Abstract
A novel method of renal denervation was developed based on electro-coagulation of tissue containing most of the sympathetic fibres travelling towards the kidney. Kidney tissue noradrenaline was decreased to 4.7 % of the content measured in the contralateral innervated kidney when studied 3 days postdenervation.
The method was utilised in anaesthetised rats to examine the effects of denervation within the heretofore unexplored first 75 min period postdenervation. Sodium excretion (UNaV) increased significantly (+82 %, P < 0.03) over the 25-50 min after denervation. In a parallel group, with a lower baseline UNaV, there was also a significant increase in UNaV (+54 %, P < 0.03) within the first 25 min. The renal perfusion pressure was maintained at a constant value and the glomerular filtration rate did not change after denervation.
Renal cortical and medullary blood flows (CBF, MBF) were estimated as laser Doppler flux and medullary tissue ion concentration was estimated as electrical admittance (Y). Following denervation, in both groups CBF increased significantly within the first 25 min (+12 %, P < 0.01 and +8 %, P < 0.05, respectively) while MBF did not change or decreased slightly; Y did not change.
The data document the development of natriuresis within the first 25-50 min after denervation. The increase in CBF indicated that, prior to denervation, the cortical, but not medullary, circulation was under a tonic vasoconstrictor influence of the renal nerves. Such a dissociation of neural effects on the renal cortical vs. medullary vasculature has not been previously described.
The renal circulation, tubular reabsorption and release of renin are under multiple control by the renal nerves, hormones and paracrine active agents (DiBona & Kopp, 1997). Therefore, removal of the neural input by renal denervation, leading to inhibition of tubular transport (denervation natriuresis) and, under some circumstances, to a decrease in renin secretion and an increase in renal blood flow may be expected to be compensated, at least in part, by control systems working in the opposite direction. For instance, humoral vasoconstrictor agents could help offset the loss of vasoconstrictor tone provided by noradrenaline released from renal nerve endings. However, it is possible that some of the compensatory mechanisms do not come into play immediately after denervation but require some time to develop. For instance, the sensitivity of the tubulo-glomerular feedback has been reported to be decreased as a function of time when measured 60-240 min after acute denervation. (Thorup et al. 1995).
The standard technique of renal denervation necessitates considerable dissection around the kidney, cutting all visible nerve fibres of the renal pedicle and painting the renal artery with phenol solution in ethanol. This procedure is extended in time and traumatising; the conspicuous signs of trauma are alternate periods of constriction and dilatation of the renal artery. Therefore, the protocols in such acute studies allowed at least one hour postdenervation before starting measurements; by that time a new steady-state has presumably been established. An exception to this standard approach have been studies in which local anaesthetics were applied to the renal pedicle for partial reversible suppression of transmission in the renal nerves (Richardson et al. 1974; Grady & Bullivant, 1992).
In the search for a method to enable assessment of the early effects of renal denervation, before potential compensatory mechanisms have come into play, we have developed a technique enabling rapid denervation by a procedure that is significantly less traumatising than the standard method. The tissue containing most of nerve fibres entering the renal hilus was surrounded with a thin flexible wire loop with minimal prior dissection. After baseline measurements of renal function, a high frequency current was applied to the wire and the tissue was cut by electro-coagulation; renal haemodynamic measurements (laser Doppler) were not interrupted and urine collection was started almost immediately after denervation. In the present study, this method was used to examine the early effects of denervation on renal circulation and renal excretion.
METHODS
Experiments were designed to examine renal haemodynamics, diuresis, natriuresis, and total ion concentration in the medullary tissue before and directly after, denervation of the left kidney using a technique newly developed developed in our laboratory. The experimental procedures were approved by the Ethical Committee of the Medical Research Centre, Polish Academy of Sciences.
Preparations and surgical procedures
Male Wistar rats were fed a standard pellet diet (Altromin GmbH, Lage, Germany) and had free access to water. The animals, weighing 280-340 g, were anaesthetised with sodium thiopenthal (Biochemie GmbH, Vienna, Austria, 100 mg kg−1, i.p.), which provided stable anaesthesia for at least the three hours needed to complete an experiment. A polyethylene tube was placed in the trachea to ensure free airways. The rats were placed on a heated surgery table to maintain rectal temperature at about 37°C. The femoral vein was cannulated for fluid infusions and the femoral artery was used for aortic blood pressure measurement and blood sampling. The left kidney was exposed via a flank incision, immobilised in a plastic holder and the ureter was cannulated to permit timed urine collections.
Subsequently, preparations were made to enable rapid renal denervation later during an experiment. Although the kidney had been dissected free from the adjacent tissue, special care was taken to leave intact the fat and connective tissue cephalad to the junction of the renal artery and the aorta. A loop of stainless steel wire, 0.1 mm in diameter, was placed around this tissue. Previous dissection studies under a dissecting microscope have consistently shown that most nerve fibres travelling from the coeliac ganglion toward the renal pedicle, and many others whose origin could not be clearly defined, also converging towards the kidney, are located in this area; we have practiced encompassing all these fibres within the wire loop without actual dissection. Both ends of the loop were threaded through a segment of thick-walled polyethylene tubing, about 0.6 mm inner diameter, and left loose. In order to effect the denervation, the loop was tightened and fixed with a bulldog clamp; immediately thereafter 0.15 ml of 1% novocaine solution was injected through the tubing, twice at 20 s intervals. This was done in order to minimize the potential trauma due to subsequent cutting of the nerves. The trauma could include a momentary efferent and afferent renal nerve stimulation leading to disturbances of intrarenal or systemic haemodynamics. The tissue within the loop, including renal nerve fibres, was electro-coagulated: a 5 MHz current generated by a neurosurgical cautery device (Famed, Warsaw, Poland) was applied for 5-10 s until the tissue was cut completely and the loop could be removed.
In all experiments a screw-controlled snare was placed around the aorta above the renal arteries. The snare was slightly tightened to lower blood pressure distal to the constriction site by 4-8 mmHg. Later during experiments the snare could be released or tightened as needed, and the mean blood pressure below the snare, equivalent to renal perfusion pressure (RPP) was maintained constant. A set of two probes used for measurement of inner medullary blood flow (laser Doppler flux, MBF) and tissue electrical admittance (Y) was inserted into the kidney and another laser Doppler probe, for measurement of cortical blood flow (CBF), was placed on the kidney surface (see below: Measurement of intrarenal blood).
During surgical procedures 3 % bovine serum albumin was infused i.v. at 2.4 ml h−1 to preserve plasma volume. Directly after insertion of the MBF/Y measuring set, this infusion was replaced by Ringer solution, 3.2 ml h−1. In one series (protocol II, see below) this solution contained [methoxy-3H] inulin given at a rate of 6 μC h−1, for measurement of the glomerular filtration rate (GFR). The details of clearance procedures as well as analytical techniques for plasma and urine osmolality, sodium, and tritiated inulin have been described previously (Kompanowska-Jezierska et al. 1994). After completion of the experimental protocols the animals were killed by an intravenous overdose of sodium pentobarbitone.
Experimental protocols
Protocol I
(n = 11). In addition to the surgical preparations described earlier, the urinary bladder was cannulated and left ureteral and right kidney (bladder) urine were collected separately. Two control 25 min bilateral urine collections were made, the left kidney was then denervated, urine collection was resumed and three further 25 min bilateral collection periods were made. Throughout the experiment RPP was maintained constant at about 120 mmHg, and CBF, MBF and medullary tissue Y were continuously recorded.
Protocol II
(n = 8). Experiments were conducted 2 months later than the other studies. Urine was collected from the left kidney only (the bladder was not cannulated). Since the rats’ baseline aortic blood pressure was lower than in the other groups, RPP was initially set at 110 instead of 120 mmHg so that adjustments needed to keep it constant could still be made as described earlier. The sequence of control and experimental urine collections was as in protocol I. In this series, tritiated inulin was infused and GFR was determined.
Protocol III
(left renal sham denervation, n = 7). The procedure was as in Protocol I except that the wire loop normally used for denervation was placed around the fat and connective tissue located caudal to the aortic-renal artery junction and this tissue was later electro-coagulated. Urine was separately collected from the left and the right kidney and RPP was maintained at 120 mmHg.
Protocol IV
(n = 6). In this study, the sequential effects of novocaine and electro-coagulation on CBF were examined. In rats destined for denervation and later determination of renal noradrenaline content, prior to denervation two surface laser Doppler probes were placed non-invasively on the dorsal surface of the left kidney: PF407 type used in all studies (optical fibre separation 0.15 mm) and PF416 type (separation: 0.25 mm); the latter probe provides a deeper penetration of the light beam i.e. measures signals within the cortex at a slightly greater depth. Initially, CBF was recorded by the two probes for about 10 min and thereafter novocaine was applied to the tissue containing the nerve fibres via the tubing segment housing the wire loop. Subsequently, about 20-25 min were allowed to observe the effect of novocaine itself on CBF before electro-coagulation was performed. This was followed by 10-15 min observation of CBF after electro-coagulation.
Measurements of intrarenal blood flow and tissue electrical admittance
Medullary blood flow (MBF, laser Doppler flux) was measured using a PF402 needle probe inserted into the kidney to the depth of the inner medulla and connected to a PeriFlux 4001 flowmeter (Perimed, Jarfalla, Sweden). Tissue electrical admittance (Y, an index of total ion concentration in medullary interstitium) was measured at about the same depth, between the tips of the stainless steel cannula housing the optical fibres of the probe and of a parallel iridium-platinum needle, both forming an admittance cell connected to a conductance meter measuring at a frequency of 24 kHz (Sadowski et al. 1997). Another laser Doppler probe (PF407) was placed on the kidney surface for measurement of superficial cortical blood flow. The laser Doppler flux values (a product of the number of blood cells moving and their mean velocity, within an area less than 1 mm3 beneath the tip of the probes) were expressed in arbitrary perfusion units (PU) or in volts of the analogue output (1000 PU = 10 V). Thus, only relative changes were measured but the calibration procedure allowed the results to be compared between animals.
Noradrenaline content of renal tissue
For verification of the effectiveness of the method described above, the noradrenaline content of renal tissue was determined 3 days after denervation. Six rats were anaesthetised with intraperitoneal sodium pentobarbitone, 50 mg (kg body wt)−1 with small intravenous supplements as required. The left kidney was exposed and a short non-invasive experiment consisting of measurements of renal cortical blood flow before and after denervation was performed (Protocol IV). Left renal denervation was performed as described above. Subsequently, ampicillin powder, about 100 mg (kg body weight)−1, was applied directly into the abdomen and the flank wound was closed. Ringer solution (5 ml) was administered subcutaneously and, for postoperative analgesia, metamizole sodium (20 mg kg−1) was given. On the third day postdenervation, animals were re-anaesthetised, both kidneys were removed through a midline abdominal incision, cut to ∼0.3 g pieces, frozen on dry ice, weighed, and stored at-80°C until analysed. The tissue was deproteinized with a mixture of perchloric acid and reduced glutathione, and homogenized. A part of the homogenate was used for determination of protein content, the rest was centrifuged and the supernatant, after appropriate dilution, was analysed for noradrenaline using a radioenzymatic method (Johnson et al. 1980).
Statistics
The significance of trends was first examined by repeat measurement analysis of variance and then differences between means were tested by Student's t test for dependent variables. The standard error of mean (s.e.m.) was used as a measure of data dispersion.
RESULTS
The mean tissue noradrenaline concentration measured 72 h after left renal denervation in six rats was 3.5 ± 0.7 pmol (mg protein)−1 in the denervated kidney which was less than 5 % of the 88.0 ± 23.6 pmol (mg protein)−1 recorded in the contralateral innervated kidney (P < 0.001, paired Student's t test).
Urine osmolality in these studies ranged from 950 to 1350 mosmol (kg H2O)−1 and was not significantly changed by denervation or sham denervation.
It can be seen in Fig. 1 that following the denervation there was a progressive increase in UNaV of the denervated kidney beginning in the first postdenervation period, which became significant at 25-50 min after denervation and further increased in the third postdenervation period. The changes in urine flow (V) (Fig. 1) roughly paralleled those of UNaV. There was no change in UNaV and V in either the sham-denervated kidneys or in the contralateral kidneys of the group of rats subjected to the renal denervation. Blood pressure remained at a constant level throughout the observation period. Figure 2 presents data from an additional group (protocol II) in which left kidney denervation was performed as in protocol I but in which the baseline conditions were slightly different. Renal perfusion pressure was set 10 mmHg below that in protocol I, and the overall surgical trauma was slightly less (the bladder was not cannulated). In this group, the baseline UNaV was approximately one half of that measured in protocol I studies, but there was a significant increase in UNaV and V within the first 25 min after denervation, i.e. earlier than in the rats subjected to protocol I. It was evident that there was no change in GFR during experiments. There was also no change in blood pressure throughout the period of study.
Figure 1. The acute effect of renal denervation (n = 11) or sham denervation (n = 7) on sodium excretion (UNaV) and urine flow (V).
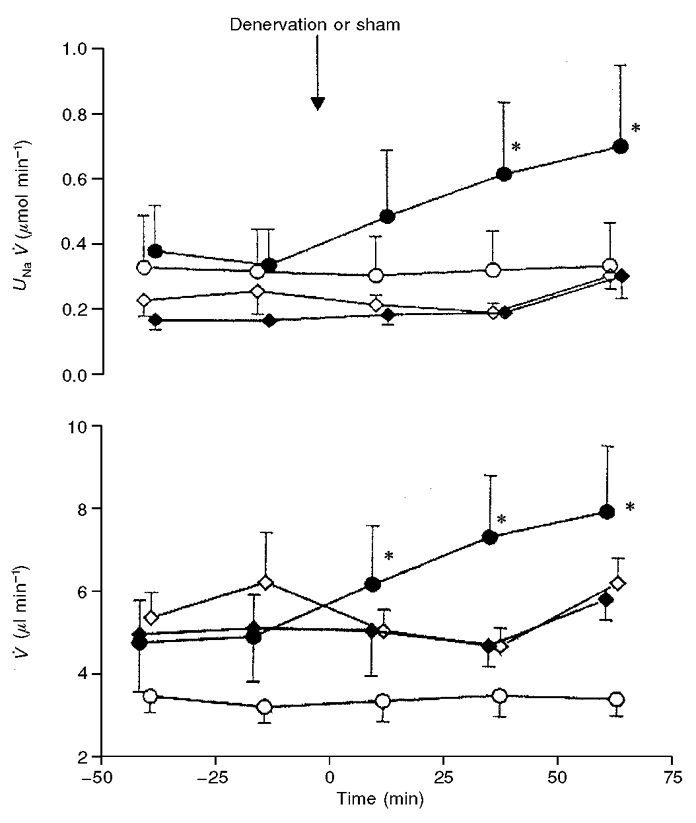
The data are means ±s.e.m. for the denervated (○) or contralateral (○) kidney (n = 11), and for the sham denervated (♦) or contralateral (⋄) kidney (n = 7). In all experiments the renal perfusion pressure was maintained constant at about 120 mmHg. *Significantly different from the predenervation period (second control period) at P < 0.05 or less.
Figure 2. The acute effect of left renal denervation on ipsilateral sodium excretion (UNaV), urine flow (V) and glomerular filtration rate (GFR) in rats with renal perfusion pressure maintained constant at ≈110 mmHg.
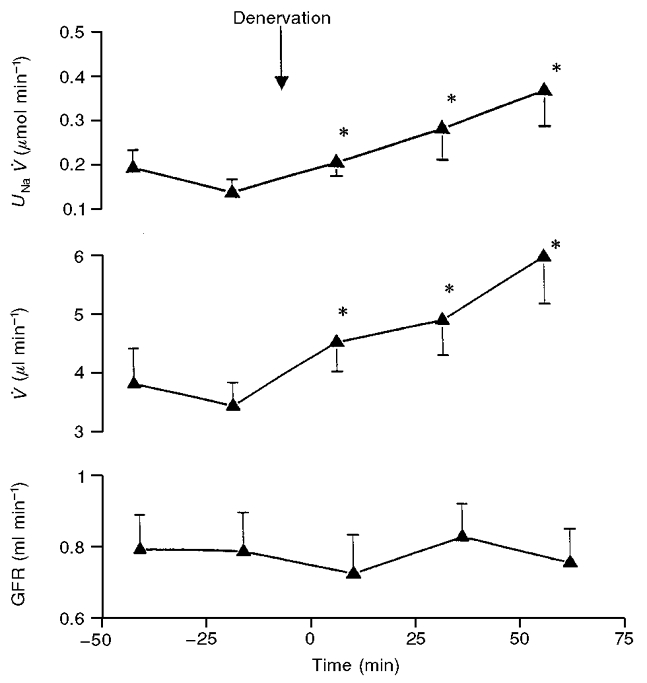
The data are means ±s.e.m., n = 8. *Significantly different from the predenervation period (second control period) at P < 0.05 or less.r
The medullary tissue admittance (Y), an index of interstitial NaCl concentration, was not significantly affected over the 75 min following renal denervation. In the group of rats subjected to protocol I the control value was 964 ± 49 μS, compared with 984 ± 40 μS measured 75 min after denervation (a 2 % difference), whilst in the group undergoing protocol II these values were 882 ± 35 and 887 ± 38 μS, respectively (less than 1 % difference). There was also no change in medullary tissue admittance in the animals in which sham denervation was performed.
Figure 3 shows the effects of denervation on CBF and MBF in the two renal denervation groups (protocols I and II) and in the sham-denervated group (protocol III). In both denervated groups, CBF increased significantly (P < 0.05) within 25 min or less and remained elevated for the subsequent 50 min; sham denervation had no effect on CBF. MBF was unchanged following denervation in both protocol I and II. Indeed, MBF tended to decrease in protocol II and at 50-75 min was even slightly but significantly (P < 0.05) lower than control (Fig. 3). However, a similar tendency to decrease over the time period of observation was also seen in the sham denervation experiments. In all these protocols, blood pressure remained at an unchanged value over the period of experimentation.
Figure 3. The acute effect of left renal denervation or sham denervation on cortical (CBF) and medullary (MBF) blood flow (laser Doppler flux).
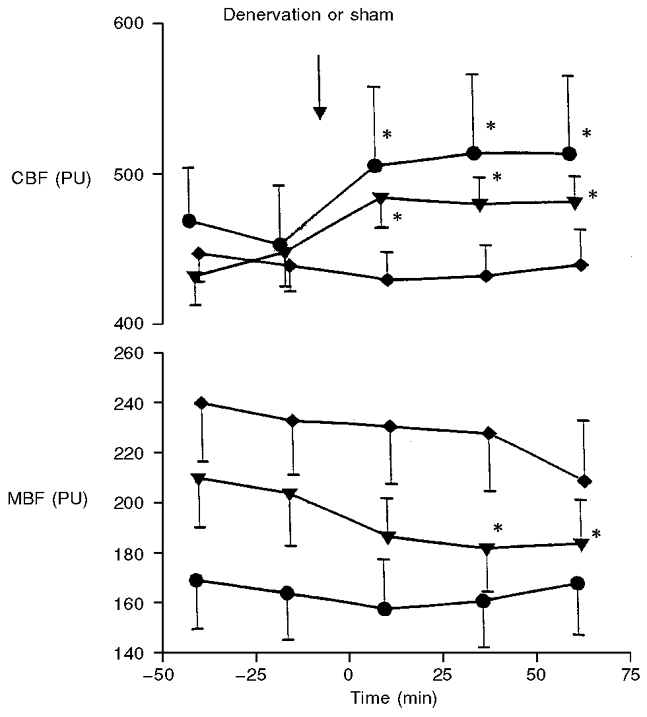
The data points represent means of the values averaged for simultaneous 25 min urine collection periods; symbols refer to denervation studies at renal perfusion pressure maintained constant at 120 mmHg (○), 110 mmHg (▾), and sham denervation at 120 mmHg (♦). *Significantly different from the predenervation period (second control period) at P < 0.05 or less.
Additional observations on the effect of inhibition of renal nerve activity were made in studies in which application of novocaine was followed by a 20-25 min observation of CBF before electro-coagulation was performed in the usual way (Table 1, Fig. 4). Novocaine caused a significant increase in CBF recorded both by the surface laser Doppler probe, used in all the definitive studies (PF407) and by the surface probe measuring slightly deeper in the cortex (PF416). However, in most cases, an initial increase in CBF was seen as soon as novocaine was applied, but then it tended to decrease after about 10 min. The subsequent electro-coagulation caused a further increase in CBF which started after about 8 min and was complete at around 18 min. The maximum rise in CBF after electro-coagulation was significantly higher (P < 0.05) than that achieved after application of novocaine. The mean overall increase after novocaine plus electro-coagulation was approximately 20 % with the PF407 probe and 15 % with the PF416 probe.
Table 1.
Effects of application of novocaine to the tissue containing renal nerve fibres, and of subsequent electro–coagulation of this tissue, on the cortical blood flow
| Control | Novocaine | Coagulation | |
|---|---|---|---|
| Probe PF 407 | 537 ± 22 | 575 ± 14* | 617 ± 14† |
| Probe PF416 | 508 ± 36 | 546 ± 30* | 610 ± 21† |
Cortical blood flow (laser Doppler flux, perfusion units) was measured on kidney surface by a standard PF 407 probe and a PF 416 probe that provided slightly deeper penetration of the laser light beam. Means ± s. e. m., n = 6.
Significantly different from control value for the same probe
significantly different from the preceding novocaine value at P < 0.05 or less (tested by paired Student's t test).
Figure 4. A record of the effects of novocaine application and subsequent electro-coagulation of the tissue encompassing most renal nerves on the cortical blood flow.

Cortical Blood Flow (CBF, laser Doppler flux in perfusion units) was measured by two surface probes: the standard PF407 probe and the PF416 probe characterized by a slightly deeper tissue penetration.
DISCUSSION
The effectiveness of our method of renal denervation was validated by determination of tissue noradrenaline content. The degree of depletion, 5 % of the concentration measured in the contralateral innervated kidney, compares satisfactorily with the data reported by authors using a standard method of renal denervation. A precise comparison would be difficult because, over the years, methods used for noradrenaline determination have varied and the results have been expressed differently: either per gram of tissue or per gram of tissue protein. In two more recent studies using the radioenzymatic method, as in our study, noradrenaline concentration after denervation equalled less than 10 % (Fernandez-Repollet et al. 1985) and less than 2 % (Greenberg et al. 1993) of the content measured in the innervated kidney.
Acute denervation effects on renal excretion and GFR
Denervation natriuresis is a well described and reproducible phenomenon, dependent on exclusion of the neural stimulatory influence on tubular reabsorption and one of the major goals of the present study was to establish its earliest point of onset. Therefore, we attempted to avoid circumstances which could alter UNaV independent of changes in renal sympathetic nerve activity. Even modest changes in RPP can affect UNaV and it is noteworthy that in our studies at RPP of 120 mmHg (protocol I) baseline UNaV was roughly double that measured at 110 mmHg (protocol II). To take into account this phenomenon, we maintained RPP precisely constant throughout experiments.
The data showed that the natriuresis began within the first 25 min and was fully expressed by 50 min after denervation, which was a time frame that had not been accessible to study using the more conventional methods of renal denervation. The different consecutive phases of classical denervation, such as severing nerve fibres in the hilus, stripping the adventitia off the renal artery and painting the vessel with phenol solution, result in alternate periods of constriction and dilatation of the renal artery which were of variable duration. It is most likely that together these manoeuvres would be associated with intrarenal vasoconstriction and vasodilatation, and fluctuations of GFR.
Here the excretion of sodium and water increased progressively throughout the 75 min observation period. It was of interest that there was no decrease in UNaV on the contralateral side, as reported by a number of workers and described as a postdenervation reno-renal reflex enhancement of the innervated kidney function (DiBona & Kopp, 1997). One possible explanation may be that there is a delay in the reflex enhancement of tubular sodium reabsorption in the innervated kidney and that the effect did not become apparent over the time course of our experiments.
Since the denervation natriuresis was not associated with any alteration in GFR, the early progressive increase in sodium and water excretion was most likely due to a progressive postdenervation decrease in tubular sodium reabsorption. This postdenervation decrease in sodium reabsorption in the proximal tubule has been documented by many groups of workers (DiBona & Kopp, 1997). This observation would also accord with early morphological data indicating the presence of nerve endings in contact with the tubular cells of the thick ascending limb of Henle's loop. Moreover, there is functional evidence from tubule microperfusion experiments of an increase in sodium transport in this segment after renal nerve stimulation (DiBona & Sawin, 1982) and a decrease in sodium transport following renal denervation (Bencsath et al. 1985). The action of the renal nerves on the thick ascending limb of Henle's loop could manifest itself as a decrease in electrolyte concentration in the medullary interstitium and, indeed, one study reported a postdenervation decrease in sodium and total solute concentration in tissue slices of the renal medulla (Kurkus et al. 1980). Therefore, in the present study we measured electrical admittance of medullary tissue (Y) as an index of local ion concentration and NaCl transport in medullary loops (Sadowski et al. 1997). We found no change in medullary tissue Y, indicating no distinct effect of renal denervation (protocols I and II) or sham denervation on salt concentration in the medullary interstitium. The reasons underlying this may be straightforward. The denervation would have reduced proximal tubular reabsorption and thereby increased delivery of fluid to the loop. Because the transport in this segment is clearly flow dependent, it is possible that any intrinsic decrease in NaCl transport in the ascending limb of Henle's loop resulting from denervation would be, at least in part, offset by an increase in transport related to a greater inflow to this segment.
Acute effects of denervation on renal cortical and medullary blood flow (CBF, MBF)
The evidence from a range of previous investigations has shown that in conscious animals renal denervation does not cause any increase in total renal blood flow, which was probably due to low baseline renal sympathetic nerve activity. In animals that were anaesthetised and/or subjected to a surgical procedure, a modest increase or no change in blood flow has been reported. The actual response would depend, presumably, on the animal species and various features of the experimental conditions which determined the level of predenervation renal nerve activity (DiBona & Kopp, 1997).
The classical methodology makes uninterrupted measurement of renal circulation before and after denervation impracticable because of the mechanical interventions and disturbances. In this study both CBF (which can be taken as a reliable index of the total renal blood flow) and MBF were measured continuously and the data before and immediately after denervation could be compared directly. The clear postdenervation increase in CBF (but not in MBF) observed under our experimental conditions suggested that the cortical circulation was under a tonic vasoconstrictor influence of the renal sympathetic nerves. There seems to be no doubt that the increase in renal cortical circulation after the electro-coagulation procedure was a consequence of the interruption of the renal sympathetic nerves. This interpretation was further supported by the studies in which novocaine application and electro-coagulation were separated in time. The CBF responses to pharmacological blockade and to surgical denervation were similar although the former were less pronounced and transient. In agreement with earlier studies (Richardson et al. 1974; Grady & Bullivant, 1992), the local anaesthetic must have partially interrupted neural traffic to the kidney. As the action of novocaine is short lasting, this would explain why the increase in CBF began to subside after about 10 min. The subsequent electro-coagulation caused a further significant and lasting increase in CBF (Table 1, Fig. 4). Indirectly, the clear increase in CBF after application of the novocaine to the area encompassed by the wire loop indicated that the tissue which was electro-coagulated contained most of renal nerve fibres. A second fibreoptic laser Doppler probe (PF416) was used in this study, which provided a greater penetration of the laser beam than that obtained with the standard PF407 probe used in the other experimental series. The increases in CBF measured with the two probes after novocaine application were comparable, which suggests that the increase in CBF was not confined to the most superficial layer of the cortex.
It was interesting that MBF did not increase after denervation, indeed, a trend for a reduction and, ultimately, a slight significant decrease was observed in one group of renal-denervated rats (Fig. 3). One possible explanation might be that the decrease in MBF could have resulted from a decrease in RPP. It is recognised that within the medulla autoregulation of the renal blood flow is not as efficient as in the cortex (Pallone et al. 1990). Accordingly, it could be argued that even a moderate decrease in RPP could have caused a depression of medullary circulation and offset any increase in MBF in response to renal denervation. However, this would not apply in the present experiments as RPP was not allowed to vary by more than 1-2 mmHg. A more likely reason for a decrease in MBF would be some deterioration of medullary circulation with time, which was presumably more pronounced in the group of rats whose RPP was maintained at a lower level (110 vs. 120 mmHg). It was apparent that the deterioration in the medullary circulation was not alleviated by renal denervation as the decreasing trend was similar to that observed in the sham-denervated kidney (Fig. 3).
A possible dissociation of the effects of renal sympathetic nerve activity on the renal cortical and medullary circulations had been proposed previously but the findings of earlier studies were questioned because of an inadequacy of the methods used for the separate measurement of blood flow in the two kidney zones (DiBona & Kopp, 1997). More recently, both afferent and efferent arterioles of juxtamedullary glomeruli in the hydronephrotic kidney preparation (a non-filtering kidney with reduced blood flow) were found to constrict after renal nerve stimulation (Chen & Fleming, 1993), compatible with a role for renal nerves in the control of medullary circulation. On the other hand, laser Doppler measurements of cortical and papillary blood flow in anaesthetised rats showed that the blood flow responses to renal nerve stimulation were much smaller in the papilla where a 4 % reduction was seen only with stimulation at the highest frequency rate of 5 Hz (Rudenstam et al. 1995). These data are consistent with the present denervation studies which suggested that the medullary circulation in anaesthetised rats was not under the tonic influence of the renal nerves. Overall, there is increasing evidence that control mechanisms of the medullary and cortical circulations may differ significantly.
In summary, we have developed a novel approach for a rapid renal denervation in the rat, which is less invasive but as effective as the classical approach, as demonstrated by the marked reduction in tissue noradrenaline level. Owing to the reduced invasiveness of our procedure, measurements of renal haemodynamics and renal excretion were not interrupted at the moment of denervation and protocols could be designed whereby an experimental intervention and measurement (including suitable controls) could be made before and then after denervation, within the same experiment in one animal. It was found that the responses of the cortical and medullary circulation were dissociated: CBF increased almost immediately after denervation whilst MBF did not. In addition, we demonstrated that a natriuresis developed within about 0.5 h after denervation, without changes in GFR.
Acknowledgments
The study was supported in part by a Polish-British joint project grant (Royal Society, UK). We are greatly indebted to Dr Józef Langfort for help in catecholamine determination in the renal tissue.
References
- Bencsath P, Szenasi G, Takacs L. Water and electrolyte transport in Henle's loop and distal tubule after renal sympathectomy in the rat. American Journal of Physiology. 1985;249:F308–314. doi: 10.1152/ajprenal.1985.249.2.F308. [DOI] [PubMed] [Google Scholar]
- Chen J, Fleming JT. Juxtamedullary afferent and efferent arterioles constrict to renal nerve stimulation. Kidney International. 1993;44:684–691. doi: 10.1038/ki.1993.301. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Kopp U. Neural control of renal function. Physiological Reviews. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Sawin LL. Effect of renal nerve stimulation on NaCl and H2O transport in Henle's loop of the rats. American Journal of Physiology. 1982;243:F576–580. doi: 10.1152/ajprenal.1982.243.6.F576. [DOI] [PubMed] [Google Scholar]
- Fernandez-Repollet E, Silva-Netto CR, Colindres RE, Gottschalk CW. Role of renal nerves in maintaining sodium balance in unrestrained conscious rats. American Journal of Physiology. 1985;249:F819–826. doi: 10.1152/ajprenal.1985.249.6.F819. [DOI] [PubMed] [Google Scholar]
- Grady HC, Bullivant EMA. Renal blood flow varies during normal activity in conscious unrestrained rats. American Journal of Physiology. 1992;262:R926–932. doi: 10.1152/ajpregu.1992.262.5.R926. [DOI] [PubMed] [Google Scholar]
- Greenberg SG, Enders C, Osborn JL. Renal nerves affect rate of achieving sodium balance in spontaneously hypertensive rats. Hypertension. 1993;22:1–8. doi: 10.1161/01.hyp.22.1.1. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Kupiecki RM, Baker CA. Single isotope derivative (radioenzymatic) method in measurement of catecholamines. Metabolism. 1980;29(suppl. 1):1106–1113. doi: 10.1016/0026-0495(80)90018-9. [DOI] [PubMed] [Google Scholar]
- Kompanowska-Jezierska E, Dobrowolski L, Sadowski J. Role of vasopressin V2 receptors in modulation of the renal cortico-papillary NaCl gradient. Pflügers Archiv. 1994;428:410–414. doi: 10.1007/BF00724525. [DOI] [PubMed] [Google Scholar]
- Kurkus J, Sadowski J, Gellert R, Krus S. Influence of renal denervation on urine concentration in awake anaesthetized dogs. European Journal of Clinical Investigation. 1980;10:463–467. doi: 10.1111/j.1365-2362.1980.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Robertson CR, Jamison RL. Renal medullary circulation. Physiological Reviews. 1990;70:885–920. doi: 10.1152/physrev.1990.70.3.885. [DOI] [PubMed] [Google Scholar]
- Richardson D, Stella A, Leonetti G, Bertorelli A, Zanchetti A. Mechanisms of renal release of renin by electrical stimulation of the brainstem in the cat. Circulation Research. 1974;34:425–434. doi: 10.1161/01.res.34.4.425. [DOI] [PubMed] [Google Scholar]
- Rudenstam J, Bergstrom G, Taghipour K, Gothberg G, Karlstrom G. Efferent renal sympathetic nerve stimulation in vivo. Effects on regional renal haemodynamics in the Wistar rat, studied by laser-Doppler technique. Acta Physiologica Scandinavica. 1995;154:387–394. doi: 10.1111/j.1748-1716.1995.tb09922.x. [DOI] [PubMed] [Google Scholar]
- Sadowski J, Kompanowska-Jezierska E, Dobrowolski L, Walkowska A, Badzynska B. Simultaneous recording of tissue ion content and blood flow in rat renal medulla: evidence on interdependence. American Journal of Physiology. 1997;273:F658–662. doi: 10.1152/ajprenal.1997.273.4.F658. [DOI] [PubMed] [Google Scholar]
- Thorup C, Kurkus J, Morsing P, Persson A E G. Acute renal denervation causes time-dependent resetting of the tubuloglomerular feedback mechanism. Acta Physiologica Scandinavica. 1995;153:43–49. doi: 10.1111/j.1748-1716.1995.tb09832.x. [DOI] [PubMed] [Google Scholar]


