Abstract
Intracellular dialysis of NIH/3T3 cells with a commercially available anti-ClC-3 polyclonal antibody (Ab) for ≈30 min completely inhibited expressed guinea-pig ClC-3 currents (IgpClC-3), while intracellular dialysis with antigen-preabsorbed anti-ClC-3 Ab failed to affect IgpClC-3.
Anti-ClC-3 Ab was used as a selective probe to examine the relationship between endogenous ClC-3 expression and native volume-sensitive outwardly rectifying anion channels (VSOACs) in guinea-pig cardiac cells, canine pulmonary arterial smooth muscle cells (PASMCs) and Xenopus laevis oocytes. Intracellular dialysis or injection of anti-ClC-3 Ab abolished native VSOAC function in cardiac cells and PASMCs and significantly reduced VSOACs in oocytes. In contrast, native VSOAC function was unaltered by antigen-preabsorbed anti-ClC-3 Ab.
It is suggested that endogenous ClC-3 represents a major molecular entity responsible for native VSOACs in cardiac and smooth muscle cells and Xenopus oocytes. Anti-ClC-3 Ab should be a useful experimental tool to directly test the relationship between endogenous ClC-3 expression and native VSOAC function, and help resolve existing controversies related to the regulation and physiological role of native VSOACs in a wide variety of different cells.
Studies of the physiological role and regulation of volume-sensitive outwardly rectifying anion channels, also referred to as volume-sensitive organic osmolyte and anion channels (VSOACs; Strange et al. 1996), have been impeded due to lack of specific pharmacological inhibitors (Doughty et al. 1998; Dick et al. 1999; Duan & Hume, 2000). The molecular identification of the protein(s) responsible for native VSOACs has been particularly difficult to resolve (Clapham, 1998; Strange, 1998; Valverde, 1999). In 1997, we proposed ClC-3, a ubiquitously expressed member of the ClC Cl− channel superfamily, as a novel molecular candidate for a VSOAC (Duan et al. 1997b), with a specific N-terminus protein kinase C (PKC) phosphorylation site which acts as the volume sensor (Duan et al. 1999). Many properties of the expressed guinea-pig (gp) IClC-3 resemble those reported for native VSOACs in heart (Duan et al. 1997a) and other tissues. Although the ClC-3 hypothesis has received additional experimental support from a variety of sources (Coca-Prados et al. 1996; Schmid et al. 1998; von Weikersthal et al. 1999; Nastrucci et al. 1999; Shimada et al. 2000), some discrepancies have been reported as well (Higgins et al. 1999; Valverde, 1999).
The availability of a specific experimental tool to inhibit endogenous ClC-3 function would be very useful to assess the relationship between endogenous ClC-3 and native VSOAC function in a wide variety of different cell types and may help to resolve some of the existing controversies in this field. In the present report, we describe a simple and specific approach to eliminate endogenous ClC-3 function and use this approach to demonstrate that ClC-3 may be the predominant molecular entity responsible for VSOACs in three different native cell types.
METHODS
Cell preparation
Single guinea-pig cardiac and canine pulmonary vascular smooth muscle cells were enzymatically dispersed as previously described (Yamazaki et al. 1998; Duan et al. 1999). Mongrel dogs were killed with sodium pentobaritone (45 mg kg−1, i.v.) whereas guinea-pigs were killed by CO2 inhalation. All experiments were carried out in accordance with the recommendations of the University of Nevada Animal Care and Use Committee. Ovarian lobes were surgically removed from Xenopus laevis anaesthesized with 1,3-aminobenzoate methane sulphonic acid salt and follicle-enclosed oocytes were removed as previously described (Yamazaki et al. 1999). NIH/3T3 cells were transiently co-transfected by electroporation (Duan et al. 1997b) with wild-type gpClC-3 (40 μg) in the Zeocin vector (pZeoSV) and a green fluorescent protein (GFP) reporter plasmid (8 μg, pcDNA3.1 CT-GFP, Invitrogen, Carlsbad, CA, USA) as a marker for transfection. Following electroporation, cells were plated out on glass coverslips for electrophysiological recordings, 24-48 h post-transfection. Western blot analysis was performed as previously described (Britton et al. 2000).
Electrophysiological recordings
Cl− currents were measured from isolated NIH/3T3 cells (American Type Culture Collection, Rockville, MD, USA), guinea-pig ventricular myocytes, canine PASMCs and oocytes at room temperature (22-24°C) as previously described (Yamazaki et al. 1998, 1999; Duan et al. 1999). Oocytes were injected with 50 nl of either anti-ClC-3 Ab or antigen-preabsorbed anti-ClC-3 Ab (Alomone Labs, Jerusuelem, Isreal) to achieve a final intraoocyte concentration of ∼15 μg ml−1 (assuming an average 1000 nl oocyte volume). Membrane currents were filtered at a frequency of 1 kHz and digitized on-line at 5 kHz using a Pentium III computer and pCLAMP 6.0 or 7.0 software (Axon Instruments, Foster City, CA, USA).
Solutions and reagents
All bath and pipette solutions were chosen to facilitate Cl− current recording. For NIH/3T3 cells, the hypotonic (250 mosmol (kg H2O)−1) bath solutions contained (mM): 125 NaCl, 2.5 MgCl2, 2.5 CaCl2, 10 N-2-hydroxyethylpiperazine-N‘-2-ethanesulphonic acid (Hepes); pH 7.2; [Cl−]o, 135 mM. The isotonic and hypertonic bath solutions were adjusted to 300 and 350 mosmol (kg H2O)−1, respectively, by adding mannitol. The pipette (internal) solution contained (mM): 135 N-methyl-D-glucamine chloride (NMDG-Cl), 2 ethylene glycol-bis(β-aminoethylether)-N,N,N’,N‘-tetra-acetic acid (EGTA), 5 Mg-ATP, 10 Hepes (pH 7.2; [Cl−]i, 135 mM; 300 mosmol (kg H2O)−1 by adding mannitol). For cardiac myocytes, the hypotonic (220 mosmol (kg H2O)−1) bath solutions contained (mM): 90 NaCl, 0.8 MgCl2, 1.0 CaCl2, 0.2 CdCl2, 2.0 BaCl2, 0.33 NaH2PO4, 10 tetraethylammonium (TEA)-Cl, 10 Hepes, 5.5 glucose; pH 7.4; [Cl−]o, 108 mM. The isotonic bath solutions were the same but adjusted to 300 mosmol (kg H2O)−1 by adding mannitol. The pipette (internal) solution contained (mM): 108 NMDG-Cl, 2.0 EGTA, 5.0 Mg-ATP, 10 Hepes (pH 7.4; [Cl−]i, 108 mM; 290 mosmol (kg H2O)−1 by adding mannitol). For PASMCs, the standard isotonic bath solution contained (mM): 107 NMG-Cl, 1.5 MgCl2, 2.5 MnCl2, 0.5 CdCl2, 10 glucose, 70 D-mannitol, 0.05 GdCl3 and 10 Hepes (pH 7.4; 300 mosmol (kg H2O)−1). Standard hypotonic (230 mosmol (kg H2O)−1) and hypertonic solutions (370 mosmol (kg H2O)−1) were made by adjusting D-mannitol. The pipette solution contained (mM): 95 CsCl, 20 TEA-Cl, 5 Mg-ATP, 5 EGTA, 80 D-mannitol and 5 Hepes (pH 7.2; 300 mosmol (kg H2O)−1). For Xenopus oocytes, isotonic bath solutions contained (mM): 72 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, 5 Hepes, 55 mannitol, 0.1 niflumic acid (220 mosmol (kg H2O)−1 and pH 7.5); D-mannitol was reduced in hypotonic bath solutions (165 mosmol (kg H2O)−1 and pH 7.5).
For intracellular dialysis experiments in NIH/3T3 cells and cardiac and smooth muscle myocytes, anti-ClC-3 Ab (Alomone Labs) was diluted in double-distilled water to 300 μg ml−1 and added into the pipette solution (final concentration of 5 μg ml−1). For preabsorbed anti-ClC-3 Ab, Ab and antigen were dissolved separately, mixed in a ratio of 1:10, stored in the refrigerator overnight, and added to the pipette solution to achieve a final concentration of Ab and antigen of 5 and 50 μg ml−1, respectively. The osmolarity of the pipette dialysis solutions was not significantly altered by inclusion of either Ab alone or preabsorbed Ab. In Figs 1-3, the onset of membrane rupture and intracellular dialysis is indicated at time 0.
Figure 1. Anti-ClC-3 Ab dialysis abolishes expressed IgpClC-3 in NIH/3T3 cells.
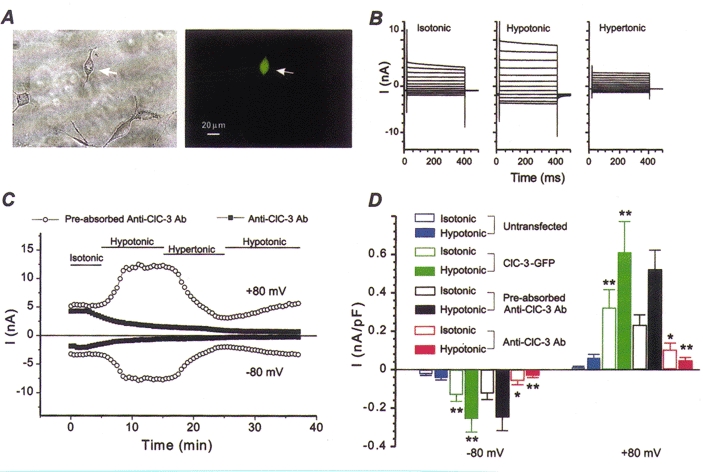
A, phase contrast (left panel) and fluorescence micrographs (right panel) of NIH/3T3 cell transfected with gpClC-3-GFP. B, representative IgpClC-3 recorded from GFP-positive NIH/3T3 cells (as shown in A) under isotonic, hypotonic and hypertonic conditions over the range -100 to +120 mV. C, effects of anti-ClC-3 Ab on IgpClC-3 at ±80 mV when pipette solutions contained preabsorbed anti-ClC-3 Ab (control, ○) or anti-ClC-3 Ab alone (▪). D, mean current densities recorded from untransfected NIH/3T3 cells (blue, n = 38), gpClC-3-GFP-transfected cells dialysed with standard intracellular pipette solutions (green, n = 9), preabsorbed anti-ClC-3 Ab (black, n = 4), and anti-ClC-3 Ab alone (red, n = 9).
Figure 3. Anti-ClC-3 Ab abolishes native VSOAC currents in canine PASMCs.
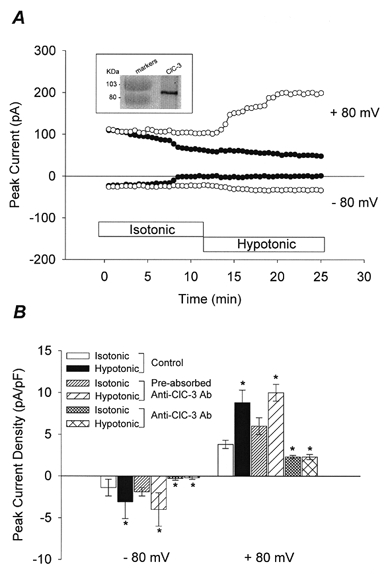
A, time course of VSOAC currents from two PASMCs dialysed with either anti-ClC-3 Ab (5 μg ml−1) preabsorbed with antigen (50 μg ml−1; ○), or anti-ClC-3 Ab alone (5 μg ml−1; •). Inset: Western blot analysis of native ClC-3 expression in isolated canine PASMCs. B, mean current densities in cells dialysed with either standard intracellular solutions (n = 11), preabsorbed anti-ClC-3 Ab (n = 4) or anti-ClC-3 Ab alone (n = 5).
Data are expressed as means ±s.e.m. (where n is the number of cells). Statistical analyses were made by Student’s paired t test and two-way analysis of variance where appropriate. Probability (P) values of less than 0.05 were considered statistically significant (*P < 0.05 and **P < 0.01).
RESULTS
Anti-ClC-3 Ab intracellular dialysis abolishes volume-sensitive IgpClC-3 expressed in NIH/3T3 cells
Figure 1A shows a phase contrast (left) and fluorescence micrograph (right) of a NIH/3T3 cell transiently co-transfected by electroporation with wild-type gpClC-3 and the GFP reporter plasmid (arrow). gpClC-3-GFP-transfected cells in symmetrical Cl− (135 mM) conditions, generated large volume-sensitive outwardly rectifying whole-cell Cl− currents (Fig. 1B) that were similar to currents previously recorded from NIH/3T3 cells transfected with gpClC-3 alone (Duan et al. 1997b). Figure 1C illustrates the effects of intracellular dialysis of either anti-ClC-3 Ab alone or antigen-preabsorbed anti-ClC-3 Ab on the amplitude and time course of IgpClC-3 activated or inhibited by exposure to hypotonic and hypertonic solutions, respectively, in gpClC-3-GFP-transfected 3T3 cells. During the initial few minutes following the onset of cell dialysis (time 0) under isotonic conditions, there was little apparent difference in the amplitude of the basally active IgpClC-3 recorded at ±80 mV in cells dialysed with either anti-ClC-3 Ab alone or antigen-preabsorbed anti-ClC-3 Ab. However, within about 3-4 min, the amplitude of the basally active IgpClC-3 recorded in isotonic solutions in the cell dialysed with anti-ClC-3 Ab began to decline at both +80 and -80 mV and this decline continued to proceed during the remainder of the experiment. Subsequent exposure of the cell to hypotonic or hypertonic bath solutions failed to alter the amplitude or time course of decline of IgpClC-3; after 35 min of intracellular dialysis with anti-ClC-3 Ab, the amplitude of any remaining IgpClC-3 became so small that it was difficult to distinguish from any residual membrane leak current. In contrast, no such decline in the amplitude of IgpClC-3 was observed in the cell dialysed with antigen-preabsorbed anti-ClC-3 Ab. The properties of IgpClC-3 in this cell appeared remarkably unaffected and similar to control cells: IgpClC-3 amplitudes were significantly augmented in response to hypotonic cell swelling, and inhibited by hypertonic cell shrinkage as previously demonstrated (Duan et al. 1999).
Figure 1D compares the differences in mean IgpClC-3 density recorded at ±80 mV in isotonic and hypotonic solutions between control untransfected NIH/3T3 cells and control gpClC-3-GFP-transfected cells dialysed with standard intracellular solutions, and gpClC-3-GFP-transfected cells dialysed with either anti-ClC-3 Ab alone or with antigen-preabsorbed anti-ClC-3 Ab. Membrane current densities of IgpClC-3±80 mV in gpClC-3-GFP-transfected cells were significantly larger than current densities measured in untransfected control cells. There was no significant difference in IgpClC-3 densities between control gpClC-3-GFP-transfected cells dialysed with standard intracellular solutions, and gpClC-3-GFP-transfected cells dialysed with antigen-preabsorbed anti-ClC-3 Ab. However, IgpClC-3 densities in gpClC-3-GFP-transfected cells dialysed with anti-ClC-3 Ab alone were significantly reduced compared with either control gpClC-3-GFP-transfected cells dialysed with standard intracellular solutions or gpClC-3-GFP-transfected cells dialysed with antigen-preabsorbed anti-ClC-3 Ab. These data strongly suggest that binding of anti-ClC-3 Ab to the carboxy terminus epitope of gpClC-3 disrupts normal Cl− channel function.
Anti-ClC-3 Ab intracellular dialysis abolishes native VSOAC function in cardiac and smooth muscle myocytes
To examine the relationship between endogenous ClC-3 expression and VSOACs in native cells, we tested the effects of intracellular dialysis of anti-ClC-3 Ab alone and antigen-preabsorbed anti-ClC-3 Ab on VSOAC currents in cardiac and smooth muscle myocytes. Endogenous ClC-3 transcripts have been identified in these tissues and the properties of native VSOACs in these cells appear to exhibit many remarkable similarities to those of expressed IgpClC-3 (Shuba et al. 1996; Duan et al. 1997b, 1999; Greenwood & Large, 1998; Yamazaki et al. 1998; Lamb et al. 1999). Furthermore, in cardiac tissues endogenous ClC-3 protein expression has been confirmed using the same anti-ClC-3 Ab (Alomone Labs) in immunoblot and immunofluorescence experiments (Britton et al. 2000). Figures 2 and 3 illustrate the effects of intracellular dialysis of anti-ClC-3 Ab alone or antigen-preabsorbed anti-ClC-3 Ab on native VSOACs in guinea-pig cardiac and canine pulmonary arterial smooth muscle myocytes, respectively.
Figure 2. Anti-ClC-3 Ab dialysis abolishes native VSOAC currents in guinea-pig cardiac myocytes.
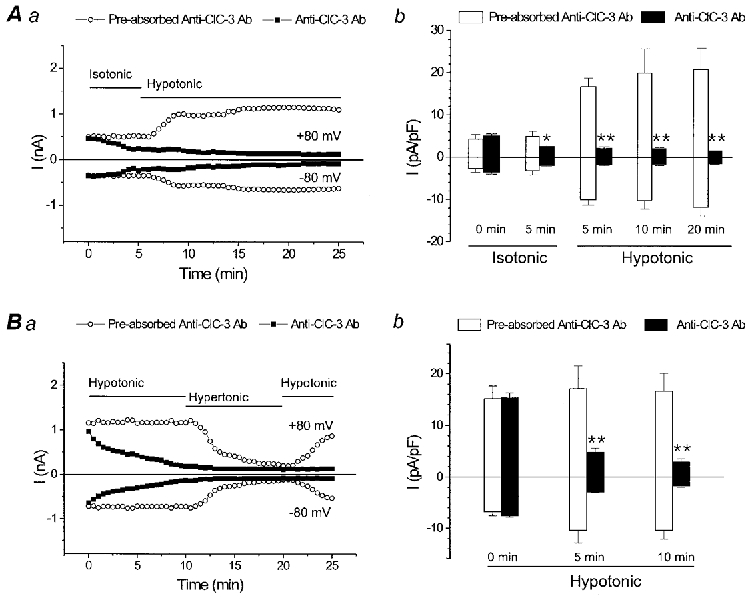
A, native VSOAC currents at ±80 mV in myocytes exposed to isotonic and hypotonic solutions. Aa, pipette solutions contained preabsorbed anti-ClC-3 Ab (control, ○) or anti-ClC-3 Ab alone (▪). Ab, mean current densities for preabsorbed anti-ClC-3 Ab (□, n = 3) or anti-ClC-3 Ab alone (▪, n = 5) at times indicated. B, same as A, except cells were preswelled by pre-exposure to hypotonic bath solutions prior to membrane rupture and dialysis; preabsorbed anti-ClC-3 Ab (□, n = 4), and anti-ClC-3 Ab alone (▪, n = 5).
In cardiac cells, during the initial few minutes following the onset of cell dialysis (time 0) under isotonic conditions, there was little apparent difference in the amplitude of the basally active VSOAC currents recorded at ±80 mV in cells dialysed with either anti-ClC-3 Ab alone or antigen-preabsorbed anti-ClC-3 Ab (Fig. 2Aa). However, within a few minutes, the amplitude of the basally active VSOAC currents recorded in isotonic solutions in the cell dialysed with anti-ClC-3 Ab began to decline at both +80 and -80 mV and this decline continued to proceed during the remainder of the experiment. Subsequent exposure of the cell to hypotonic bath solutions failed to alter the amplitude or time course of decline of VSOAC current; after 25 min of intracellular dialysis with anti-ClC-3 Ab, the amplitude of any remaining VSOAC current became very small and difficult to distinguish from any residual membrane leak current. In contrast, no such decline in the amplitude of native VSOAC current was observed in the cell dialysed with antigen-preabsorbed anti-ClC-3 Ab. The properties of VSOAC currents in this cell appeared remarkably unaffected and similar to control cells (Duan et al. 1999): VSOAC current amplitudes were significantly augmented in response to exposure to hypotonic bath solutions. Figure 2Ab compares the mean VSOAC current densities at ±80 mV under isotonic and hypotonic conditions between groups of cells dialysed with anti-ClC-3 Ab alone or antigen-preabsorbed anti-ClC-3 Ab at individual time points. VSOAC current densities were significantly reduced after 5 min anti-ClC-3 Ab dialysis in isotonic solutions compared with cells dialysed with antigen-preabsorbed anti-ClC-3 Ab and were also significantly attenuated at all time points following exposure to hypotonic bath solutions. Similar results were observed in a complementary series of experiments, in which ventricular myocytes were first pre-swelled by exposure to hypotonic bath solutions prior to patch clamping (Fig. 2B a).
Figure 3A (inset) illustrates Western blot analysis of native ClC-3 expression in canine PASMCs using anti-ClC-3 Ab as a primary Ab. A major band was detected for ClC-3, corresponding to approximately 90 kDa. The properties of native VSOACs in PASMCs also appeared remarkably unaffected by dialysis with antigen-preabsorbed anti-ClC-3 Ab (Fig. 3; Yamazaki et al. 1998), whereas dialysis with anti-ClC-3 Ab significantly abolished native VSOAC current. There was no significant difference in VSOAC current densities between control PASMCs dialysed with standard intracellular solutions, and PASMCs dialysed with antigen-preabsorbed anti-ClC-3 Ab. However, native VSOAC current densities in PASMCs dialysed with anti-ClC-3 Ab alone were significantly reduced compared with either control cells dialysed with standard intracellular solutions or cells dialysed with antigen-preabsorbed anti-ClC-3 Ab.
Anti-ClC-3 Ab injection significantly attenuates native VSOAC function in Xenopus oocytes
Figure 4A illustrates Western blot analysis of native ClC-3 expression in oocytes using anti-ClC-3 Ab as a primary Ab. A major band was detected for ClC-3, corresponding to approximately 80 kDa, with several weaker bands at higher molecular masses, corresponding to different glycosylated forms of ClC-3 (Schmieder et al. 1999). Control experiments using antigen-preabsorbed anti-ClC-3 Ab or secondary Ab alone confirmed the absence of significant non-specific anti-ClC-3 Ab binding. Figure 4B illustrates a family of native oocyte VSOAC currents activated by hypotonic bath solutions over the voltage range of -100 to +120 mV (Satterwhite et al. 1999). In agreement with a recent report (Souktani et al. 2000), native oocyte VSOAC currents appear to be strongly modulated by 4-phorbol 12,13-dibutyrate (Fig. 4B, right panel), in a manner very similar to expressed IgpClC-3 and native VSOAC currents in cardiac myocytes and some other mammalian cells (Duan et al. 1999).
Figure 4. Anti-ClC-3 Ab injection inhibits native VSOAC currents in Xenopus laevis oocytes.

A, Western blot analysis of native ClC-3 expression in oocytes. B, 4-phorbol 12,13-dibutyrate (PDBu) inhibition of native VSOAC currents in oocytes (-100 to +120 mV). C, time course of VSOAC activation in response to hypotonic bath solutions from a non-injected control oocyte (○) and an oocyte injected with anti-ClC-3 Ab (15 μg ml-1; •) at time shown. Cells were held at -30 mV for 30 ms, hyperpolarized to -100 mV for 210 ms and then depolarized to +100 mV for 210 ms, repetitively at 2 Hz. D, mean current densities from control oocytes (n = 10), oocytes injected with anti-ClC-3 Ab alone (15 μg ml-1) (n = 4) or injected with preabsorbed anti-ClC-3 Ab (150 μg ml-1) (n = 4). Peak current densities were measured after 5 min exposure to isotonic solutions, and after 85 min exposure to hypotonic solutions.
Figure 4C illustrates the time course of activation of native VSOAC currents in two oocytes during exposure to hypotonic bath solutions. In one oocyte, following activation of VSOAC currents, injection of anti-ClC-3 Ab using a second pipette produced a slow, but marked, reduction in the amplitude of VSOAC currents at ±100 mV (filled circles), whereas the amplitude of VSOAC currents remained sustained over a similar time course in an uninjected control oocyte (open circles). Figure 4D summarizes the results from a number of similar experiments. Native oocyte VSOAC current was unaffected by injection of antigen-preabsorbed anti-ClC-3 Ab and was similar to that of control, uninjected oocytes. However, native VSOAC current densities in oocytes injected with anti-ClC-3 Ab were significantly reduced compared with either control uninjected oocytes, or oocytes injected with antigen-preabsorbed anti-ClC-3 Ab. Less than complete inhibition of VSOAC current by anti-ClC-3 Ab may be due to (1) relatively slow diffusion and intracellular equilibration of anti-ClC-3 Ab due to the relatively large intraoocyte volume, or (2) a separate protein, in addition to endogenous ClC-3, being responsible for a subpopulation of oocyte VSOACs.
DISCUSSION
Although many of the known properties of ClC-3 appear to satisfy several of the criteria originally proposed by Okada (Okada et al. 1998) for the molecular identification of VSOACs, the ClC-3 hypothesis needs to be more thoroughly tested in a wider variety of cell types, and controversies regarding several key aspects of the regulation of native VSOACs need to be resolved (Duan & Hume, 2000). The effects of ClC-3 on endogenous VSOAC function have to date only been tested in bovine epithelial cells, where ClC-3 antisense treatment delayed the rate of activation of native VSOACs and reduced its amplitude by up to ∼60 % in a dose-dependent manner (Wang et al. 2000). It was suggested that the remaining component of VSOAC current, which appeared to be resistant to ClC-3 antisense, might be attributable to another protein, but it is not known if 48 h exposure to ClC-3 antisense causes complete elimination of endogenous ClC-3 protein levels.
In the present report, we describe a simple and specific alternative approach, which does not depend upon endogenous protein turnover rate, to selectively eliminate endogenous ClC-3 function. We first demonstrate that intracellular dialysis with a commercially available antibody which targets a specific 70 amino acid epitope on the carboxyl terminus of rat ClC-3 (anti-ClC-3 Ab) abolishes expressed IgpClC-3 in transfected NIH/3T3 cells, and then demonstrate that intracellular dialysis or injection of anti-ClC-3 Ab into native cardiac and vascular smooth muscle myocytes and Xenopus oocytes abolishes or significantly attenuates native VSOAC function. The 70 amino acid anti- ClC-3 Ab epitope appears to be unique among known proteins and also appears to be highly conserved across species. For example, gpClC-3 and Xenopus (x)ClC-3 have 69/70 and 61/70 identity to rat ClC-3 in this epitope, but considerably less sequence homology exists with related ClC proteins such as ClC-4 (45/70) and ClC-5 (47/70). This suggests that the anti-ClC-3 Ab used in our study will exhibit strong reactivity across species for ClC-3, but little cross-reactivity with ClC-4 and ClC-5. Indeed, anti-ClC-3 Ab has been shown to display no cross-reactivity to either ClC-4 or ClC-5 expressed in Xenopus oocytes (Schmieder et al. 1999) or to ClC-5 in mouse kidney (Luyckx et al. 1999). Thus, our data provide strong evidence that endogenous ClC-3 is a major molecular entity responsible for native VSOACs in native cardiac and vascular smooth muscle myocytes and Xenopus oocytes. Although it is possible that ClC-3 may instead function as a regulator of endogenous VSOACs, this possibility seems unlikely for a number of reasons: (1) ClC-3 is a member of a bonafide Cl− channel family, (2) expression of ClC-3 gives rise to outwardly rectifying Cl− currents with properties very similar to many native VSOACs, (3) ClC-3 antisense oligonucleotides reduce endogenous ClC-3 and the magnitude of native VSOACs (Wang et al. 2000), and (4) specific amino acid mutations alter the anion selectivity and rectification properties of expressed gpClC-3 (Duan et al. 1997b).
Anti-ClC-3 Ab should be a useful, selective experimental tool in future studies to establish unequivocally the relationship between endogenous ClC-3 expression and native VSOACs in a wide variety of different cells. It may also prove to be a particularly useful tool to help resolve controversies related to the regulation and physiological role of ClC-3 and native VSOACs in some cell types (Welsh et al. 2000) and the atypical biophysical and pharmacological properties recently attributed to ClC-3 expression in CHO cells (Li et al. 2000).
Acknowledgments
This work was supported by HL 49254, P2015581 and Fondecyt 3980043.
References
- Britton FC, Hatton WJ, Rossow CF, Duan D, Hume JR, Horowitz B. Molecular distribution of volume regulated chloride channels (ClC-2, ClC-3) in cardiac tissues. American Journal of Physiology. 2000;279:H2225–2233. doi: 10.1152/ajpheart.2000.279.5.H2225. [DOI] [PubMed] [Google Scholar]
- Clapham DE. The list of potential volume-sensitive chloride currents continues to swell. Journal of General Physiology. 1998;111:623–624. doi: 10.1085/jgp.111.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca-Prados M, Sanchez-Torres J, Peterson-Yantorno K, Civan MM. Association of ClC-3 channel with Cl− transport by human nonpigmented ciliary epithelial cells. Journal of Membrane Biology. 1996;150:197–208. doi: 10.1007/s002329900044. [DOI] [PubMed] [Google Scholar]
- Dick GM, Kong ID, Sanders KM. Effects of anion channel antagonists in canine colonic myocytes: comparative pharmacology of Cl−, Ca2+ and K+ currents. British Journal of Pharmacology. 1999;127:1819–1831. doi: 10.1038/sj.bjp.0702730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty JM, Miller AL, Langton PD. Non-specificity of chloride channel blockers in rat cerebral arteries: block of the L-type calcium channel. The Journal of Physiology. 1998;507:433–439. doi: 10.1111/j.1469-7793.1998.433bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Cowley S, Horowitz B, Hume JR. A serine residue in ClC-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell volume. Journal of General Physiology. 1999;113:57–70. doi: 10.1085/jgp.113.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Hume JR. NO and the regulation of VSOACs. The Journal of Physiology. 2000;528:2. doi: 10.1111/j.1469-7793.2000.t01-1-00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Hume JR, Nattel S. Evidence that outwardly rectifying Cl− channels underlie volume-regulated Cl− currents in heart. Circulation Research. 1997a;80:103–113. doi: 10.1161/01.res.80.1.103. [DOI] [PubMed] [Google Scholar]
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997b;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Properties of a Cl− current activated by cell swelling in rabbit portal vein vascular smooth muscle cells. American Journal of Physiology. 1998;275:H1524–1532. doi: 10.1152/ajpheart.1998.275.5.H1524. [DOI] [PubMed] [Google Scholar]
- Higgins CF, Weylandt KH, Nastrucci C, Sardini A, Linton K, Diaz M, Valverde MA. Swelling-activated chloride channels and their regulation by P-glycoprotein. In: Kozlowski R, editor. Chloride Channels. Oxford: Isis Medical Media Limited; 1999. pp. 35–46. [Google Scholar]
- Lamb FS, Clayton GH, Liu BX, Smith RL, Barna TJ, Schutte BC. Expression of CLCN voltage-gated chloride channel genes in human blood vessels. Journal of Molecular and Cell Cardiology. 1999;31:657–666. doi: 10.1006/jmcc.1998.0901. [DOI] [PubMed] [Google Scholar]
- Li X, Shimada K, Showalter LA, Weinman SA. Biophysical properties of ClC-3 differentiate it from swelling-activated chloride channels in CHO-K1 cells. Journal of Biological Chemistry. 2000;275:35994–35998. doi: 10.1074/jbc.M002712200. [DOI] [PubMed] [Google Scholar]
- Luyckx VA, Leclercq B, Dowland LK, Yu ASL. Diet-dependent hypercalciuria in transgenic mice with reduced ClC5 chloride channel expression. Proceedings of the National Academy of Sciences of the USA. 1999;96:12174–12179. doi: 10.1073/pnas.96.21.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastrucci C, Diaz M, Weylandt KH, Sardini A, Higgins CF. Expression of human ClC-3 in NIH3T3 fibroblasts is associated with volume-activated, outwardly rectifying chloride currents. The Journal of Physiology. 1999;517:P74–75P. [Google Scholar]
- Okada Y, Oiki S, Hazama A, Morishima S. Criteria for the molecular identification of the volume-sensitive outwardly rectifying Cl− channel. Journal of General Physiology. 1998;112:365–367. doi: 10.1085/jgp.112.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite CM, Britton F, Duan D, Horowitz B, Hume JR. Functional and molecular characterization of an endogenous volume-sensitive chloride current in Xenopus oocytes. Biophysical Journal. 1999;76:403A. [Google Scholar]
- Schmid A, Blum R, Krause E. Characterization of cell volume-sensitive chloride currents in freshly prepared and cultured pancreatic acinar cells from early postnatal rats. The Journal of Physiology. 1998;513:453–465. doi: 10.1111/j.1469-7793.1998.453bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder S, Lindenthal S, Idelson G, Ehrenfeld J. ClC-3 chloride channels distribution in mammalian tissue. The Journal of Physiology. 1999;517.P:15P. [Google Scholar]
- Shimada K, Li X, Xu G, Nowak DE, Showalter LA, Weinman SA. Expression and canalicular localization of two isoforms of the ClC-3 chloride channel from rat hepatocytes. American Journal of Physiology. 2000;279:G268–276. doi: 10.1152/ajpgi.2000.279.2.G268. [DOI] [PubMed] [Google Scholar]
- Shuba LM, Ogura T, Mcdonald TF. Kinetic evidence distinguishing volume-sensitive chloride current from other types in guinea-pig ventricular myocytes. The Journal of Physiology. 1996;491:69–80. doi: 10.1113/jphysiol.1996.sp021197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souktani R, Berdeaux A, Ghaleh B, Giudicelli JF, Guize L, Le Heuzey JY, Henry P. Induction of apoptosis using sphingolipids activates a chloride current in Xenopus laevis oocytes. American Journal of Physiology. 2000;279:C158–165. doi: 10.1152/ajpcell.2000.279.1.C158. [DOI] [PubMed] [Google Scholar]
- Strange K. Molecular identity of the outwardly rectifying, swelling-activated anion channel: time to reevaluate pICln. Journal of General Physiology. 1998;111:617–622. doi: 10.1085/jgp.111.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. American Journal of Physiology. 1996;270:C711–730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Valverde MA. CIC channels: leaving the dark ages on the verge of a new millennium. Current Opinion in Cell Biology. 1999;11:509–516. doi: 10.1016/s0955-0674(99)80074-x. [DOI] [PubMed] [Google Scholar]
- von Weikersthal SF, Barrand MA, Hladky SB. Functional and molecular characterization of a volume-sensitive chloride current in rat brain endothelial cells. The Journal of Physiology. 1999;516:75–84. doi: 10.1111/j.1469-7793.1999.075aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Chen L, Jacob TJ. The role of ClC-3 in volume-activated chloride currents and volume regulation in bovine epithelial cells demonstrated by antisense inhibition. The Journal of Physiology. 2000;524:63–75. doi: 10.1111/j.1469-7793.2000.t01-1-00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DG, Nelson MT, Eckman DM, Brayden JE. Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure. The Journal of Physiology. 2000;527:139–148. doi: 10.1111/j.1469-7793.2000.t01-1-00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki J, Britton F, Collier ML, Horowitz B, Hume JR. Regulation of recombinant cardiac CFTR chloride channels by protein kinase C. Biophysical Journal. 1999;76:1972–1987. doi: 10.1016/S0006-3495(99)77356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki J, Duan D, Janiak R, Kuenzli K, Horowitz B, Hume JR. Functional and molecular expression of volume-regulated chloride channels in canine vascular smooth muscle cells. The Journal of Physiology. 1998;507:729–736. doi: 10.1111/j.1469-7793.1998.729bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


