.877. [DOI] [PubMed] [Google Scholar]
- Collins DF, Prochazka A. Movement illusions evoked by ensemble cutaneous input from the dorsum of the human hand. Journal of Physiology. 1996;496:857–871. doi: 10.1113/jphysiol.1996.sp021733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordo P, Gurfinkel VS, Bevan L, Kerr GK. Proprioceptive consequences of tendon vibration during movement. Journal of Neurophysiology. 1995;4:1675–1688. doi: 10.1152/jn.1995.74.4.1675. [DOI] [PubMed] [Google Scholar]
- Craske B. Perception of impossible limb positions induced by tendon vibration. Science. 1977;196:71–73. doi: 10.1126/science.841342. [DOI] [PubMed] [Google Scholar]
- Edin BB. Finger joint movement sensitivity of non-cutaneous mechanoreceptor afferents in the human radial nerve. Experimental Brain Research. 1990;82:417–422. doi: 10.1007/BF00231261. [DOI] [PubMed] [Google Scholar]
- Edin BB. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. Journal of Neurophysiology. 1992;67:1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. Journal of Neurophysiology. 1991;65:657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin BB, Bäckström PA, Bäckström LO. Single unit retrieval in microneurography: a microprocessor-based device controlled by an operator. Journal of Neuroscience Methods. 1988;24:137–144. doi: 10.1016/0165-0270(88)90057-x. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. Journal of Physiology. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo Å B. Dynamic response of human muscle spindle afferent to stretch. Journal of Neurophysiology. 1990;63:1297–1306. doi: 10.1152/jn.1990.63.6.1297. [DOI] [PubMed] [Google Scholar]
- Finger S. Origins of Neuroscience. New York: Oxford University Press; 1994. pp. 203–204. [Google Scholar]
- Gandevia SC, Hall LA, McCloskey DI, Potter EK. Proprioceptive sensation at the terminal joint of the middle finger. Journal of Physiology. 1983;335:507–517. doi: 10.1113/jphysiol.1983.sp014547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhodes JC, Roll JP, Tardy-Gervet MF. Perceptual and motor effects of agonist-antagonist muscle vibration in man. Experimental Brain Research. 1986;61:395–402. doi: 10.1007/BF00239528. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PBC. The contribution of muscle afferents to kinesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Gracco VL, Abbs JH. Dynamic control of the perioral system during speech: kinematic analyses of autogenic and nonautogenic sensorimotor processes. Journal of Neurophysiology. 1985;54:418–432. doi: 10.1152/jn.1985.54.2.418. [DOI] [PubMed] [Google Scholar]
- Grill SE, Hallett M. Velocity sensitivity of human muscle spindle afferents and slowly adapting type II cutaneous mechanoreceptors. Journal of Physiology. 1995;489:593–602. doi: 10.1113/jphysiol.1995.sp021075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth KE, Hongell A, Hallin RG, Torebjörk HE. Afferent impulses in median nerve fascicles evoked by tactile stimuli of the human hand. Brain Research. 1970;24:423–442. doi: 10.1016/0006-8993(70)90183-6. [DOI] [PubMed] [Google Scholar]
- Hensel H, Boman KKA. Afferent impulses in cutaneous sensory nerves in human subjects. Journal of Neurophysiology. 1960;23:564–578. doi: 10.1152/jn.1960.23.5.564. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Thelin A-E, Vallbo Å B. The response of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. Journal of Physiology. 1979;291:233–249. doi: 10.1113/jphysiol.1979.sp012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M, Vallbo Å B. The response of muscle spindle afferents during voluntary tracking movements in man. Load dependent servo assistance? Brain Research. 1979;166:401–404. doi: 10.1016/0006-8993(79)90227-0. [DOI] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. Journal of Physiology. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis JT, Frank JS, Inglis B. The effect of muscle vibration on human position sense during movements controlled by lengthening muscle contraction. Experimental Brain Research. 1991;84:631–634. doi: 10.1007/BF00230975. [DOI] [PubMed] [Google Scholar]
- Jerosch J, Prymka M. Knee joint proprioception in normal volunteers and patients with anterior cruciate ligament tears, taking special account of the effect of a knee bandage. Archives of Orthopaedic and Trauma Surgery. 1996;115:162–166. doi: 10.1007/BF00434546. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Trulsson M, Olsson K Å, Abbs JH. Mechanoreceptive afferent activity in the infraorbital nerve in man during speech and chewing movements. Experimental Brain Research. 1988;72:209–214. doi: 10.1007/BF00248519. [DOI] [PubMed] [Google Scholar]
- Jürgens R, Boss T, Becker W. Estimation of self-turning in the dark: comparison between active and passive rotation. Experimental Brain Research. 1999;128:491–504. doi: 10.1007/s002210050872. [DOI] [PubMed] [Google Scholar]
- Knibestöl M, Vallbo Å B. Single unit analysis of mechanoreceptive activity from the human glabrous hand. Acta Physiologica Scandinavica. 1970;80:178–195. doi: 10.1111/j.1748-1716.1970.tb04783.x. [DOI] [PubMed] [Google Scholar]
- Lackner JR. Some proprioceptive influences on the perceptual representation of body shape and orientation. Brain. 1988;111:281–297. doi: 10.1093/brain/111.2.281. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Kinesthetic sensibility. Physiological Reviews. 1978;58:763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- Mackel R. Conduction of neural impulses in human mechanoreceptive cutaneous afferents. Journal of Physiology. 1988;401:597–615. doi: 10.1113/jphysiol.1988.sp017182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Proprioceptors and their contribution to somatosensory mapping: complex messages require complex processing. Canadian Journal of Physiology and Pharmacology. 1988;66:430–438. doi: 10.1139/y88-073. [DOI] [PubMed] [Google Scholar]
- Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. Journal of Physiology. 1990;426:229–240. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin M, Thomander L. Intrafascicular multi-unit recordings from the human infra-orbital nerve. Acta Physiologica Scandinavica. 1989;135:139–148. doi: 10.1111/j.1748-1716.1989.tb08561.x. [DOI] [PubMed] [Google Scholar]
- Perlau R, Frank C, Fick G. The effect of elastic bandages on human knee proprioception in the uninjured population. American Journal of Sports Medicine. 1995;23:251–255. doi: 10.1177/036354659502300221. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man studied by tendon vibration and microneurography. Experimental Brain Research. 1982;47:177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Experimental Brain Research. 1989;76:213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- Roll R, Velay JL, Roll JP. Eye and neck proprioceptive messages contribute to the spatial coding of retinal input in visually oriented activities. Experimental Brain Research. 1991;85:423–431. doi: 10.1007/BF00229419. [DOI] [PubMed] [Google Scholar]
- Sandström G, Båckström A, Olsson K Å. REMAC: a video-based motion analyser interfacing to an existing flexible sampling system. Journal of Neuroscience Methods. 1996;69:205–211. doi: 10.1016/S0165-0270(96)00079-9. [DOI] [PubMed] [Google Scholar]
- Trulsson M, Essick GK. Low-threshold mechanoreceptive afferents in the human lingual nerve. Journal of Neurophysiology. 1997;77:737–748. doi: 10.1152/jn.1997.77.2.737. [DOI] [PubMed] [Google Scholar]
- Vallbo Å, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Research. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s. [DOI] [PubMed] [Google Scholar]
- Vallbo Å B, Hagbarth K-E. Activity from skin mechanoreceptors recorded percutaneously in awake human subjects. Experimental Neurology. 1968;21:270–289. doi: 10.1016/0014-4886(68)90041-1. [DOI] [PubMed] [Google Scholar]
- Vallbo Å B, Hulliger M, Nordh E. Do spindle afferents monitor joint position in man? A study with active position holding. Brain Research. 1981;204:209–213. doi: 10.1016/0006-8993(81)90666-1. [DOI] [PubMed] [Google Scholar]
- Vallbo Å B, Johansson RS. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Human Neurobiology. 1984;3:3–14. [PubMed] [Google Scholar]
- Vallbo Å B, Olausson H, Wessberg J, Kakuda N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. Journal of Physiology. 1995;483:783–795. doi: 10.1113/jphysiol.1995.sp020622. [DOI] [PMC free article] [PubMed] [Google Scholar]


