Abstract
The physiological importance of l-tryptophan transport for placental indoleamine 2,3-dioxygenase-mediated degradation of l-tryptophan has been studied using human placental chorionic villous explants.
l-Tryptophan influx into villous explants is supported exclusively by transport system L and is substantially inhibited by the L-system-specific substrate 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) and also by 1-methyl-tryptophan which is also an inhibitor of indoleamine 2,3-dioxygenase. l-Tryptophan influx is enhanced 2.3-fold following in vitro culture of the villous explant. Interferon-γ, which increases villous explant indoleamine 2,3-dioxygenase expression, has no effect on l-tryptophan influx.
In explants both BCH and 1-methyl-tryptophan inhibit indoleamine 2,3-dioxygenase-mediated l-tryptophan degradation. This also applies when l-tryptophan degradation has been stimulated by interferon-γ.
These findings show transport of l-tryptophan into the trophoblast to be a rate-limiting step for indoleamine 2,3-dioxygenase-mediated l-tryptophan degradation and therefore for the normal physiology of mammalian pregnancy.
In the accompanying paper (Kudo & Boyd, 2001) we have described membrane transport pathways available for l-tryptophan to supply the substrate for indoleamine 2,3-dioxygenase in placental tissue. The enzyme is found both in trophoblasts (Yamazaki et al. 1985; Kudo & Boyd, 2000a) and macrophages (Munn et al. 1999); the latter are known to be present in the placental villous core. Indoleamine 2,3-dioxygenase is the major l-tryptophan-catabolising enzyme and is responsible for suppressing the maternal immune response to the allogeneic murine fetus (Munn et al. 1998). Since the enzyme is intracellular, for it to be effective the substrate l-tryptophan must enter the cell. We have now examined the relationship between l-tryptophan transport and indoleamine 2,3-dioxygenase activity in the intact human chorionic villus using a well-established explant preparation (Watson et al. 1995). In this paper, transport has been manipulated and overall indoleamine 2,3-dioxygenase-mediated l-tryptophan degradation has been determined in the intact tissue, thus permitting us to see whether physiologically transport is rate limiting for the overall enzyme activity. The results show unequivocally that if transport is reduced by competitive inhibition then l-tryptophan degradation is very substantially suppressed, particularly when enzyme expression is stimulated by cytokines (Kudo et al. 2000). This is important since it adds a new dimension to the key finding of Munn et al. (1998) that placental indoleamine 2,3-dioxygenase is required to deplete l-tryptophan at the feto-maternal interface and thus locally abrogate T-cell activity, hence suppressing the immune response mounted by the mother against the allogeneic fetus. Our findings suggest that l-tryptophan transport is required for this process and therefore for normal viviparity. Disorders of transport may therefore logically be associated with immune-mediated pathophysiology of pregnancy.
METHODS
Culture of villous tissue
Normal term placentae were obtained (with ethical committee approval) within 15 min of delivery and chilled on ice. The placenta was cut into cotyledons and the decidual surface was removed. The tissue was washed three times with ice-cold phosphate-buffered saline (PBS) containing 100 units ml−1 penicillin and 100 units ml−1 streptomycin and chorionic villi were dissected into small pieces (a single piece was approximately 5 mg). All these procedures were carried out below 4 °C. Three pieces of chorionic villi were placed on a polyester mesh (Netwell 500 μm mesh, Costar, NY, USA) and cultured in 35 mm plastic culture dishs at 37 °C in an atmosphere of 5 % CO2 and 95 % air for the indicated time. The culture medium used was RPMI medium 1640 with addition of 5 % fetal bovine serum, 100 units ml−1 penicillin, 100 units ml−1 streptomycin and interferon-γ or vehicle. Other additions are described in the figure legends. The conditioned medium was collected at the time indicated and was mixed completely by vortexing with one-tenth volume of ice-cold 2.4 m perchloric acid. The mixture was chilled on ice for 15 min and centrifuged at 10 000 g for 3 min. The clear protein-free supernatant was used for high-performance liquid chromatography (HPLC) analysis. Cultures were conducted in triplicate for each set of experiments to assess reproducibility. The number of placentae used in each study is mentioned in the figure and table legends.
HPLC analysis of l-tryptophan catabolism
Concentrations of l-tryptophan and l-kynurenine in the conditioned medium were analysed by an HPLC system consisting of a Kontron 420 pump, a Kontron 460 autosampler and a Kontron 432 variable wavelength detector (Watford, Herts, UK) with the Spherisorb S5-ODS1 column (4.6 mm x 150 mm; Waters, Milford, MA, USA). The mobile phase consisted of 40 mm citrate buffer (pH 2.25), 50 % methanol and 0.4 mm sodium dodecylsulphate, which were used after filtration through a 0.45 μm membrane filter and degassed by a vacuum aspirator. A 20 μl volume of protein-free extract was injected on the column, chromatographed at a flow rate of 2.0 ml min−1 and detected at 365 nm for l-kynurenine and at 280 nm for l-tryptophan. The minimum amount of l-kynurenine and l-tryptophan reproducibly detected was 20 pmol and calibration was linear up to 10 nmol of l-kynurenine or of l-tryptophan.
Assay of indoleamine 2,3-dioxygenase
The cultured pieces of chorionic villi were washed twice, suspended in ice-cold PBS and disrupted by sonication for 30 s in an ice bath at a power of 100 W. The homogenate was centrifuged at 800 g for 10 min at 4 °C to remove unbroken fragments of the tissue. The supernatant was then centrifuged at 15 000 g for 15 min at 4 °C. The resultant supernatant was used for the colorimetric assay of indoleamine 2,3-dioxygenase activity, as described previously (Kudo & Boyd, 2000a).
Amino acid influx studies
At least three placentae were used for each experiment shown here. In each individual experiment three fragments were used per well, and three or five wells were used for each experimental condition. Chorionic villi were washed twice with pre-warmed (37 °C) PBS. The influx of amino acid was initiated by placing tissue in prewarmed PBS containing 2 μml-[3H]tryptophan, followed by further incubation at 37 °C. Other additions are described in the figure legends. When sodium-free PBS was required for determination of influx in the absence of sodium, NaCl, NaHCO3 and NaH2PO4 were replaced by choline chloride, choline bicarbonate and KH2PO4, respectively. Chorionic villi were quickly washed with ice-cold PBS with 10 mm unlabelled l-tryptophan, suspended in PBS and disrupted by sonication for 30 s in an ice bath at a power of 100 W. Aliquots were taken for liquid scintillation counting and protein determination.
Protein estimation
Protein concentration of the vesicle preparation was determined by the method of Lowry et al. (1951) using bovine serum albumin as a standard.
Statistical analysis
Differences between groups were analysed using Student's t test and results were considered statistically significant at P < 0.05.
Chemicals
l-[5-3H]Tryptophan (31.0 Ci mmol−1 or 1.15 TBq mmol−1) was purchased from Amersham Life Sciences (Amersham, Buckinghamshire, UK). Human recombinant interferon-γ, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH) and 1-methyl-d, l-tryptophan were obtained from Sigma-Aldrich Chemicals (Poole, Dorset, UK), tissue culture supplements were from Gibco BRL (Paisley, UK). All other chemicals were of the highest purity commercially available.
RESULTS
Effects of 1-methyl-tryptophan and BCH on indoleamine 2,3-dioxygenase activity of placental tissue extract
We have previously shown that 1-methyl-tryptophan is a competitive inhibitor of human placental indoleamine 2,3-dioxygenase (Kudo & Boyd, 2000a). In contrast BCH (at a concentration of 2 mm) has no effect on indoleamine 2,3-dioxygenase activity of tissue extract. The activities measured in three placental extracts (nmol (mg protein)−1 min−1) were: control, 0.52 ± 0.02; in the presence of 2 mm 1-methyl-tryptophan, 0.15 ± 0.01; in the presence of 2 mm BCH, 0.52 ± 0.01. This negative finding with BCH therefore allows us to distinguish between the effect of inhibitors of l-tryptophan transport (BCH, 1-methyl-tryptophan) and inhibitors acting at the enzyme active site (1-methyl-tryptophan but not BCH).
Effect of 1-methyl-tryptophan and BCH on l-tryptophan transport in placental explants
Figure 1 shows the time course of l-tryptophan influx into fresh villous explants. There was no significant difference between l-tryptophan influx in either the presence or the absence of sodium from the influx medium. The uptake of 2 μml-tryptophan measured under these conditions was linear for at least the first 60 s of incubation. The effect of different concentrations of 1-methyl-tryptophan, BCH and l-tryptophan itself on the initial rate of l-tryptophan influx into fresh villous explants was studied (Fig. 2). Amino acids tested showed a concentration-dependent inhibition of l-tryptophan influx and almost completely inhibited at 10 mm. The concentration giving half-maximal inhibition was found to be 0.43 ± 0.09 mm for l-tryptophan, 0.63 ± 0.11 mm for 1-methyl-tryptophan and 0.44 ± 0.10 mm for BCH. The extent of the inhibition by BCH was the same as that found with l-tryptophan suggesting that system L accounts for all of the l-tryptophan influx into villous explants. Figure 3 compares 2 μml-tryptophan influx into fresh villous explants and into explants cultured with or without interferon-γ for 36 h. As predicted from earlier work (Kudo & Boyd, 2000b) mediated l-tryptophan influx was stimulated approximately 2.3-fold following culture; this additional activity was not sodium dependent. The pattern of inhibition of l-tryptophan influx by the amino acids tested was the same in fresh and cultured tissue suggesting that system L is solely responsible for l-tryptophan influx in cultured as in fresh villous explants. Interferon-γ showed no effect on l-tryptophan influx. It is therefore probable that the increased activity of system L accounts for the observed stimulation of the total l-tryptophan influx following culture.
Figure 1. Time course of l-tryptophan influx into placental explants.
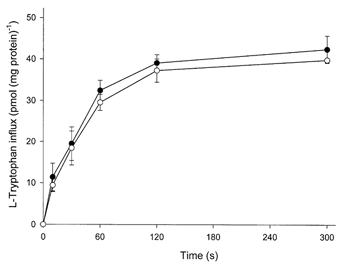
Radioactive l-tryptophan content of explants of villous tissue was measured as a function of time over the period indicated. Medium contained 2 μml-[3H]tryptophan (2 μCi ml−1 or 74 KBq ml−1) in the presence or absence (choline) of sodium as described in Methods. •, sodium; ○, choline. Data represent the means ±s.d. of three separate experiments with triplicate assays from three different placentae.
Figure 2. Effect of BCH and 1-methyl-tryptophan and l-tryptophan on radioactive l-tryptophan influx into placental explants.
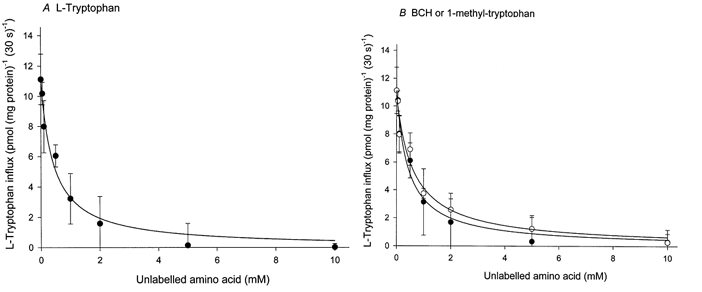
l-Tryptophan influx over a 30 s period was measured in medium containing 2 μml-[3H]tryptophan (2 μCi ml−1 or 74 KBq ml−1) with indicated concentrations of unlabelled l-tryptophan (A) and BCH (BCH) or 1-methyl-tryptophan (B) in the absence (choline) of sodium as described in Methods. Data show carrier-mediated influx rate defined by subtracting the diffusional component from the total influx. The diffusional component was determined by measuring the influx of l-[3H]tryptophan in the presence of 20 mm unlabelled l-tryptophan. The lines were fitted using least-square regression analysis. Each point represents the mean ±s.d. of one representative of three separate experiments from three different placentae with five replicate assays. •, l-tryptophan (A) or BCH (B); ○, 1-methyl-tryptophan.
Figure 3. Effect of in vitro culture on l-tryptophan influx in placental explants.
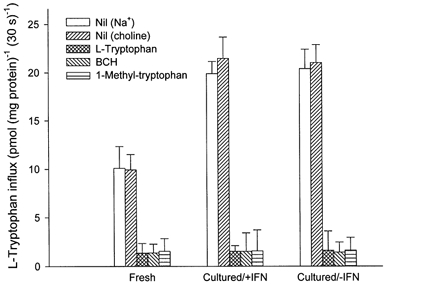
l-Tryptophan flux into either fresh or cultured (with or without 1000 units ml−1 interferon-γ (IFN)) explant of villous tissue over a 30 s period was measured in medium containing 2 μml-[3H]tryptophan (2 μCi ml−1 or 74 KBq ml−1) with or without unlabelled amino acid (2 mm for l-tryptophan, BCH or 1-methyl-tryptophan, final concentration) in the presence or absence (choline) of sodium as described in Methods. Data show carrier-mediated influx defined by subtracting the diffusional component from the total influx (in either the presence or absence of sodium). The diffusional component was determined by measuring the influx of l-[3H]tryptophan in the presence of 20 mm unlabelled l-tryptophan. Data represent the means ±s.d. of three separate experiments with triplicate assays from three different placentae. The mediated BCH-sensitive fluxes in fresh and in cultured tissue with or without interferon-γ are, respectively, 8.56 ± 1.58, 19.98 ± 2.20 and 19.64 ± 1.85 pmol (mg protein)−1 (30 s)−1. The rate following culture is significantly greater than for fresh samples (P value was less than 0.01 in both control and interferon-γ group).
Effect of 1-methyl-tryptophan and BCH on l-tryptophan catabolism by indoleamine 2,3-dioxygenase in placental explants
Concentrations of l-tryptophan and of l-kynurenine, the major indoleamine 2,3-dioxygenase degradation product, in the conditioned medium following in vitro culture of placental villous explants were determined. The presence of interferon-γ in the culture medium stimulated l-tryptophan degradation markedly, consequently showing a marked increase in l-kynurenine concentration (Fig. 4A and B). A significant stimulatory effect of interferon-γ on l-tryptophan catabolism was observed at 12 h after the initiation of culture. Indoleamine 2,3-dioxygenase activity in cultured villous explants was also stimulated by interferon-γ in a time-dependent manner (Fig. 4C). Therefore the stimulatory effect of interferon-γ on l-tryptophan catabolism could be attributed to interferon-γ-induced stimulation of indoleamine 2,3-dioxygenase activity. The indoleamine 2,3-dioxygenase inhibitor 1-methyl-tryptophan showed inhibition of l-tryptophan catabolism in either the presence or absence of interferon-γ; both the decrease in l-tryptophan concentration and the associated increase in l-kynurenine concentration were reduced by the presence of 1-methyl-tryptophan. In contrast to these effects on l-tryptophan catabolism in the intact explant, if 1-methyl-tryptophan was removed before the activity of the isolated enzyme was studied there was no inhibition as compared with controls not exposed to the inhibitor. This shows that culture in medium containing 1-methyl-tryptophan had no effect on the functional expression of indoleamine 2,3-dioxygenase. Figure 5 shows the equivalent experiment for BCH. There is, in the presence or absence of interferon-γ, inhibition by BCH although this is somewhat less than that found with 1-methyl-tryptophan. Exposure to BCH during culture of villous explants showed no effect on total indoleamine 2,3-dioxygenase activity measured subsequently, indicating that similarly there is no effect on functional indoleamine 2,3-dioxygenase expression of this inhibitor.
Figure 4. Effect of 1-methyl-tryptophan on l-tryptophan catabolism by indoleamine 2,3-dioxygenase in placental explants.

Villous explants were cultured with 1000 units ml−1 interferon-γ and/or 2 mm 1-methyl-tryptophan or vehicle (control) for the time indicated. Concentrations of l-tryptophan (A) and l-kynurenine (B) in the conditioned medium were analysed by HPLC and indoleamine 2,3-dioxygenase activity (C) in the tissue extract was determined colorimetrically as described in Methods. •, control; ○, interferon-γ; ▾, 1-methyl-tryptophan; ▿, interferon-γ and 1-methyl-tryptophan. Data represent the means ±s.d. of three separate experiments with triplicate assays from three different placentae.
Figure 5. Effect of BCH on l-tryptophan catabolism by indoleamine 2,3-dioxygenase in placental explants.
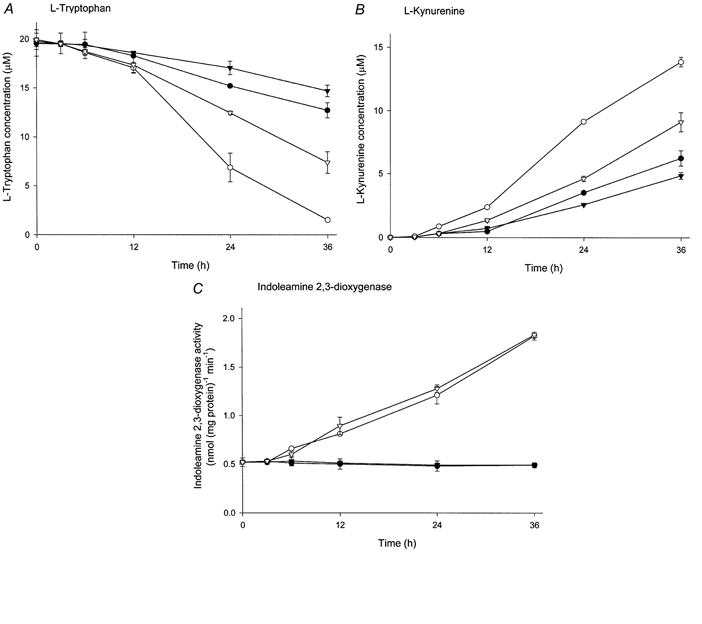
Villous explants were cultured with 1000 units ml−1 interferon-γ and/or 2 mm BCH or vehicle (control) for the time indicated. Concentrations of l-tryptophan (A) and l-kynurenine (B) in the conditioned medium were analysed by HPLC and indoleamine 2,3-dioxygenase activity (C) in the tissue extract was determined colorimetrically as described in Methods. •, control; ○, interferon-γ; ▾, BCH; ▿, interferon-γ and BCH. Data represent the means ±s.d. of three separate experiments with triplicate assays from three different placentae.
DISCUSSION
In this paper we have described findings which show that BCH is an excellent tool for inhibiting l-tryptophan transport into the trophoblast since entry of this amino acid into placental explants is exclusively through system L. BCH is an excellent competitive substrate for this transporter and moreover is not metabolised (Christensen et al. 1969) and is without effect on the indoleamine 2,3-dioxygenase enzyme itself. Therefore the findings that BCH reduces l-tryptophan removal and l-kynurenine appearance in the conditioned media unambiguously show that in intact tissue, substrate delivery to this cytoplasmic enzyme is a necessary step for indoleamine 2,3-dioxygenase-mediated l-tryptophan degradation. Although Knowles et al. (1989) have previously speculated that in fibroblasts ‘cellular tryptophan degradation is limited by something other than indoleamine 2,3-dioxygenase activity, perhaps tryptophan transport into the cells’, our findings on the placenta provide the first direct evidence for this. Since in the accompanying paper (Kudo & Boyd, 2001) we have shown using membrane vesicles that system L is exclusively responsible for l-tryptophan transport at the brush border membranes and since in the present paper we have shown that BCH totally inhibits tryptophan transport into intact fresh or cultured villous explant, BCH is an ideal tool for inhibiting l-tryptophan delivery to cytoplasmic indoleamine 2,3-dioxygenase.
The molecular basis of the system L transporter has been elucidated recently (Kanai et al. 1998; Boado et al. 1999; Pineda et al. 1999; Rossier et al. 1999; Rajan et al. 2000). Intriguingly it is a member of the heterodimeric membrane transport family comprising both a heavy chain (CD98 hc) and a catalytic light chain system L-amino acid transporter (LAT) (-1 or -2). The heavy chain is known to have other functions (Devés & Boyd, 2000), including cell fusion. This raises the question of whether syncytium formation (perhaps CD98 hc mediated) is required for trophoblastic indoleamine 2,3-dioxygenase functional expression (system L dependent).
1-Methyl-tryptophan (like BCH) is also an inhibitor of l-tryptophan transport; it also acts directly (unlike BCH) on indoleamine 2,3-dioxygenase (Kudo & Boyd, 2000a). Therefore, unsurprisingly, in the intact explant system, 1-methyl-tryptophan showed greater inhibition of indoleamine 2,3-dioxygenase-mediated l-tryptophan degradation (Fig. 4). Neither of these molecules altered functional enzyme expression following culture. Following culture with interferon-γ, indoleamine 2,3-dioxygenase activity was stimulated as predicted from an earlier study (Kudo et al. 2000); after this, however, the relative potencies of BCH and of 1-methyl-tryptophan as inhibitors of l-tryptophan degradation were altered. This is compatible with the nature (mechanism) of inhibition being different. Additionally, it seems likely that in the explant there is more than a single pool of indoleamine 2,3-dioxygenase activity, hence BCH may be less able to inhibit interferon-γ-induced indoleamine 2,3-dioxygenase activity than is 1-methyl-tryptophan. Macrophages in the villous core could be one major source of the indoleamine 2,3-dioxygenase activity which is upregulated by interferon-γ.
Our results raise the possibility that therapeutic intervention aimed at regulating indoleamine 2,3-dioxygenase activity and hence the maternal immune response to pregnancy may usefully be focused on manipulation of l-tryptophan transport through system L. Specifically, manipulation of l-tryptophan transport might be of value in the maternal syndrome of pre-eclampsia which is characterised by an exaggerated immune response (reviewing by Redman et al. 1999). Interestingly in the cord blood of intrauterine growth-retarded babies the concentrations of a number of amino acids including those which are supported by transport system L are found to be lowered (Cetin et al. 1990; Marconi et al. 1999); this is compatible with the suggestion that in such pregnancies placental indoleamine 2,3-dioxygenase is less effective at local manipulation of l-tryptophan concentrations. This may be a novel connection between intrauterine growth retardation and pre-eclampsia (Lin et al. 1991).
Acknowledgments
We thank Dr C. M. Siman and Professor C. P. Sibley, Academic Unit of Child Health, St Mary's Hospital, University of Manchester for valuable advice on the explant assay, staff at the John Radcliffe Hospital, Oxford for assistance with obtaining placentae and Action Research for financial support.
References
- Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Procceedings of the National Academy of Sciences of the USA. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin I, Corbetta C, Sereni LP, Marconi AM, Boettizz P, Pardi G, Battaglia FC. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. American Journal of Obstetrics and Gynecology. 1990;162:253–261. doi: 10.1016/0002-9378(90)90860-a. [DOI] [PubMed] [Google Scholar]
- Christensen HN, Handlogten ME, Lam I, Tager HS, Zand R. A bicyclic amino acid to improve discriminations among transport systems. Journal of Biological Chemistry. 1969;244:1510–1520. [PubMed] [Google Scholar]
- Devés R, Boyd CAR. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. Journal of Membrane Biology. 2000;173:165–177. doi: 10.1007/s002320001017. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) Journal of Biological Chemistry. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Knowles RG, Salter M, Pogson CI. Tryptophan degradation and indoleamine 2,3-dioxygenase in MRC-5 fibroblasts. Biochemical Society Transactions. 1989;17:539–540. [Google Scholar]
- Kudo Y, Boyd CAR. Human placental indoleamine 2,3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochimica et Biophysica Acta. 2000a;1500:119–124. doi: 10.1016/s0925-4439(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. Heterodimeric amino acid transporters: expression of heavy but not light chains of CD98 correlates with induction of amino acid transport systems in human placental trophoblast. Journal of Physiology. 2000b;523:13–18. doi: 10.1111/j.1469-7793.2000.t01-1-00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. Characterisation of l-tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. Journal of Physiology. 2001;531:405–416. doi: 10.1111/j.1469-7793.2001.0405i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR, Sargent IL, Redman CWG. Modulation of indoleamine 2,3-dioxygenase by interferon-γ in human placental chorionic villi. Molecular Human Reproduction. 2000;6:369–374. doi: 10.1093/molehr/6.4.369. [DOI] [PubMed] [Google Scholar]
- Lin CC, Su SJ, River LP. Comparison of associated high-risk factors and perinatal outcome between symmetric and asymmetric fetal intrauterine growth retardation. American Journal of Obstetrics and Gynecology. 1991;164:1535–1541. doi: 10.1016/0002-9378(91)91433-w. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Marconi AM, Paolini CL, Stramare L, Cetin I, Fennessey PV, Pardi G, Battaglia FC. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatric Research. 1999;46:114–119. doi: 10.1203/00006450-199907000-00019. [DOI] [PubMed] [Google Scholar]
- Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. Journal of Experimental Medicine. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Pineda M, FernandeZ E, Torrents D, EsteveZ R, LopeZ C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. Journal of Biological Chemistry. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- Rajan DP, Kekuda R, Huang W, Devoe LD, Leibach FH, Prasad PD, Ganapathy V. Cloning and functional characterization of Na+-independent, broad-specific neutral amino acid transporter from mammalian intestine. Biochimica et Biophysica Acta. 2000;1463:6–14. doi: 10.1016/s0005-2736(99)00224-2. [DOI] [PubMed] [Google Scholar]
- Redman CWG, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. American Journal of Obstetrics and Gynecology. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, Kuhn LC. LAT2, a new basolateral 4F2 hc/CD98-associated amino acid transporter of kidney and intestine. Journal of Biological Chemistry. 1999;274:34948–34954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]
- Watson AL, Palmer ME, Burton G. Human chorionic gonadotrophin release and tissue viability in placental organ culture. Human Reproduction. 1995;10:2159–2164. doi: 10.1093/oxfordjournals.humrep.a136253. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Kuroiwa T, Takikawa O, Kido R. Human indolylamine 2,3-dioxygenase. Its tissue distribution, and characterization of the placental enzyme. Biochemical Journal. 1985;230:635–638. doi: 10.1042/bj2300635. [DOI] [PMC free article] [PubMed] [Google Scholar]


