Abstract
The present study investigated the effects of hibernation and hibernating body temperature (10°C) on the relative changes that may occur in adrenergic and purinergic perivascular neurotransmission of the golden hamster. The hindlimb resistance vessels and the tibial artery of age-matched controls, cold exposed controls and hibernated hamsters were examined by pharmacological and electrophysiological techniques.
At 34°C, electrical field stimulation (EFS; supramaximal voltage, 0.5 ms; for 10 s) in all three groups evoked only twitch responses at 1-5 Hz, which were inhibited by piridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), a 2PX receptor antagonist. At 10-50 Hz the twitch responses were followed by sustained contractile responses, which were inhibited by prazosin, an α1-adrenoceptor antagonist. These responses were markedly enhanced at higher frequencies in hibernated tissues. At 10°C, EFS evoked only the PPADS-sensitive transient responses in all the three groups, and this was markedly enhanced in hibernated tissues.
At 34°C, a single stimulus evoked a PPADS-sensitive excitatory junction potential (EJP) in all three groups but a train of pulses (e.g. ≥0.5) evoked EJPs and prazosin-sensitive sustained depolarizations. These responses were markedly enhanced in hibernated cells. At 10°C, either a single stimulus or a train of stimuli evoked only transient PPADS-sensitive EJPs, which were markedly enhanced in hibernated cells.
The contractile responses and electrical membrane responses to exogenous ATP (1-1000 μm) and noradrenaline (0.1-100 μm) were unchanged in the three groups at 34 and at 10°C.
These results suggest that during hibernation enhancement of ATP release from the sympathetic perivascular nerves may occur, leading to an efficient means for maintenance of vascular tone and peripheral resistance.
Mammalian hibernation is an unusual physiological phenomenon which occurs under stress conditions such as hypothermia, hypoxia or ischaemia. In hibernation, animals undergo a dramatic drop in body temperature and a fall in heart and respiratory rates (Lyman, 1965; Nedergaard & Cannon, 1990; Nürnberger, 1995). Despite this decline, the peripheral vascular resistance increases with deepening hibernation to keep the blood pressure within a reasonable range, and it is thought that the enhancement of the sympathetic mediation is one of the major contributing factors for this regulation (Lyman & O'Briben, 1963; Ralevic et al. 1997; Karoon et al. 1998). Sympathetic perivascular nerves play an important role in the control of peripheral circulation, and it is now widely accepted that in many of these nerves, ATP is a co-transmitter with noradrenaline (NA) (Kennedy et al. 1986; Burnstock, 1995; Thapaliya et al. 1999). Some pathophysiological studies, on the other hand, indicate that the pattern of the relative contribution of adrenergic and purinergic components in the sympathetic perivascular neurotransmission may alter considerably under certain stressed conditions. In canine cutaneous veins exposed to a cold environment, a reflex enhancement of sympathetic tone is blocked by P2-purinoreceptor desensitization, enabling the prevention of excessive heat loss (Flavahan & Vanhoutte, 1986). In the hypertensive rat tail artery and the induced hypertensive rabbit saphenous artery, increased transmitter release (predominantly ATP) has been claimed (Vidal et al. 1986; Bulloch & McGrath, 1992).
Thus, our interest is focused on determining whether the relative contribution of the adrenergic and purinergic involvement in sympathetic perivascular neurotransmission is (1) altered during hibernation in the peripheral resistance vessels, and (2) varies with temperature. We have used microelectrode techniques to investigate the fast excitatory junction potentials (EJPs) since in many isolated arteries it is this response which provides the material evidence for the purinergic involvement in the most accurate way (McLaren et al. 1995; Brock & Cunnane, 1999; Thapaliya et al. 1999). Besides, we have considered that it is indispensable to investigate responses under hypothermic condition (10°C) as well as under normothermic conditions to compare hibernating and non-hibernating animals, since the body temperature of the hibernating hamster remains between 8 and 12°C (Lyman, 1965; Ralevic et al. 1997; Karoon et al. 1998). A preliminary account of these data has been presented previously (Saito et al. 2000).
METHODS
Animals
Golden hamsters (Mesocricetus auratus) of either sex from our closed colony weighing 120-150 g were employed in these investigations. The experimental procedures were approved by Gifu University, Animal Care and Use Committee and were in accordance with Japanese Department of Agriculture guidelines. The hamsters were killed by exposure to diethyl ether followed by exsanguination.
Three groups of animals were used. The first group were left to hibernate for 8-10 weeks and were used in experiments while undergoing the fifth successive bout of deep hibernation for at least 48 h. The second group, referred to as cold control animals, were animals that did not themselves hibernate but were exposed to the same conditions of temperature, photoperiod and total duration as those that did hibernate. The third group, control animals, were hamsters of the same age (5-7 months) as those that had undergone hibernation but had been kept at room temperature (22-24°C). It remains unclear why some hamsters kept under conditions conducive to hibernation fail to hibernate. However, preparation for hibernation is accompanied by altered endocrine function (Hoffman et al. 1965) which may vary with different individuals.
Acclimatization for hibernation
Animals for hibernation were placed in a ventilated, refrigerated incubator initially set at 17°C and with a light:dark photoperiod of 8:16 h. The temperature was gradually reduced by 1°C per day together with a reduction of 30 min of light per day, to reach final conditions of an ambient temperature of 5°C and a photoperiod of 2:22 h. The hamsters were housed individually and nesting material was supplied. Food and water were provided ad libitum. Hibernation was judged by the lack of response to a physical stimulus, such as the sprinkling of sawdust onto the back of the hamster. A sleeping hamster always awoke to this stimulus, whereas a hibernating animal did not. Animals were in hibernation for 8-10 weeks.
In vivo procedures
During a deep hibernation bout of 1-4 days, some animals were used to monitor heart beat, respiratory rate, and regional blood flow. The heart rate was obtained from an electrocardiogram (ECG) recorded by using standard electrophysiological techniques via three standard limb leads and a ground lead. Stainless steel electrodes were gently attached to the planta region of corresponding limbs. The respiratory rate was counted visually by the movement of the thorax. The blood flow was obtained using Laser-Doppler flowmetry (LDF, ALF 21N, Advance, Japan) on the surface of the planta region of the hindlimb. These data were compared with those of control and cold control animals, which were achieved under anaesthesia (pentobarbital sodium, 50 mg kg−1, i.p.). Rectal temperature from control, cold control and hibernating animals was also measured, as opportunity permitted.
Experimental temperature protocol
To determine the effect of tissue temperature this investigation was conducted under two separate experimental conditions namely acclimatization for the normothermic state and a hypothermic state (i.e. similar to the hibernating body temperature). Each animal group aforementioned was assigned to one of these two conditions for investigation. Normally each animal was only used for a single experiment at one of those temperatures. In some cases, after a series of tests the bath temperature was changed over a 30 min period from the euthermic temperature to the hypothermic one or vice versa and the procedure was repeated at the alternative temperature to identify whether any of the responses were due to the effect of the order in which temperature was altered. The hibernating animals were removed from the cold room and quickly transferred to a thermostatically controlled bath (model LE-100, Advantec, Japan) maintained at hibernating body temperature (see Results), in which all procedures were carried out unless stated otherwise.
Mechanical recordings
After the animals were killed, the hindquarters were used for mechanical recording by the method of Wisnes et al. (1979a,b). Thus, the animals were eviscerated, and the abdominal aorta and caval vein were freed from the surrounding connective tissue for about 5-6 mm distal to the renal branch. A physiological saline solution (PSS)-filled polyethylene cannula (0.8 mm bore) was inserted into the aorta and connected to a peristaltic pump (model SJ-1211, ATTO, Japan), and perfused at a constant flow rate of about 0.6 ml min−1 with oxygenated PSS. The outflow was allowed to leak out freely through a polyethylene cannula (1 mm bore) inserted into the caval vein. PSS contained (mm): 133 NaCl, 4.7 KCl, 1.3 Na2PO4, 2.5 CaCl2, 0.6 MgSO4, 16 NaHCO3 and 7.8 glucose, gassed with 95 % O2-5 % CO2. Maintaining a constant flow rate through the hindlimb circulation meant that changes in perfusion pressure (mmHg) were indicative of changes in arterial resistance of the site. Responses (mmHg) were measured as changes in perfusion pressure via a pressure transducer (Uniflow, Baxter, USA) on a side arm of the perfusion cannula and recorded on a polygraph (model RTA-1100M, Nihon Kohden, Japan). To eliminate any contribution of endothelium-dependent vasodilatation, endothelial cells were removed by perfusion of warmed PSS containing collagenase (1 mg ml−1) into the lumen for 15 min (Bolton et al. 1984; Thapaliya et al. 1999). Preparations were then allowed to equilibrate for at least 1 h. The removal of endothelium cells was considered to be successful when no vasodilatation was elicited by a concentration of acetylcholine (10 μm) that would give a maximal response to NA (1 μm) in the pre-contracted artery beds. In all the preparations, the constant basal perfusion pressure and outflow were checked every 30 min for 4 h after the onset, and if this was not the case during an experiment the tissue was discarded. Electrical field stimulation (EFS) was achieved by passing a current (model SEN-2201 stimulator, Nihon Kohden, Japan) between a pair of parallel, platinum wire electrodes which were hooked under the abdominal aorta. The two electrodes were separated by a distance of 3-4 mm, and the distance between the femoral bifurcation and the downward electrode was set as 1-2 mm. EFS was carried out in all the experiments using a square-wave pulse (supramaximal voltage for 0.5 ms) for 10 s with frequencies ranging from 0.5 to 50 Hz. Reproducible vascular responses were obtained with nerve stimulation for 10 s. Vasoconstrictor responses to ATP and NA were recorded at basal tone. The raised tone was allowed to return to baseline before a subsequent injection was given. Doses were applied as 100 μl bolus injections through a side tube proximal to the preparation. In some experiments, piridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) was added at a final concentration of 5 μm to the reservoir 20 min prior to construction of a frequency-response curve, then PPADS together with prazosin (1 μm) was administered via the perfusate reservoir for a further 20 min and another frequency-response curve established. The stability of mechanical responses to the EFS and exogenous drugs (NA, 1 μm; ATP, 100 μm) was checked every hour in all preparations, and if the responses were changed greatly the preparation was discarded.
Electrophysiological recordings
The tibial arteries were dissected from the site and placed in a Petri dish filled with PSS. The artery was cannulated and gently flushed with 1 ml of PSS in order to eliminate blood in the vessels. Subsequently, preparations were fixed to a rubber base attached to the bottom of a 3 ml experimental partition chamber, and perfused at a constant flow rate of 3 ml min−1 with oxygenated PSS gassed with 95 % O2-5 % CO2. Intracellular recordings of membrane potential were made from small intact segments (0.5-1.0 cm) of the tibial artery of 200-350 μm diameter, using glass filament microelectrodes filled with 3 m KCl with tip resistance ranging from 60 to 80 MΩ. In all experiments, perivascular nerves were stimulated with a square-wave pulse (0.5 ms in duration) delivered at supramaximal voltage via two platinum electrodes, one being a platinum wire upon which the preparation was rested and the other about 1-2 mm distal to the tissue. Repetitive stimulation for nerves was applied utilizing frequencies ranging from 0.25 to 20 Hz with a train of 6-20 pulses. The electrical activities were monitored on an oscilloscope (CS 4026, Kenwood, Tokyo, Japan) and recorded on a thermal-array recorder (RTA-1100 M, Nihon Kohden, Tokyo, Japan). Impalements were made on the adventitial side, within 2 mm of the plate electrode.
Drugs
The drugs used were as follows (Sigma, USA): noradrenaline (NA) hydrochloride, ATP (disodium salt), PPADS, acetylcholine chloride, guanethidine, prazosin, tetrodotoxin, pentobarbital sodium acid and collagenase.
NA (0.1 mm) was dissolved in ascorbic acid (0.1 mm). Prazosin (10 mm) was dissolved in methanol (100 %). All the other drugs were dissolved in distilled water. The drugs were serially diluted in PSS to the required final concentrations immediately before the experiments.
Data analysis
All data are expressed as means ±s.e.m. Statistical differences between the groups were determined by analysis of variance (ANOVA) and Student's unpaired t test as appropriate for individual points and one-way ANOVA for the entire curve. In each group, comparisons between the temperatures were also determined by analysis of variance (ANOVA) and Student's unpaired t test as appropriate for individual points and one-way ANOVA for the entire curve. A value of P less than 0.05 was considered statistically significant.
RESULTS
Induction into hibernation
Hamsters started hibernating after 7-11 weeks in the cold room and were allowed to hibernate for a further 8 weeks. During this time, bouts of 1-4 days of deep hibernation occurred. There was a significant weight loss in both hibernating and cold control groups compared with the control group (Table 1). Mean heart and respiratory rate, regional blood flow and rectal body temperature of the hibernated animals were significantly lower than those of control and cold control animals (Table 1).
Table 1.
Body weight, heart rate, respiratory rate, blood flow and body temperature of control, cold control and hibernating hamsters
| Control | Cold control | Hibernating | |
|---|---|---|---|
| Weight (g) | 161 ± 4.8 (15) | 130 ± 4.8 (12)* | 101 ± 2.7 (8)*† |
| Heart rate (beats min−1) | 438 ± 48 (5) | 412 ± 51 (5) | 8.4 ± 1.2 (4)*† |
| Respiratory rate (breaths min−1) | 88 ± 14 (5) | 82 ± 15 (4) | 1.75 ± 0.5 (4)*† |
| Peripheral blood flow§ (LDF unit) | 25.9 ± 0.49 (5) | 26.4 ± 0.52 (3) | 3.28 ± 0.17 (3)*† |
| Rectal temperature (°C) | 34.5 ± 0.2 (8) | 33.8 ± 0.3 (6) | 9.8 ± 0.6 (5)*† |
Measurements for hibernating and cold control hamsters were made at 4–6 weeks after hibernation and after 11–13 weeks in a refrigerated incubator, respectively.
On the surface of the planta region. Data are means ± S.E.M. (number of animals).
P < 0.05vs. control
P < 0.05vs. cold control.
Contractile responses in the hindlimb vasculature evoked by electrical field stimulation (EFS) at 34 and 10°C
The experimental temperatures were determined according to the mean rectal temperatures from control, cold control and hibernated animals, that is 34°C for the euthermic state and 10°C for the hypothermic state. At 34°C, at a constant flow rate of about 0.6 ml min−1, there was no significant difference in the basal perfusion pressure among control, cold control and hibernated animals. Basal perfusion pressure was 23.8 ± 1.2 (n = 15), 23.6 ± 1.0 (n = 22) and 24.8 ± 0.7 mmHg (n = 10) in control, cold control and hibernated animals, respectively. There was no difference in basal perfusion pressure before and after removal of the endothelium (data not shown). EFS at 0.5-5 Hz evoked a rapid transient response. At frequencies of 10-50 Hz, electrical stimulation evoked the twitch response, with a subsequent slow sustained response (Fig. 1). The sensitivity and the maximum height of the twitch responses to EFS (10-50 Hz) in hibernated animals were significantly larger than those of control and cold control animals, and the sensitivity and the maximum height of the sustained responses to EFS (20-50 Hz) in hibernated animals were also significantly larger than those of control and cold control animals (Fig. 1D). In cold control animals, the twitch response to EFS at 20 Hz was significantly greater than that of control animals.
Figure 1. Hindlimb perfusion: contractile responses to electrical field stimulation (EFS) at 34 °C.
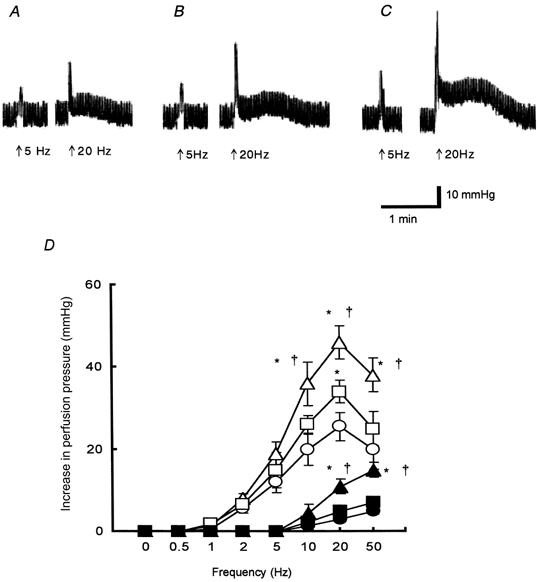
A-C, representative traces showing vasoconstrictor responses (expressed as increase in perfusion pressure, mmHg) to EFS (arrow: supramaximal voltage, 0.5 ms; 5 or 20 Hz for 10 s) from a control animal (A), a cold control animal (B) and a hibernated animal (C). The fluctuations in perfusion pressure were due to the pulsatile movements of the pump. Responses in all preparations at 20 Hz are biphasic, being twitch-like components and sustained components. D, summary of the different components elicited by the nerve stimulation: the twitch-like component from controls (○), cold controls (□) and hibernated animals (▵), and the sustained one from controls (•), cold controls (▪) and hibernated animals (▴). All results are expressed as means ±s.e.m.; n = 5-7. *P < 0.05vs. control; †P < 0.05vs. cold control.
At 10°C, there was no significant difference in the basal pressure among control, cold control and hibernated animals, although the values differed significantly from the respective groups at 34°C (P < 0.05). Basal perfusion pressure was 41.1 ± 1.1 (n = 15), 40.3 ± 0.8 (n = 12), and 40.6 ± 1.0 mmHg (n = 8) in control, cold control and hibernated animals, respectively. EFS at basal tone evoked frequency-dependent contractile responses in the three groups, but at all frequencies (0.5-50 Hz) there were rapid transient contractile responses as shown in Fig. 2. The sensitivity and the maximum height of twitch responses to EFS (0.5-50 Hz) in hibernated animals were significantly larger than in both control and cold control animals (Fig. 2D). In contrast, there was no significant difference in response in control and cold control animals (Fig. 2D).
Figure 2. Hindlimb perfusion: contractile responses to electrical field stimulation (EFS) at 10°C and effects of altering the bath temperature.
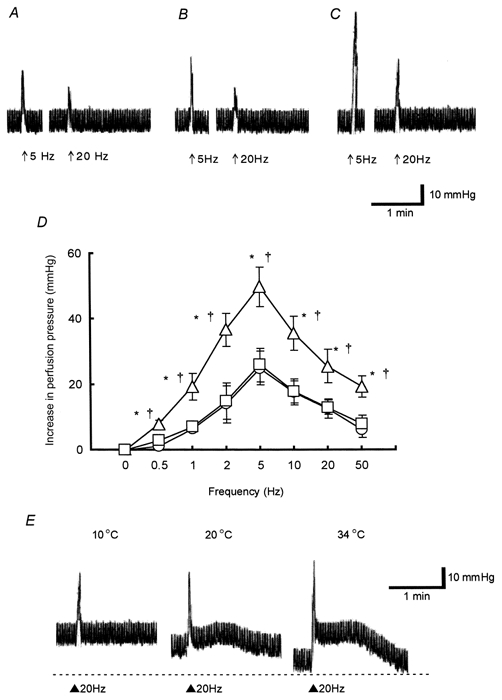
A-C, representative traces showing vasoconstrictor responses (expressed as increase in perfusion pressure, mmHg) to EFS (arrow: supramaximal voltage, 0.5 ms; 5 or 20 Hz for 10 s) from a control animal (A), a cold control animal (B) and a hibernated animal (C). Note that all the responses are monophasic, being twitch-like at 10°C. D, summary of the effects of the nerve stimulation from controls (○), cold controls (□) and hibernated animals (▵). E, effects of alteration of temperature on responses elicited by the nerve stimulation from a hibernated animal. Note that the mean basal perfusion pressure was influenced by temperature; the rapid components are not changed by cooling whereas the sustained ones are reduced at the lower temperature (20°C) and undetectable at 10°C. Fluctuations in perfusion pressure were due to the pulsatile movements of the pump. All results are expressed as means ±s.e.m.; n = 5-7. *P < 0.05vs. control; †P < 0.05vs. cold control.
The frequency-response curves of the twitch responses from all three groups at 10°C were significantly shifted to the left in comparison with those obtained at 34°C. The maximum height of the twitch responses from the hibernated animals was the same at both temperatures, whereas the values obtained from control and cold control animals were significantly reduced at 10°C (Fig. 1 and 2, n = 5-7, P < 0.01).
The effect of altering bath temperature from 10 to 34°C was investigated using hibernated tissues. Warming from 10 to 34°C caused a decrease in basal perfusion pressure (Fig. 2E, at 20°C, 31.6 ± 1.2 mmHg, n = 4 and at 34°C, 23.2 ± 1.1 mmHg, n = 4). The sustained response evoked by EFS (20 Hz), which was undetectable at 10°C, was observed at 20°C (Fig. 2E).
All the responses evoked by EFS in the control, cold control and hibernated animals were blocked by guanethidine (5 μm, n = 5-6, data not shown) or tetrodotoxin (1 μm, n = 6-8, data not shown).
Effects of PPADS and prazosin on responses in the hindlimb vasculature evoked by electrical field stimulation (EFS) at 34 and 10°C
Experiments were conducted with PPADS, a 2PX receptor antagonist, and prazosin, an α1-adrenoceptor antagonst. At 34°C, in control, cold control and hibernated animals, the twitch responses evoked by EFS (20 Hz) were significantly inhibited in the presence of PPADS (5 μm, Fig. 3, 0.5 ± 0.2, 0.6 ± 0.2 and 0.5 ± 0.2 mmHg, respectively, n = 6-7, P < 0.01, cold control data not shown). Furthermore, in the presence of PPADS (5 μm) and prazosin (1 μm), the residual sustained responses were significantly inhibited in all three groups tested (Fig. 3, 0.4 ± 0.1, 0.3 ± 0.2 and 0.3 ± 0.1 mmHg, respectively, n = 6-7, P < 0.01). At 10°C, addition of PPADS (5 μm) inhibited significantly the twitch responses evoked by EFS (20 Hz) in all three groups (Fig. 3, 0.4 ± 0.2, 0.5 ± 0.2 and 0.4 ± 0.2 mmHg, respectively, n = 5-7, P < 0.01), after which residual responses were negligible (Fig. 3, 0.3 ± 0.1, 0.3 ± 0.2 and 0.3 ± 0.1 mmHg, respectively,n = 4-6).
Figure 3. Effects of piridoxal phosphate-6-azophenyl-2’,4’-disulphonic acid (PPADS) and prazosin on the rapid and sustained responses evoked by electrical field stimulation (EFS) in hamster hindlimb perfusion at 34 and 10°C.
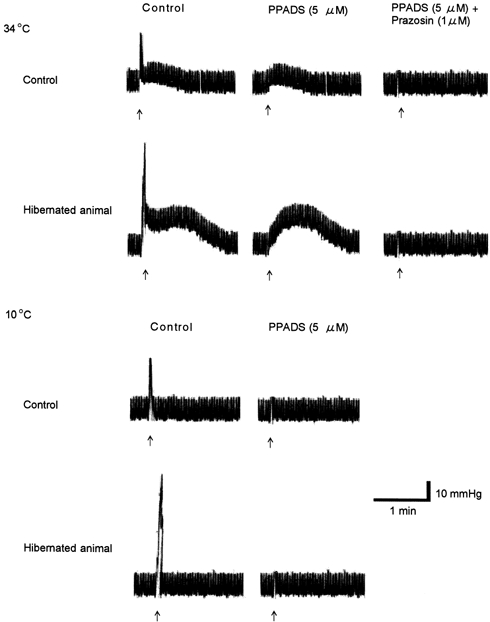
The inhibitions of control responses to EFS (arrow: supramaximal voltage, 0.5 ms; 20 Hz for 10 s) by PPADS (5 μm) and subsequently prazosin (1 μm) from a control animal and a hibernated animal at 34 and 10°C are shown. Note that at 34°C the twitch-like and sustained components of the biphasic response were almost completely abolished by PPADS and prazosin, respectively. At 10°C the monophasic response to nerve stimulation was PPADS sensitive, and the sustained component observed at 34°C, which was blocked by prazosin, was not detected.
Contractile responses in the hindlimb vasculature evoked by exogenous ATP and NA at 34 and 10°C
At 34°C, exogenous ATP (1-1000 μm) and NA (0.1-100 μm) evoked vasoconstrictor responses in a concentration-dependent manner. The sensitivity of responses to the drugs and the maximum heights of the responses among control, cold control and hibernated animals were unchanged (Fig. 4). At 10°C, exogenous ATP (1-1000 μm) and NA (0.1-100 μm) also evoked vasoconstrictor responses in control, cold control and hibernated animals in a concentration-dependent manner. There was no significant difference in the sensitivity of responses or the maximum height of the responses to both drugs among all three groups tested (Fig. 4). In contrast, in all three groups the maximum heights of the responses to ATP at 34°C did not differ from those obtained at 10°C, whilst the responses to NA were significantly reduced at 10°C (Fig. 4, n = 7-9, P < 0.05).
Figure 4. Concentration-response relationship for ATP and noradrenaline (NA) in hamster hindlimb perfusion at 34 and 10°C.
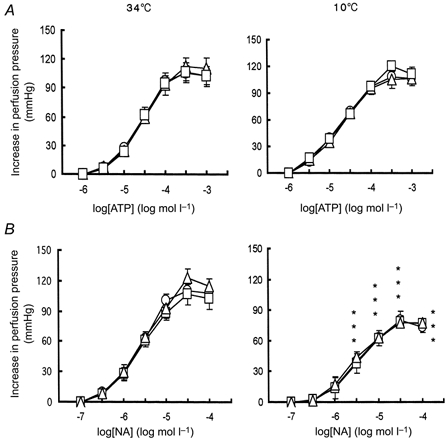
Concentration-response curves showing vasoconstrictor responses (expressed as increase in perfusion pressure, mmHg) to ATP (A) and NA (B) of hindlimb perfusion from control animals (○), cold control animals (□) and hibernated animals (▵). All results are means ±s.e.m.; n = 7-9. *P < 0.05vs. 34°C of respective groups.
Excitatory junction potentials (EJPs) and slow depolarizations evoked by nerve stimulation at 34 and 10°C
At 34°C, the mean values of the resting membrane potential of tibial arteries did not significantly alter among control, cold control and hibernated animals (Table 2). When unstimulated, the smooth muscle cells in all three groups were electrically quiescent. The electrical response of the arteries from the three groups to a single stimulus was a transient depolarization, namely an EJP (Fig. 5Aa, n = 8-9) since it was blocked by tetrodotoxin (1 μm). When a train of pulses at ≥ 0.5 Hz was applied, each pulse evoked an EJP which facilitated. At 1 Hz, a slow maintained depolarization also appeared, which persisted after the cessation of the stimulation and then slowly returning to control level (Fig. 5Ac and d, n = 7-15). This phenomenon was also observed in control and cold control groups (n = 11-15, data not shown). The mean peak amplitude of the EJPs evoked by the single nerve stimulation from hibernated animals was significantly larger than those of control and cold control animals (Table 2). There was no significant difference in the mean values of latencies, time to peak and total duration of the EJPs among the three groups (Table 2). The slow depolarization amplitude evoked by a train of pulses (20 Hz, 20 pulses) from hibernated animal was also greater than those of control and cold controls animals (6.8 ± 0.3, 3.8 ± 0.2 and 3.9 ± 0.3 mV, n = 7-11, respectively, P < 0.05). The values of latency, time to peak amplitude and total duration recorded at 34°C did not differ significantly among control, cold control and hibernated animals (Table 2).
Table 2.
Electrical responses to single stimulus pulse and ATP and NA in tibial artery smooth muscle from control, cold control and hibernated hamsters at 34 and 10°C
| Control | Cold control | Hibernated | |
|---|---|---|---|
| 34°C | |||
| Resting membrane potential (mV) | −64.5 ± 0.5 (15) | −63.9 ± 1.4 (11) | −62.5 ± 5.8 (7) |
| EJP | |||
| amplitude (mV) | 6.4 ± 0.2 (11) | 6.5 ± 0.3 (8) | 9.6 ± 0.5(6)*† |
| latency (ms) | 21.1 ± 0.8 (11) | 22.1 ± 1.4 (8) | 21.8 ± 1.2 (6) |
| time to peak amplitude (ms) | 43.9 ± 1.8 (11) | 44.5 ± 2.4 (8) | 44.9 ± 2.5(6) |
| total duration (ms) | 684.1 ± 34.0 (11) | 675.5 ± 36.2 (8) | 691.5 ± 35.0 (6) |
| Vm after ATP (100 μM) (mV) | 13.5 ± 1.4 (6) | 13.4 ± 1.3 (4) | 13.5 ± 1.2 (5) |
| Vm after NA (100 μM) (mV) | 6.1 ± 0.2 (5) | 5.9 ± 0.3 (4) | 6.0 ± 0.3 (4) |
| 10°C | |||
| Resting membrane potential (mV) | −59.0 ± 2.2 (8) | −59.0 ± 2.2 (7) | −55.5 ± 8.3 (7) |
| EJP | |||
| amplitude (mV) | 1.2 ± 0.4 (7)‡ | 1.3 ± 0.5 (6)‡ | 6.6 ± 0.8 (6)*†‡ |
| latency (ms) | 229.7 ± 5.3 (7)‡ | 224.7 ± 6.3 (6)‡ | 230.7 ± 6.3 (6)‡ |
| time to peak amplitude (ms) | 738.6 ± 31.2 (7)‡ | 734.4 ± 29.5 (6)‡ | 726.4 ± 30.2 (6)‡ |
| total duration (ms) | 2907.7 ± 42.9 (7)‡ | 3007.7 ± 37.9 (6)‡ | 3433.1 ± 42.0 (6)*‡ |
| Vm after ATP (100 μM) (mV) | 13.1 ± 1.6 (6) | 13.1 ± 1.3 (4) | 13.3 ± 1.5 (6) |
| Vm after NA (100 μM) (mV) | 0.3 ± 0.2 (5)‡ | 0.2 ± 0.3 (4)‡ | 0.2 ± 0.1 (4)‡ |
Data are means ± S.E.M. (number of observations).
P < 0.05vs. control
P < 0.05vs. cold control
P < 0.05vs. 34°C of respective groups. Stimulation paramer: supramaximal voltage, 0.5ms, single pulse.
Figure 5. Effect of temperature on excitatory junction potentials (EJPs) and slow depolarizations in hamster tibial artery smooth muscle cells during hibernation.
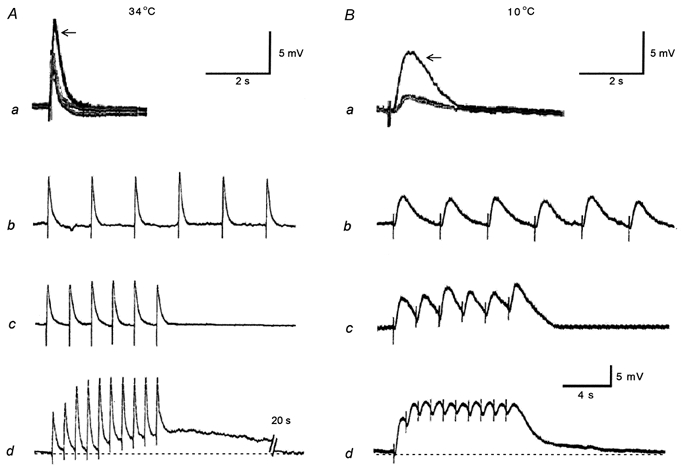
Aa and Ab, representative traces showing superimposed EJPs to a single stimulus (supramaximal voltage, 0.5 ms) from a control, a cold control and a hibernated animal (arrow) at 34°C (Aa) and 10°C (Ba). The traces from control and cold control animals were nearly identical. Ab-d and Bb-d, membrane responses to electrical field stimulation (supramaximal voltage, 0.5 ms, 6-10 pulses) from hibernated animals at 0.25 Hz (Ab and Bb), 0.5 Hz (Ac and Bc) and 1 Hz (Ad and Bd) at 34°C (A) and 10°C (B). Note that at both temperatures the amplitude of the EJPs from hibernated animals (arrow) are greater than those of control and cold control animals (Aa and Ba), and at 10°C as the frequency progresses (Bd), summations appear which were not present at 34°C (Ad). At 1 Hz at 34°C a slow depolarization emerges (Ad). Each vertical bar shows an electrical stimulus. Resting membrane potentials for Aa and Ba were -62 (control), -61 (cold control) and -60 mV (hibernated) at 34°C, and -59 (control), -60 (cold control) and -59 mV (hibernated) at 10°C, and for Ab, Ac, Ad, Bb, Bc and Bd were -64, -62, -62, -59, -60 and -59 mV, respectively. The lowest scale bar refers to Ab-d and Bb-d.
Under hypothermia (10°C), the mean values of resting membrane potential did not significantly differ between control, cold controls and hibernated animals (Table 2). The electrical response of the arteries from the three groups to a single stimulus was also a depolarization (Fig. 5Ba, n = 7-8), which was blocked by tetrodotoxin (1 μm), and therefore can be considered to be an EJP. When a train of pulses was applied, each pulse evoked an EJP and, as the train progressed (0.25-1 Hz), summation occurred; this was most conspicuously observed in hibernated cells (n = 7-9). But even at 1 Hz with a train of 10 pulses, nerve stimulation evoked only EJPs which were not followed by depolarization in any of the three experimental groups (n = 7-8, Fig. 5Bb-d from the hibernated animals; data from the other groups not shown). The mean peak amplitude and the total duration of EJPs in hibernated animals were significantly larger than in controls and cold controls at 10°C (Table 2). The latency and time to peak amplitude recorded at 10°C did not differ significantly from values of control and cold control animals (Table 2).
No significant difference between resting membrane potentials at 34 and 10°C was observed. The amplitude of EJPs was reduced significantly at 10°C compared with 34°C in all three groups. However, the reduction was greater in control and cold control than hibernated groups. Summation, which at 10°C we could observe in a stable manner at 1 Hz, did not occur at 34°C in any group. The latency, time to peak amplitude and total duration of EJPs at 34°C in control, cold control and hibernated animals were significantly increased at 10°C (Table 2).
Effects of PPADS and prazosin on EJPs and slow depolarizations evoked by nerve stimulation at 34 and 10°C
At 34 and 10°C, in control, cold control and hibernated animals, the EJPs evoked by a single stimulus were significantly inhibited in a concentration-dependent manner in the presence of PPADS (Fig. 6A and C). Addition of prazosin (0.1-1 μm) also significantly inhibited the slow depolarizations evoked by a train of pulses (20 Hz, 10 pulses) at 34°C in a concentration dependent-manner in control, cold control and hibernated animals (Fig. 6B and C). Neither PPADS nor prazosin caused any change in resting membrane potentials in all the preparations (data not shown).
Figure 6. Effects of piridoxal phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) and prazosin on the responses to electrical field stimulation (EFS) in the hamster tibial artery smooth muscle cells.
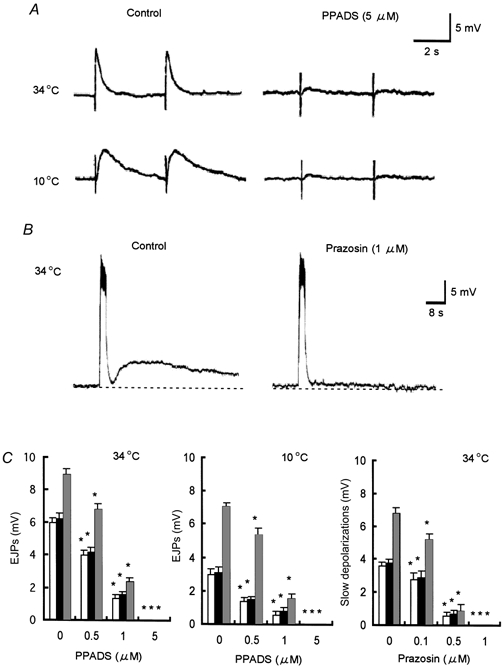
A, effects of PPADS (5 μm) on the EJPs evoked by a single stimulus (supramaximal voltage, 1 ms) at 34 and 10°C from hibernated animals. B, effect of prazosin (1 μm) on the slow depolarization evoked by a train of pulses (supramaximal voltage, 0.5 ms; 20 Hz, 20 pulses) at 34°C from a hibernated animal. Each vertical bar indicates a stimulus. C, shows summary of the effects of both antagonists on EJPs and slow depolarizations from control (□), cold control (▪) and hibernated animals ( ) All results are expressed as means ±s.e.m.; n = 5-7. *P < 0.05vs. control. Note that the EJPs and slow depolarizations from three preparations were completely abolished by PPADS and prazosin, respectively. Membrane potential for A at 34 and 10°C was -63, -57 mV, respectively, and for B was -64 mV.
) All results are expressed as means ±s.e.m.; n = 5-7. *P < 0.05vs. control. Note that the EJPs and slow depolarizations from three preparations were completely abolished by PPADS and prazosin, respectively. Membrane potential for A at 34 and 10°C was -63, -57 mV, respectively, and for B was -64 mV.
Depolarization responses by exogenous ATP and NA at 34 and 10°C
At 34°C, adventitial application of ATP (100 μm) for 1 min evoked a depolarization response in control, cold control and hibernated animals of similar amplitude (Table 2). Adventitial application of NA (5 μm) for 1 min also evoked a depolarization response in all three groups, which was not significantly different in amplitude (Table 2). At 10°C, application of both ATP (100 μm) and NA (5 μm) for 1 min also evoked a depolarization response of similar amplitude (Table 2). The depolarization responses evoked by ATP at 34 and 10°C did not differ between any groups. In contrast, the responses to NA were greatly reduced at 10°C in all groups.
DISCUSSION
The present study provides, for the first time, evidence for a temperature-dependent change in the adrenergic and purinergic components of sympathetic neurotransmission in the golden hamster hindlimb resistance vessels during hibernation. Our results show that the sympathetic perivascular neurotransmission in the hindlimb involves a prominent purinergic component and a noticeable adrenergic component. During hypothermia, this proportion changes. In hibernated animals, the purinergic component showed a marked enhancement, especially at 10°C (hibernating body temperature), compared with normothermic and cold-acclimated animals. This suggests an important purinergic involvement for maintenance of peripheral circulation during hibernation.
An increase in sympathetic perivascular neurotransmission of hamster in hibernation has been reported previously, but whether this augmentation is a result of prejunctional or postjunctional changes was not clearly defined (Ralevic et al. 1997, 1998; Karoon et al. 1998). However, the present study strongly suggests that the increase of the twitch-like and sustained responses to EFS may be caused by changes occurring prejunctionally in hibernating hamsters, at least in the resistance vessels of the hindlimb, given that there was no significant difference in responsiveness to exogenous ATP and NA among the three groups. It has been previously shown that amplitude of EJPs may indicate indirectly the release of ATP (Komori & Suzuki, 1987; McLaren et al. 1995; Brock & Cunnane, 1999). Therefore, the increased amplitude of EJPs observed in this study may also reflect an increase in ATP release.
Vascular responsiveness was also affected by cooling (Wisnes et al. 1979a;Cassell et al. 1988), as in the present experiments. The amplitudes of EJPs in animals from all the three groups were reduced significantly by cooling to 10°C although the reduction was smaller in hibernated animals. On the other hand, the frequency-response curves of the twitch responses in all groups were significantly shifted to the left by cooling to 10°C. The maximal height of the response from hibernated tissue remained much the same while that of the control and cold control animals was reduced markedly. At present, we cannot explain why the EJP in hibernated animals decreased in amplitude from 34 to 10°C whereas the maximum height of twitch response remained the same. However, we believe that the prompted summation of EJPs observed in hibernated tissues at 10°C may contribute to the shift to the left of the frequency- response curves of the twitch response. The low frequency stimulus which evoked summation at 10°C never evoked summation at 34°C, similar to the situation in the guinea-pig vas deferens (Blakeley & Cunnane, 1982). The summation is due to prolongation of the time course of the EJP, and this alteration could result from a number of factors: decreases in membrane conductance (Cassell et al. 1988; Cunnane & Manchanda, 1988), an enzymatic inactivation of ATP (Cunnane & Manchanda, 1988; Westfall et al. 1996), or the asynchronous release of quanta at low temperature (Katz & Miledi, 1965). As a consequence of summation, the total depolarization amplitude of summed EJPs increases, and it should be noted that in hibernated cells, the total amplitude is always markedly greater than those of the other groups. Furthermore, this increased depolarization could increase Ca2+ influx through ATP-gated channels more effectively, leading to vasoconstriction (Benham, 1989).
From our experiments at 10°C, it is strongly suggested that during hibernation the adrenergic involvement in the sympathetic neurotransmission may be negligible. The decline of adrenergic components is most likely to be a consequence of a progressive depression of the noradrenaline release under hypothermia, as previously shown in the dog cutaneous veins (Vanhoutte & Verbeuren, 1976; Rusch et al. 1981). However, this does not exclude the possibility that the raised tone at 10°C, which may be due to the cold-induced myogenic contraction (Smith, 1952; Cassell et al. 1988), could be strong enough to mask the adrenergic component evoked by EFS, since responses to exogenous NA evoking contractions of similar amplitude were attenuated by cooling. In either case, the adrenergic involvement may have a minor role while the purinergic component of the sympathetic neurotransmission clearly predominates under these conditions.
Our findings from the experiments at 10°C, in which we detected the enhancement in perivascular purinergic neurotransmission, and the depression of adrenergic neurotransmission from hibernated animals, strongly suggested that the experimental temperature when studying hibernation might be an important consideration. Ralevic et al. (1998) showed that an increase in the sensitivity of sympathetic perivascular neurotransmission of hibernating hamster rapidly reverted to normal during arousal from hibernation. Whether this reversibility is associated with the rising body temperature remains to be determined. However, taken together with our results, it is likely that there may be some changes in hibernation that could not be detected under normothermic conditions.
In the present study, we have detected a significant difference between control animals and cold exposed animals only in the mechanical twitch response obtained at 20 Hz at 34°C. Although this was mimicked to a lesser degree by hibernation (Karoon et al. 1998), our results obtained under the hypothermic condition, where we have not detected any significant difference between these two groups, may show a different feature of the hibernation from that of cold acclimation.
It is important to recognize that hibernation occurs under conditions of extremely low body temperature, and how this lowered temperature, fatal to other animals, affects physiological, biochemical and metabolic phenomena of hibernating animals (Lyman, 1965; Nedergaard & Cannon, 1990). Thus, a rather surprising observation in our results was that the release of ATP still elicited a functional response. In peripheral vasculature during hibernation, however, the concept of the enhancement of ATP release, the increased duration of the effect of the purinergic component, and the increase in vascular tension due to myogenic constriction under hypothermia as a compensatory means of maintaining the vascular resistance are attractive as they could explain the following phenomena: during hibernation the activities of smooth muscle membranes or various enzymes and spontaneous activity in the autonomic nerve can be depressed, remarkably as observed in the cerebral cortex of hibernating golden hamster (Chatfield et al. 1951), the neuromuscular junction of golden hamster (Moravec et al. 1973; Vyskočil, 1976) and the portal vein of the hedgehog (Eliassen & Helle, 1975). The purinergic component known as a short-lasting twitch (Burnstock & Warland, 1987) is favourably reactive to a short burst of electrical impulses (Kügelgen & Starke, 1985; Kennedy et al. 1986; Bulloch & Starke, 1990; Todorov et al. 1994). We observed it to be more sensitive during hibernation, and therefore it is ideal for precisely timed regulation of the circulatory system. Furthermore, the prolongation of the purinergic effect could help the vessel maintain resistance, offsetting the absence of sustained adrenergic involvement. Thirdly, when blood pressure and blood flow remain depressed considerably in hibernation (Wells, 1971; Zatzman, 1984) (Table 1), the myogenic constriction of arteries, especially muscle arteries (Kirkebö, 1968), may avoid congestion, helping to raise the resistance and the blood flow.
It has been proposed that under normal condition NA plays a dominant role, but under stress condition such as spontaneous hypertension the purinergic component may emerge prominently (Burnstock, 1995, 1988). Thus, we believe that in hibernation, which could be one of the stressed conditions (Hochachka, 1986; Kevelaitis et al. 1999), the proportional alteration in adrenergic and purinergic components may contribute to the compensatory change for the adaptation.
In conclusion, it is suggested that enhancement of sympathetic neurotransmission, mainly increase of ATP release, in synergism with cold-induced constriction of vascular smooth muscles, may account for the increased peripheral resistance which contributes to maintenance of vascular tone during hibernation.
Acknowledgments
The authors are grateful to Dr Richard J. Lang and Dr M. F. Klemm for helpful comments on the manuscript, as well as to Dr H. Shiomi and Dr N. Ibuka for technical suggestions for inducing hibernation.
References
- Blakeley AGH, Cunnane TC. An electrophysiological analysis of the effects of cooling on autonomic neuromuscular transmission in the guinea-pig vas deferens. Quarterly Journal of Experimental Physiology. 1982;67:617–628. doi: 10.1113/expphysiol.1982.sp002681. [DOI] [PubMed] [Google Scholar]
- Benham CD. ATP-activated channels gate calcium entry in single smooth muscle cells dissociated from rabbit ear artery. Journal of Physiology. 1989;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton TB, Lang RJ, Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric arteries. Journal of Physiology. 1984;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, Cunnane TC. Effects of Ca2+ concentration and Ca2+ channel blockers on noradrenaline release and purinergic neuroeffector transmission in rat tail artery. British Journal of Pharmacology. 1999;126:11–18. doi: 10.1038/sj.bjp.0702256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloch JM, McGrath JC. Evidence for increased purinergic contribution on hypertensive blood vessels exhibiting co-transmission. British Journal of Pharmacology. 1992;107(suppl.):145P. [Google Scholar]
- Bulloch JM, Starke K. Presynaptic α2-autoinhibition in a vascular neuroeffector junction where ATP and noradrenaline act as co-transmitters. British Journal of Pharmacology. 1990;99:279–284. doi: 10.1111/j.1476-5381.1990.tb14694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Sympathetic purinergic transmission in small blood vessels. Trends in Pharmacological Sciences. 1988;9:116–117. doi: 10.1016/0165-6147(88)90185-x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Noradrenaline and ATP: Co-transmitters and neuromodulators. Journal of Physiology and Pharmacology. 1995;46:365–384. [PubMed] [Google Scholar]
- Burnstock G, Warland JJI. A pharmacological study of the rabbit saphenous artery in vivo: a vessel with a large purinergic contractile response to symapathetic nerve stimulation. British Journal of Pharmacology. 1987;90:111–120. doi: 10.1111/j.1476-5381.1987.tb16830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell JF, McLachlane EM, Sittiracha T. The effects of temperature on neuromuscular transmission in the main caudal artery of the rat. Journal of Physiology. 1988;397:31–49. doi: 10.1113/jphysiol.1988.sp016986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield PO, Lyman CP, Purpura DP. The effects of temperature on the spontaneous and induced electrical activity in the cerebral cortex of the golden hamster. Electroencephalography and Clinical Neurophysiology. 1951;3:225–230. doi: 10.1016/0013-4694(51)90015-6. [DOI] [PubMed] [Google Scholar]
- Cunnane TC, Manchanda R. Electrophysiological analysis of the inactivation of symapathetic transmitter in the guinea-pig vas deferens. Journal of Physiology. 1988;404:349–364. doi: 10.1113/jphysiol.1988.sp017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliassen E, Helle KB. Spontaneous activity in smooth muscle of the portal vein of the hedgehog Erinaceus Europaeus, L. Comparative Biochemistry and Physiology. 1975;52:C119–125. doi: 10.1016/0306-4492(75)90025-8. [DOI] [PubMed] [Google Scholar]
- Flavahan NA, Vanhoutte PM. Sympathetic purinergic vasoconstriction and thermosensitivity in a canine cutaneous vein. Journal of Pharmacology and Experimental Therapeutics. 1986;239:784–789. [PubMed] [Google Scholar]
- Hochachka PW. Defense strategies against hypoxia and hypothermia. Science. 1986;231:234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hoffman RA, Hester RJ, Towns C. Effect of light and temperature on the endocrine system of the golden hamster (Mesocricetus auratus Waterhouse) Comparative Biochemistry and Physiology. 1965;15:525–533. doi: 10.1016/0010-406x(65)90152-0. [DOI] [PubMed] [Google Scholar]
- Karoon P, Knight G, Burnstock G. Enhanced vasoconstrictor responses in renal and femoral arteries of the golden hamster during hibernation. Journal of Physiology. 1998;512:927–938. doi: 10.1111/j.1469-7793.1998.927bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. Journal of Physiology. 1965;181:656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Saville V, Burnstock G. The contribution of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerves stimulation depend on the parameters of stimulation. European Journal of Pharmacology. 1986;122:291–300. doi: 10.1016/0014-2999(86)90409-7. [DOI] [PubMed] [Google Scholar]
- Kirkebö A. Temperature effects on the viscosity of blood and the aorta distension from a hibernator, Einaceus europaeus L. Acta Physiologica Scandinavica. 1968;73:385–393. doi: 10.1111/j.1365-201x.1968.tb10877.x. [DOI] [PubMed] [Google Scholar]
- Komori K, Suzuki H. Heterogeneous distribution of muscarinic receptors in the rabbit saphenous artery. British Journal of Pharmacology. 1987;92:657–664. doi: 10.1111/j.1476-5381.1987.tb11369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kügelgen IV, Starke K. Noradrenaline and adenosine triphosphate as co-transmitters of neurogenic vasoconstriction in rabbit mesenteric artery. Journal of Physiology. 1985;367:435–455. doi: 10.1113/jphysiol.1985.sp015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman CP, O'Brien RC. Autonomic control of circulation during the hibernating cycle in ground squirrels. Journal of Physiology. 1963;168:477–499. doi: 10.1113/jphysiol.1963.sp007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman CP. Circulation in mammalian hibernation. In: Hamilton WF, editor. Handbook of Physiology, Circulation. III. Washington, DC: American Physiological Society; 1965. pp. 1967–1990. section 2, chap. 56. [Google Scholar]
- McLaren GJ, Kennedy C, Sneddon P. Effects of suramine on purinergic and noradrenergic neurotransmission in rat isolated tail artery. European Journal of Pharmacology. 1995;277:57–61. doi: 10.1016/0014-2999(95)00065-s. [DOI] [PubMed] [Google Scholar]
- Moravec J, Melichar I, Janský L, Vyskočil F. Effect of hibernation and noradrenaline on the resting state of neuromuscular junction of golden hamster (Mesocricetus auratus) Pflügers Archiv. 1973;345:93–106. doi: 10.1007/BF00585833. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. Mammalian hibernation. Philosophical Transactions of the Royal Society. 1990;B326:669–686. doi: 10.1098/rstb.1990.0038. [DOI] [PubMed] [Google Scholar]
- Nürnberger F. The neuroendocrine system in hibernating mammals: present knowledge and open questions. Cell and Tissue Research. 1995;281:391–412. doi: 10.1007/BF00417858. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Hill B, Crowe R, Knight G, Burnstock G. Effects of hibernation on neural and endothelial control of mesenteric arteries of the golden hamster. American Journal of Physiology. 1997;273:H148–155. doi: 10.1152/ajpheart.1997.273.1.H148. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Knight G, Burnstock G. Effects of hibernation and arousal from hibernation on mesenteric arterial responses of the golden hamster. Journal of Pharmacology and Experimental Therapeutics. 1998;287:521–526. [PubMed] [Google Scholar]
- Rusch NJ, Shepherd JT, Vanhoutte PM. The effects of profound cooling on adrenergic neurotransmission in canine cutaneous vein. Journal of Physiology. 1981;311:57–65. doi: 10.1113/jphysiol.1981.sp013572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Thapaliya S, Takewaki T. The enhancement of ATP release from hindlimb sympathetic perivascular nerve of hibernating hamster. Japanese Journal of Pharmacology. 2000;82:351P. doi: 10.1111/j.1469-7793.2001.0495i.x. suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ. Constriction of isolated arteries and their vasa vasorum produced by low temperature. American Journal of Physiology. 1952;171:528–537. doi: 10.1152/ajplegacy.1952.171.3.528. [DOI] [PubMed] [Google Scholar]
- Thapaliya S, Matsuyama H, Takewaki T. ATP released from perivascular nerves hyperpolarizes smooth muscle cells by releasing an endothelium-derived factor in hamster mesenteric arteries. Journal of Physiology. 1999;521:191–199. doi: 10.1111/j.1469-7793.1999.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov LD, Bjur RA, Westfall DP. Temporal dissociation of the release of the sympathetic co-transmitters ATP and noradrenaline. Clinical and Experimental Pharmacology and Physiology. 1994;21:931–932. doi: 10.1111/j.1440-1681.1994.tb02469.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Verbeuren TJ. Depression by local cooling of 3H-norepinephrine release evoked by nerve stimulation in cutaneous veins. Blood Vessels. 1976;13:92–99. doi: 10.1159/000158082. [DOI] [PubMed] [Google Scholar]
- Vidal M, Hicks PE, Langer SZ. Differential effects of α-β-methylene ATP on responses to nerve stimulation in SHR and WKY tail arteries. Naunyn-Schmiedebergs’ Archives of Pharmacology. 1986;332:384–390. doi: 10.1007/BF00500092. [DOI] [PubMed] [Google Scholar]
- Vyskočil F. Miniature end-plate potential and sensitivity to acetylcholine in the fast and slow limb muscles of hibernating golden hamsters. Pflügers Archiv. 1976;361:165–167. doi: 10.1007/BF00583461. [DOI] [PubMed] [Google Scholar]
- Wells LA. Circulatory patterns of hibernators. American Journal of Physiology. 1971;221:1517–1520. doi: 10.1152/ajplegacy.1971.221.5.1517. [DOI] [PubMed] [Google Scholar]
- Westfall TD, Kennedy C, Sneddon P. Enhancement of sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens by the novel ecto-ATPase inhibitor ARL 67156. British Journal of Pharmacology. 1996;117:867–872. doi: 10.1111/j.1476-5381.1996.tb15273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnes AR, Stene-Larsen G, Eliassen E. The effects of low temperature on the pressor response to noradrenaline in a hibernating (hedgehog) and a nonhibernating mammal (rat) Cryobiology. 1979a;16:78–82. doi: 10.1016/0011-2240(79)90014-2. [DOI] [PubMed] [Google Scholar]
- Wisnes AR, Eliassen E, Helle KB, Stene-Larsen G. Effects of extracellular potassium on the pressure response to noradrenaline in the perfused hindquarters of the rat. Clinical and Experimental Pharmacology and Physiology. 1979b;6:487–493. doi: 10.1111/j.1440-1681.1979.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Zatzman ML. Renal and cardiovascular effects of hibernation and hypothermia. Cryobiology. 1984;21:593–614. doi: 10.1016/0011-2240(84)90220-7. [DOI] [PubMed] [Google Scholar]


