Abstract
Our goal was to describe the in situ responses in rats of single-unit carotid body chemoreceptors to changes in arterial PO2 and PCO2. We identified single-unit carotid chemoreceptor activity in male, adult Sprague-Dawley rats by their rapid responses to i.v. NaCN (20 μg) and transient (10 s) asphyxia.
Single-unit chemoreceptor responses to isocapnic changes in oxygenation within the arterial oxygen pressure range 34–114 mmHg were described by the power function: fdis= 74010(Pa,O2)−2.5; (r2= 0.6), where fdis is the discharge frequency (spikes s−1), Pa,O2 is the arterial oxygen partial pressure (mmHg) and r2 is the correlation coefficient.
The responses to iso-oxic changes in CO2, assumed to be linear, had a slope of 0.089 spikes s−1 (mmHg Pa,CO2)−1 (r2= 0.7).
We conclude that carotid body chemoreceptors in adult rats have responses to changes in Pa,O2 and Pa,CO2 similar to those of other species.
Knowledge of the response properties of arterial chemoreceptors is important to our understanding of the basic mechanisms underlying the control of breathing. While the in situ responses of carotid body chemoreceptors at the single-unit level have been described in several species, such a description is absent from the database of rats, a species which is currently widely employed in studies of the control of breathing (Schwarzacher et al. 1991; Ling et al. 1998). We reasoned that having this information in rats would be an extension and enhancement of what is already known about the carotid body chemoreceptors in this species. Our goal then was to measure in situ single-unit carotid body chemoreceptor responses to hypoxia and carbon dioxide.
METHODS
We studied male, adult (4-6 months of age) Sprague-Dawley rats (Harlan Sprague-Dawley Inc., Madison, WI, USA). The animal procedures were approved by the animal care and use committee of the University of Wisconsin at Madison. They were anaesthetized initially with intraperitoneal isotonic (0.329 M) urethane (4 ml (100 g body wt)−1), supplemented as needed (i.v.) to eliminate the tail pinch reflex, that is, any movement and blood pressure responses to the tail pinch. A catheter was placed in a femoral artery (for sampling and pressure measurement) and vein (for drug administration) and a rectal temperature probe was inserted. The trachea was cannulated and that cannula attached to a mechanical ventilator (Harvard rodent ventilator, Harvard Apparatus, Holliston, MA, USA). The tidal volume and frequency of the ventilator were adjusted to maintain arterial oxygen pressure (Pa,O2) near 90 mmHg (in some cases supplemental oxygen was given to achieve this) and arterial CO2 pressure (Pa,CO2) near 40 mmHg. Bicarbonate (8.4 % solution) was added as needed to prevent acidification of arterial blood. A heating pad kept rectal temperature near 38°C. We monitored end-tidal CO2 continuously (Capnograph, Novametrix, Wallingford, CT, USA) as an index of adequate ventilation, but the ultimate adjustments of the ventilator settings required to produce the blood gases necessary for the experimental protocol were guided by arterial blood gas measurements. Arterial blood samples (approximately 0.25 ml) were collected anaerobically and analysed for the partial pressures of O2 (Pa,O2), CO2 (Pa,CO2) and pH (ABL 300, Radiometer, Copenhagen, Denmark), with a correction based on rectal temperature.
The left carotid sinus region was exposed and the tissues overlying the left carotid sinus nerve resected or retracted. The sinus nerve was separated from the glossopharyngeal nerve as far centrally as possible and then cut at its junction with it. Before proceeding, rats were paralysed with gallamine triethiodide (i.v. 5 mg kg−1; Flaxedil, American Cyanamid, Wayne, NJ, USA). This dosage usually produced a paralysis lasting 45-60 min. The anaesthetic state of the rat was tested hourly as above after paralysis had dissipated, the dissipation being indicated by a noticeable return of spontaneous breathing efforts. Supplemental urethane was administered if necessary before giving another dose of Flaxedil to restore paralysis. At the end of the experiment the rat was killed with an overdose of urethane (10 ml of the isotonic solution, i.v.) followed by a saturated solution of KCl (1 ml, i.v.).
Recording techniques
Even though the sinus nerves of rats are much smaller than those in larger species, we used the same recording techniques as those used in cats (Sampson et al. 1976), dogs (Vidruk & Sorkness, 1994) and goats (Nielsen et al. 1988). Preliminary studies were done to adapt our dissecting techniques and recording apparatus from these larger species to the rat. All recordings were made by placing filaments of the nerve onto one leg of a bipolar electrode (platinum wire, 30 gauge), the other leg being earthed to the rat. This technique is identical to that used in larger species. These initial experiments left us with the impression that the sinus nerve in rats was more delicate than in the other species and that the quality of recording could be readily affected by trauma during the dissection process. We learned that handling of the nerve with techniques commonly used in larger species, though without any obvious effects in larger species, seemed to damage the rat nerves. Extreme care, therefore, was taken to avoid traumatizing the sinus nerve during the dissection process. In the best preparations, the audible display (Grass AM8 audiomonitor, Quincy, MA, USA) of the whole nerve activity was dominated by the distinctive and obvious cardiac-modulated bursting pattern of baroreceptors (Vidruk & Sorkness, 1994). In these preparations, a standardized intravenous dose of NaCN (20 μg in 0.2 ml) evoked a prompt and large response from chemoreceptors whose activity could also be detected audibly. By comparison, in some preparations which may have been inadvertently traumatized, baroreceptor activity was not audible initially. In these nerve preparations, NaCN rarely produced a detectable chemoreceptor response. When this was the case, either a thorough dissection of all extraneous connective tissues from the sheath of the nerve or removal of the nerve trunk from the sheath was attempted. This usually resulted in an improved signal-to-noise ratio such that both the spontaneous baroreceptor activity and chemoreceptor response to NaCN were obvious.
We tested for the presence of chemoreceptors in two ways: first, with a standardized i.v. dose of NaCN; second, with a standardized 10 s period of asphyxia, produced by turning the ventilator off during the deflation phase. We chose a 10 s period because this stimulus elicited an obvious (i.e. doubling of baseline discharge) chemoreceptor response (Fig. 1). The whole nerve was divided into smaller strands with each strand being tested for the presence of asphyxia- and NaCN-induced chemoreceptor activity. Strands showing the presence of chemoreceptor activity were further subdivided until single-unit chemoreceptor activity could be monitored unequivocally (Fig. 1). Monitoring of single units was continued for at least 5 min at the prevailing blood-gas levels. During the 5th minute, an arterial blood sample was collected. All the action potentials from the unit during the 5 min steady-state period were counted and converted to a discharge rate (spikes per second). This rate of discharge was subsequently expressed as a function of Pa,O2. Once data collection was completed, we changed the composition of the ventilator gases to produce a Pa,O2 near 50 mmHg. In order to maintain isocapnia with this degree of hypoxaemia, we found that either the ventilator frequency had to be reduced or CO2 added. Arterial CO2 proved more labile and more difficult to control as precisely in rats as in other species. After an initial 5 min equilibration period, action potentials from the unit were counted for 5 min (arterial blood sampled during the 5th minute). Gases were then changed to produce an intermediate degree of hypoxaemia (between the control and hypoxic levels) and data collected as above. When the conditions of both the rat and the nerve strand being recorded permitted, additional levels of oxygenation or carbon dioxide were tested. We avoided very low levels (below 35 mmHg) of hypoxaemia in these studies because we had found from preliminary experiments that rats failed to recover completely from these exposures, that is, when blood pressure fell below a mean value of 30 mmHg, it remained low and acidosis ensued. The studies in which hypoxaemic exposures were kept above ∼35 mmHg were accompanied by a mean blood pressure of 81 ± 21 mmHg (mean ± 1 s.d.) which was significantly lower (P= 0.05) than the normoxic level of 111 ± 27 mmHg, but a level to which blood pressure quickly returned following hypoxic exposure. Elevated CO2 produced no significant change in blood pressure (control, 119.5 ± 23 mmHg; hypercapnia, 119.8 ± 24 mmHg).
Figure 1. Effects of 10 s of asphyxia (A), and an intravenous bolus of NaCN (20 μg, B) upon a chemoreceptor unit.
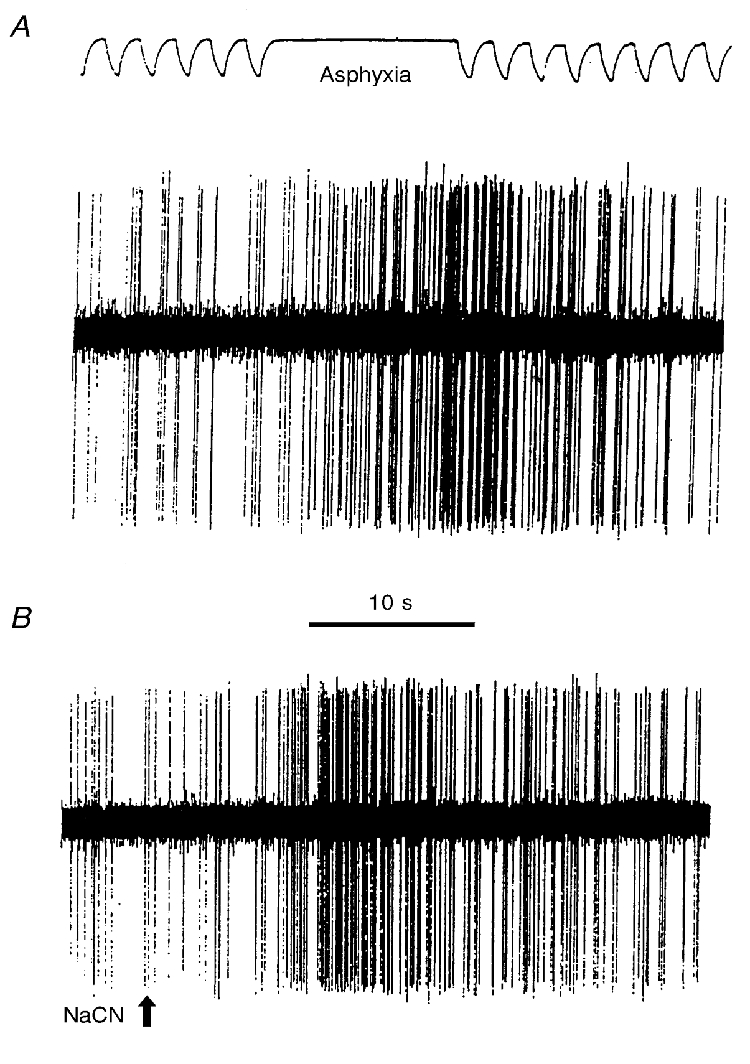
In A, the upper trace is the tracheal tidal pressure with lung inflation indicated by a downward deflection. Asphyxia was produced by turning the ventilator off, allowing the lungs to remain deflated.
Data analysis
Single-unit responses to both O2 and CO2 were fitted utilizing the least squares method (Microsoft Excel 97). Statistical analysis was performed using Student's t test. Data are presented as means ± 1 s.d. Differences were considered to be statistically significant when P < 0.05.
RESULTS
Oxygen responses of 20 single-unit chemoafferent fibres were measured in 15 rats (Fig. 2 and Fig. 3). The target Pa,CO2 ranged between 30 and 45 mmHg. The relationship between discharge frequency (fdis) and Pa,O2 was best described by a power function: fdis= 74010(Pa,O2)−2.5; (r2= 0.6), where the discharge frequency is in spikes per second and Pa,O2 in millimetres of mercury. We also fitted these data with linear, polynomial, logarithmic and exponential models (Microsoft Excel 97) but they produced r2 values which were not as large as that obtained for the power function. The unit chemoreceptor discharge at a Pa,O2 of 50 ± 9 mmHg was 6.1 (± 3.5)-fold that at a Pa,O2 of 95 ± 12 mmHg.
Figure 2. The effects of three different levels of oxygenation upon a chemoreceptor unit.
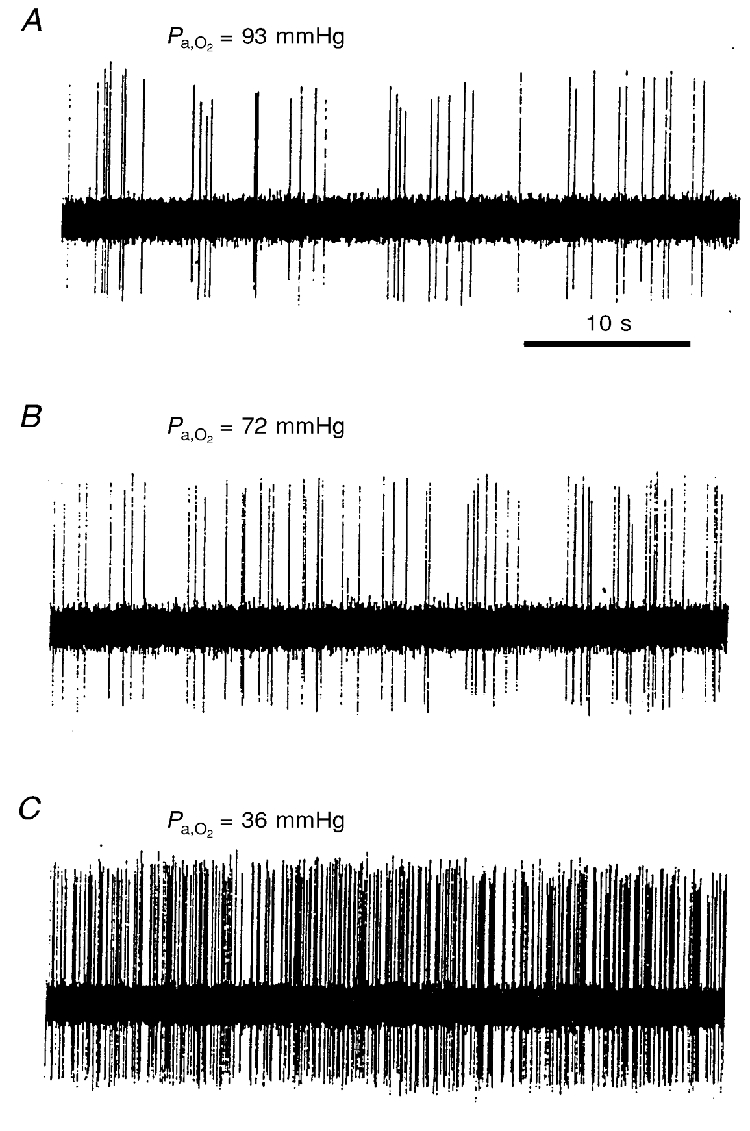
The Pa,CO2/pHa values for A, B and C are 35 mmHg/7.3, 34 mmHg/7.3 and 43 mmHg/7.23, respectively. This unit differs from the one shown in Fig. 1. These records were made after the rat had been at the appropriate inspired oxygen fraction for at least 5 min. Arterial blood was sampled subsequently, during the 5th minute of a steady-state level of discharge.
Figure 3. Effect of different levels of oxygenation upon single-unit chemoreceptor activity.
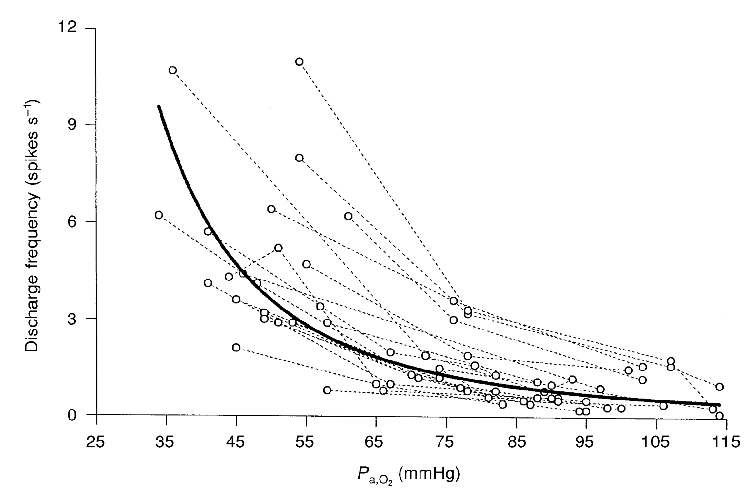
Each of the dashed lines connects the data points for a given unit (n= 20 units). The continuous line is the best fit (r2= 0.6) for all points and represents a function in which fdis= 74010 (Pa,O2)−2.5, where fdis is the discharge frequency (spikes s−1) and Pa,O2 is recorded in mmHg. r2 is the correlation coefficient.
Ten single units were subjected to an elevated level of CO2 during normoxia (Fig. 4). The Pa,O2 (86 ± 10 mmHg) during normocapnia was not significantly different from that during hypercapnia (Pa,O2= 89 ± 7 mmHg) thus, the CO2- response curves were iso-oxic. The average slope of these CO2-response curves was 0.089 spikes s−1 (mmHg Pa,CO2)−1 (r2= 0.7).
Figure 4. Effect of CO2 upon single-unit chemoreceptor activity.
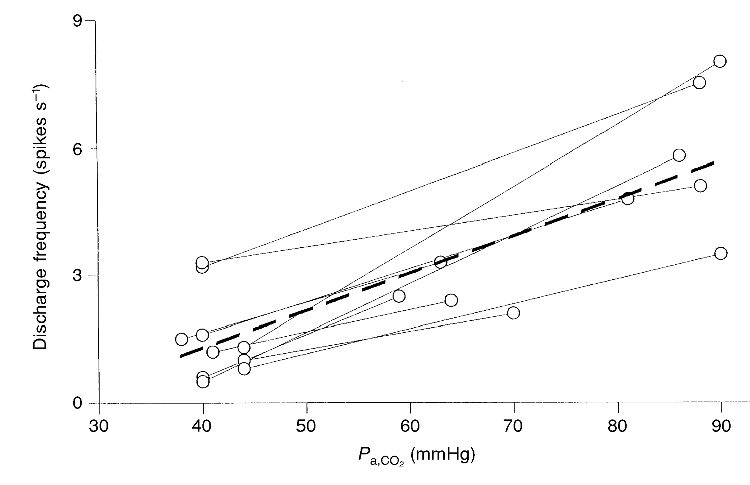
The thick dashed line represents the best linear fit to these data (fdis= 0.089(Pa,CO2)-2.3; r2= 0.69, where fdis is discharge frequency in spikes s−1 and Pa,CO2 is in mmHg; n= 10). The mean Pa,O2 (89 mmHg) during hypercapnia was not significantly different from that (86 mmHg) at the lowest CO2 level.
DISCUSSION
This is the first description of exclusively single-unit responses of rat carotid body chemoreceptors recorded in an in situ preparation and as such contributes unique information to the existing database on rat chemoreceptors. By recording single-unit activity from carotid sinus nerves, we found that carotid body chemoreceptors in adult rats have response characteristics that are, in general, similar to those in other mammalian species. A change in oxygenation above a Pa,O2 of ∼60 mmHg has a smaller effect on chemoreceptor activity than the same change below a Pa,O2 of ∼60 mmHg. This relationship between the change in oxygenation and chemoreceptor responsiveness results in a hyperbolic oxygen-response curve, a feature which is well documented for chemoreceptors in all species studied including in situ (Eden & Hanson, 1986; Fukuda et al. 1987) and in vitro (Landauer et al. 1995; Pepper et al. 1995) whole nerve and multifibre rat preparations. Single-unit chemoreceptor activity in rats increased by an average of 6.1-fold with a fall in Pa,O2 from 95 to 50 mmHg. This was a response within the range of relative responses observed previously by us in cats (Vidruk & Dempsey, 1980) and goats (Nielsen et al. 1988: Engwall et al. 1988) and in responses estimated from data published by others (Biscoe et al. 1967, 1970; Dick et al. 1974; Lahiri & DeLaney, 1975; Bisgard et al. 1979; Bisgard, 1981; Lahiri et al. 1981; Fitzgerald & Dehghani, 1982; Eden & Hanson, 1986; Fukuda et al. 1987; Blanco et al. 1988; Hanson et al. 1989; Landauer et al. 1995).
While the relative hypoxaemia-induced change in rat chemoreceptor responses falls within the range reported for other species, there is some suggestion that absolute discharge rates during hypoxaemic stimulation may be species dependent. At a Pa,O2 of ∼35 mmHg, the line of best fit through our oxygen-response curves shows that single units discharge at ∼9 spikes s−1. This rate approximates the discharge, under similar conditions, reported for goats (Nielsen et al. 1988; 5.8 impulses s−1, Pa,O2= 40.7 Torr) and for dogs (Bisgard, 1981; ∼6 impulses s−1, Pa,O2∼43 Torr). It is, however, well below that reported for cats by Biscoe et al. (1967) (20-25 impulses s−1, Pa,O2= 25-50 mmHg), Lahiri & DeLaney (1975) and Lahiri et al. (1981) (selected examples which show rates near to or within the range 20-25 impulses s−1) and Vidruk & Dempsey (1980) (18 spikes s−1, Pa,O2= 40 Torr). The hypoxaemic responsivity of mature rat chemoreceptors therefore appears to be closer to those of the less-than-fully developed 10- to 17-day-old kittens (Marchal et al. 1992; 8.8 impulses s−1, Pa,O2 estimated to be 44 Torr) and adult dogs and goats rather than mature cats. A factor which complicates attempts to identify species differences is the recently reported effect of gallamine triethiodide (Flaxedil) on chemoreceptor responsiveness to hypoxia (Fitzgerald et al. 1997). While the effects were identified experimentally in cats, it seems plausible that the chemoreceptors in other species could be similarly affected. Except for the study of Marchal et al. (1992) gallamine was used in all of the above-cited studies. If on the one hand, one assumes that gallamine in these studies affected all species in the same way, it could be argued that the identified differences among the hypoxic responses above are due to species differences in the chemoreceptor responsiveness to hypoxia. If, on the other hand, the effects of gallamine vary from species to species, the variable modulatory effects of gallamine could be mistaken for a species difference in hypoxia responsiveness. Until the effects of gallamine are elucidated in all the species being compared, the idea of a species-dependent hypoxia responsiveness might be best viewed as a highly speculative one.
Fukuda et al. (1987) argued that the chemoreceptor responsiveness to CO2 was greater in cats than in rats. At first glance, our findings seem to support this idea because the slope of our rat CO2-response curves, 0.089 spikes s−1 (mmHg Pa,CO2)−1, is clearly less than that reported for cats by Lahiri et al. (1981) (0.1-0.15 impulses s−1 (mmHg Pa,CO2)−1) and Vidruk (1973) (0.2 impulses s−1 (mmHg Pa,CO2)−1). When, however, one examines the large variability associated with these mean values, the means clearly fall within each other's confidence limits indicating that there is no clear difference between cats and rats. Interestingly, goat chemoreceptors respond almost identically (0.11 impulses s−1 (mmHg Pa,CO2)−1; Engwall et al. 1988). Although our observations raise doubts about any difference in the CO2 responsiveness of rats and cats, the question may not be answered unequivocally without a systematic study. Such a systematic study in a single-unit preparation could control for differences in factors which could confound results leading to the appearance of a species difference where none exists. With the above-reported effects of gallamine in mind, the selection of a paralytic agent deserves serious deliberation. Other factors to be controlled would be the length of time each test stimulus is presented, the time relative to the presentation of the stimulus when the response is measured, the length of time over which the response is measured and the method of calculating the response (i.e. one could use an average in the steady state, the steady-state peak rate of discharge calculated from either a selected burst or interspike intervals, the peak or average of any transient response or all of these previous methods) and finally reliable measurement of all potential stimuli, blood gases and acid-base status with carefully calibrated instruments. It is not always clear to what extent these significant factors have been controlled in published studies and this presents serious limitations to any interpretation of results which requires inter-study data. While statistical comparisons are mathematically precise, they may give an inaccurate impression unless accompanied by a detailed knowledge of the experimental methods underlying data acquisition. This caveat, we think, applies particularly to any idea that chemoreceptor responsiveness has a species difference component.
Finally, although the whole nerve data from the in vitro rat preparation is qualitatively similar to our single-unit in situ oxygen-response curves, there is an obvious difference. The in vitro preparations (Landauer et al. 1995; Pepper et al. 1995) achieve relatively higher rates of discharge at higher PO2 values than the in situ curves. This rightward shift of the in vitro curves could be related to the fact that neither blood gas values nor perfusate values precisely represent the actual stimulus level at the receptor site. This reasoning was presented to explain the right shift in the rat in vitro oxygen-response curves compared with cat in vivo curves (Pepper et al. 1995) and we think this also explains the obvious rightward shift of the in vitro rat curve compared with the in situ rat curve.
In summary, this study demonstrates that single-unit carotid body chemoreceptors in adult rats respond to changes in oxygenation and carbon dioxide in a similar manner to other mammalian species.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL53319 and NIH Training Grant HL07654).
References
- Biscoe TJ, Purves MJ, Sampson SR. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. The Journal of Physiology. 1970;208:121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe TJ, Sampson SR, Purves MJ. Stimulus response curves of single carotid chemoreceptor afferent fibres. Nature. 1967;215:654–655. doi: 10.1038/215654a0. [DOI] [PubMed] [Google Scholar]
- Bisgard GE. The response of few-fiber carotid chemoreceptor preparations to almitrine in the dog. Canadian The Journal of Physiology and Pharmacology. 1981;59:396–401. doi: 10.1139/y81-063. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Mitchell RA, Herbert DA. Effects of dopamine, norepinephrine and 5-hydroxytryptamine on the carotid body of the dog. Respiration Physiology. 1979;37:61–80. doi: 10.1016/0034-5687(79)90092-6. [DOI] [PubMed] [Google Scholar]
- Blanco CE, Hanson MA, McCooke HB. Effects on carotid chemoreceptor resetting of pulmonary ventilation in the fetal lamb in utero. Journal of Developmental Physiology. 1988;10:167–174. [PubMed] [Google Scholar]
- Dick DE, Meyer JR, Weil JV. A new approach to quantification of whole nerve bundle activity. Journal of Applied Physiology. 1974;36:393–397. doi: 10.1152/jappl.1974.36.3.393. [DOI] [PubMed] [Google Scholar]
- Eden GJ, Hanson MA. Effect of hyperoxia on the carotid chemoreceptor and ventilatory responses of rats to acute hypoxia. The Journal of Physiology. 1986;374:24. P. [Google Scholar]
- Engwall MJ, Vidruk EH, Nielsen AM, Bisgard GE. Response of the goat carotid body to acute and prolonged hypercapnia. Respiration Physiology. 1988;74:335–344. doi: 10.1016/0034-5687(88)90041-2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Dehghani GA. Neural responses of the cat carotid and aortic bodies to hypercapnia and hypoxia. Journal of Applied Physiology. 1982;52:596–601. doi: 10.1152/jappl.1982.52.3.596. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Shirahata M, Ide T. Further cholinergic aspects of carotid chemotransduction of hypoxia in cats. Journal of Applied Physiology. 1997;82:819–827. doi: 10.1152/jappl.1997.82.3.819. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Sato A, Trzebski A. Carotid chemoreceptor discharge responses to hypoxia and hypercapnia in normotensive and spontaneously hypertensive rats. Journal of the Autonomic Nervous System. 1987;19:1–11. doi: 10.1016/0165-1838(87)90139-1. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Eden GJ, Nijhuis JG, Moore PJ. Peripheral chemoreceptors and other oxygen sensors in the fetus and newborn. In: Lahiri S, Forster RE, Davies RO, Pack AI, editors. Chemoreceptors and Reflexes in Breathing: Cellular and Molecular Aspects. New York: Oxford University Press; 1989. pp. 113–120. [Google Scholar]
- Lahiri S, DeLaney RG. Stimulus interaction in the responses of carotid chemoreceptor single afferent fibres. Respiration Physiology. 1975;24:249–266. doi: 10.1016/0034-5687(75)90017-1. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Mokashi A, Mulligan E, Nishino T. Comparison of aortic and carotid chemoreceptor responses to hypercapnia and hypoxia. Journal of Applied Physiology. 1981;51:55–61. doi: 10.1152/jappl.1981.51.1.55. [DOI] [PubMed] [Google Scholar]
- Landauer RC, Pepper DR, Kumar P. Effect of chronic hypoxaemia from birth upon chemosensitivity in the adult rat carotid body in vitro. The Journal of Physiology. 1995;485:543–550. doi: 10.1113/jphysiol.1995.sp020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Olson EB, Jr, Vidruk EH, Mitchell GS. Slow recovery of impaired phrenic responses to hypoxia following perinatal hyperoxia in rats. The Journal of Physiology. 1998;511:599–603. doi: 10.1111/j.1469-7793.1998.599bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal F, Bairam A, Haouzi P, Crance JP, Di Giulio C, Vert P, Lahiri S. Carotid chemoreceptor response to natural stimuli in the newborn kitten. Respiration Physiology. 1992;87:183–193. doi: 10.1016/0034-5687(92)90058-5. [DOI] [PubMed] [Google Scholar]
- Nielsen AM, Bisgard GE, Vidruk EH. Carotid chemoreceptor activity during acute and sustained hypoxia in goats. Journal of Applied Physiology. 1988;65:1796–1802. doi: 10.1152/jappl.1988.65.4.1796. [DOI] [PubMed] [Google Scholar]
- Pepper DR, Landauer RC, Kumar P. Postnatal development of CO2-O2 interaction in the rat carotid body in vitro. The Journal of Physiology. 1995;485:531–541. doi: 10.1113/jphysiol.1995.sp020749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson SR, Aminoff MJ, Jaffe RA, Vidruk EH. A pharmacological analysis of neurally induced inhibition of carotid body chemoreceptor activity in cats. Journal of Pharmacology and Experimental Therapeutics. 1976;197:119–125. [PubMed] [Google Scholar]
- Schwarzacher SW, Wilhelm Z, Anders K, Richter DW. The medullary respiratory network in the rat. The Journal of Physiology. 1991;435:631–644. doi: 10.1113/jphysiol.1991.sp018529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidruk EH. Madison, WI, USA: University of Wisconsin; 1973. Carotid body chemoreceptor activity as recorded from the petrosal ganglion of cats. PhD Thesis. [DOI] [PubMed] [Google Scholar]
- Vidruk EH, Dempsey JA. Carotid body chemoreceptor activity as recorded from the petrosal ganglion in cats. Brain Research. 1980;181:455–459. doi: 10.1016/0006-8993(80)90629-0. [DOI] [PubMed] [Google Scholar]
- Vidruk EH, Sorkness RL. The circumsinus branch: a convenient source of baro- and chemoreceptor activity in dogs. Journal of Applied Physiology. 1994;76:1384–1387. doi: 10.1152/jappl.1994.76.3.1384. [DOI] [PubMed] [Google Scholar]


