Abstract
The inwardly rectifying K+ channel current (IK(IR)) recorded from isolated retinal pigmented epithelial (RPE) cells showed poor dependence on external K+ ([K+]o) and low sensitivity to block by Ba2+. We examined the molecular identity and specific subcellular localization of the KIR channel in RPE cells.
The Kir7.1 channel current heterologously expressed in HEK293T cells (human embryonic kidney cell line) showed identical properties to those of the RPE IK(IR), i.e. poor dependence on [K+]o and low sensitivity to Ba2+ block.
Expression of Kir7.1 mRNA and protein was detected in RPE cells by RT-PCR and immunoblot techniques, respectively.
Immunohistochemical studies including electron microscopy revealed that the Kir7.1 channel was localized specifically at the proximal roots of the apical processes of RPE cells, where Na+,K+-ATPase immunoreactivity was also detected.
The middle-distal portions of apical processes of RPE cells in the intact tissue exhibited immunoreactivity of Kir4.1, a common KIR channel. In the isolated RPE cells, however, Kir4.1 immunoreactivity was largely lost, while Kir7.1 immunoreactivity remained.
These data indicate that the only IK(IR) recorded in isolated RPE cells is derived from the functional Kir7.1 channel localized at the root of apical processes. Co-localization with Na+,K+-ATPase suggests that the Kir7.1 channel may provide the pathway for recycling of K+ to maintain pump activity and thus is essential for K+ handling in RPE cells.
The retinal pigment epithelium (RPE) is a single-layered epithelium separating the neural retina and the choroidal capillaries. The control of K+ concentration ([K+]) in the subretinal space between the apical side of the RPE and photoreceptor outer segments is thought to be essential for normal retinal function. The apical membrane of the RPE possesses Na+,K+-ATPase, which transports K+ ions from the subretinal space into RPE cells (Miller & Edelman, 1990; Newman, 1994; Hughes et al. 1998). In addition to Na+,K+-ATPase, electrophysiological studies have indicated that the RPE apical membrane expresses inwardly rectifying K+ (KIR) channels (Newman, 1994; Hughes et al. 1995; Hughes et al. 1998), which may provide the pathway to return K+ to the subretinal space (Miller & Edelman, 1990; Newman 1994; Hughes et al. 1998). Thus far, KIR channels in RPE cells have been studied mainly in freshly isolated RPE cells using the whole-cell patch-clamp technique (Hughes & Steinberg, 1990; Segawa & Hughes, 1994; Hughes & Takahira, 1996). The IK(IR) recorded in isolated RPE cells shows a unique property in that it does not alter significantly as extracellular [K+] ([K+]o) is increased. This property is unusual because the conductance of most KIR channels increases approximately in proportion to the root value of [K+]o (Hagiwara et al. 1976; Standen & Stanfield, 1978). So far the detailed properties of the RPE KIR channel, such as its molecular identity and subcellular localization, have not been examined.
We recently reported that a KIR channel, Kir4.1, is functionally expressed and localized at the apical processes of RPE cells (Kusaka et al. 1999). The current through theKir4.1 channel recorded in the cell-attached membrane patch configuration on the apical side of intact RPE sheets became larger as the [K+]o was increased and was effectively blocked by submillimolar concentrations of Ba2+. Therefore, the Kir4.1 channel current did not share the characteristics of the whole-cell IK(IR) recorded from isolated RPE cells. Recently, a novel KIR channel, Kir7.1, was cloned (Krapivinsky et al. 1998; Döring et al. 1998). It was reported that the Kir7.1 channel shows poor dependence on [K+]o and low sensitivity to Ba2+ block. In this study, therefore, we examined the possibility that Kir7.1 is expressed and responsible for the unique property of whole-cell IK(IR) in isolated RPE cells, using electrophysiological, molecular biological and immunological techniques.
METHODS
Preparation of isolated retinal pigment epithelial cells
All experiments were carried out in accordance with the Guidelines for the Use of Laboratory Animals of Osaka University Graduate School of Medicine and with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The RPE cells were isolated from eyes of neonatal Long-Evans rats of 10-14 postnatal days (Nippon Doubutsu, Kyoto, Japan) using a method described by Ueda & Steinberg (1993) with modifications. In brief, animals were anaesthetized by intraperitoneal injection of an overdose of pentobarbital (100 mg (kg body weight)−1) and the eyes were enucleated. The rat eyeballs were incubated for ∼60 min at 37°C in Ca2+-, Mg2+-free Hanks’ balanced salt solution (HBSS; Gibco BRL, Grand Island, NY, USA) containing collagenase type IV (0.5 mg ml−1; Worthington, Lakewood, NJ, USA). Then, the eyeballs were incubated in HBSS containing 0.05 % trypsin and 0.53 mM EDTA (Gibco BRL) for 40-50 min at 37°C. After the second incubation, the eyeballs were transferred to and rinsed well in a solution (bathing solution A) containing (mM): 136 NaCl, 5.4 KCl, 1.8 CaCl2, 0.53 MgCl2, and 5 Hepes-NaOH, pH 7.4. Under a microscope, a circumferential incision was made just below the ora serrata, and the retina-RPE complex was carefully extracted. Then, sheets of RPE were gently removed from the retina. The sheets of RPE were incubated in HBSS containing 0.05 % trypsin for ∼2 min at 37°C. The RPE cells were transferred to and washed well in bathing solution A and isolated mechanically by repeated gentle suction and trituration with a Pasteur pipette. The RPE cells were kept in bathing solution A at 4°C until use.
Transfection of Kir7.1 into HEK293T cells
Rat Kir7.1 cDNA was subcloned into the expression vector, pcDNA3 (Invitrogen, San Diego, CA, USA) and transfected with LipofectAMINE Plus Reagent (Gibco BRL) into HEK293T cells (human embryonic kidney cell line) as described previously (Horio et al. 1997). Two days after transfection, the cells were used for immunocytochemistry, immunoblotting or electrophysiological recording.
Electrophysiological recordings
Whole-cell currents of isolated RPE cells and HEK293T cells transfected with rat Kir7.1 were measured at room temperature using a patch-clamp amplifier (Axon 200A, Axon Instruments Inc., Foster City, CA, USA) and recorded on videocassette tapes using a PCM converter system (VR-10B, Instrutech Corp., New York, NY, USA). The tips of the patch electrodes were coated with Sylgard (Dow Corning) and fire polished. The tip resistance of the electrodes was 3-5 MΩ. For analysis, data were reproduced, low-pass filtered at 1 kHz (-3 dB) by an 8-pole Bessel filter (Frequency Devices, Haverhill, MA, USA), sampled at 5 kHz, and analysed off-line on a computer (PowerMac G3, Apple Computer Inc., Cupertino, CA, USA) using commercially available software (Patch Analyst Pro, MT Corporation, Nishinomiya, Hyogo, Japan). The bathing solution contained (mM): 136 NaCl, 5.4 KCl, 1.8 CaCl2, 0.53 MgCl2, and 5 Hepes-NaOH, pH 7.4, and the pipette solution contained (mM): 140 KCl, 3 K2ATP, 1 MgCl2, 1 CaCl2, and 5 Hepes-KOH, pH 7.4. The concentration of K+ in the bathing solution was varied by replacing NaCl with equimolar KCl. Data are expressed as means ± s.e.m.
PCR amplification of Kir7.1 cDNA
Sheets of RPE cells were isolated as described previously (Kusaka et al. 1999). Total RNA of a freshly dissociated sheet of RPE cells was extracted with RNeasy Kit (Quiagen, Stanford, CA, USA) and reverse transcribed with Superscript II (Gibco BRL) according to the manufacturer’s protocol. Polymerase chain reaction (PCR) was performed with the cDNA and primer pair specific for Kir7.1; 5′-TCTCCTTCTCTCTGGAGACACAA (329-351) and 3′-AAGACT- GTTCCTGAACATCCAAC (958-980) (Döring et al. 1998). PCR amplification was performed for 30 cycles at 95°C for 45 s, 55°C for 45 s and 72°C for 60 s followed by 72°C for 8 min. The products were electrophoresed on a 1 % agarose gel. The nucleotide sequence of the amplified PCR products was confirmed using dye-primer method and DNA sequencer (A-381, The Perkin-Elmer Corp., Foster City, CA, USA) after TA cloning (TA Cloning Kit, Invitrogen, Carlsbad, CA, USA).
Immunological studies
A polyclonal antibody specific for rat Kir7.1 (anti-Kir7.1-1H1) was raised in rabbit against a synthetic peptide corresponding to amino acid residues 345-361 (NGQSIDNFQIAETGLTE) in the C-terminal region of rat Kir7.1. The rabbits were killed at the end of the experiment by intravenous injection with an overdose of sodium pentobarbital. A polyclonal antibody for Kir4.1 (anti-KAB-2C2), which recognizes the C-terminal region of rat Kir4.1, was also used (Ito et al. 1996). Both antibodies were purified through protein A-coupled (Seikagaku Corp., Tokyo, Japan) and antigenic peptide-coupled Sulfolink coupling resins (Pierce, Rockford, IL, USA). Mouse monoclonal antibodies against rabbit Na+,K+-ATPase α1-subunit and pan cytokeratin were purchased from Upstate Biotechnology Inc. (clone C464.6; Lake Placid, NY, USA) and Sigma (clone C11; St Louis, MO, USA), respectively.
HEK293T cells transfected with Kir7.1 or freshly isolated RPE cells were fixed with 4 % (w/v) paraformaldehyde in 0.1 M sodium phosphate (PA solution), pH 7.4. Wistar rats weighing about 250 g were deeply anaesthetized with pentobarbital (100 mg kg−1i.p.) and perfused with 100 ml PBS and then with 250 ml PA solution. Eyes and brains were dissected, postfixed with PA solution for 48 h, dehydrated with 30 % (w/v) sucrose solution and then frozen with O.C.T. compound (Tissue-Tek, Sakura Finetechnology Inc., Tokyo, Japan). Sections (12 μm) were cut on a cryostat and thaw mounted on gelatin-coated slides. Fresh frozen sections (12 μm) fixed in ice-cold anhydrous methanol for 10 min were also prepared for the detection of Na+,K+-ATPase α-subunit.
Samples were washed twice with PBS containing 0.1 % Triton X-100 (PBST) for 5 min each, treated with 5 % (w/v) goat serum and 5 % (w/v) bovine serum albumin in PBST (IH solution) at room temperature for 60 min, and then incubated with their first antibody, i.e. anti-Kir7.1 (0.5 μg ml−1), anti-KAB-2C2 (0.15 μg ml−1), anti-Na+,K+-ATPase α1-subunit (5 μg ml−1) or anti-pan cytokeratin (45 μg ml−1) in the IH solution at 4°C overnight. The samples were washed three times with PBST at room temperature for 30 min each and exposed to their second antibody, i.e. fluorescein isothiocyanate (FITC)-labelled anti-rabbit IgG (EY Laboratories, San Mateo, CA, USA) or Texas Red-labelled anti-mouse IgG (Protos Immunoresearch, San Francisco, CA, USA). For experiments in which anti-Na+,K+-ATPase antibody was used, FITC-labelled anti-mouse IgG (EY Laboratories) and anti-rabbit IgG (Alexa Fluor 568; Molecular Probes, Eugene, OR, USA) were used. The samples were examined with a confocal microscope (MRC-1024, Bio-Rad, Hertfordshire, England). Specificity of the anti-Kir7.1 antibody (1H1) was confirmed with antibody which was preincubated with a 100-fold amount of antigenic peptides.
Membrane fractions of RPE sheet, sensory retina, choroid plexus, cerebellum and cerebrum from Long-Evans rats of 10-14 postnatal days and that of HEK cells were prepared as described previously (Inanobe et al. 1995). The membrane proteins were separated by SDS-polyacrylamide gel (11 %) electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes. The PVDF membranes were overlaid with anti-Kir7.1 (1H1) at a concentration of 0.2 μg ml−1 in buffer A containing 5% (w/v) skimmed milk and 0.2% (w/v) Lubrol PX in 50 mM Tris-HCl (pH 8.0) and 80 mM NaCl. After being washed three times with buffer A for 10 min each, the membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (EnVision Plus; Dako, Carpinteria, CA, USA) diluted to 1:2000 (v/v) in buffer A, followed by three washes with 2 % Lubrol PX and 0.2 % (w/v) SDS in 50 mM Tris-HCl (pH 8.0) and 150 mM NaCl. Immunoreactive bands were developed with ECL Plus chemiluminescence immunostaining kit (Amersham Pharmacia Biotech, Buckinghamshire, UK) according to the manufacturer’s protocol.
Electron microscopy
Immunogold electron microscopy was performed as described previously (Fujita et al. 1999). In brief, Wistar rats weighing about 250 g were deeply anaesthetized with pentobarbital (100 mg kg−1i.p.) and perfused with the following phosphate-buffered (1) and bicarbonate-buffered (2) fixatives: (1) 4 % paraformaldehyde in 0.1 M phosphate buffer, pH 7.4; (2) 4 % paraformaldehyde, pH 6.0, followed by 4 % paraformaldehyde, pH 10.5 (pH shift protocol; 0.2 % picric acid was added to both solutions). After fixation small blocks of the posterior segment of the eye were cryoprotected by immersion in graded concentrations of glycerol (10, 20, 30 %) in phosphate buffer and plunged into liquid propane (-170°C) in a cryofixation unit (KF 80; Reichert, Wien, Austria). The samples were then immersed in 1.5 % uranyl acetate dissolved in anhydrous methanol (-90°C) in a cryosubstitution unit (AFS; Reichert). The temperature was raised in steps of 4°C h−1 to -45°C. Samples were washed with anhydrous methanol and infiltrated with Lowicryl HM20 resin to methanol. Polymerization was carried out with UV light (360 nm) for 48 h.
Ultrathin sections were cut with a Reichert ultramicrotome, mounted on nickel grids and processed for immunogold cytochemistry as described by Matsubara et al. (1996). Briefly, the sections were treated with a saturated solution of NaOH in absolute ethanol (2-3 s), rinsed in phosphate buffer and incubated sequentially in the following solutions (at room temperature): (1) 0.1 % sodium borohydride and 50 mM glycine in Tris-buffer (5 mM) containing 0.1 % Triton X-100 and 50 mM NaCl (TBST; 10 min); (2) 2 % human serum albumin in TBST (10 min); (3) primary antibody (anti-Kir7.1, 1.5 μg ml−1; anti-Kir4.1, 1.5 μg ml−1) diluted in the solution used in the preceding step (2 h); (4) same solution as in step (2) for 10 min; (5) gold-conjugated Fab fragment (10 min; EM.GFAR10; BioCell Research Laboratories, Cardiff, UK), diluted 1:20 in TBST containing human serum albumin and polyethylene glycol (0.5 mg ml−1, 2 h). Finally, the sections were counterstained and examined with a Hitachi 7100-α electron microscope.
RESULTS
Whole-cell currents recorded from RPE cells and HEK293T cells transfected with Kir7.1
Whole-cell currents of isolated rat RPE cells (Fig. 1Ab) were recorded in the presence of various concentrations of extracellular K+ ([K+]o) (Fig. 1A). Under whole-cell voltage clamp, voltage steps from a holding potential of −30 mV elicited currents which were essentially time independent. The current-voltage relationship of isolated RPE cells showed a weak inward rectification irrespective of [K+]o (Fig. 1Ac). The slope conductance relative to the cell capacitance of RPE cells in the range −130 to −90 mV was 0.14 ± 0.03 nS pF−1 (n = 11), in the presence of 5.4 mM [K+]o. When [K+]o was increased to 14.7, 47 and 147 mM, the slope conductance relative to the cell capacitance was 0.12 ± 0.02, 0.13 ± 0.01 and 0.15 ± 0.01 nS pF−1 (n = 11), respectively. Thus, the slope conductance of the whole-cell current showed poor dependency on [K+]o (Fig. 1Ac). In bathing solution containing 5.4 mM [K+]o, the total membrane current crossed the zero-current level at -61.6 ± 6.9 mV (n = 13). When measured in bathing solutions containing 14.7, 47 and 147 mM [K+]o, the reversal potential (Vrev) was -41.6 ± 5.9, -22.0 ± 6.4 and 1.3 ± 6.2 mV, respectively (n = 11). Thus, Vrev of the total membrane currents shifted positively as [K+]o was raised. The slope of the Vrevversus [K+]o relationship was approximately 46 mV decade−1 (n = 11). This value is slightly smaller than the 58 mV decade−1 predicted for an ideally K+-selective membrane.
Figure 1. Whole-cell recordings from freshly isolated rat retinal pigment epithelial (RPE) cells and HEK293T cells transfected with Kir7.1.
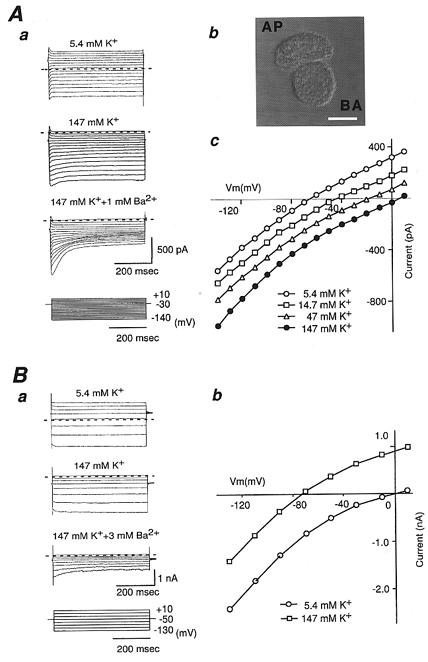
A, whole-cell currents were recorded from a freshly isolated rat RPE cell in response to voltage-clamp steps from the holding potential (-30 mV) to voltages between −140 mV and +10 mV (Δ = 10 mV) in 5.4, 14.7, 47 and 147 mM [K+]o. Aa, whole-cell currents in 5.4 and 147 mM [K+]o and 147 mM [K+]o in the presence of 1 mM Ba2+. Horizontal dashed lines mark the zero-current level. Whole-cell currents were partially blocked by 1 mM Ba2+. Ab, phase-contrast photograph of an isolated RPE cell. AP, apical membrane; BA, basolateral membrane. Scale bar = 10 μm. Ac, I-V relationships of the steady-state currents measured isochronally between 450 and 480 ms of 500 ms pulse. Note that the conductance of these currents shows poor dependency on [K+]o. Ba, whole-cell currents were recorded from HEK293T cells transfected with Kir7.1 in response to voltage-clamp steps from the holding potential (-50 mV) to voltages between −130 mV and +10 mV (Δ = 20 mV) in 5.4, 147 mM [K+]o, and 147 mM [K+]o in the presence of 3 mM Ba2+. Horizontal dashed lines mark the zero-current level. Bb, steady-state I-V relationships from the recordings in Ba. Current was plotted as the mean current measured isochronally between 450 and 480 ms of 500 ms pulse. Note that these curves show weak inward rectification and that the conductance does not increase in proportion to the increase in [K+]o.
When 1 mM Ba2+ was added to bathing solution containing 147 mM K+, inward and outward currents were both partially blocked in a voltage- and time-dependent manner (Fig. 1Aa). The inward current at the end of the voltage step to −110 mV was blocked to 69.3 ± 2.2 % of the control by 1 mM [Ba2+]o (n = 4). As the RPE IK(IR) was thus poorly sensitive to Ba2+ block, the Vrev of IK(IR) could not be measured accurately. Instead, in this study, the potential at the zero-current level of total whole-cell current was used as a measure of Vrev. The Vrev was therefore determined not only by the RPE IK(IR) but also by ‘leak’ currents. Taking this into account, it seems reasonable to conclude that the whole-cell current whose Vrev showed 46 mV decade−1 alteration for [K+]o change is mainly carried by K+. These features of the K+ dominant whole-cell RPE cell current, i.e. poor dependence on [K+]o and poor sensitivity to Ba2+, are similar to those reported for currents through Kir7.1 channels (Krapivinsky et al. 1998; Döring et al. 1998).
We therefore examined the properties of rat Kir7.1 channel currents expressed in HEK293T cells (Fig. 1B). In the presence of 5.4 or 147 mM [K+]o, voltage-clamp steps from a holding potential of −50 mV elicited currents which showed weak inward rectification. The Vrev in 5.4 and 147 mM [K+]o was -71.1 ± 6.7 and 3.6 ± 4.2 mV, respectively (n = 9). The slope of the Vrevversus [K+]o relationship was approximately 53 mV decade−1 (n = 9). The slope conductance relative to the cell capacitance measured over the range −130 to −90 mV was -1.10 ± 0.12 nS pF−1 with 5.4 mM [K+]o and 0.78 ± 0.05 nS pF−1 with 147 mM [K+]o (n = 9). Thus, the conductance of Kir7.1 channels expressed in HEK293T cells showed a poor dependence on [K+]o. When 3 mM Ba2+ was added to the bathing solution, the current through the Kir7.1 channel was partially blocked in a voltage- and time-dependent manner. The current was almost completely blocked by 30 mM [Ba2+]o (not shown). The EC50 for Ba2+ block at −110 mV was ∼3 mM (n = 4). These properties of the Kir7.1 channel current expressed in HEK293T cells were very similar to those of the K+ dominant RPE whole-cell current.
Expression of Kir7.1 mRNA and protein in RPE cells
To examine whether Kir7.1 channels are expressed in RPE cells, we performed RT-PCR analysis of the total mRNA obtained from rat RPE sheets (Fig. 2A). A single band of DNA with the expected size (682 bp) was amplified from the RPE. The product was identified as Kir7.1 with sequencing. No band was detected from the cDNA of the sensory retina or distilled water (negative control).
Figure 2. Expression of Kir7.1 mRNA in RPE cells and immunological analysis of Kir7.1.

A, RT-PCR amplification of Kir7.1 cDNA from a sheet of RPE cells. Kir7.1 fragments (682 bp) were amplified from cDNA/mRNA from the RPE cells (RPE). Retina, rat sensory retina; -, negative control (distilled water instead of cDNA); +, positive control (rat Kir7.1 cDNA). Numbers on the right indicate the positions of molecular weight markers in base pairs. Ba, immunoblot analysis of an affinity-purified, polyclonal rabbit antibody against the C-terminal region of rat Kir7.1. Membrane fractions obtained from HEK293T cells with (lanes 1 and 3) or without (lane 2) transfection of Kir7.1 were separated by SDS-PAGE, transferred to PVDF membranes, and then immunoblotted with the anti-Kir7.1 antibody. In lane 3 the antibody was preincubated with antigenic peptide. Numbers on the left indicate the positions of molecular mass markers in kilodaltons. Immunolabelling was blocked by preincubation with the antigenic peptides. Bb, immunofluorescence images of HEK293T cells transfected with Kir7.1 obtained with anti-Kir7.1 antibody followed by FITC-conjugated anti-rabbit IgG (green) and analysed with confocal microscopy. Nuclei staining was obtained with propidium iodide (red). Scale bar = 10 μm. Ca, membrane fractions obtained from rat RPE cells (RPE), the sensory retina (Retina), choroid plexus (CP), cerebellum and cerebrum were separated by SDS-PAGE, transferred to PVDF membranes, and then immunoblotted with the anti-Kir7.1 antibody. Numbers on the left indicate the positions of molecular mass markers in kilodaltons. Cb, double staining of a sagittal section of rat brain with anti-Kir7.1 antibody followed by FITC-conjugated anti-rabbit IgG (green) and monoclonal anti-pan cytokeratin antibody followed by Texas Red-labelled anti-mouse IgG (red). Cc, a Nomarski image of the same sagittal section as in Cb. Scale bar = 50 μm.
To further analyse the expression of Kir7.1 protein in RPE cells, we first developed a rabbit polyclonal antibody against rat Kir7.1. The antibody was affinity-purified and evaluated by immunoblot and immunocytochemical experiments using HEK293T cells transfected with Kir7.1. In immunoblot analysis (Fig. 2Ba), the antibody detected one major band at 52 kDa and several minor bands migrating faster in the membrane fraction of HEK293T cells expressing Kir7.1 (lane 1). No signal could be developed in the lane loaded with control cell membrane (lane 2). Preincubation of the antibody with antigenic peptide prevented the detection of any immunoreactivity (lane 3). The major band migrated much slower than Kir7.1 with the expected size (40.6 kDa), and several additional bands were detected by the antibody. Nakamura et al. (1999) also reported that in immunoblot analysis a 54 kDa band was detected by antisera to Kir7.1 in rat thyroid and small intestine. This is possibly due to post-translational modifications, such as glycosylation and phosphorylation, since Kir7.1 possesses a potential N-glycosylation site, Asn-His-Thr at residues 95-97, and protein kinase C phosphorylation sites, Ser-Gln-Arg at residues 14-16, Ser-Ile-Arg at residues 169-171, and Ser-Val-Arg at residues 201-203.
In immunocytochemical analysis, immunoreactivity to the antibody was clearly detected in HEK293T cells transfected with Kir7.1 cDNA (Fig. 2Bb) but not in those without transfection (not shown). These data suggest that the affinity-purified polyclonal rabbit IgG antibody for rat Kir7.1 can specifically recognize the Kir7.1 protein and can be used for immunoblot and immunocytochemical analyses.
Figure 2Ca shows the immunoblot analysis of Kir7.1 proteins using this antibody in the membrane fractions of rat tissues such as RPE, sensory retina, choroid plexus, cerebellum and cerebrum. A single band of ∼52 kDa could be detected in the RPE and choroid plexus (CP) but not in the neuroretina, cerebellum or cerebrum (Döring et al. 1998; Nakamura et al. 1999). In Fig. 2Cb and Cc, we further confirmed that the Kir7.1 immunoreactivity was detected specifically in the apical membrane of the choroid plexus. No immunoreactivity was observed after preincubation of the anti-Kir7.1 antibody with antigenic peptide (not shown).
Confocal image and immunogold electron microscopy analyses of Kir7.1 immunoreactivity in the RPE cells
Figure 3A depicts the distributions of immunoreactivities of Kir4.1, Kir7.1 and Na+,K+-ATPase in sections of Wistar rat retina. Kir4.1 immunoreactivity was detected in the photoreceptor outer segment layer (OS), where apical processes of RPE cells and photoreceptor outer segments are localized (see the schema in Fig. 3A). On the other hand, Kir7.1 immunoreactivity was detected in the proximal region of OS and apical side of RPE cells. The immunogold electron microscopic study further showed that many gold particles for Kir4.1 were detected on the middle-distal portions of apical processes (Fig. 3Ba, Kir4.1) as reported previously (Kusaka et al. 1999). In contrast, gold particles for Kir7.1 were not detected at all on the cell membrane in the middle-distal portions of apical processes (Fig. 3Ba, Kir7.1), while they were clearly detected on the cell membrane in the root portion and bottom of the apical processes (Fig. 3Bb). No gold particles were detected on the basolateral membrane of RPE cells for both Kir4.1 (not shown) and Kir7.1 (Fig. 3Bc). Thus, within the apical processes of RPE, Kir4.1 and Kir7.1 were differently distributed. Furthermore, double staining of retinal sections using anti-Kir7.1 (green) and anti-Na+,K+-ATPase (red) antibodies produced prominent yellow signals at the proximal portion of processes and apical membrane (Fig. 3A), which strongly suggests that Kir7.1 and Na+,K+-ATPase are localized in close vicinity.
Figure 3. Immunohistochemical and immunoelectron microscopy analyses of Kir4.1 and Kir7.1 in the retina.

A, a 12 μm sagittal section from a paraformaldehyde-fixed rat retina was stained with affinity-purified anti-Kir4.1 antibody (left) and affinity-purified anti-Kir7.1 antibody (middle) followed by FITC-conjugated anti-rabbit IgG (green). Kir4.1 is expressed mainly in the apical processes of RPE cells. In contrast, Kir7.1 is expressed mainly in the apical membrane, especially at the root of apical processes of RPE cells. The right panel shows double staining of a sagittal section of methanol-fixed rat retina with anti-Kir7.1 antibody followed by anti-rabbit IgG (Alexa Fluor 568; red) and monoclonal anti-Na+,K+-ATPase α1-subunit antibody followed by FITC-labelled anti-mouse IgG (green). ONL, outer nuclear layer; OS, outer segment layer; RPE, retinal pigment epithelial cell layer. Scale bars = 10 μm. B, ultra-thin sections were stained with anti-Kir4.1 antibody or anti-Kir7.1 antibody, and anti-rabbit IgG coupled to colloidal gold particles. The portions from which electron microscope images were obtained are indicated as a, b and c in the schematic drawing on the right. Positive gold particles (arrows) coupled to Kir4.1 antibodies were detected on the membranes of middle-distal portions of apical processes (a, Kir4.1). In contrast, positive gold particles (arrows) coupled to Kir7.1 antibodies were not detected at all on the middle of the processes (a, Kir7.1). They were abundantly detected on the root of apical processes (b, Kir7.1). Gold particles coupled to either Kir4.1 or Kir7.1 antibodies were not detected on the basolateral membrane (c, Kir7.1). OS, photoreceptor outer segment; AP (in a), apical process; P (in b), apical process; F, basal infoldings; B, basement membrane of RPE. Scale bars = 200 nm.
Immunoreactivity of Kir7.1 and Kir4.1 in enzymatically isolated RPE cells
Although the inward-rectifier K+ channel Kir4.1 exists in the middle-distal portions of apical processes of RPE cells, the whole-cell current recorded from freshly isolated RPE cells exhibited only the properties of the Kir7.1 channel current (Fig. 1A). Therefore, we next looked for the immunoreactivity of Kir7.1 and Kir4.1 in isolated RPE cells.
Figure 4 depicts isolated RPE cells, which had been prepared in the same manner as for patch-clamp recording. Many isolated RPE cells seemed to have lost their long apical processes. The immunoreactivities to Kir7.1, Kir4.1, and Na+,K+-ATPase antibodies were examined in these cells. Strong immunoreactivity of Kir7.1 (green) was localized at the apical side of RPE cells (Fig. 4A). The immunoreactivity of Na+,K+-ATPase (red) was detected in the same region (Fig. 4B). On the other hand, the immunoreactivity of Kir4.1 (green) was much less remarkable or undetectable in most RPE cells examined (Fig. 4C and D). Therefore, it seems likely that during the isolation procedure the RPE cells lost most of the middle-distal portions of apical processes where Kir4.1 channels were localized.
Figure 4. Immunocytochemical analyses of Kir4.1 and Kir7.1 in enzymatically isolated RPE cells.

Enzymatically isolated rat RPE cells were stained with affinity-purified anti-Kir7.1 (A, green) or Kir4.1 (C and D, green) antibody followed by FITC-conjugated anti-rabbit IgG and monoclonal anti-α1-subunit of Na+,K+-ATPase antibody followed by Texas Red-labelled anti-mouse IgG (B, red). Immunoreactivity of Kir7.1 and Na+,K+-ATPase was observed in the apical membrane of RPE cells. In contrast, immunoreactivity of Kir4.1 was remarkably weak in isolated RPE cells that seemed to have lost most of their apical processes. AP, apical membrane; BA, basolateral membrane. Scale bar = 10 μm.
DISCUSSION
The major findings of this study are as follows. (1) Whole-cell currents of freshly enzymatically isolated RPE cells were comprised mainly of a IK(IR) whose properties were similar to those of Kir7.1 channel current. (2) Expression of Kir7.1 in RPE cells was confirmed at mRNA and protein levels using RT-PCR, immunoblotting and immunostaining techniques. (3) Kir7.1 immunoreactivity was localized at the root-bottom portions of apical processes of RPE cells, where Na+,K+-ATPase immunoreactivity was also detected. (4) Enzymatically isolated RPE cells almost completely lost their middle-distal portions of apical processes where Kir4.1 channels were localized.
The unique IK(IR) recorded in isolated RPE cells flows through Kir7.1 channels
In a previous report (Kusaka et al. 1999), we showed that Kir4.1 channels are expressed and localized in the apical processes of RPE cells. In single-channel recordings from the apical side of intact RPE sheets, we could detect only one type of KIR channel whose properties were identical to those of Kir4.1 channels. The unitary conductance of the Kir4.1 channel increases in proportion to the root value of [K+]o and the channel current is effectively blocked by external Ba2+, which is common for most KIR channels (Hagiwara et al. 1976; Standen & Stanfield, 1978; Takumi et al. 1995; Tada et al. 1998). As the IK(IR) recorded in the isolated RPE cells exhibits poor dependence on [K+]o and low sensitivity to block by Ba2+ (Hughes & Steinberg, 1990; Wen et al. 1993; Segawa & Hughes, 1994; Hughes & Takahira, 1996; present study), the Kir4.1 channel could not explain the unique isolated RPE cell KIR channel current.
The properties of the isolated RPE cell KIR channel current were very similar to those reported for the Kir7.1 channel current (Krapivinsky et al. 1998; Döring et al. 1998), which was confirmed in this study. We showed that Kir7.1 channels are actually expressed in RPE cells. Expression of Kir7.1 in bovine RPE has also been reported recently (Yuan et al. 2000). We further showed that Kir7.1 channels are localized specifically at the root-bottom portions of apical processes, and that in enzymatically isolated RPE cells Kir7.1 channels are retained while Kir4.1 channels are mostly lost, probably because the middle-distal portions of apical processes are torn away during isolation.
Taken together, it may be reasonable to conclude that the IK(IR) in isolated RPE cells flows through Kir7.1 channels at the apical membrane. Probably because the single-channel conductance of Kir7.1 is too small (∼50 fS with 150 mM [K+]o) to be detected with the single-channel recording technique (Krapivinsky et al. 1998), we could not observe currents from Kir7.1 channels in previous cell-attached patch-clamp recordings from the apical side of intact RPE sheets (Kusaka et al. 1999).
Segregated distribution of Kir7.1 and Kir4.1 channels within apical processes of RPE cells
It was rather a surprise that two KIR channels, Kir4.1 and Kir7.1, were localized differentially within the apical processes of RPE cells. This may suggest distinct functional roles of these two KIR channels in the handling of K+ at the apical side of the RPE.
It is well known that Na+,K+-ATPase is localized at the apical membrane of RPE. Na+,K+-ATPase transports Na+ from inside to outside the cell and vice versa for K+, which is essential in maintaining the electrochemical gradients of Na+ and K+ across the plasma membrane in all mammalian cells (Jorgensen, 1982). Because the extracellular K+ is essential for activation of Na+,K+-ATPase, recycling of K+ is thought to be important to maintain the activity of the pump. It may also be essential for the [K+] homeostasis in the subretinal space by balancing the K+ influx through Na+,K+-ATPase. Because Kir7.1 channels and Na+,K+-ATPases seemed to be localized in close vicinity at the root-bottom portions of apical processes, these two membrane proteins may be functionally linked. In addition, because the Kir7.1 channel exhibits weak inward rectification, it allows K+ to exit the cell more readily than KIR channels with mild- strong rectification, including Kir4.1. This feature of the Kir7.1 channel may thus be beneficial for the recycling of K+. Therefore, it may be reasonable to propose that this channel is responsible for the K+ recycling for Na+,K+-ATPase at the apical membrane of RPE cells.
In a study on the frog RPE-choroid preparation, when the [K+] at the apical membrane of RPE cells was increased, the cells depolarized and passive K+ efflux from the basolateral membrane increased (Immel & Steinberg, 1986). Thus, in RPE cells, apical Ko+ seems to be transferred to the basolateral side through both apical and basolateral K+ conductances by a mechanism called ‘spatial buffering’. Both Kir4.1 and Kir7.1 could be involved in this mechanism. However, because Kir4.1 is more broadly distributed on the apical processes than Kir7.1, the Kir4.1 channel would be more suitable for this spatial buffering action.
Recent studies showed that Kir4.1 can interact through the sequence Ser-Asn-Val in its C-terminus with anchoring proteins containing PDZ domains (Horio et al. 1997; Kruschner et al. 1998). It has therefore been postulated that the subcellular distribution of Kir4.1 is regulated by PDZ domain-containing anchoring proteins in various tissues including retinal Müller cells (Horio et al. 1997), although it is not yet known which anchoring proteins are expressed in RPE cells. On the other hand, little is known about the mechanism controlling the subcellular localization of Kir7.1 which does not possess specific motifs for interaction with intracellular anchoring proteins or the cytoskeleton. Because Kir7.1 is distributed differently from Kir4.1 and is co-localized with Na+,K+-ATPase specifically at the root-bottom of apical processes in RPE cells (this study) and possibly also in choroid plexus (Nakamura et al. 1999), an unidentified mechanism controls the distribution of Kir7.1 proteins. Further studies are definitely needed to elucidate the molecular mechanisms responsible for the segregated distribution of Kir4.1 and Kir7.1 within the apical membrane of RPE cells.
In conclusion, the present study indicates that Kir7.1 is functionally expressed and co-localized with Na+,K+-ATPase at the root-bottom of apical processes in RPE cells where it may play a pivotal role in the handling of K+.
Acknowledgments
We thank Dr Ian Findlay (Université de Tours, Tours, France) for the critical reading of the manuscript, Ms Keiko Tsuji for secretarial work, and Ms Kadue Takahashi for technical assistance. This work was supported partly by grants from the Ministry of Education, Culture, Sports, and Science of Japan, from Research for the Future Programme (JSPS-RFTF96L00302) of The Japan Society for the Promotion of Science, and from the Human Frontier Science Programme (RG0158/1997-B).
References
- Döring F, Derst C, Wischmeyer E, Karschin C, Schneggenburger R, Daut J, Karschin A. The epithelial inward rectifier channel Kir7.1 displays unusual K+ permeation properties. Journal of Neuroscience. 1998;18:8625–8636. doi: 10.1523/JNEUROSCI.18-21-08625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Horio Y, Nielsen S, Nagelhus EA, Hata F, Ottersen OP, Kurachi Y. High-resolution immunogold cytochemistry indicates that AQP4 is concentrated along the basal membrane of parietal cell in rat stomach. FEBS Letters. 1999;459:305–309. doi: 10.1016/s0014-5793(99)01256-9. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Rosenthal NP. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. Journal of General Physiology. 1976;67:621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio Y, Hibino H, Inanobe A, Yamada M, Ishii M, Tada M, Satoh E, Hata Y, Takai Y, Kurachi Y. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4.1, by an anchoring protein, PSD-95/SAP90. Journal of Biological Chemistry. 1997;272:12885–12888. doi: 10.1074/jbc.272.20.12885. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Gallemore RP, Miller SS. Transport mechanism in the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. New York: Oxford University Press; 1998. pp. 103–134. [Google Scholar]
- Hughes BA, Shaikh A, Ahmad A. Effect of Ba2+ and Cs+ on apical membrane K+ conductance in toad retinal pigment epithelium. American Journal of Physiology C. 1995;268:1164–1172. doi: 10.1152/ajpcell.1995.268.5.C1164. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Steinberg RH. Voltage-dependent currents in isolated cells of the frog retinal pigment epithelium. The Journal of Physiology. 1990;428:273–297. doi: 10.1113/jphysiol.1990.sp018212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes BA, Takahira M. Inwardly rectifying K+ currents in isolated human retinal pigment epithelial cells. Investigative Ophthalmology and Visual Science. 1996;37:1125–1139. [PubMed] [Google Scholar]
- Immel J, Steinberg RH. Spatial buffering of K+ by the retinal pigment epithelium in frog. Journal of Neuroscience. 1986;6:3197–3204. doi: 10.1523/JNEUROSCI.06-11-03197.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inanobe A, Ito H, Ito M, Hosoya Y, Kurachi Y. Immunological and physical characterization of the brain G protein-gated muscarinic potassium channel. Biochemical and Biophysical Research Communications. 1995;217:1238–1244. doi: 10.1006/bbrc.1995.2901. [DOI] [PubMed] [Google Scholar]
- Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, KAB-2 (Kir4.1), in the basolateral membrane of distal renal tubular epithelia. FEBS Letters. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- Jorgensen PL. Mechanism of the Na+,K+-ATP pump. Protein structure and conformation of the pure (Na+,K+) ATPase Biochimica et Biophysica Acta. 1982;694:27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Eng L, Krapivinsky L, Yang Y, Clapham DE. A novel inward rectifier K+ channel with unique pore properties. Neuron. 1998;20:995–1005. doi: 10.1016/s0896-6273(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Kurschner C, Mermelstein PG, Holden WT, Surmeier J. CIPP, a novel multivalent PDZ domain protein, selectively interacts with Kir4.0 family members, NMDA receptor subunits, neurexins, and neuroligins. Molecular and Cellular Neuroscience. 1998;11:161–172. doi: 10.1006/mcne.1998.0679. [DOI] [PubMed] [Google Scholar]
- Kusaka S, Horio Y, Fujita A, Matsushita K, Inanobe A, Gotow T, Uchiyama Y, Tano Y, Kurachi Y. Expression and polarized distribution of an inwardly rectifying K+ channel, Kir4.1, in rat retinal pigment epithelium. The Journal of Physiology. 1999;520:373–381. doi: 10.1111/j.1469-7793.1999.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: Quantitative immunogold analysis of hair cell synapses in the rat organ of corti. Journal of Neuroscience. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SS, Edelman JL. Active ion transport pathways in the bovine retinal pigment epithelium. The Journal of Physiology. 1990;424:283–300. doi: 10.1113/jphysiol.1990.sp018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Suzuki Y, Sakuta H, Ookata K, Kawahara K, Hirose S. Inwardly rectifying K+ channel Kir7.1 is highly expressed in thyroid follicular cells, intestinal epithelial cells and choroid plexus epithelial cells: implication for a functional coupling with Na+,K+-ATPase. Biochemical Journal. 1999;342:329–336. [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Müller cells and the retinal pigment epithelium. In: Albert DM, Jacobiec FA, editors. Principle and Practice of Ophthalmology, Basic Science. Philadelphia, PA: Saunders; 1994. pp. 398–419. [Google Scholar]
- Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO Journal. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- Segawa Y, Hughes BA. Properties of the inwardly rectifying K+ conductance in the toad retinal pigment epithelium. The Journal of Physiology. 1994;476:41–53. [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Stanfield PR. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibers by barium and strontium ions. The Journal of Physiology. 1978;289:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Horio Y, Kurachi Y. Inwardly rectifying K+ channel in retinal Müller cells: Comparison with the KAB-2/Kir4.1 channel expressed in HEK293T cells. Japanese Journal of Physiology. 1998;48:71–80. doi: 10.2170/jjphysiol.48.71. [DOI] [PubMed] [Google Scholar]
- Takumi T, Ishii T, Horio Y, Morishige K, Takahashi N, Yamada M, Yamashita T, Kiyama H, Sohmiya K, Nakanishi S, Kurachi Y. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. Journal of Biological Chemistry. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Steinberg RH. Voltage-operated calcium channels in fresh and cultured rat retinal pigment epithelial cells. Investigative Ophthalmology and Visual Science. 1993;34:2408–3418. [PubMed] [Google Scholar]
- Wen R, Lui GM, Steinberg RH. Whole-cell K+ currents in fresh and cultured cells of the human and monkey retinal pigment epithelium. The Journal of Physiology. 1993;465:121–147. doi: 10.1113/jphysiol.1993.sp019669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Chang JT, Shimura M, Campochiaro PA, Zack DJ, Hughes BA. Molecular cloning and functional expression of Kir7.1, an inwardly rectifying K+ channel from the retinal pigment epithelium (RPE) Investigative Ophthalmology and Visual Science. 2000;41(suppl.):613. [Google Scholar]


