Abstract
To elucidate the hepatic microvascular response upon the hepatic arterial buffer response (HABR), we analysed blood flow (ultrasonic flowprobes) of the hepatic artery (HA) and portal vein (PV), microcirculation (intravital microscopy), and tissue oxygenation (polarography) in anaesthetized Sprague-Dawley rats and re-evaluated the role of adenosine in mediating the HABR by using 8-phenyltheophylline as a competitive antagonist.
Upon restriction of PV blood flow to 11 ± 3 % of baseline values, HA blood flow increased by a factor of 1.77 (P < 0.05), thus confirming HABR. Strikingly, red blood cell velocity and volumetric blood flow in terminal hepatic arterioles (THAs) did not increase but were even found to be slightly decreased, by 8 and 13 %, respectively. In contrast, red blood cell velocity and volumetric blood flow in terminal portal venules (TPVs) decreased to only 66 % (P < 0.05), indicating upstream hepatic arteriolo-portal venular shunting. As a consequence, red blood cell velocity and volumetric blood flow in sinusoids were found to be reduced to only 66–68 % compared with baseline (P < 0.05). Diameters of neither of those microvessels changed, thus excluding THA-, TPV-, and sinusoid-associated mechanisms of vasomotor control in HABR.
Tissue PO2 and hepatocellular NADH fluorescence remained unchanged, indicating HABR-mediated maintenance of adequate oxygen delivery, despite the marked reduction of total liver blood flow. Further, hepatic arteriolo-portal venular shunting guaranteed homogeneity of nutritive blood flow upon HABR, as given by an unchanged intra-acinar coefficient of variance of sinusoidal perfusion.
Pretreatment of animals with the adenosine antagonist 8-phenyltheophylline completely blocked the hepatic arterial buffer response with the consequence of decreased tissue oxygenation and increased heterogeneity of sinusoidal perfusion.
In conclusion, hepatic microhaemodynamics, in particular unchanged diameters of THAs, TPVs and sinusoids, during HABR indicate that reduction in resistance to HA flow is located upstream and functions via hepatic arteriolo-portal venular shunts resulting in equal distribution of microvascular blood flow and oxygen delivery under conditions of restricted PV blood supply.
Hepatic blood circulation is unique in that the supply comes from both a vein, the portal vein (PV), contributing 70-80 %, and an artery, the hepatic artery (HA), contributing 20-30 % to the total hepatic blood flow (Greenway & Stark, 1971). The intimate relationship between the two systems, as shown by the ability of the HA to produce reciprocal compensatory flow changes in response to changes in PV flow, is termed the ‘hepatic arterial buffer response’ (HABR) (Lautt, 1981) and is implicated in holding total hepatic oxygen consumption constant (Mathie & Blumgart, 1983). Morphologically, the HA and the PV branches run in parallel to each other within the liver and both vessels supply blood to the hepatic sinusoids via their terminal branches (THAs and TPVs). Whereas portal venules end directly in the sinusoids, drainage of hepatic arterioles is highly species dependent. In the rat, hepatic arterioles first support the peribiliary plexus, which then drains into sinusoids finally conducting mixed blood (for review see Ballet, 1990). This complex situation evokes the question of regulation of the HABR with respect to the distinct segments of the hepatic microvasculature.
Adenosine has been repeatedly advocated as the putative mediator together with the space of Mall as the potential site of the mediator-driven communication between the HA and the PV (Lautt et al. 1985). However this view does not include our recently achieved knowledge on the regulation of the hepatic microcirculation by perisinusoidal stellate cells and the actions of various vasoactive substances at both sinusoidal and extrasinusoidal sites (Kawada et al. 1992; Bauer et al. 1994; Suematsu et al. 1995; Rockey, 1995; Rockey & Weisiger, 1996). Therefore, we have analysed in detail the hepatic microvasculature to clarify the site of reduction in resistance to HA flow during HABR using fluorescence microscopy of the rat liver in vivo and re-evaluated the role of adenosine by using 8-phenyltheophylline as a competitive antagonist.
METHODS
Surgical procedure
Experiments were performed on Sprague-Dawley rats (Charles River, Fa. Wiga, Sulzfeld, Germany). They were performed in accordance with the German legislation on protection of animals and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council; NIH Guide, vol. 25, no. 28, 1996). At the end of the experiments, the animals were killed in deep pentobarbital sodium anaesthesia by intravenous injection of saturated potassium chloride solution.
After overnight fasting, but free access to tap water, rats of either sex (body weight (bw) 328 ± 80 g) were anaesthetized with pentobarbital sodium (50 mg (kg bw−1) i.p.; Narcoren; Braun, Melsungen, Germany), and supplemental doses (10 mg (kg bw−1) i.p.) were given during the experiment as required. Tracheotomy was performed to facilitate spontaneous breathing and the animals were placed in a supine position on a heating pad, maintaining body temperature at 36-37 °C. Catheters (PE-50, 0.58 mm i.d.; Portex, Hythe, UK) were placed in the right carotid artery and jugular vein for continuous monitoring of mean arterial blood pressure and heart rate and for fluid substitution. Depth of anaesthesia was maintained according to blood pressure, heart rate and respiration rate as well as lack of withdrawal of paw pinch. After transverse laparotomy, microsurgical preparation for assessment of liver blood flow was performed in a way similar to the method described by Lautt et al. (1985) in cats. An ultrasonic perivascular flow probe (0.5 V; Transonic Systems, Ithaca, NY, USA) was placed around the coeliac artery, and all other branches including the splenic artery, the left gastric artery and the gastroduodenal artery were ligated, so that all blood entering the HA was derived from the coeliac artery. Likewise, a second flow probe (1.5R; Transonic Systems) was positioned around the superior mesenteric artery, which, after ligation of all other inlet arteries to the splanchnic system (inferior mesenteric artery, anastomoses with rectal arteries), conducted blood flow solely representative of the PV. This experimental approach allowed for simultaneous assessment of HA and PV blood flow without the risk of mechanical obstruction or kinking of the referring vessels. The flow probes were connected to a flowmeter (T206 Animal Research Flowmeter, Transonic Systems), and the blood flow data and mean arterial blood pressure were recorded using a computerized data aquisition system (Dasylab; Datalog, Mönchengladbach, Germany). An additional PE-10 catheter was advanced into the PV via the splenic vein for selective portal-venous injection of fluorescent dyes into the hepatic microcirculation, allowing differentiation of afferent vessels as either TPVs or THAs.
Intravital fluorescence microscopy and microcirculatory parameters
After microsurgical instrumentation with placement of the perivascular flow probes, rats were positioned on their left side and the livers were prepared for intravital fluorescence microscopy by placing the left lobe on a plasticine disk held by an adjustable stage that was attached to the heating pad. Thereby, the lower surface of the liver was situated horizontal to the microscope, which guaranteed an adequate homogeneous focus level for the microscopic procedure of the area of liver surface under investigation. In addition, the adjustment of the plasticine disk allowed us to avoid mechanical obstruction of feeding and draining macrovessels and to minimize respiratory movements of the lobe. The exposed area of the left liver lobe was immediately covered with an oxygen-impermeable foil to prevent drying of tissue and the influence of ambient oxygen.
In vivo microscopy was performed using a modified Zeiss Axio-Tech microscope (Zeiss, Oberkochen, Germany) and the epi-illumination technique with a 100 W mercury lamp, as described previously (Vollmar et al. 1999). Microscope images were registered by a charge-coupled device video camera (FK 6990; Prospective Measurements Inc., San Diego, CA, USA) and were transferred to a video system (VO-5800 PS; Sony, Munich, Germany). With the use of a ×10 objective (×10/0.30, Zeiss) or water immersion objective (W ×20/0.5, Zeiss) magnifications of ×350 and ×700 were achieved on the videoscreen (PVM-1442 QM, diagonal: 330 mm, Sony). For contrast enhancement, the plasma marker FITC-dextran (relative molecular mass 150 000; 5 %, 0.1 ml (100 g bw)−1; Sigma) was used and visualized by a blue filter set (450-490 nm/> 520 nm excitation/ emission wavelength) to assess red blood cell velocity within the individual microvessels, i.e. hepatic sinusoids, TPVs and THAs. Volumetric blood flow (VQ) in the individual microvessels was estimated from red blood cell velocity (V) and microvascular cross-sectional area (πr2) according to the equation of Gross & Aroesty (1972), i.e. VQ =Vπr2. The equation is very simplistic inasmuch as a cylindrical shape of the respective microvessel is assumed. Therefore, the values may not accurately reflect the actual flow but allow assessment of relative changes between different interventions within the group. Small bolus injections of FITC-dextran (0.05 ml) via either the carotid arterial or the PV catheter were used to differentiate between THAs and TPVs by assessing the time delay between injection and intravascular appearance of the dye. An ultraviolet filter set (330-390 nm/> 430 nm) allowed - after photobleaching of stellate cell-associated vitamin A - for clear assessment of sinusoidal boundaries at the site of stellate cell location as well as for identification of hepatocellular NADH fluorescence as a measure of mitochondrial redox state (Chance et al. 1962; Vollmar et al. 1997).
Quantification of microhaemodynamic parameters was performed offline by frame-to-frame analysis of the videotaped images using a computer-assisted image analysis system (Cap-Image; Zeintl, Heidelberg, Germany). In each animal, five sinusoidal observation fields were recorded with each filter set at ×700 and red blood cell velocity and diameter in midzonal regions (classification according to Rappaport, 1973) were assessed in a total of five to ten individual sinusoids per observation field. A total of two to five THAs and TPVs per animal were recorded and analysed for diameter and red blood cell velocity. Hepatocellular NADH fluorescence in midzonal regions was densitometrically assessed by computer-assisted grey level determination (CapImage) at the end of a 20 s light exposure of the observation area (Burkhardt et al. 1998).
polarographic tissue pO2 measurements
Hepatic tissue oxygenation was assessed by means of a flexible polyethylene microcatheter, Clark-type PO2 probe (diameter 470 μm, length 300 mm; LICOX System, GMS, Kiel-Mielkendorf, Germany), which was positioned beneath the oxygen-impermeable foil on the liver surface. This allowed the LICOX probe to integrate local tissue PO2 values over the tissue area in contact with the 5 mm long PO2-sensitive area near the catheter tip without interference with the ambient air (polarization voltage of 795 mV). On-line temperature compensation was performed by a temperature probe (type K thermocouple probe; LICOX System) which was also positioned between the hepatic surface and the oxygen-impermeable foil.
Hepatic arterial buffer response
After placing tourniquets (5-0 Ethibond; Ethicon, Norderstedt, Germany) around the superior mesenteric and coeliac artery, reduction of PV blood flow to 10-20 % of baseline values was performed using a micromanipulator-controlled constrictor. Reduced blood flow conditions were kept constant over a 15 min time period for fluorescence microscopic assessment of hepatic microhaemodynamic parameters followed by a sufficient recovery time for regaining baseline haemodynamics. To analyse the reciprocal relationship of the HABR, HA blood flow was reduced to 10-20 % of baseline values for a 15 min period followed by release of the tourniquet. In all animals studied, first HABR was evoked by PV flow restriction, followed by tourniquet release and regaining baseline haemodynamics, then subsequent restriction of the HA blood flow. Intravital fluorescence microscopic analysis of the hepatic microcirculation was performed at each baseline as well as during the 15 min time period of flow restriction of either the PV or HA. Average tissue oxygenation and PV and HA blood flow data were continuously monitored during baseline and the time periods of either PV or HA tourniquets.
In an additional set of experiments (n= 5), animals underwent the protocol described above, but were pretreated with 8-phenyltheophylline (8 mg (kg bw)−1, Sigma), a potent and long-acting competitive antagonist of adenosine (Lautt & Légaré, 1985). 8-Phenyltheophylline was applied into the hepatic artery via a splenic arterial catheter 15 min prior to initiation of HABR.
Statistical analysis
All values are expressed as means ± standard error of the mean (s.e.m.). As a measure of heterogeneity, the coefficient of variance of sinusoidal red blood cell velocity and volumetric blood flow was calculated as standard deviation/mean. Statistically significant differences between baseline values and those obtained during vessel tourniquet were calculated using the Student’s paired t test with P < 0.05 taken as the level of significance. Statistics were performed using the software package SigmaStat (SPSS Inc., Chicago, IL, USA).
RESULTS
Animals exhibited physiological values of mean arterial blood pressure (100-120 mmHg) and heart rate (400- 450 beats min−1) throughout the experimental period without signs of transient awareness due to insufficient anaesthetic depth as assessed by lack of withdrawal of paw pinch.
During baseline conditions, PV and HA inflow ranged between 8.6 ± 2.5 and 9.3 ± 2.5 ml min−1 and 5.0 ± 1.9 and 5.5 ± 2.0 ml min−1, respectively (Fig. 1A and B), resulting in a HA/PV flow ratio of 0.52 ± 0.17. As expected, reduction of PV blood flow to a mean value of 1.0 ± 0.6 ml min−1 (11 ± 3 % of baseline values) resulted in a significant increase of HA inflow averaging 8.9 ± 2.5 ml min−1 (Fig. 1B). Polarographically assessed tissue PO2 was found to be unchanged, with 16.6 ± 2.0 mmHg upon induction of HABR, compared with a baseline value of 14.5 ± 2.1 mmHg (Fig. 2A). In parallel, maintenance of adequate oxygen delivery was indicated by unchanged values of parenchymal NADH fluorescence (Fig. 2B).
Figure 1. Effect of either a portal venous or a hepatic arterial tourniquet on portal venous and hepatic arterial blood flow.

Mean values ±s.e.m. of portal venous (A) and hepatic arterial (B) blood flow (ml min−1) are displayed during baseline (open bars) and application of a tourniquet (filled bars) to either the portal vein (PV) or the hepatic artery (HA) with flow reduction to 10-20 % of baseline values. Paired t test, *P < 0.05vs. baseline.
Figure 2. Effect of either a portal venous or a hepatic arterial tourniquet on hepatic tissue oxygenation.

Mean values ±s.e.m. of hepatic tissue PO2 (mmHg) (A) and hepatocellular NADH fluorescence (arbitrary units, aU) (B) are given during baseline (open bars) and application of a tourniquet (filled bars) to either the portal vein (PV) or the hepatic artery (HA) with flow reduction to 10-20 % of baseline values. Paired t test, *P < 0.05vs. baseline.
In order to localize the site of the reduction of resistance to HA inflow, we monitored the diameter response of TPVs and THAs as well as that of sinusoids using in vivo fluorescence microscopy. None of the vessels significantly changed diameter during the restriction of PV inflow (Table 1). Despite the increase of HA inflow to the liver by a factor of 1.77 (Fig. 1B), red blood cell velocity and volumetric blood flow within THAs were not found to be increased, but even tended to decrease, with mean values of 463 ± 132 μm s−1 and 129 ± 31pl s−1, respectively, when compared with 504 ± 66 μm s−1 and 148 ± 26 pl s−1, respectively, at baseline (Fig. 3A and B). At the same time, TPVs exhibited a reduction of red blood cell velocity and volumetric blood flow to only 66 % (Fig. 4A and B), despite the overall PV inflow reduction to ∼11 % of baseline values (Fig. 1A). Sinusoidal red blood cell velocity (Fig. 5A) and sinusoidal volumetric blood flow (Fig. 5B) were also found to be decreased, to 66-68 % of baseline values prior to HABR. Whereas the interacinar coefficient of variance of both sinusoidal red blood cell velocity (Fig. 6A) and sinusoidal volumetric blood flow (data not shown) showed a statistically significant increase, intra-acinar coefficients of variance remained unchanged (Fig. 6B), indicating a homogeneous perfusion pattern upon PV inflow restriction-induced HABR within individual acini.
Table 1.
Diameters (μm) of terminal hepatic arterioles (THAs), terminal portal venules (TPVs) and sinusoids during baseline and application of a tourniquet to either the portal vein (PV) or the hepatic artery (HA) with flow reduction to 10–20% of baseline values
| Baseline | PV tourniquet | Baseline | HA tourniquet | |
|---|---|---|---|---|
| THAs | 20.7 ± 3.8 | 21.1 ± 4.3 | 19.2 ± 1.6 | 19.4 ± 2.0 |
| TPVs | 25.5 ± 2.3 | 25.6 ± 2.8 | 24.5 ± 2.2 | 24.0 ± 2.1 |
| Sinusoids | 6.77 ± 0.17 | 6.72 ± 0.16 | 6.81 ± 0.19 | 6.77 ± 0.20 |
Data are means ± s.e.m.
Figure 3. Effect of either a portal venous or a hepatic arterial tourniquet on red blood cell velocity and volumetric blood flow in terminal hepatic arterioles.

Means ±s.e.m. of red blood cell velocity (μm s−1) (A) and volumetric blood flow (pl s−1) (B) in terminal hepatic arterioles (THAs) are given during baseline (open bars) and application of a tourniquet (filled bars) to either the portal vein (PV) or the hepatic artery (HA) with flow reduction to 10-20 % of baseline values. Paired t test, *P < 0.05vs. baseline.
Figure 4. Effect of either a portal venous or a hepatic arterial tourniquet on red blood cell velocity and volumetric blood flow in terminal portal venules.
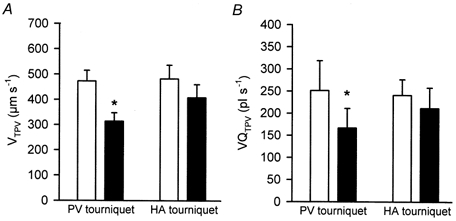
Means ±s.e.m. of red blood cell velocity (μm s−1) (A) and volumetric blood flow (pl s−1) (B) in terminal portal venules (TPVs) are given during baseline (open bars) and application of a tourniquet (filled bars) to either the portal vein (PV) or the hepatic artery (HA) with flow reduction to 10-20 % of baseline values. Paired t test, *P < 0.05vs. baseline.
Figure 5. Effect of either a portal venous or a hepatic arterial tourniquet on hepatic sinusoidal perfusion.

Means ±s.e.m. of red blood cell velocity (μm s−1) (A) and volumetric blood flow (pl s−1) (B) in hepatic sinusoids are given during baseline (open bars) and application of a tourniquet (filled bars) to either the portal vein (PV) or the hepatic artery (HA) with flow reduction to 10-20 % of baseline values. Paired t test, *P < 0.05vs. baseline.
Figure 6. Effect of either a portal venous or a hepatic arterial tourniquet on heterogeneity of sinusoidal perfusion.

Means ±s.e.m. of interacinar (A) and intra-acinar (B) coefficient of variance of sinusoidal red blood cell velocity are given as a measure of heterogeneity (relative dispersion, standard deviation/mean) during baseline (open bars) and application of a tourniquet (filled bars) to either the portal vein (PV) or the hepatic artery (HA) with flow reduction to 10-20 % of baseline values. Paired t test, *P < 0.05vs. baseline.
In the case of pretreatment with 8-phenyltheophylline, HA blood flow did not compensate for restricted inflow of PV blood flow, but was found to be almost unchanged at 103 ± 8 % of baseline values. Concomitantly, volumetric blood flow in hepatic sinusoids fell markedly from 10.4 ± 0.6 to 4.9 ± 0.1 pl s−1 and hepatic tissue PO2 decreased by ∼34 %. Lack of the hepatic arterial buffer response by antagonizing adenosine was further characterized by a considerable heterogeneity of sinusoidal perfusion within the individual acini, as given by the significant (P < 0.01) rise of the coefficient of variance from 0.20 ± 0.01 to 0.49 ± 0.01.
After release of a PV tourniquet and the regaining of baseline haemodynamics, HA inflow was restricted in order to test PV capacity to compensate for reduced HA blood flow. Reduction of HA inflow to 16 ± 5 % of baseline values did not provoke PV flow changes (Fig. 1A). Again, no changes of microvessel diameters could be observed upon restricted HA blood flow (Table 1). Red blood cell velocity and volumetric blood flow were found to be reduced in THAs to only 56 and 59 %, respectively (Fig. 3A and B), and decreased slightly in TPVs (Fig. 4A and B). However, this did not result in adequate oxygen supply to tissue as reflected by a significant decline of tissue oxygenation from 16.8 ± 3.0 to 9.5 ± 1.5 mmHg and an increase of hepatocellular NADH fluorescence (Fig. 2A and B), which is probably due to the marked reduction of the fraction of well-oxygenated (hepatic arterial) blood in sinusoids. Animals pretreated with the adenosine antagonist 8-phenyltheophylline showed identical macrohaemodynamic and microvascular responses upon HA flow restriction as described above in animals without adenosine blockade (data not shown).
DISCUSSION
This study has defined the intrahepatic microvascular consequences of either PV or HA blood flow reduction in the rat. Our results confirm the adenosine-mediated HABR already described in other models (Mathie & Blumgart, 1983; Lautt, 1983, 1985; Lautt et al. 1985) but, more importantly, extend these observations, providing the first evidence (1) that HABR is not associated with changes in microvessel diameters, disproving local modulation of microvascular tone upon acute PV inflow restriction, and (2) that HABR results in maintainence of homogeneity of nutritional microvascular supply using hepatic arteriolo-portal venular shunts.
The magnitude or efficiency of the buffer response varies widely depending on the condition of the animal and on the technique employed. The methodology used here for selective recording of HA and PV blood flow requires splenectomy, which reduces portal venous inflow and might therefore activate the buffer response. Moreover, the simultaneous exterioration of the left liver lobe for intravital microscopy of the hepatic microcirculation might unavoidably lead to some perturbations of liver blood flow, as in part reflected by a higher HA to PV blood flow ratio when compared to values obtained in less invasive experimental approaches.
The hepatic microvasculature consists of four components, i.e. the TPVs, THAs, sinusoids (corresponding to the capillary bed) and postsinusoidal efferent venules, each of which have been postulated to modulate liver blood flow. Pre- and postsinusoidal flow resistance may be controlled by contraction/dilatation of vascular smooth muscle cells surrounding THAs and TPVs as well as postsinusoidal venules. Sinusoidal endothelial (Oda et al. 1990) and perisinusoidal stellate cells (Kawada et al. 1992; Rockey, 1995) have been implicated in the regulation of sinusoidal dimensions and vascular tone. Supplemental to a recent study by Sherman et al. (1996) demonstrating relatively constant diameters of postsinusoidal venules regardless of HA to PV flow ratios, we emphasized with our intravital microscopic approach the analysis of the response of hepatic terminal afferent vessels and sinusoids to HABR. Importantly, these vessels did not change in diameter upon the HABR. Therefore, sites upstream from the terminal afferent vessels have to be considered as effective locations of reduction in resistance to HA blood flow. In favour of that, it has been postulated that the factors responsible for the HA dilatation were acting upstream from both the sinusoids and the HA closing pressure (Ayuse et al. 1994). The complete inhibition of the hepatic arterial buffer response using 8-phenyltheophylline reinforces the concept by Lautt et al. (1985) that intrinsic HABR is primarily mediated by local adenosine concentrations and disproves a pivotal role of other vasoactive substances in effecting the HABR.
The net change of volumetric blood flow in TPV to only 66 % despite an overall PV inflow reduction to ∼11 % of baseline values strongly suggests the existence and function of hepatic arteriolo-portal venular shunts. The existence of these shunts can also be confirmed by intravital microscopy (Fig. 7), although in most acini they are not located at the liver surface but in hepatic tissue not approachable for epi-illumination fluorescence microscopy. From our results showing that increased HA blood flow covers reduced TPV flow to the extent of 54 % of its baseline values, shunt volume can be calculated to be 136 pl s−1. The sum of the volumetric blood flow in THAs (129 pl s−1) and shunt volume (136 pl s−1) is 178 % of baseline volumetric blood flow in THAs, and thus ideally corresponds with the extent of the HABR. Our results indicate that these hepatic arteriolo-portal venular shunts aim at exchanging blood to equalize overall sinusoidal perfusion of the liver even at lower levels (Fig. 8A and B). As such, upon HABR preferential perfusion of those sinusoids which are also supplied by a hepatic arteriolar branch should not occur. In support of this hypothesis, intra-acinar coefficients of variance of sinusoidal red blood cell velocity remained low (0.22 ± 0.02 vs. baseline 0.23 ± 0.02), indicating maintained homogeneity of nutritive perfusion during HABR despite an average reduction of volumetric blood flow in hepatic sinusoids to approximately 67 %. By contrast, under conditions of blockade of HABR by the adenosine antagonist 8-phenyltheophylline, the marked increase of the intra-acinar coefficient of variance of sinusoidal perfusion characteristically displays the dissociation of perfusion of sinusoids solely depending on portal venous blood flow and those being additionally provided by an arterial branch. Whether these shunts operate equally effectively or are present within every single acinus cannot be definitely answered, but the significantly increased interacinar coefficients of variance of sinusoidal red blood cell velocity and volumetric blood flow indicate certain variation of these aspects from acinus to acinus. In line with that, intravital microscopy of dually perfused rat liver preparations revealed that FITC-labelled red blood cells injected into the PV were distributed fairly uniformly within each acinus and between acini regardless of the HA to PV flow ratios, whereas FITC-labelled red blood cells delivered via the HA were found to enter mainly selected acinar regions that constituted 30 % of the total sinusoidal bed (Sherman et al. 1996). Thus, the fact that portal venous flow reduction leads to interacinar, but not intra-acinar heterogeneity of flow strongly suggests that the site of the HABR-mediating mechanism is upstream from the microvasculature of each acinus but downstream from branch points supplying the different acini (Fig. 8A and B). This concept in turn completely fits with the proposed site of action of adenosine, i.e. the space of Mall where the most terminal hepatic vessels are in intimate proximity, and allows us to state that adenosine released from the space of Mall serves to open adjacent shunt channels.
Figure 7.
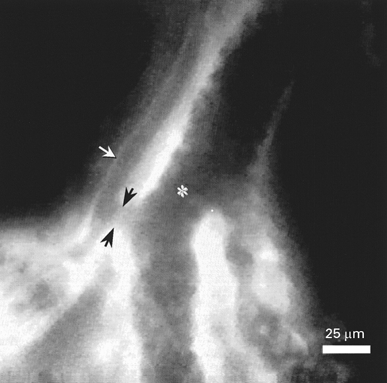
Intravital fluorescence microscopic image of the surface of a normal rat liver displaying the most terminal hepatic arteriole (white arrow) and portal venule (asterisk) running parallel to each other. Note the hepatic arteriolo-portal venular shunt (black arrows) located in the distal part of the terminal afferent vessels, but upstream from the sinusoidal network of the acinus.
Figure 8. Schematic representation of hepatic arterial and portal venous blood supply to sinusoids with indication of the hepatic arteriolo-portal venular shunt under normal conditions (A) and under conditions of portal venous flow reduction with induction of the hepatic arterial buffer response (HABR) (B).

Preferential shunt perfusion upon HABR leads to a disproportionately increased contribution of perfusion of sinusoids with hepatic artery-derived blood, which guarantees maintenance of oxygen supply despite an overall reduction of nutritive blood flow.
Preferential shunt perfusion upon HABR leads to a disproportionately increased contribution of perfusion of sinusoids with hepatic artery-derived blood, which guarantees maintenance of oxygen supply despite an overall reduction of nutritive blood flow.
Despite an overall reduction of liver blood flow upon PV flow restriction, HABR is capable of meeting hepatic tissue oxygen demand, as indicated by maintained values of both tissue PO2 and NADH fluorescence. This can be easily understood because (1) the HA provides a larger relative contribution to basal total hepatic oxygen consumption than to basal total blood flow, (2) the extraction efficiency of oxygen from HA blood is greater than from PV blood, entailing less requirement for HA flow to increase in order to compensate fully for any decrease in PV flow and (3) hepatic oxygen consumption from the HA blood increases as PV flow is reduced with the resultant effect of minimal changes in total hepatic oxygen consumption (Mathie & Blumgart, 1983).
In contrast to the true HABR which made up for a decrease of PV blood flow, a compensation of reduced HA flow by PV flow increase could not be observed, as originally reported by Lautt et al. (1985). Despite the fact that both total liver blood flow and volumetric blood flow in sinusoids were found to be comparably reduced regardless of the restriction of either HA or PV, only the HABR with a disproportionately increased contribution of arterial perfusion of sinusoids can provide sufficient oxygen supply (Fig. 8B), whereas major sinusoidal perfusion by PV blood during HA flow restriction results in deterioration of tissue oxygenation, as given by a decreased tissue PO2 and an increased parenchymal NADH fluorescence.
In conclusion, our results strongly support the idea that there is a site located upstream of the terminal afferent vessels where HABR takes place and which serves to give homogeneous blood flow distribution and adequate oxygen delivery to tissue by hepatic arteriolo-portal venular shunting.
Acknowledgments
This study is supported by a grant of the Deutsche Forschungsgemeinschaft, Bonn-Bad Godesberg (Me 900/1-3 and 900/1-4). B.V. is a recipient of a Heisenberg Stipendium of the Deutsche Forschungsgemeinschaft, Bonn-Bad Godesberg (Vo 450/6-1).
S. Richter and B. Vollmar contributed equally to this work.
References
- Ayuse T, Brienza N, O’Donnell C, Robotham JL. Pressure-flow analysis of portal vein and hepatic artery interactions in porcine liver. American Journal of Physiology. 1994;267:H1233–1242. doi: 10.1152/ajpheart.1994.267.4.H1233. [DOI] [PubMed] [Google Scholar]
- Ballet F. Hepatic circulation: potential for therapeutic intervention. Pharmacology and Therapeutics. 1990;47:281–328. doi: 10.1016/0163-7258(90)90091-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Zhang JX, Bauer I, Clemens MG. ET-1 induced alterations of hepatic microcirculation: sinusoidal and extrasinusoidal sites of action. American Journal of Physiology. 1994;267:G143–149. doi: 10.1152/ajpgi.1994.267.1.G143. [DOI] [PubMed] [Google Scholar]
- Burkhardt M, Vollmar B, Menger MD. vivo analysis of hepatic NADH fluorescence: Methodological approach to exclude Ito cell vitamin A-derived autofluorescence. Advances in Experimental Medicine and Biology. 1998;454:83–89. [PubMed] [Google Scholar]
- Chance B, Cohen P, Jöbsis F, Schoener B. Intracellular oxidation-reduction states in vivo. The microfluorometry of pyridine nucleotide gives a continuous measurement of the oxidation state. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- Greenway CV, Stark RD. Hepatic vascular bed. Physiological Reviews. 1971;51:23–65. doi: 10.1152/physrev.1971.51.1.23. [DOI] [PubMed] [Google Scholar]
- Gross JF, Aroesty J. Mathematical models of capillary flow. A critical review. Biorheology. 1972;9:225–264. doi: 10.3233/bir-1972-9402. [DOI] [PubMed] [Google Scholar]
- Kawada N, Klein H, Decker K. Eicosanoid-mediated contractility of hepatic stellate cells. Biochemical Journal. 1992;285:367–371. doi: 10.1042/bj2850367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautt WW. Role and control of the hepatic artery. In: Lautt WW, editor. Hepatic Circulation in Health and Disease. New York: Raven Press; 1981. pp. 203–226. [Google Scholar]
- Lautt WW. Relationship between hepatic blood flow and overall metabolism: the hepatic arterial buffer response. Federation Proceedings. 1983;42:1662–1666. [PubMed] [Google Scholar]
- Lautt WW. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: hepatic arterial buffer response. American Journal of Physiology. 1985;249:G549–556. doi: 10.1152/ajpgi.1985.249.5.G549. [DOI] [PubMed] [Google Scholar]
- Lautt WW, Légaré DJ. The use of 8-phenyl theophylline as a competitive antagonist of adenosine and an inhibitor of the intrinsic regulatory mechanims of the hepatic artery. Canadian Journal of Physiology. 1985;63:717–722. doi: 10.1139/y85-117. [DOI] [PubMed] [Google Scholar]
- Lautt WW, Légaré DJ, D’Almeida MS. Adenosine as putative regulator of hepatic arterial flow (the buffer response) American Journal of Physiology. 1985;248:Hs331–338. doi: 10.1152/ajpheart.1985.248.3.H331. [DOI] [PubMed] [Google Scholar]
- Mathie RT, Blumgart LH. The hepatic haemodynamic response to acute portal venous blood flow reductions in the dog. Pflügers Archiv. 1983;399:223–227. doi: 10.1007/BF00656719. [DOI] [PubMed] [Google Scholar]
- Oda M, Azuma T, Watanabe N, Nishizaki Y, Nishida J, Ishii K, Suzuki H, Kaneko H, Komatsu H, Tsukada N, Tsuchiya M. Regulatory mechanism of hepatic microcirculation: Involvement of the contraction and dilatation of sinusoids and sinusoidal endothelial fenestrae. In: Messmer K, Hammersen F, editors. Gastrointestinal Microcirculation, Progress of Applied Microcirculation. Vol. 17. Basel, Switzerland: Karger; 1990. pp. 103–128. [Google Scholar]
- Rappaport AM. The microcirculatory hepatic unit. Microvascular Research. 1973;6:212–228. doi: 10.1016/0026-2862(73)90021-6. [DOI] [PubMed] [Google Scholar]
- Rockey DC. Characterization of endothelin receptors mediating rat hepatic stellate cell contraction. Biochemical and Biophysical Research Communications. 1995;207:725–731. doi: 10.1006/bbrc.1995.1247. [DOI] [PubMed] [Google Scholar]
- Rockey DC, Weisiger RA. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology. 1996;24:233–240. doi: 10.1002/hep.510240137. [DOI] [PubMed] [Google Scholar]
- Sherman IA, Dlugosz JA, Barker F, Sadeghi FM, Pang KS. Dynamics of arterial and portal venous flow interactions in perfused rat liver: an intravital microscopic study. American Journal of Physiology. 1996;271:G201–210. doi: 10.1152/ajpgi.1996.271.1.G201. [DOI] [PubMed] [Google Scholar]
- Suematsu M, Goda N, Sano T, Kashiwagi S, Egawa T, Shinoda Y. Carbon monoxide: an endogeneous modulator of sinusoidal tone in the perfused rat liver. Journal of Clinical Investigation. 1995;96:2431–2437. doi: 10.1172/JCI118300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar B, Burkhardt M, Minor T, Klauke H, Menger MD. High-resolution microscopic determination of hepatic NADH fluorescence for in vivo monitoring of tissue oxygenation during hemorrhagic shock and resuscitation. Microvascular Research. 1997;54:164–173. doi: 10.1006/mvre.1997.2028. [DOI] [PubMed] [Google Scholar]
- Vollmar B, Siegmund S, Richter S, Menger MD. Microvascular consequences of Kupffer cell modulation in rat liver fibrogenesis. Journal of Pathology. 1999;189:85–91. doi: 10.1002/(SICI)1096-9896(199909)189:1<85::AID-PATH399>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]


