Abstract
Angiotensin II (ANGII) acting on ANGII type 1 (AT1) receptors in the solitary tract nucleus (NTS) depresses the baroreflex. Since ANGII stimulates the release of nitric oxide (NO), we tested whether the ANGII-mediated depression of the baroreflex in the NTS depended on NO release.
In a working heart-brainstem preparation (WHBP) of rat NTS microinjection of either ANGII (500 fmol) or a NO donor (diethylamine nonoate, 500 pmol) both depressed baroreflex gain by -56 and -67 %, respectively (P < 0.01). In contrast, whilst ANGII potentiated the peripheral chemoreflex, the NO donor was without effect.
NTS microinjection of non-selective NO synthase (NOS) inhibitors (l-NAME; 50 pmol) or (l-NMMA; 200 pmol) prevented the ANGII-induced baroreflex attenuation (P > 0.1). In contrast, a neurone-specific NOS inhibitor, TRIM (50 pmol), was without effect.
Using an adenoviral vector, a dominant negative mutant of endothelial NOS (TeNOS) was expressed bilaterally in the NTS. Expression of TeNOS affected neither baseline cardiovascular parameters nor baroreflex sensitivity. However, ANGII microinjected into the transfected region failed to affect the baroreflex.
Immunostaining revealed that eNOS-positive neurones were more numerous than those labelled for AT1 receptors. Neurones double labelled for both AT1 receptors and eNOS comprised 23 ± 5.4 % of the eNOS-positive cells and 57 ± 9.2 % of the AT1 receptor-positive cells. Endothelial cells were also double labelled for eNOS and AT1 receptors.
We suggest that ANGII activates eNOS located in either neurones and/or endothelial cells to release NO, which acts selectively to depress the baroreflex.
There are a number of pathologies of the cardiorespiratory system that are associated with raised activity of the renin-angiotensin II system. Some forms of hypertension, which have been related to heightened activity of angiotensin II (ANGII), are associated with a depressed baroreceptor reflex gain. In this regard, several groups including ourselves have shown that low physiological doses of ANGII acting in the NTS, the central termination site of cardiorespiratory afferents (e.g. see Blessing, 1997, for review), reversibly depressed the cardiac component of the baroreceptor reflex (Casto & Phillips, 1986; Michelini & Bonagamba, 1990; Luoh & Chan, 1998; Paton & Kasparov, 1999).
In the present study we sought to determine a possible transduction mechanism that could account for the depressant effect of ANGII in the NTS on the baroreceptor reflex. Since ANGII can stimulate release of nitric oxide (NO) from endothelial cells in peripheral vascular beds (see Millatt et al. 1999, for review), we considered whether a similar mechanism operates in the NTS. That is, does exogenously applied ANGII activate nitric oxide synthase (NOS), located in either endothelial cells or neurones in the NTS, to release NO, which acts as an intermediate to attenuate the baroreceptor reflex?
The ‘neuronal’ isoform of NOS (nNOS) has been found in the NTS (Ruggiero et al. 1996; Lawrence et al. 1998; Batten et al. 2000) including NTS neurones expressing c-fos in response to induced hypertension (Chan & Sawchenko, 1998). In addition to NTS neurones, vagal afferents also contain nNOS immunoreactivity (Lin et al. 1998). Moreover, some second order NTS neurones innervated by primary vagal afferents were immunopositive for nNOS (Batten et al. 2000). Based on this anatomical substrate it is not surprising that NO in the NTS influences circulatory control (see Lawrence & Jarrott, 1996, for review). Indeed, NO donors in the NTS caused bradycardia and hypotension (Tseng et al. 1996; Vitagliano et al. 1996; Lin et al. 1999) whereas NOS inhibitors produced an opposite pattern of response (Harada et al. 1993). The latter is consistent with the hypertension produced following a NTS injection of antisense oligonucleotides for nNOS (Maeda et al. 1999). Although these effects may or may not be mediated by NTS circuitry subserving the baroreceptor reflex, other studies have investigated the effects of NO on this reflex directly. The data, however, are inconsistent. Both a blockade of NOS in the NTS and microinjection of NO donors failed to affect the baroreceptor reflex (Harada et al. 1993; Pontieri et al. 1998; Zanzinger et al. 1995). However, Hironaga et al. (1998) showed that intracisternal blockade of NOS reduced the duration of baroreceptor reflex-induced inhibition of renal sympathetic nerve activity suggesting a tonic potentiating effect of NO on the sympathetic limb of the reflex. Results obtained in the spontaneously hypertensive rat model are also inconsistent: Pontieri et al. (1998) found no effect of inhibiting NOS activity in the NTS on baroreceptor reflex gain whereas an increase was observed by Kumagai et al. (1993). The latter result might support a tonic release of NO suppressing baroreflex function in hypertensive rats.
In the light of the contrasting reports regarding the actions of NO in the NTS on baroreceptor reflex gain we have re-assessed its role in an unanaesthetised decerebrate rat model to circumvent problems related to anaesthesia. Both pharmacological and in vivo gene transfer experiments support a major role for endothelial NOS (eNOS) in the NTS in the regulation of baroreceptor reflex gain.
Preliminary reports of this study were presented previously (Paton & Kasparov, 2000b; Paton et al. 2000).
METHODS
Setting up a working heart-brainstem preparation (WHBP)
Male Wistar rats between 80 and 120 g were anaesthetised deeply with halothane to achieve a complete loss of reflex withdrawal responses following a pinch of a hindpaw or the tail. Subsequently, rats were transected sub-diaphragmatically, decerebrated at the precollicular level, cerebellectomised (to fully expose the fourth ventricle) and skinned in ice-chilled artificial cerebrospinal fluid (ACSF). The thorax and head were perfused with ACSF containing Ficoll (1.25 %) at 31°C, which was gassed with carbogen (95 % O2 and 5 % CO2). The perfusate was pumped at a constant flow via a double lumen catheter inserted into the aorta retrogradely with the second lumen connected to a pressure transducer to measure the aortic pressure directly.
The left phrenic nerve was isolated and its activity recorded via a glass suction electrode. Phrenic nerve activity was integrated using a time constant of 100 ms. Using a window discriminator, the R-wave of the electrocardiogram (ECG) present in the phrenic nerve activity was used to generate transistor-transistor logic (TTL) pulses, which together with the digitised arterial pressure signal were acquired by a CED 1401plus interface, displayed on a computer using Spike2 software (CED, UK) and stored on a hard drive. The instantaneous frequency of the TTL pulses was computed to give heart rate in beats per minute (bpm). Over an initial period of 10-15 min perfusion pressure was adjusted so that phrenic nerve motor outflow showed stable rhythmic discharges with an augmenting pattern.
Baro- and chemoreceptor reflex stimulation
The baroreceptor reflex was evoked by increasing the rate of perfusion for 2-3 s, which elevated perfusion pressure at a constant rate in any individual preparation as recently described (Paton & Kasparov, 1999). In all experiments a series of increases in perfusion pressure of various amplitudes were made. Peak changes (Δ) in perfusion pressure and heart rate were measured on-line and baroreceptor reflex gain calculated as the ratio Δheart rate/Δperfusion pressure (expressed as bpm mmHg−1). These values were plotted to produce reflex function curves. At least 5 min intervals between the pressure ramp tests were necessary to obtain reproducible gain values. To activate peripheral arterial chemoreceptors, three doses of sodium cyanide (CN; 0.03 % solution, 7.5, 15 or 22.5 μg) were injected directly into the aorta via the perfusion cannula. Our previous studies showed that these doses give repeatable submaximal responses consistently (Paton & Kasparov, 1999). The peripheral chemoreceptor reflex response consisted of an increase in central inspiratory drive and a powerful bradycardia; the latter was evident since lung stretch receptors are not activated in the WHBP.
Bilateral microinjections
The colourless perfusate and cerebellectomy in the WHBP allow clear visualisation of surface landmarks on the dorsal medulla. Calamus scriptorius was used as a reference for the orientation of micropipettes into sites known to contain baroreceptive NTS neurones in the rat (Deuchars et al. 2000) and baroreceptor afferent terminals (see Blessing, 1997, for example). Drugs were applied bilaterally using pressure injection from a 3-barrelled micropipette (tip diameter 30-40 μm approximately), which was driven into the medulla to a depth of 400-450 μm ventral to the dorsal surface, ±400 μm rostro-caudal relative to calamus scriptorius and between 250 and 500 μm from midline where the medial-most injections were most caudal. The injected volume (50 nl) was measured by observing the movement of the meniscus through a binocular microscope fitted with a calibrated eye piece graticule. Sequential bilateral microinjections were made with the second injection completed within 45 s of the first. In experiments where NOS inhibitors were used, these were microinjected prior to the microinjection of ANGII. Only one type of NOS inhibitor was used per preparation and no more than four microinjections were made into one side of the NTS in any given preparation.
Experimental protocol
At least three stable reflex control responses were obtained before a NTS microinjection of either ANGII or a NO donor alone was given. Baro- and chemoreceptor reflexes were re-tested in random order within 60-90 s following completion of a bilateral microinjection and further reflex testing was performed at 5 min intervals until reflex sensitivity was comparable to control levels. In all cases the effects of the microinjectates were reversible and washed out over a period of 10-20 min. Where a NOS inhibitor was used, the effects of ANGII were tested first. Once reflexes had recovered to control levels, bilateral microinjections of the inhibitor followed immediately by one of ANGII were given. Bilateral NTS microinjections of 0.9 % NaCl alone did not cause any measurable effects on resting cardiorespiratory parameters or reflexes. At the end of the experiment the position of the micropipette was marked by injection of 50 nl of 2 % Pontamine Sky Blue. The brainstem was removed, fixed and subsequently sectioned (50 μm) and counterstained with Neutral Red. Microinjection sites were documented on pre-drawn sections from Paxinos & Watson (1986).
TeNOS adenoviral vectors
Ad-TeNOS was the kind gift of Drs Erin Schuman and Norman Davidson (California Institute of Technology, Pasadena, CA, USA). Ad-TeNOS is a replication-deficient recombinant adenoviral vector that directs the expression of a truncated form of eNOS (TeNOS) under the constitutive control of the cytomegalovirus (CMV) promoter-enhancer (Kantor et al. 1996). TeNOS lacks catalytic activity yet retains the NH2-terminal sequences required for cotranslational NH2-terminal glycine myristoylation (Liu & Sessa, 1994) and membrane localisation (Busconi & Michel, 1993). TeNOS acts as a dominant negative inhibitor of wild-type eNOS activity through heterodimerisation with the native protein (Lee et al. 1995). To visualise the infected areas Ad-TeNOS was mixed with the tetracycline-regulated binary adenoviral system (Harding et al. 1997, 1998) expressing enhanced green fluorescent protein (eGFP). The two viruses that comprise this system co-infect target cells. The first virus (Ad-CMV-TetOFF) drives the expression of the Tet-OFF transcriptional activator under the control of the CMV promoter. The second virus (Ad-TRE-eGFP) drives the expression of eGFP under the control of a basal mammalian promoter and tetracycline responsive elements (TREs). In the absence of tetracycline, Tet-OFF binds to the TREs and directs the expression of eGFP. Using standard techniques (Graham & Prevec, 1995), adenoviral vectors were purified by CsCl centrifugation to the following titres (plaque-forming units (pfu) per ml): Ad-TeNOS, 3 x 109 pfu ml−1; Ad-CMV-Tet-OFF, 1 x 1010 pfu ml−1; Ad-TRE-eGFP, 2.8 x 109 pfu ml−1. For injections, Ad-TeNOS was mixed with Ad-CMV-Tet-OFF and Ad-TRE-eGFP in a ratio of viral particles of 2.0:3.6:1.0 to give a total final titre of 4.7 x 109 pfu ml−1. For control injections, Ad-CMV-Tet-OFF was mixed with Ad-TRE-eGFP in a ratio of viral particles of 4:1 to give a final titre of 6.4 x 109 pfu ml−1.
In vivo gene transfer in the NTS
Weaned male Wistar rats (60-70 g) were deeply anaesthetised with a mixture of halothane (1.5 %) and nitrous oxide (80 %). They were placed in a stereotaxic head holder and through a midline incision in the dorsal neck the caudal dorsal medulla was exposed. Bilateral microinjections (300 nl over 1.5 min, 3 injections on either side) were made at the level of calamus scriptorius and 300 μm rostral and caudal to it, which were 300-400 μm from midline and 350-500 μm below the dorsal surface of the medulla. Two groups of animals were injected. The first received NTS microinjections of Ad-TeNOS, Ad-CMV-TetOFF and Ad-TRE-eGFP. A second control group of animals received bilateral microinjections of Ad-CMV-TetOFF and Ad-TRE-eGFP only. The wound was sutured, cleaned and treated with neomycin powder, and the rats allowed to recover. All animals received non-steroidal analgesia and a course of antibiotics (i.m.). Rats were inspected daily but there were never any signs of discomfort. Further, there were never any infections around the wound, which healed cleanly, and no signs of rough coats, loss of weight or lethargy post-operatively. Following 5-6 days, rats were used for physiological experiments using the WHBP in which the effects of ANGII microinjection into the NTS on the baroreceptor reflex gain were tested (see above). The latter studies were performed ‘blind’ so that the experimenter was unaware of the combination of vectors used. NTS regions expressing eGFP were documented following fixation of the brain and cutting of transverse sections as described above. Transfected regions were plotted and microinjection sites superimposed on representative transverse sections from Paxinos & Watson (1986).
Solutions and drugs
The ACSF contained the following (mm): 10 dextrose, 125 NaCl, 24 NaHCO3, 5 KCl, 2.5 CaCl2, 1.25 MgSO4, 1.25 KH2PO4. Chemicals were obtained from Sigma-Aldrich (UK) unless stated otherwise. Based on our previous study we used 500 fmol of ANGII as a standard to produce a significant attenuation of the baroreceptor reflex-induced bradycardia (Paton & Kasparov, 1999). l-Arginine (100 pmol) was microinjected as a NO precursor. Two NO donors were used: diethylamine nonoate (NONOate, 50 and 500 pmol; Calbiochem) and sodium nitroprusside (SNP, 50 pmol). Three NOS inhibitors were used: NG-nitro-l-arginine methyl ester hydrochloride (l-NAME, 50 pmol), NG-monomethyl-l-arginine monoacetate (l-NMMA, 200 pmol; RBI) and 1-(2-trifluoromethylphenyl)imidazole (TRIM, 50 pmol; RBI). All drugs were dissolved in 0.9 % NaCl.
Statistical analysis
All data were analysed using Spike2 software with custom-written scripts. To obtain the baroreceptor and chemoreceptor reflex input-output curves data were fitted using Prism (GraphPad) and Excel (Microsoft) as described previously (Paton & Kasparov, 1999). The integral of rectified phrenic nerve activity was measured for a period covering three to five control cycles. This was compared with the phrenic nerve integral during the reflex response using an identical time window. To standardise data across preparations phrenic nerve activity integrals were expressed as percentages of control. The significance of effects was assessed by Student’s paired two-tailed t test to the raw data. All values are quoted as the mean ± standard error of mean (s.e.m.) and n is the number of preparations. Differences were taken as significant at the 95 % confidence limit.
Immunocytochemical studies
Male rats (100-150 g) were anaesthetised with an intraperitoneal injection of 60 mg kg−1 pentobarbitone (Sagatal) and perfused transcardially with 100 ml of 0.9 % saline followed by 500 ml of ice-cold fixative containing 0.01-0.5 % glutaraldehyde and 4 % paraformaldehyde. Brainstems were removed and post-fixed for 4-12 h at 4°C before sectioning on a vibrating microtome (Leica) at 50 μm; sections were collected in phosphate-buffered saline (PBS) in glass vials. To differentiate between NTS neurones and dorsal vagal motoneurones we pre-labelled vagal preganglionic neurones in two rats by injecting 100 μl of 2 % FluoroGold intraperitoneally for 7 days.
Fluorescence microscopy
Following 3 x 10 min washes in PBS the sections were transferred to PBS with 0.1 % Triton, containing rabbit anti-AT1 receptor antibody (1:2000 to 1:10 000; Giles et al. 1999) and one of two mouse anti-eNOS antibodies (1:500 to 1:2000 for both; Sigma, clone NOS-E1; Transduction Laboratories) for 12-48 h at 4°C. The anti-AT1 receptor antibody was a generous gift from Dr A. Allen (Howard Florey Institute, University of Melbourne, Australia). Sections were then washed for 3 x 30 min in PBS and incubated in donkey anti-rabbit antibody conjugated to Cy3 (1:500; Jackson Laboratories) for 4-12 h, followed by 3 x 30 min washes in PBS and incubation in biotinylated anti-mouse antibody (1:500, Vector Laboratories) for 3-12 h. Following 3 x 30 min washes in PBS the sections were incubated in streptavidin-Alexa488 (1:500 to 1:1000; Molecular Probes) for 2-6 h. Finally the sections were washed in PBS and mounted on glass slides with coverslips using VectaMount (Vector Laboratories). To control for possible non-specific labelling of secondary antibodies, some sections were incubated in only one of the primary antibodies, but processed through the rest of the procedure as above. Sections were then examined on a fluorescence microscope and Cy3 visualised with a Cy3 filter set (red), Alexa488 with a fluorescein isothiocyanate (FITC) filter set (green) and FluoroGold with a UV filter set. Images were obtained by using an Acquis Image Capture system (Synoptics) equipped with a JVC KYF55 camera and manipulated in Corel Photopaint 8.
Neurones and vessels singly labelled for eNOS and AT1 receptors could be observed, indicating that there was minimal bleed through of fluorescence from one filter set to the other. To determine the degree of double labelling, sections from four different rostro-caudal levels of the medulla were examined at x 40 magnification and the number of double labelled neurones calculated as a percentage of immunoreactive cells. To further confirm that double labelling was not due to bleed through of fluorescent dyes, co-localisation of markers was verified with scanning laser confocal microscopy (Leica TCS).
Transmitted light microscopy
To map the distribution of labelled neurones some sections were incubated with either the anti-AT1 receptor antibody or the anti-eNOS antibodies as described above. However, the biotinylated secondary antibody was localised with avidin-biotin-peroxidase complex (Vector ABC kit), which was visualised with diaminobenzidine. The results were documented using a drawing tube attached to a light microscope.
RESULTS
Baroreceptor reflex studies in naive rats
Baseline cardiorespiratory variables (n = 18)
In all preparations phrenic nerve activity displayed an augmenting motor pattern with ramps of 0.66 ± 0.02 s. This pattern conforms to Cohen’s (1979) definition of eupnoea and is indicative of an adequately perfused brainstem. The basal levels of perfusion pressure, heart rate, phrenic nerve activity cycle length and baroreceptor reflex gain are shown in Table 1. Baroreceptor reflex stimulation also depressed phrenic nerve activity (Table 1). A sinus arrhythmia was also observed (see Table 1).
Table 1.
Baseline cardiorespiratory variables in naive, TeNOS- and eGFP-transfected WHBPs
| Perfusion pressure (mmHg) | HR (bpm) | PNA cycle length (s) | Respiratory sinus arrhythmia (bpm) | Baroreflex gain (bpm mmHg−1) | |
|---|---|---|---|---|---|
| Naive (18) | 76 ± 1 | 339 ± 24 | 3.7 ± 0.3 | −14 ± 3 | 1.6 ± 0.1 |
| TeNOS (14) | 80 ± 3 | 295 ± 22 | 3.7 ± 0.2 | −12 ± 2 | 1.8 ± 0.2 |
| eGFP (7) | 76 ± 3 | 293 ± 23 | 4.6 ± 0.6 | −11 ± 1 | 1.5 ± 0.2 |
n is given in parentheses. HR, heart rate; PNA, phrenic nerve activity.
Effect of a NO precursor and NO donors in the NTS on the baroreceptor reflex
Exogenous l-arginine microinjected into the NTS did not affect resting levels of the cardiorespiratory variables monitored but caused a consistent and significant reduction in the baroreceptor reflex bradycardic response, reducing the gain from 1.66 ± 0.16 to 0.80 ± 0.17 bpm mmHg−1 (Fig. 1Fig. 2; n = 5, P < 0.01). This was accompanied by a decrease in the reflex reduction of phrenic nerve activity integral from -43.4 ± 6 to -22.7 ± 5 % (Fig. 1Fig. 2; n = 5, P < 0.01). Both effects were reversible after 10-15 min (Fig. 1Fig. 2).
Figure 1. NO in the NTS attenuates the baroreceptor reflex.
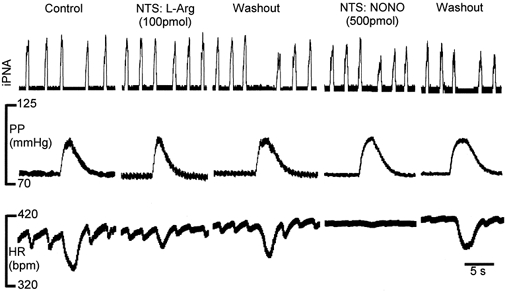
Raw data showing that in the NTS NO derived from either a precursor (l-arginine, l-Arg) or a donor (diethylamine nonoate, NONO) reduced both baroreceptor reflex-induced cardiac and phrenic nerve activity responses. The baroreceptor reflex was stimulated by increases in perfusion pressure. Both effects reversed after 10 min approximately. Note the reduction in respiratory sinus arrhythmia, which may represent a reduced excitatory drive from the NTS to cardiac vagal motoneurones or possible spread of injectate to cardiac vagal motoneurones in the dorsal vagal nucleus. Abbreviations: HR, heart rate in beats per minute (bpm); iPNA, integrated phrenic nerve activity; PP, perfusion pressure.
Figure 2. NO precursor and two NO donors in the NTS attenuate the baroreceptor reflex.
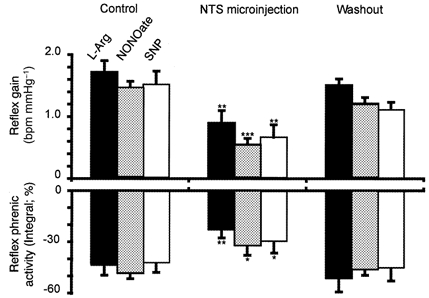
Group data summarising the effects of bilateral NTS microinjections of l-arginine (l-Arg, 100 pmol), diethylamine nonoate (NONOate, 500 pmol) and sodium nitroprusside (SNP, 50 pmol) on the baroreceptor reflex responses in heart rate and integrated phrenic nerve activity. The reflex cardiac response is expressed as the gain (see Methods) and data are taken from the linear part of the input-output function curve. Washout values were obtained up to 20 min after a bilateral microinjection. *P < 0.05, **P < 0.01, ***P < 0.001.
Similar effects were observed with both NO donors used: a bilateral NTS microinjection of NONOate (500 pmol) did not affect baseline heart rate or phrenic nerve activity. However, it depressed significantly both the baroreceptor reflex gain from 1.5 ± 0.04 to 0.5 ± 0.03 bpm mmHg−1 (Fig. 1, 2 and 3A; n = 6, P < 0.001) and the reflex-evoked reduction in phrenic nerve activity integral from -48.2 ± 3 to -32 ± 5 % (Fig. 1Fig. 2; n = 6, P < 0.05). This effect occurred within 1-2 min following the injections, persisted for 5-10 min and thereafter was fully reversible (Fig. 1Fig. 2). Microinjection of a lower dose of NONOate (50 pmol) was without effect (n = 6, data not shown). SNP (50 pmol) microinjections yielded qualitatively similar data. There was no change to baseline cardiorespiratory variables but baroreceptor reflex gain was reversibly reduced from 1.5 ± 0.3 to 0.65 ± 0.2 bpm mmHg−1 (P < 0.01) and the reflex reduction in phrenic nerve activity integral fell from -42.3 ± 5 to -29.5 ± 6 % (Fig. 2; n = 4, P < 0.05).
Figure 3. Effects of NO and ANGII on baroreceptor reflex input-output function.
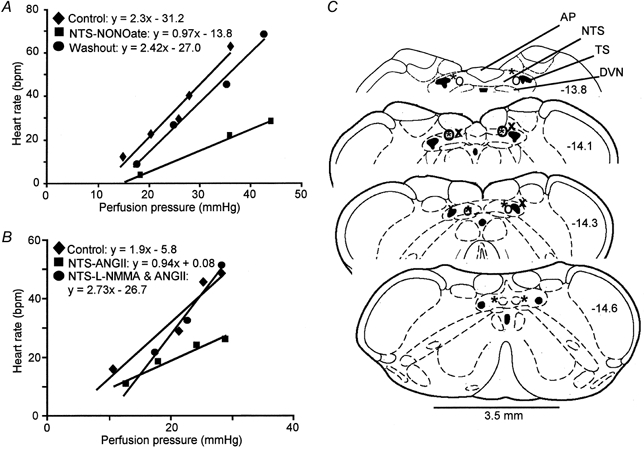
A, a representative preparation illustrating the reflex bradycardiac responses to incrementing ramp increases in perfusion pressure. Bilateral NTS microinjection of NONOate (500 pmol) reduced the gain of the baroreceptor reflex. This effect reversed after 10 min (Washout). B, an example showing the baroreceptor reflex response curve under control conditions, after a bilateral NTS microinjection of ANGII (500 fmol) alone and after rapid consecutive injections of l-NMMA (200 pmol) and ANGII (500 fmol). The ANGII-induced depression of the baroreceptor reflex was completely prevented by the NOS inhibitor. Note that the rate of rise of the increases in perfusion pressure were comparable throughout the experiment. C, representative serial transverse sections through the medulla indicating many of the sites of microinjection for NONOate and l-Arg (left-hand side) and l-NAME-ANGII and l-NMMA-ANGII (right-hand side). The different symbols (*, ○ and x) denote different experiments. Numbers refer to the distance from bregma. Abbreviations: AP, area postrema, DVN, dorsal vagal motor nucleus; NTS, nucleus of the solitary tract; TS, solitary tract.
Effect of ANGII in the NTS on the baroreflex and its antagonism by NOS inhibitors
Bilateral microinjections of 500 fmol ANGII into the NTS caused transient effects (≈30 s) on heart rate (tachycardia) and phrenic nerve activity (tachypnoea), comparable to those described recently by us (see Paton & Kasparov, 1999). These transient effects were not quantified further in the present study. Once baseline cardiorespiratory variables returned to their control levels, both the baroreceptor reflex gain and the reflex fall in the respiratory response were attenuated (see Table 2 and Fig. 3BFig. 4). Thus, the gain fell significantly from 1.6 ± 0.06 to 0.7 ± 0.03 bpm mmHg−1 (P < 0.01) and phrenic nerve discharge integral was reduced from -41.1 ± 5.2 to -23.6 ± 5.3 % (n = 7, P < 0.05).
Table 2.
Effects of three different NOS inhibitors microinjected into the NTS on the ANGII-induced depression of the baroreceptor reflex
| Control | ANGII | L-NAME | L-NAME + ANGII | |
|---|---|---|---|---|
| Gain (bpm mmHg−1) | 1.5 ± 0.2 | 0.8 ± 0.1 *** | 1.47 ± 0.2 | 1.3 ± 0.3 |
| PNA (% integral) | −39 ± 5 | −24 ± 5 * | −40.1 ± 4 | −35 ± 1.9 |
| Control | ANGII | L-NMMA | L-NMMA + ANGII | |
|---|---|---|---|---|
| Gain (bpm mmHg−1) | 1.8 ± 0.2 | 1.0 ± 0.2 *** | 1.6 ± 0.2 | 1.6 ± 0.3 |
| PNA (% integral) | −32 ± 6 | −24 ± 5 * | −34 ± 3 | −32 ± 5 |
| Control | ANGII | TRIM | TRIM + ANGII | |
|---|---|---|---|---|
| Gain (bpm mmHg−1) | 1.7 ± 0.3 | 0.9 ± 0.2 *** | 1.8 ± 0.3 | 1.0 ± 0.3 ** |
| PNA (% integral) | −39 ± 4 | −23 ± 3 ** | −41 ± 5 | −22 ± 5 ** |
ANGII, 500 fmol; L-NAME, 50 pmol; L-NMMA, 200 pmol; TRIM, 50 pmol. n = 6 for all.
P < 0.05
P < 0.01
P < 0.001
Figure 4. NOS inhibitor blocks the depressant effect of ANGII in the NTS on the baroreceptor reflex bradycardia.
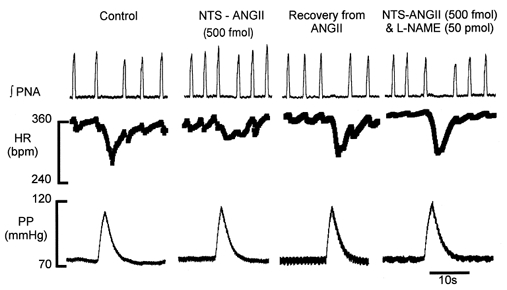
Original recordings of integrated phrenic nerve activity (∫PNA), heart rate (HR) and perfusion pressure (PP). A bilateral NTS microinjection of ANGII into the NTS attenuated the reflex cardiac and phrenic nerve responses. This effect of ANGII was prevented by a prior microinjection of a NOS inhibitor (l-NAME).
NTS microinjection of any of the NOS inhibitors used (l-NAME, l-NMMA and TRIM) did not effect baseline cardiorespiratory variables or baroreceptor reflex gain (Table 2). However, a bilateral NTS microinjection of either l-NAME (n = 6) or l-NMMA (n = 6), prior to ANGII, prevented the attenuation of the baroreceptor reflex cardiac and phrenic nerve activity responses seen when ANGII was microinjected alone (Table 2, Fig. 3BFig. 4). In contrast, TRIM, acting preferentially on nNOS, did not prevent the ANGII-induced reflex depression of the baroreceptor reflex (Table 2). In six preparations both the baroreceptor reflex gain and the reduction in the reflex-induced decrease in phrenic nerve activity integral were significantly attenuated when ANGII was microinjected following TRIM (Table 2).
Specificity of NO modulation in the NTS
To assess whether the effect of NO in the NTS was specific for the baroreceptor reflex pathway, we investigated whether NONOate perturbed the peripheral chemoreceptor reflex. Three doses of CN were tested (7.5, 15 and 22.5 μg). NONOate affected neither the reflex bradycardia nor the phrenic nerve activity response to all CN doses (n = 6, P > 0.3 in all cases). For example, under control conditions stimulation of peripheral chemoreceptors with 22.5 μg CN evoked a bradycardia of -188 ± 20 bpm and a 33 ± 5 % increase in phrenic nerve activity integral, which was not different to that after bilateral NTS microinjections of NONOate (i.e. -184 ± 17 bpm and 28.5 ± 6 %; P > 0.9 and P > 0.6, respectively).
Since ANGII acting in the NTS potentiated the chemoreceptor reflex bradycardia (see Paton & Kasparov, 1999), we determined whether antagonism of NOS activity in the NTS influenced this finding. Following microinjection of ANGII into the NTS there was a potentiation of the chemoreceptor reflex bradycardia (evoked with 50 μl of 0.03 % CN) from -67.0 ± 4 to -106.7 ± 5 bpm but this was not obviously affected when ANGII was co-microinjected with either l-NAME or l-NMMA (bradycardias were -108.5 ± 7 and -112 ± 8 bpm, respectively; n = 4, data not shown).
TeNOS transfection studies
To more specifically investigate a role for eNOS in mediating the ANGII-induced baroreceptor reflex depression, dominant negative TeNOS protein was expressed in the NTS using replication-deficient adenoviral vectors.
Baseline cardiorespiratory variables in TeNOS-transfected rats
Baseline perfusion pressure, heart rate, phrenic nerve activity pattern and frequency, and an ongoing respiratory sinus arrhythmia in these preparations were not different to those of naive rats (see Table 1).
Effect of ANGII in the NTS of TeNOS-transfected rats
In 12 of 14 transfected rats, bilateral NTS microinjections of ANGII produced no change in the baroreceptor reflex (i.e. 1.8 ± 0.2 versus 1.9 ± 0.1 bpm mmHg−1; Fig. 5A, n = 12). Moreover, there was no change in the reflex reduction in phrenic nerve activity integrals (control -46.6 ± 6 versus -40.0 ± 4 %; P = 0.2). However, in two rats, ANGII did produce a reduction in baroreceptor reflex gain (controls 1.44 and 1.88 bpm mmHg−1versus 0.47 and 0.81 bpm mmHg−1 after ANGII) and the reflex decrease in phrenic nerve activity was also reduced.
Figure 5. eNOS blockade in the NTS using in vivo gene transfer prevents the attenuating action of ANGII and l-arginine on baroreceptor reflex function.
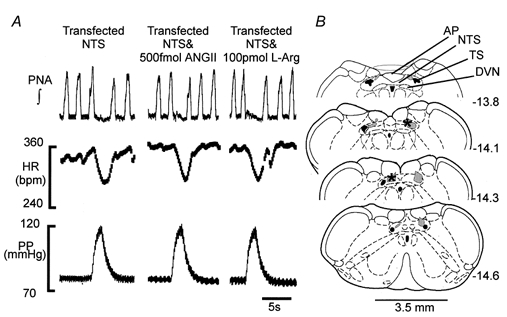
A, WHBPs were prepared 5-6 days following NTS infection with adenovirus expressing TeNOS. In these preparations, bilateral NTS microinjections of either ANGII or the NO precursor l-arginine (l-Arg) failed to affect the baroreceptor reflex. Note that baseline cardiorespiratory variables including the respiratory sinus arrhythmia were not different from those of naive animals (see Table 1). B, representative transverse sections through the medulla indicating both the extent of transfection within the NTS as indicated by enhanced green fluorescent protein (hatched shading) and the microinjection sites of ANGII and l-arginine. Data are from a single preparation. Numbers refer to the distance from bregma. Abbreviations: AP, area postrema; DVN, dorsal vagal motor nucleus; NTS, nucleus of the solitary tract; TS, solitary tract.
Effect of l-arginine in the NTS of TeNOS-transfected rats
Figure 5A shows that as with ANGII, l-arginine microinjected into the NTS also failed to affect both the baroreceptor reflex gain (control 1.82 ± 0.2 versus 2.1 ± 0.3 bpm mmHg−1) and phrenic nerve activity integral (-51.3 ± 6.5 versus -42.4 ± 6.6 %; n = 7, P = 0.2).
eGFP-transfected rats
In seven animals in which eGFP only was expressed in the NTS the baseline variables measured were not different to those of naive rats (Table 1). Baroreceptor reflex gain was similar to that of untransfected rats (i.e. 1.5 ± 0.2 bpm mmHg−1) and was attenuated significantly with bilateral microinjections of ANGII (to 0.59 ± 0.15 bpm mmHg−1; P < 0.001).
Histological verification of microinjection and transfection sites
Eighty-two per cent of the microinjection sites for NONOate and l-arginine were recovered successfully (Fig. 3C). Figure 3C also shows the microinjection sites for l-NMMA-ANGII and l-NAME-ANGII, which were recovered in 67 % of animals. These loci were restricted to the caudal regions of the NTS, co-existent with area postrema, and extending 0.6 mm caudal to it into the commissural NTS. We believe these sites are representative of all other microinjection loci that used identical co-ordinates.
In transfected rats, we saw eGFP expressed in the NTS and also in restricted regions of the gracile nucleus and dorsal vagal motor nucleus. Figure 5B depicts the transfected regions in a representative animal together with microinjection sites of ANGII and l-arginine. In all transfected rats, microinjection sites were within NTS regions caudal to the obex extending caudally into the commissural NTS (Fig. 5B). In two transfected rats NTS microinjections of ANGII produced a reduction in baroreceptor reflex gain (see above). This might be accounted for by the fact that in one case eGFP expression was sparse and only ipsilateral whereas in the other no fluorescence was found at all.
Immunohistochemical studies
Specificity of antibodies and procedures
The specificity of the antibody against the AT1 receptor has been described (Giles et al. 1999). To verify our eNOS staining we used two different primary antibodies, generated against different epitopes of the C-terminal end of the eNOS. The pattern of staining with both antibodies was identical and most subsequent studies were conducted with the anti-eNOS antibody from Sigma. The secondary antibodies did not cross-react since: (1) in control sections where one of the primary antibodies had been omitted but the sections otherwise processed as normal the staining corresponded only to that of the primary antibody present and (2) in double labelling studies neurones labelled for only one of the antigens were clearly observed.
Immunoreactivity in the NTS
Immunoreactivity for eNOS and AT1 receptors was observed in both neurones and endothelial cells lining blood vessels throughout the NTS (Fig. 6). Since these cells were not labelled with FluroGold they were considered to be neurones of the NTS. In NTS neurones, immunoreactivity for eNOS was diffuse, apparently spread throughout the cytoplasm of the soma of labelled cells (Fig. 6) with limited dendritic labelling. In contrast, AT1 receptor immunoreactivity was punctate in appearance and could be detected in somata and dendrites of labelled neurones (Fig. 6). There were many more neurones immunoreactive for eNOS than for AT1 receptors. However, there were no clear patterns of labelling for either antigen, since immunoreactive neurones were observed throughout the various sub-nuclei of the NTS (Fig. 6). In the NTS double labelled neurones were distributed throughout the sub-nuclei (Fig. 6C and D) and were approximately 23 ± 5.4 % of the eNOS-immunoreactive neurones and 57 ± 9.2 % of the AT1 receptor-immunoreactive neurones. There were no differences in the number of double labelled neurones in the different rostro-caudal levels of the NTS. However, it must be noted that the limitations of immunohistochemistry undoubtedly make such measurements of double labelling an underestimate.
Figure 6. Co-localisation of immunoreactivity for eNOS and AT1 receptors in neurones and blood vessels in the NTS.
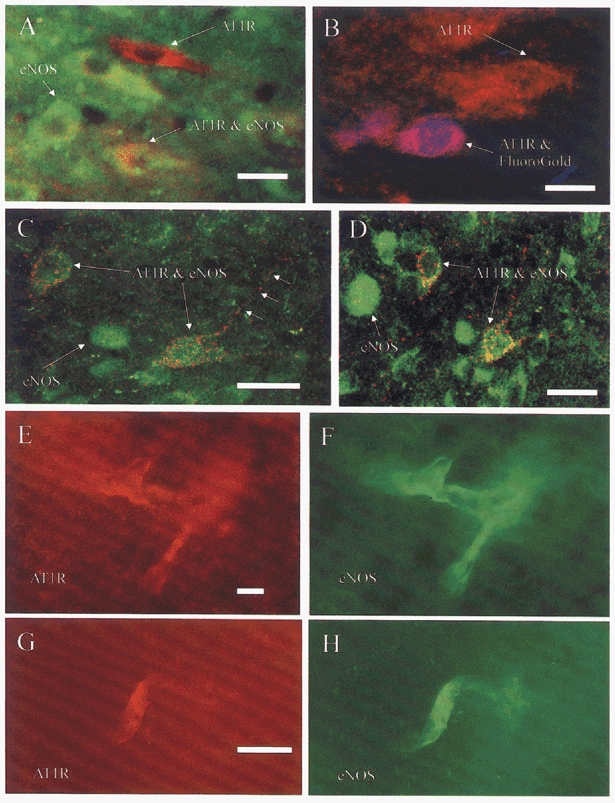
Fluorescence image of neurones in the medial NTS, digitally combined from images captured whilst viewing under single filter sets. A, neurones immunoreactive solely for eNOS (green) or AT1 receptors (red) were observed, as well as double labelled neurones (red and green). The presence of neurones immunoreactive for only one antigen as well as both indicates that there was no cross-reactivity in the procedure and no bleed through from one filter set to the other. B, AT1 receptor-immunoreactive neurones in the dorsal vagal nucleus were vagal preganglionic neurones (blue and red), shown by the presence of FluoroGold following intraperitoneal injections 7 days previously. AT1 receptor-immunoreactive neurones dorsal to the DVN did not contain FluoroGold, indicating that they were NTS neurones. C and D, confocal microscope image of dual labelled neurones in the commissural NTS. Images were obtained by single laser scans and combined digitally. Neurones labelled for eNOS (green) and eNOS and AT1 receptor (green and red) were observed. E-H, fluorescent images of immunoreactivity observed in blood vessels in the caudal NTS. Images were obtained by viewing and capturing AT1 receptor immunoreactivity (E, G), separately from the eNOS labelling (F, H). In G, AT1 receptor immunoreactivity was observed before the tissue was incubated with antibodies to eNOS (H), precluding the possibility of cross-reactions or bleed through. Scale bars, 20 μm.
Immunoreactivity for eNOS and AT1 receptors was also observed in capillaries in the NTS (Fig. 6E and H). To further check for antibody cross-reactivity some sections were incubated with the two primary antibodies sequentially. The first incubation with the AT1 receptor antibody visualised with Cy3 revealed labelled capillaries when viewed with the Cy3 filter, but no labelling with the FITC filter (Fig. 6E and G). The sections were then washed and incubated in anti-eNOS antibody and visualised with FITC, revealing immunoreactivity in endothelial cells lining the capillaries (Fig. 6F and H).
DISCUSSION
We report that the effect of ANGII in the NTS on baroreceptor reflex function is dependent on the release of NO. Our pharmacological evidence, together with in vivo gene transfer studies both suggest that ANGII stimulates eNOS in the NTS. The immunocytochemical data presented here indicate that some neurones and endothelial cells in the NTS exhibit both AT1 receptors and eNOS immunoreactivity, thereby revealing two possible cellular targets for ANGII.
As alluded to in the Introduction, there is inconsistency between studies regarding the effects of NO in the NTS on baroreceptor reflex function and this might reflect whether studies were performed on anaesthetised or conscious animals. The WHBP used in the present study lacks circulating ANGII and is also unanaesthetised, which are important advantages. Anaesthesia not only affects numerous neurotransmitter systems directly, but in vivo also increases renin and ANGII plasma levels, which could affect the function of the NTS (Keeton & Campbell, 1980).
NO in the NTS and cardiovascular control
There are reports describing increases in systemic pressure following blockade of NOS in the NTS. Maeda et al. (1999) inhibited nNOS by microinjecting antisense oligonucleotides whereas others used non-selective pharmacological NOS inhibitors (see Harada et al. 1993, for example). In contrast, we found no consistent effects on perfusion pressure in the WHBP following NTS microinjections of three different NOS inhibitors. This may reflect the limited ability of the WHBP to generate changes in vascular resistance due to the low peripheral resistance.
From the present study it appears that in the WHBP there is no tonic NO modulation of NTS circuitry regulating the cardiac component of the baroreceptor reflex. This finding is consistent with that of several other studies where NTS microinjections of NOS inhibitors in both anaesthetised (Harada et al. 1993; Zanzinger et al. 1995) and conscious animals (Pontieri et al. 1998) were without effect on the baroreceptor reflex. In contrast, we found that the baroreceptor reflex was attenuated by NO at the level of the NTS. The NO precursor l-arginine as well as two NO donors (sodium nitroprusside and diethylamine nonoate) all depressed reversibly the baroreceptor reflex gain. Our data are consistent with previous reports in conscious rats (spontaneously hypertensive) and rabbits where NOS inhibitors enhanced the reflex gain (Kumagai et al. 1993; Liu et al. 1996). However, neither of the latter studies identified the central site of action. In studies where comparable techniques of NTS microinjection were employed, no effect of NO donors was found (Harada et al. 1993; Zanzinger, 1995). However, these studies were performed in anaesthetised animals (rabbits). In our preliminary studies we found that low doses of pentobarbitone administered into the perfusate of the WHBP severely reduced or abolished the effect of a NO donor in the NTS (J. F. R. Paton & S. Kasparov, unpublished data); this may provide a plausible explanation for the difference. It should also be noted that the effects of NO in this study were reflex specific as the peripheral chemoreceptor reflex was unaffected. This further substantiates the notion of sensory channel-specific modulation in the NTS (Paton & Kasparov, 2000a).
Role for eNOS in the NTS in the regulation of the baroreceptor reflex
The present study supports a role for eNOS rather than nNOS in mediating the ANGII-depressant effect on the baroreceptor reflex at the level of the NTS. The pharmacological blocker TRIM, which acts preferentially on nNOS (Handy & Moore, 1997), failed to prevent baroreceptor reflex depression induced by ANGII. In contrast, both l-NAME and l-NMMA, which have a higher affinity for eNOS than nNOS, prevented the depressant effects of ANGII in the NTS on the baroreceptor reflex. It may be argued that the dose of TRIM was not sufficient and that both l-NAME and l-NMMA are not completely selective for eNOS. To substantiate a role for eNOS we employed a gene transfer strategy to selectively disable eNOS in the NTS; this is the first demonstration of such technology in vivo. In 89 % of preparations transfected with TeNOS, both l-arginine and ANGII were ineffective in perturbing the baroreceptor reflex gain.
Our functional data are supported by immunocytochemical evidence. eNOS immunoreactivity was found in caudal regions of the NTS; these areas are comparable to those in which we performed our microinjections. Interestingly, some neurones and endothelial cells showed co-localisation of eNOS with ANGII type 1 receptors (AT1 receptors). However, further experiments are required to estimate the relative importance of eNOS in NTS neurones versus capillary endothelial cells in the mechanism of ANGII action on the baroreceptor reflex. Indeed, we must also consider the potential for a change in arterial perfusion within the NTS in mediating the effects we observed with NO donors.
Plausible mechanism of interaction between ANGII and NO in the NTS
The cellular effects of ANGII on synaptic transmission within the NTS in vitro were described recently (Kasparov & Paton, 1999). This included an ANGII-mediated enhancement of evoked IPSPs in a population of neurones. We also provided evidence that ANGII might act pre-synaptically to enhance GABA release. This mechanism could explain the effect of ANGII on intracellularly recorded functionally characterised baroreceptive NTS neurones where carotid sinus pressure-evoked synaptic responses were reduced (Paton, 1999; Paton & Kasparov, 2000a).
Since the ANGII-induced attenuation of the baroreceptor reflex was dependent upon release of NO, the cellular mechanisms of action in the NTS are of interest. Two previous in vitro extracellular recording studies described that l-arginine or sodium nitroprusside increased the activity of some NTS neurones (Tagawa et al. 1994) whereas it was decreased by l-NAME (Ma et al. 1995). Further, in three cells recorded in vivo that responded to increases in arterial pressure, NO donors also increased cell firing (Ma et al. 1995). Based on this latter finding it might be expected that NO release would increase baroreceptor reflex sensitivity, rather than decrease it as demonstrated here. The situation, however, would be opposite if the cells excited by NO are local inhibitory interneurones. Indeed, this scenario applies to the paraventricular nucleus (PVN) of the hypothalamus where ANGII induces NO release to increase IPSPs recorded from magnocellular neurones in vitro (Ferguson & Latchford, 2000). In the PVN ANGII is thought to release NO, which excites parvocellular GABAergic neurones impinging on magnocellular cells. It remains to be established whether NO positively drives GABAergic interneurones in the NTS. However, in the NTS AT1 receptors were found on neurones expressing eNOS (discussed above) and ANGII increased intracellular calcium in a proportion of neurones (Kasparov & Paton, 2000). Thus it is plausible that Ca2+-dependent release of NO may follow. NO may diffuse to stimulate guanylyl cyclase in the terminals of local GABAergic NTS interneurones. A NO-mediated increase in GABA transmission is supported in several other areas of the brain, for example, striatum (Hanania & Johnson, 1998) and periaqueductal grey matter (Hall & Behbehani, 1998). Interestingly, in eNOS-knockout mice the NMDA-evoked GABA release was significantly reduced in all areas of the brain, implying that this NOS isoform might be particularly important for regulating GABAergic transmission (Kano et al. 1998).
Plausible mechanism allowing alteration in baroreflex sensitivity without affecting baseline cardiorespiratory activity
The finding of reduced baroreflex sensitivity in the absence of a change in cardiorespiratory variables during NTS exposure to ANGII is interesting and requires discussion. In previous studies inhibitory synaptic responses to afferent stimulation were potentiated by ANGII without affecting input resistance (Kasparov & Paton, 1999). We also showed in baroreceptive NTS neurones that ANGII applied focally decreased evoked baroreceptor-mediated synaptic inputs but again failed to affect input resistance or resting membrane potential (Paton & Kasparov, 2000a). We suggest that ANGII does not evoke a tonic release of large quantities of GABA from the GABAergic terminals. Rather, we speculate that ANGII causes a selective ‘deafferentation’ of baroreceptor inputs to NTS neurones. This will depend on the site of contact of afferents and the relative position of GABAA receptors on NTS neurones. If dendritic it may explain the absence of changes in input resistance and resting membrane potential given the relatively long thin dendrites of baroreceptive NTS neurones (Deuchars et al. 2000) and limited space clamp capacity of recording techniques. It is therefore highly conceivable that the excitability of the soma will only be affected slightly. Such a mode of action would explain why inhibition of the baroreceptor reflex with ANGII may occur without an expected tachycardia (Michelini & Bonagamba, 1990; Paton & Kasparov, 1999) or why a microinjection of ANGII antagonists into the NTS evokes little or no bradycardia although potentiating the baroreceptor reflex (Campagnole-Santos et al. 1988; Michelini & Bonagamba, 1990; Kasparov et al. 1998).
Possible mechanisms by which ANGII and NO acting in the NTS may cause hypertension
It is well known that increasing plasma ANGII increases arterial pressure but, unlike other pressor agents, it does not induce a reflex bradycardia (see Reid, 1992). It might be inferred from this result that ANGII depresses baroreceptor reflex function. This is supported by the fact that exogenous ANGII in the NTS attenuates the baroreceptor reflex (Casto & Phillips, 1986; Luoh & Chan, 1998; Michelini & Bonagamba, 1990; Paton & Kasparov, 1999). The question arises as to a possible transduction mechanism as it is unlikely that plasma ANGII freely crosses the blood-brain barrier. The present data may shed some light on this issue. Circulating ANGII might stimulate AT1 receptors located on the endothelial cells in the NTS to produce NO, which diffuses to affect NTS neurones and depresses the baroreceptor reflex. Our finding of co-localisation of AT1 receptors on endothelial cells containing eNOS supports this idea. Notwithstanding, circulating ANGII can also affect cells within the area postrema which when activated can enhance inhibitory transmission within the NTS (e.g. Cai et al. 1994).
The finding of eNOS immunoreactivity and AT1 receptors co-localised on NTS neurones in the present study creates an alternative possibility. Centrally produced ANGII could trigger release of NO from NTS neurones and account for the hypertension caused by increases in central ANGII. Indeed, ANGII can be synthesised by the brain independently of peripheral sources (Richards et al. 1989; Phillips et al. 1993) and is viewed as a peptidergic transmitter by some (Ferguson & Washburn, 1998). In this context, in the spontaneously hypertensive rat brainstem levels of ANGII are higher compared to controls (Morishita et al. 1995) and AT1 receptors more dense in the NTS compared with normotensive controls (Gutkind et al. 1988). The ANGII could originate from NTS cells (see Fuxe et al. 1994) or be secreted from axonal terminals arriving from remote sources.
In conclusion, the data presented here suggests that ANGII acting within the NTS depresses the baroreceptor reflex via release of NO and this depends primarily on the activation of eNOS. The structures containing eNOS in the NTS include both endothelial cells and neurones but the relative importance of each of these in mediating the ANGII depressant effect is unknown.
Acknowledgments
The authors wish to thank Drs Erin Schuman and Norman Davidson (California Institute of Technology, Pasadena, CA, USA) for their generous gift of Ad-TeNOS and Dr D. Knight for preparation of the adenoviral vectors. We are most appreciative of the financial support from the British Heart Foundation (BS/93003 and PG/99055), the Wellcome Trust (042603, 044994 and 057761) and the BBSRC (24/S111296).
References
- Batten TFC, Atkinson L, Deuchars J. Nitric oxide systems in the medulla oblongata and their involvement in autonomic control. In: Steinbusch HWM, De Vente J, editors. Handbook of Chemical Neuroanatomy, Functional Neuroanatomy of the Nitric Oxide System. XIV. Amsterdam: Elsevier; 2000. pp. 177–213. [Google Scholar]
- Blessing WW. The Lower Brainstem and Bodily Homeostasis. New York: Oxford University Press; 1997. Anatomy of the lower brainstem; pp. 29–99. [Google Scholar]
- Busconi L, Michel T. Endothelial nitric oxide synthase. N-terminal myristoylation determines subcellular localization. Journal of Biological Chemistry. 1993;268:8410–8413. [PubMed] [Google Scholar]
- Cai Y, Hay M, Bishop V1S. Stimulation of area postrema by vasopressin and angiotensin II modulates neuronal activity in the nucleus tractus solitarius. Brain Research. 1994;647:242–248. doi: 10.1016/0006-8993(94)91323-4. [DOI] [PubMed] [Google Scholar]
- Campagnole-Santos MJ, DiZ DI, Ferrario CM. Baroreceptor reflex modulation by angiotensin II at the nucleus tractus solitari. Hypertension. 1988;11(suppl. I):I167–171. doi: 10.1161/01.hyp.11.2_pt_2.i167. [DOI] [PubMed] [Google Scholar]
- Casto R, Phillips MI. Angiotensin II attenuates baroreflexes at nucleus tractus solitarius of rats. American Journal of Physiology. 1986;250:R193–198. doi: 10.1152/ajpregu.1986.250.2.R193. [DOI] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: Implications for understanding baroreceptor reflex circuitry. Journal of Neuroscience. 1998;18:371–187. doi: 10.1523/JNEUROSCI.18-01-00371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI. Neurogenesis of respiratory rhythm in the mammal. Physiological Reviews. 1979;59:1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- Deuchars J, Li YW, Kasparov S, Paton JFR. Morphological and electrophysiological properties of neurones in the dorsal vagal complex of the rat activated by arterial baroreceptors. Journal of Comparative Neurology. 2000;417:233–249. [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ. Local circuitry regulates the excitability of rat neurohypophyseal neurones. Experimental Physiology. 2000;85S:153S–162S. doi: 10.1111/j.1469-445x.2000.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Washburn DLS. Angiotensin II: A peptidergic neurotransmitter in central autonomic pathways. Progress in Neurobiology. 1998;54:169–192. doi: 10.1016/s0301-0082(97)00065-8. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Covenas R, NorvaeZ JA, Bunnemann B, Bjelke B. Volume transmission in transmitter peptide costoring neurons in the medulla oblongata. In: Barraco IRA, editor. Nucleus of the Solitary Tract. Boca Raton, Ann Arbor, London, Tokyo: CRC Press; 1994. pp. 75–89. [Google Scholar]
- Giles ME, Fernley RT, Nakamura Y, Moeller I, Aldred GP, Ferraro T, Penschow JD, McKinley MJ, Oldfield BJ. Characterization of a specific antibody to the rat angiotensin II AT1 receptor. Journal of Histochemistry and Cytochemistry. 1999;47:507–516. doi: 10.1177/002215549904700409. [DOI] [PubMed] [Google Scholar]
- Graham FL, Prevec L. Methods for construction of adenovirus vectors. Molecular Biotechnology. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- Gutkind JS, Kurihara M, Castren E, Saavedra JM. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. Journal of Hypertension. 1988;6:79–84. doi: 10.1097/00004872-198801000-00012. [DOI] [PubMed] [Google Scholar]
- Hall CW, Behbehani MM. Synaptic effects of nitric oxide on enkephalinergic, GABAergic, and glutamatergic networks of the rat periaqueductal gray. Brain Research. 1998;805:69–87. doi: 10.1016/s0006-8993(98)00648-9. [DOI] [PubMed] [Google Scholar]
- Hanania T, Johnson KM. Regulation of neurotransmitter release by endogenous nitric oxide in striatal slices. European Journal of Pharmacology. 1998;359:111–117. doi: 10.1016/s0014-2999(98)00636-0. [DOI] [PubMed] [Google Scholar]
- Handy RLC, Moore PK. Mechanisms of the inhibition of neuronal nitric oxide synathase by 1-(2-trifluoromethylphenyl) imidazole (TRIM) Life Sciences. 1997;60:389–394. doi: 10.1016/s0024-3205(97)00295-6. [DOI] [PubMed] [Google Scholar]
- Harada S, Tokunaga S, Momohara M, Masaki H, Tagawa T, Imaizumi T, Takeshita A. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circulation Research. 1993;72:511–516. doi: 10.1161/01.res.72.3.511. [DOI] [PubMed] [Google Scholar]
- Harding TC, Geddes BJ, Murphy D, Knight D, Uney JB. Switching transgene expression in the brain using an adenoviral tetracycline-regulatable system. Nature Biotechnology. 1998;16:553–555. doi: 10.1038/nbt0698-553. [DOI] [PubMed] [Google Scholar]
- Harding TC, Geddes BJ, Noel JD, Murphy D, Uney JB. Tetracycline-regulated transgene expression in hippocampal neurones following transfection with adenoviral vectors. Journal of Neurochemistry. 1997;69:2620–2623. doi: 10.1046/j.1471-4159.1997.69062620.x. [DOI] [PubMed] [Google Scholar]
- Hironaga K, Hirooka Y, Matsuo I, Shihara M, Tagawa T, Harasawa Y, Takeshita A. Role of endogenous nitric oxide in the brain stem on the rapid adaptation of baroreflex. Hypertension. 1998;31:27–31. doi: 10.1161/01.hyp.31.1.27. [DOI] [PubMed] [Google Scholar]
- Kano T, Shimizu-Sasamata M, Huang PL, MoskowitZ MA, Lo EH. Effects of nitric oxide synthase gene knockout on neurotransmitter release in vivo. Neuroscience. 1998;86:695–699. doi: 10.1016/s0306-4522(98)00179-1. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Lanzrein M, Stary SJ, Sandoval GM, Smith WB, Sullivan BM, Davidson N, Schuman EM. A role for endothelial NO synthase in LTP revealed by adenovirus-mediated inhibition and rescue. Science. 1996;274:1744–1748. doi: 10.1126/science.274.5293.1744. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Butcher JW, Paton JFR. Angiotensin II receptors within the nucleus of the solitary tract mediate the developmental attenuation of baroreceptor vagal reflex in pre-weaned rats. Journal of the Autonomic Nervous System. 1998;74:160–168. doi: 10.1016/s0165-1838(98)00149-0. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Paton JFR. Differential effects of angiotensin II in the nucleus tractus solitarii of the rat – plausible neuronal mechanisms. Journal of Physiology. 1999;521:227–238. doi: 10.1111/j.1469-7793.1999.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasparov S, Paton JFR. Angiotensin II and increases in intracellular calcium in NTS neurones. Autonomic Neuroscience: Basic and Clinical. 2000;82:57. [Google Scholar]
- Keeton TK, Campbell WB. The pharmacological alteration of renin release. Pharmacological Reviews. 1980;31:81–227. [PubMed] [Google Scholar]
- Kumagai H, Averill DB, Khosla MC, Ferrario CM. Role of nitric oxide and angiotensin II in the regulation of sympathetic nerve activity in spontaneously hypertensive rats. Hypertension. 1993;21:476–484. doi: 10.1161/01.hyp.21.4.476. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Castillo-MelendeZ M, McLean K, Jarrott B. The distribution of nitric oxide synthase-, adenosine deaminase- and neuropeptide Y-immunoreactivity through the entire rat nucleus tractus solitarius. Effects of unilateral nodose ganglionectomy. Journal of Chemical Neuroanatomy. 1998;15:27–40. doi: 10.1016/s0891-0618(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Progress in Neurobiology. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Lee CM, Robinson LJ, Michel T. Oligomerization of endothelial nitric oxide synthase. Evidence for a dominant negative effect of truncation mutants. Journal of Biological Chemistry. 1995;270:27403–27406. doi: 10.1074/jbc.270.46.27403. [DOI] [PubMed] [Google Scholar]
- Lin H-C, Wan F-J, Tseng C-J. Modulation of cardiovascular effects produced by nitric oxide and ionotropic glutamate receptor interaction in the nucleus tractus solitarii of rats. Neuropharmacology. 1999;38:935–941. doi: 10.1016/s0028-3908(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Lin L-H, Cassell MD, Sandra A, Talman WT. Direct evidence for nitric oxide synthase in vagal afferents to the nucleus tractus solitarii. Neuroscience. 1998;84:549–558. doi: 10.1016/s0306-4522(97)00501-0. [DOI] [PubMed] [Google Scholar]
- Liu J, Sessa WC. Identification of covalently bound amino-terminal myristic acid in endothelial nitric oxide synthase. Journal of Biological Chemistry. 1994;269:11691–11694. [PubMed] [Google Scholar]
- Liu J-L, Murakami H, Zucker IH. Effects of NO on baroreflex control of heart rate and renal nerve activity in conscious rabbits. American Journal of Physiology. 1996;270:R1361–1370. doi: 10.1152/ajpregu.1996.270.6.R1361. [DOI] [PubMed] [Google Scholar]
- Luoh HF, Chan SHH. Participation of AT1 and AT2 receptor subtypes in the tonic inhibitory modulation of baroreceptor reflex response by endogenous angiotensins at the nucleus tractus solitarii in the rat. Brain Research. 1998;782:73–82. doi: 10.1016/s0006-8993(97)01198-0. [DOI] [PubMed] [Google Scholar]
- Ma S, Abboud FM, Felder RB. Effects of L-arginine-derived nitric oxide synthesis on neuronal activity in nucleus tractus solitarius. American Journal of Physiology. 1995;268:R487–491. doi: 10.1152/ajpregu.1995.268.2.R487. [DOI] [PubMed] [Google Scholar]
- Maeda M, Hirano H, Kudo H, Doi Y, Higashi K, Fujimoto S. Injection of antisense oligos to nNOS into nucleus tractus solitarii increases blood pressure. NeuroReport. 1999;10:1957–1960. doi: 10.1097/00001756-199906230-00030. [DOI] [PubMed] [Google Scholar]
- Michelini LC, Bonagamba LH. Angiotensin II as a modulator of baroreceptor reflexes in the brainstem of conscious rats. Hypertension. 1990;15:I45–50. doi: 10.1161/01.hyp.15.2_suppl.i45. [DOI] [PubMed] [Google Scholar]
- Millatt LJ, Abdel-Rahman EM, Siragy HM. Angiotensin II and nitric oxide: a question of balance. Regulatory Peptides. 1999;81:1–10. doi: 10.1016/s0167-0115(99)00027-0. [DOI] [PubMed] [Google Scholar]
- Morishita R, Higaki J, Nakamura Y, Aoki M, Yamada K, Moriguchi A, Rakugi H, Tomita N, Tomita S, Yu H, Nakamura F, Mikami H, Ogihara T. Effect of an antihypertensive drug on brain angiotensin II levels in renal and spontaneously hypertensive rats. Clinical and Experimental Pharmacology and Physiology. 1995;22:665–669. doi: 10.1111/j.1440-1681.1995.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Paton JFR. Nucleus tractus solitarii: integrating structures. The Sharpey-Schaffer Prize Lecture. Experimental Physiology. 1999;84:815–833. [PubMed] [Google Scholar]
- Paton JFR, Kasparov S. Differential effects of angiotensin II on cardiorespiratory reflexes mediated by nucleus tractus solitarii – a microinjection study in the rat. Journal of Physiology. 1999;521:213–225. doi: 10.1111/j.1469-7793.1999.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR, Kasparov S. Sensory channel specific modulation in the nucleus of the solitary tract. Journal of the Autonomic Nervous System. 2000a;80:117–129. doi: 10.1016/s0165-1838(00)00077-1. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Kasparov S. Nitric oxide mediates baroreflex depression induced by angiotensin II in the solitary tract nucleus of rat. Journal of Physiology. 2000b;523.P:256–257P. [Google Scholar]
- Paton JFR, Wong L-F, Murphy D, Kasparov S. Angiotensin II induced baroreceptor reflex depression in the nucleus tractus solitarius (NTS) is mediated by nitric oxide (NO) FASEB Journal. 2000;14:460.8. [Google Scholar]
- Paxinos G, Watson C. The Rats Brain in Stereotaxic Coordinates. London: Academic Press Ltd; 1986. [Google Scholar]
- Pontieri V, Venezuela MK, Scavone C, Michelini LC. Role of endogenous nitric oxide in the nucleus tractus solitarii on baroreflex control of heart rate in spontaneously hypertensive rats. Journal of Hypertension. 1998;16:1993–1999. doi: 10.1097/00004872-199816121-00021. [DOI] [PubMed] [Google Scholar]
- Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regulatory Peptides. 1993;43:1–20. doi: 10.1016/0167-0115(93)90403-u. [DOI] [PubMed] [Google Scholar]
- Reid IA. Interactions between Ang II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. American Journal of Physiology. 1992;262:E763–778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- Richards EM, Hermann K, Sumners C, Raizada MK, Phillips MA. Release of immunoreactive angiotensin II from neuronal cultures: Adrenergic influences. American Journal of Physiology. 1989;257:C588–595. doi: 10.1152/ajpcell.1989.257.3.C588. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Mtui EP, Otake K, Anwar M. Central and primary visceral afferents to nucleus tractus solitarii may generate nitric oxide as a membrane-permeant neuronal messenger. Journal of Comparative Neurology. 1996;361:51–67. doi: 10.1002/(SICI)1096-9861(19960101)364:1<51::AID-CNE5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Imaizumi T, Harada S, Endo T, Shiramoto M, Hirooka Y, Takeshita A. Nitric oxide influences neuronal activity in the nucleus tractus solitarius of brainstem slices. Circulation Research. 1994;75:70–76. doi: 10.1161/01.res.75.1.70. [DOI] [PubMed] [Google Scholar]
- Tseng C-J, Liu H-Y, Lin H-C, Ger L-P, Tung C-S, Yen M-H. Cardiovascular effects of nitric oxide in the brainstem nuclei of rats. Hypertension. 1996;27:36–42. doi: 10.1161/01.hyp.27.1.36. [DOI] [PubMed] [Google Scholar]
- Vitagliano S, Berrino L, D’Amico M, Maione S, De Nvellis V, Rossi F. Involvement of nitric oxide in cardiorespiratory regulation in the nucleus tractus solitarii. Neuropharmacology. 1996;35:625–631. doi: 10.1016/0028-3908(96)84633-8. [DOI] [PubMed] [Google Scholar]
- Zanzinger J, Czachurski J, Seller H. Effects of nitric oxide on sympathetic baroreflex transmission in the nucleus tractus solitarii and caudal ventrolateral medulla in cats. Neuroscience Letters. 1995;197:199–202. doi: 10.1016/0304-3940(95)11929-q. [DOI] [PubMed] [Google Scholar]


